Oxysterol-Induced Inflammation in Human Diseases: Strategies for Treatment with Natural Compounds and Synthetic Molecules
Abstract
1. Introduction
1.1. Oxysterols: Origins and Biogenesis
1.1.1. Dietary Origin of Oxysterols
1.1.2. Enzymatic Formation of Oxysterols
1.1.3. Formation of Oxysterols by Autoxidation
2. Involvement of Oxysterols in Inflammatory Human Diseases
2.1. Cardiovascular Diseases
2.2. Neurodegenerative Diseases
2.2.1. Alzheimer’s Disease
2.2.2. Parkinson’s Disease
2.2.3. Multiple Sclerosis
2.2.4. Amyotrophic Lateral Sclerosis
2.2.5. X-Linked Adrenoleukodystrophy
2.2.6. Autism Spectrum Disorder (ASD)
2.3. Eye Diseases
2.3.1. Cataract
2.3.2. Age-Related Macular Degeneration (AMD)
2.4. Osteoporosis
2.5. Sarcopenia
2.6. Bowel Diseases
2.7. Lung Diseases
2.7.1. Tuberculosis
2.7.2. SARS-CoV-2 and Respiratory Diseases
2.7.3. Silicosis
2.8. Behcet’s Disease
3. Mechanisms Associated with Oxysterol-Induced Inflammation
3.1. Cytokinic/TLR4 Pathway
3.1.1. Membrane Receptors
3.1.2. Nuclear Receptors
3.2. Non-Cytokinic Pathways: Leukotrienes, and Prostaglandins
3.3. Contributions of Calcium and Potassium to Oxysterol-Induced Inflammation
3.3.1. Calcium Channels
3.3.2. Potassium Channel
4. Prevention of Oxysterol-Induced Inflammation
4.1. Natural Molecules
4.1.1. Tocopherols
4.1.2. Carotenoids and Other Terpenoids
4.1.3. Phenolic Compounds
4.1.4. Betalains
4.2. Plant Extracts
4.3. Edible Oils and Fatty Acids
4.3.1. Edible Oils
4.3.2. Fatty Acids
4.4. Probiotics and Microbial Enzymes
4.5. Synthetic Molecules
4.5.1. Monomethyl Fumarate and Dimethyl Fumarate
4.5.2. UDP-003
4.5.3. Sulfo-N-Succinimidyl Oleate
4.5.4. Other Molecules
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canzoneri, F.; Leoni, V.; Rosso, G.; Risso, D.; Menta, R.; Poli, G. Oxysterols as reliable markers of quality and safety in cholesterol containing food ingredients and products. Front. Nutr. 2022, 9, 853460. [Google Scholar] [CrossRef] [PubMed]
- Ghzaiel, I.; Sassi, K.; Zarrouk, A.; Ghosh, S.; Dias, I.H.; Nury, T.; Ksila, M.; Essadek, S.; Joutey, M.T.; Brahmi, F.; et al. Sources of 7-ketocholesterol, metabolism and inactivation strategies: Food and biomedical applications. Redox Exp. Med. 2022, 2022, R40–R56. [Google Scholar] [CrossRef]
- Brown, A.J.; Sharpe, L.J.; Rogers, M.J. Oxysterols: From physiological tuners to pharmacological opportunities. Br. J. Pharmacol. 2021, 178, 3089–3103. [Google Scholar] [CrossRef] [PubMed]
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef]
- Poli, G.; Leoni, V.; Biasi, F.; Canzoneri, F.; Risso, D.; Menta, R. Oxysterols: From redox bench to industry. Redox Biol. 2022, 49, 102220. [Google Scholar] [CrossRef]
- Boselli, E.; Velazco, V.; Caboni, M.F.; Lercker, G. Pressurized liquid extraction of lipids for the determination of oxysterols in egg-containing food. J. Chromatogr. A 2001, 917, 239–244. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Sottero, B.; Poli, G. Oxidized products of cholesterol: Dietary and metabolic origin, and proatherosclerotic effects. J. Nutr. Biochem. 2002, 13, 700–710. [Google Scholar] [CrossRef]
- Iuliano, L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem. Phys. Lipids 2011, 164, 457–468. [Google Scholar] [CrossRef]
- Sabolová, M.; Pohořelá, B.; Fišnar, J.; Kouřimská, L.; Chrpová, D.; Pánek, J. Formation of oxysterols during thermal processing and frozen storage of cooked minced meat. J. Sci. Food Agric. 2017, 97, 5092–5099. [Google Scholar] [CrossRef]
- Nguyen, C.; Saint-Pol, J.; Dib, S.; Pot, C.; Gosselet, F. 25-Hydroxycholesterol in health and diseases. J. Lipid Res. 2024, 65, 100486. [Google Scholar] [CrossRef]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Vine, D.; Croft, K.; Beilin, L.; Mamo, J. Absorption of dietary cholesterol oxidation products and incorporation into rat lymph chylomicrons. Lipids 1997, 32, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Brzeska, M.; Szymczyk, K.; Szterk, A. Current knowledge about oxysterols: A review. J. Food Sci. 2016, 81, R2299–R2308. [Google Scholar] [CrossRef] [PubMed]
- Addis, P.B.; Emanuel, H.A.; Bergmann, S.D.; Zavoral, J.H. Capillary GC quantification of cholesterol oxidation products in plasma lipoproteins of fasted humans. Free Radic. Biol. Med. 1989, 7, 179–182. [Google Scholar] [CrossRef]
- Viens, L.; Athias, A.; Lizard, G.; Simard, G.; Gueldry, S.; Braschi, S.; Gambert, P.; Lallemant, C.; Lagrost, L. Effect of lipid transfer activity and lipolysis on low density lipoprotein (LDL) oxidizability: Evidence for lipolysis-generated non-esterified fatty acids as inhibitors of LDL oxidation. J. Lipid Res. 1996, 37, 2179–2192. [Google Scholar] [CrossRef]
- Babiker, A.; Diczfalusy, U. Transport of side-chain oxidized oxysterols in the human circulation. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1998, 1392, 333–339. [Google Scholar] [CrossRef]
- Brown, R.B. Phospholipid packing defects and oxysterols in atherosclerosis: Dietary prevention and the French paradox. Biochimie 2019, 167, 145–151. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Yutuc, E.; Abdel-Khalik, J.; Crick, P.J.; Hearn, T.; Dickson, A.; Bigger, B.W.; Wu, T.H.-Y.; Goenka, A.; Ghosh, A.; et al. Metabolism of non-enzymatically derived oxysterols: Clues from sterol metabolic disorders. Free Radic. Biol. Med. 2019, 144, 124–133. [Google Scholar] [CrossRef]
- Walker, B.R.; Andrew, R. Tissue production of cortisol by 11β-hydroxysteroid dehydrogenase type 1 and metabolic disease. Ann. N. Y. Acad. Sci. 2006, 1083, 165–184. [Google Scholar] [CrossRef]
- Nury, T.; Samadi, M.; Zarrouk, A.; Riedinger, J.M.; Lizard, G. Improved synthesis and in vitro evaluation of the cytotoxic profile of oxysterols oxidized at C4 (4α-and 4β-hydroxycholesterol) and C7 (7-ketocholesterol, 7α-and 7β-hydroxycholesterol) on cells of the central nervous system. Eur. J. Med. Chem. 2013, 70, 558–567. [Google Scholar] [CrossRef]
- Meaney, S. Epigenetic regulation of oxysterol formation. Biochimie 2013, 95, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Wang, Y. Oxysterols as lipid mediators: Their biosynthetic genes, enzymes and metabolites. Prostaglandins Other Lipid Mediat. 2020, 147, 106381. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, C.; Iuliano, L. Cholesterol and related sterols autoxidation. Free Radic. Biol. Med. 2017, 111, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Jessup, W. Oxysterols: Sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol. Asp. Med. 2009, 30, 111–122. [Google Scholar] [CrossRef]
- Brown, A.J.; Dean, R.T.; Jessup, W. Free and esterified oxysterol: Formation during copper-oxidation of low density lipoprotein and uptake by macrophages. J. Lipid Res. 1996, 37, 320–335. [Google Scholar] [CrossRef]
- Anderson, A.; Campo, A.; Fulton, E.; Corwin, A.; Jerome III, W.G.; O’Connor, M.S. 7-Ketocholesterol in disease and aging. Redox Biol. 2020, 29, 101380. [Google Scholar] [CrossRef]
- De Médina, P.; Paillasse, M.R.; Segala, G.; Voisin, M.; Mhamdi, L.; Dalenc, F.; Lacroix-Triki, M.; Filleron, T.; Pont, F.; Saati, T.A.; et al. Dendrogenin A arises from cholesterol and histamine metabolism and shows cell differentiation and anti-tumour properties. Nat. Commun. 2013, 4, 1840. [Google Scholar] [CrossRef]
- Poirot, M.; Soules, R.; Mallinger, A.; Dalenc, F.; Silvente-Poirot, S. Chemistry, biochemistry, metabolic fate and mechanism of action of 6-oxo-cholestan-3β, 5α-diol (OCDO), a tumor promoter and cholesterol metabolite. Biochimie 2018, 153, 139–149. [Google Scholar] [CrossRef]
- de Medina, P.; Diallo, K.; Huc-Claustre, E.; Attia, M.; Soulès, R.; Silvente-Poirot, S.; Poirot, M. The 5, 6-epoxycholesterol metabolic pathway in breast cancer: Emergence of new pharmacological targets. Br. J. Pharmacol. 2021, 178, 3248–3260. [Google Scholar] [CrossRef]
- Zarrouk, A.; Vejux, A.; Mackrill, J.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.; Lizard, G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 2014, 18, 148–162. [Google Scholar] [CrossRef]
- Testa, G.; Rossin, D.; Poli, G.; Biasi, F.; Leonarduzzi, G. Implication of oxysterols in chronic inflammatory human diseases. Biochimie 2018, 153, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Marcello, A.; Civra, A.; Bonotto, R.M.; Alves, L.N.; Rajasekharan, S.; Giacobone, C.; Caccia, C.; Cavalli, R.; Adami, M.; Brambilla, P.; et al. The cholesterol metabolite 27-hydroxycholesterol inhibits SARS-CoV-2 and is markedly decreased in COVID-19 patients. Redox Biol. 2020, 36, 101682. [Google Scholar] [CrossRef]
- Foo, C.X.; Bartlett, S.; Ronacher, K. Oxysterols in the immune response to bacterial and viral infections. Cells 2022, 11, 201. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Khabour, O.F.; Yousuf, A.; Mohammedsaeed, W.; Alahmadi, N.F.; Alshangeety, A.M.; Alotaibi, O.H.; Alhaidary, A.A. The relationships between 7-kchol, 7β-ohchol, chol-triol, Lp (A) and PON1 with coronary heart disease in patients with diabetes mellitus T1DM and T2DM. Pak. J. Pharm. Sci. 2022, 35, 761–768. [Google Scholar] [PubMed]
- Dias, I.H.; Shokr, H. Oxysterols as Biomarkers of Aging and Disease. In Implication of Oxysterols and Phytosterols in Aging and Human Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 307–336. [Google Scholar]
- Samadi, A.; Isikhan, S.Y.; Tinkov, A.A.; Lay, I.; Doşa, M.D.; Skalny, A.V.; Skalnaya, M.G.; Chirumbolo, S.; Bjørklund, G. Zinc, copper, and oxysterol levels in patients with type 1 and type 2 diabetes mellitus. Clin. Nutr. 2020, 39, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Nury, T.; Zarrouk, A.; Ragot, K.; Debbabi, M.; Riedinger, J.-M.; Vejux, A.; Aubourg, P.; Lizard, G. 7-Ketocholesterol is increased in the plasma of X-ALD patients and induces peroxisomal modifications in microglial cells: Potential roles of 7-ketocholesterol in the pathophysiology of X-ALD. J. Steroid Biochem. Mol. Biol. 2017, 169, 123–136. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Sterolomics in biology, biochemistry, medicine. TrAC Trends Anal. Chem. 2019, 120, 115280. [Google Scholar] [CrossRef]
- Sitarska, D.; Ługowska, A. Laboratory diagnosis of the Niemann-Pick type C disease: An inherited neurodegenerative disorder of cholesterol metabolism. Metab. Brain Dis. 2019, 34, 1253–1260. [Google Scholar] [CrossRef]
- Poli, G.; Biasi, F.; Leonarduzzi, G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013, 1, 125–130. [Google Scholar] [CrossRef]
- Prunet, C.; Montange, T.; Véjux, A.; Laubriet, A.; Rohmer, J.F.; Riedinger, J.M.; Athias, A.; Lemaire-Ewing, S.; Néel, D.; Petit, J.M.; et al. Multiplexed flow cytometric analyses of pro-and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytom. Part A 2006, 69, 359–373. [Google Scholar] [CrossRef]
- Joffre, C.; Leclère, L.; Buteau, B.; Martine, L.; Cabaret, S.; Malvitte, L.; Acar, N.; Lizard, G.; Bron, A.; Creuzot-Garcher, C.; et al. Oxysterols induced inflammation and oxidation in primary porcine retinal pigment epithelial cells. Curr. Eye Res. 2007, 32, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Glass, C.K. Macrophages, oxysterols and atherosclerosis. Circ. J. 2010, 74, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Lizard, G. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Asp. Med. 2009, 30, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Zmysłowski, A.; Szterk, A. Oxysterols as a biomarker in diseases. Clin. Chim. Acta 2019, 491, 103–113. [Google Scholar] [CrossRef]
- Prunet, C.; Petit, J.; Ecarnot-Laubriet, A.; Athias, A.; Miguet-Alfonsi, C.; Rohmer, J.; Steinmetz, E.; Neel, D.; Gambert, P.; Lizard, G. High circulating levels of 7β-and 7α-hydroxycholesterol and presence of apoptotic and oxidative markers in arterial lesions of normocholesterolemic atherosclerotic patients undergoing endarterectomy. Pathol. Biol. 2006, 54, 22–32. [Google Scholar] [CrossRef]
- Ziedén, B.; Kaminskas, A.; Kristenson, M.; Kucinskienê, Z.; Vessby, B.; Olsson, A.G.; Diczfalusy, U. Increased plasma 7β-hydroxycholesterol concentrations in a population with a high risk for cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 967–971. [Google Scholar] [CrossRef]
- Björkhem, I. Five decades with oxysterols. Biochimie 2013, 95, 448–454. [Google Scholar] [CrossRef]
- Phair, I.R.; Sovakova, M.; Alqurashi, N.; Nisr, R.B.; McNeilly, A.D.; Lamont, D.; Rena, G. In-depth proteomic profiling identifies potentiation of the LPS response by 7-ketocholesterol. J. Mol. Cell. Cardiol. Plus 2025, 11, 100285. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Li, M.; Chen, M.; Sun, C. Multifaceted functions of CH25H and 25HC to modulate the lipid metabolism, immune responses, and broadly antiviral activities. Viruses 2020, 12, 727. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, L.; Xu, S.; Zhang, T.; Wang, K. Cholestane-3β, 5α, 6β-triol promotes vascular smooth muscle cells calcification. Life Sci. 2004, 76, 533–543. [Google Scholar] [CrossRef]
- Testa, G.; Gamba, P.; Badilli, U.; Gargiulo, S.; Maina, M.; Guina, T.; Calfapietra, S.; Biasi, F.; Cavalli, R.; Poli, G.; et al. Loading into nanoparticles improves quercetin’s efficacy in preventing neuroinflammation induced by oxysterols. PLoS ONE 2014, 9, e96795. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Cedazo-Minguez, A.; Leoni, V.; Meaney, S. Oxysterols and neurodegenerative diseases. Mol. Asp. Med. 2009, 30, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, M.; Younger, D.; Ravula, A.R.; Alay, E.; Rama Rao, K.V.; Chandra, N. Synergistic role of oxidative stress and blood-brain barrier permeability as injury mechanisms in the acute pathophysiology of blast-induced neurotrauma. Sci. Rep. 2019, 9, 7717. [Google Scholar] [CrossRef]
- Phan, H.T.; Shimokawa, N.; Sharma, N.; Takagi, M.; Mun’delanji, C.V. Strikingly different effects of cholesterol and 7-ketocholesterol on lipid bilayer-mediated aggregation of amyloid beta (1-42). Biochem. Biophys. Rep. 2018, 14, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Iriondo, A.; Garcia-Sebastian, M.; Arrospide, A.; Arriba, M.; Aurtenetxe, S.; Barandiaran, M.; Clerigue, M.; Ecay-Torres, M.; Estanga, A.; Gabilondo, A.; et al. Cerebrospinal fluid 7-ketocholesterol level is associated with amyloid-β 42 and white matter microstructure in cognitively healthy adults. J. Alzheimer’s Dis. 2020, 76, 643–656. [Google Scholar] [CrossRef]
- Debbabi, M.; Nury, T.; Zarrouk, A.; Mekahli, N.; Bezine, M.; Sghaier, R.; Grégoire, S.; Martine, L.; Durand, P.; Camus, E.; et al. Protective effects of α-tocopherol, γ-tocopherol and oleic acid, three compounds of olive oils, and no effect of trolox, on 7-ketocholesterol-induced mitochondrial and peroxisomal dysfunction in microglial BV-2 cells. Int. J. Mol. Sci. 2016, 17, 1973. [Google Scholar] [CrossRef]
- Izumi, Y.; O’Dell, K.A.; Mennerick, S.; Zorumski, C.F. Effects of acute pro-inflammatory stimulation and 25-hydroxycholesterol on hippocampal plasticity and learning involve NLRP3 inflammasome and cellular stress responses. Sci. Rep. 2025, 15, 6149. [Google Scholar] [CrossRef]
- Ademowo, O.S.; Dias, I.H. Circulating oxysterols in Alzheimer’s disease: A systematic review and meta-analysis. Redox Exp. Med. 2022, 2022, R116–R126. [Google Scholar] [CrossRef]
- Minagawa, H.; Gong, J.S.; Jung, C.G.; Watanabe, A.; Lund-Katz, S.; Phillips, M.C.; Saito, H.; Michikawa, M. Mechanism underlying apolipoprotein E (ApoE) isoform-dependent lipid efflux from neural cells in culture. J. Neurosci. Res. 2009, 87, 2498–2508. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.M.; Lee, C.; Jo, Y.S.; Muradillaevna, M.S.; Kim, J.H.; Yoon, J.H.; Song, P. Pathophysiological role of 27-hydroxycholesterol in human diseases. Adv. Biol. Regul. 2022, 83, 100837. [Google Scholar] [CrossRef]
- Gamba, P.; Giannelli, S.; Staurenghi, E.; Testa, G.; Sottero, B.; Biasi, F.; Poli, G.; Leonarduzzi, G. The controversial role of 24-S-hydroxycholesterol in Alzheimer’s disease. Antioxidants 2021, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Popp, J.; Meichsner, S.; Kölsch, H.; Lewczuk, P.; Maier, W.; Kornhuber, J.; Jessen, F.; Lütjohann, D. Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer’s disease. Biochem. Pharmacol. 2013, 86, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.M.; Kuller, L.H.; Lopez, O.L.; Becker, J.T.; Evans, R.W.; Sutton-Tyrrell, K.; Rosano, C. Markers of cholesterol metabolism in the brain show stronger associations with cerebrovascular disease than Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 30, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Joaquim, H.P.; Nunes, V.S.; Kerr, D.S.; Ferreira, G.S.; Forlenza, O.V.; Gattaz, W.F.; Talib, L.L. Donepezil effects on cholesterol and oxysterol plasma levels of Alzheimer’s disease patients. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 501–507. [Google Scholar] [CrossRef]
- Roy, D.; Chakrabarti, S.S.; Banerjee, A.; Sharma, P.; Biswas, A.; Chakrabarti, S. Serum 24-hydroxycholesterol in probable Alzheimer’s dementia: Reexploring the significance of a tentative Alzheimer’s disease biomarker. Aging Med. 2019, 2, 74–81. [Google Scholar] [CrossRef]
- An, Y.; Xi, Y.; Wang, T.; Ju, M.; Feng, W.; Guo, Z.; Sun, X.; Yang, K.; Qi, C.; Xiao, R. A panel of altered blood oxysterols in patients with mild cognitive impairment: A novel combined diagnostic marker. Pharmacol. Res. 2025, 213, 107661. [Google Scholar] [CrossRef]
- Zhang, D.-D.; Yu, H.-L.; Ma, W.-W.; Liu, Q.-R.; Han, J.; Wang, H.; Xiao, R. 27-Hydroxycholesterol contributes to disruptive effects on learning and memory by modulating cholesterol metabolism in the rat brain. Neuroscience 2015, 300, 163–173. [Google Scholar] [CrossRef]
- Liu, Q.; An, Y.; Yu, H.; Lu, Y.; Feng, L.; Wang, C.; Xiao, R. Relationship between oxysterols and mild cognitive impairment in the elderly: A case–control study. Lipids Health Dis. 2016, 15, 1–6. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouda, S.; Ghzaiel, I.; Hammami, S.; Khamlaoui, W.; Ahmed, S.H.; Lizard, G.; Hammami, M. Association between oxidative stress and altered cholesterol metabolism in Alzheimer’s disease patients. Curr. Alzheimer Res. 2020, 17, 823–834. [Google Scholar] [CrossRef]
- Mateos, L.; Ismail, M.-A.-M.; Gil-Bea, F.-J.; Leoni, V.; Winblad, B.; Bjoerkhem, I.; Cedazo-Minguez, A. Upregulation of brain renin angiotensin system by 27-hydroxycholesterol in Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 24, 669–679. [Google Scholar] [CrossRef]
- Iuliano, L.; Monticolo, R.; Straface, G.; Spoletini, I.; Gianni, W.; Caltagirone, C.; Bossù, P.; Spalletta, G. Vitamin E and enzymatic/oxidative stress-driven oxysterols in amnestic mild cognitive impairment subtypes and Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 21, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Gamba, P.; Guglielmotto, M.; Testa, G.; Monteleone, D.; Zerbinati, C.; Gargiulo, S.; Biasi, F.; Iuliano, L.; Giaccone, G.; Mauro, A.; et al. Up-regulation of β-amyloidogenesis in neuron-like human cells by both 24-and 27-hydroxycholesterol: Protective effect of N-acetyl-cysteine. Aging Cell 2014, 13, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, K.; Zhang, Y.; Mustapha, O.; Weigel, T.K.; Upchurch, C.M.; Tian, X.; Herbert, F.; Huang, W.; Leitinger, N.; Eyo, U.B.; et al. 7-ketocholesterol contributes to microglia-driven increases in astrocyte reactive oxygen species in Alzheimer’s disease. arxiv 2025. [Google Scholar] [CrossRef]
- Choi, H.; Kim, H.J.; Lee, S.-E.; Song, H.H.; Kim, J.; Han, J.; Jeong, J.-H.; Lee, D.Y.; Chang, S.; Mook-Jung, I. 25-Hydroxycholesterol modulates microglial function and exacerbates Alzheimer’s disease pathology: Mechanistic insights and therapeutic potential of cholesterol esterification inhibition. J. Neuroinflamm. 2025, 22, 50. [Google Scholar] [CrossRef]
- Papotti, B.; Palumbo, M.; Adorni, M.P.; Elviri, L.; Chiari, A.; Tondelli, M.; Bedin, R.; Baldelli, E.; Lancellotti, G.; Lupo, M.G.; et al. Influence of APOE4 genotype on PCSK9-lipids association in cerebrospinal fluid and serum of patients in the Alzheimer’s disease continuum. J. Alzheimer’s Dis. 2024, 102, 162–172. [Google Scholar] [CrossRef]
- Jahn, T.; Clark, C.; Kerksiek, A.; Lewczuk, P.; Lütjohann, D.; Popp, J. Cholesterol metabolites and plant sterols in cerebrospinal fluid are associated with Alzheimer’s cerebral pathology and clinical disease progression. J. Steroid Biochem. Mol. Biol. 2021, 205, 105785. [Google Scholar] [CrossRef]
- Dias, I.H.; Shokr, H.; Shephard, F.; Chakrabarti, L. Oxysterols and oxysterol sulfates in Alzheimer’s disease brain and cerebrospinal fluid. J. Alzheimer’s Dis. 2022, 87, 1527–1536. [Google Scholar] [CrossRef]
- Doria, M.; Maugest, L.; Moreau, T.; Lizard, G.; Vejux, A. Contribution of cholesterol and oxysterols to the pathophysiology of Parkinson’s disease. Free Radic. Biol. Med. 2016, 101, 393–400. [Google Scholar] [CrossRef]
- Alnaaim, S.A.; Al-Kuraishy, H.M.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E. Role of Brain Liver X Receptor in Parkinson’s Disease: Hidden Treasure and Emerging Opportunities. Mol. Neurobiol. 2024, 61, 341–357. [Google Scholar] [CrossRef]
- Cheng, D.; Kim, W.S.; Garner, B. Regulation of α-synuclein expression by liver X receptor ligands in vitro. Neuroreport 2008, 19, 1685–1689. [Google Scholar] [CrossRef]
- Marwarha, G.; Ghribi, O. Does the oxysterol 27-hydroxycholesterol underlie Alzheimer’s disease–Parkinson’s disease overlap? Exp. Gerontol. 2015, 68, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tang, K.; Wu, S.; Liu, L.; Qiang, C.; Lin, X.; Liu, B. Astragalus polysaccharides lowers plasma cholesterol through mechanisms distinct from statins. PLoS ONE 2011, 6, e27437. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Ghzaiel, I.; Nury, T.; Schneider, V.; Charrière, K.; Sghaier, R.; Zarrouk, A.; Leoni, V.; Moreau, T.; Lizard, G. Oxysterols and multiple sclerosis: Physiopathology, evolutive biomarkers and therapeutic strategy. J. Steroid Biochem. Mol. Biol. 2021, 210, 105870. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Dijkstra, C.; Polman, C. Biological markers in CSF and blood for axonal degeneration in multiple sclerosis. Lancet Neurol. 2005, 4, 32–41. [Google Scholar] [CrossRef]
- McComb, M.; Browne, R.W.; Bhattacharya, S.; Bodziak, M.L.; Jakimovski, D.; Weinstock-Guttman, B.; Kuhle, J.; Zivadinov, R.; Ramanathan, M. The cholesterol autoxidation products, 7-ketocholesterol and 7β-hydroxycholesterol are associated with serum neurofilaments in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 50, 102864. [Google Scholar] [CrossRef]
- Luu, W.; Sharpe, L.J.; Capell-Hattam, I.; Gelissen, I.C.; Brown, A.J. Oxysterols: Old tale, new twists. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 447–467. [Google Scholar] [CrossRef]
- Nury, T.; Zarrouk, A.; Mackrill, J.J.; Samadi, M.; Durand, P.; Riedinger, J.-M.; Doria, M.; Vejux, A.; Limagne, E.; Delmas, D.; et al. Induction of oxiapoptophagy on 158N murine oligodendrocytes treated by 7-ketocholesterol-, 7β-hydroxycholesterol-, or 24 (S)-hydroxycholesterol: Protective effects of α-tocopherol and docosahexaenoic acid (DHA; C22: 6 n-3). Steroids 2015, 99, 194–203. [Google Scholar] [CrossRef]
- van de Kraats, C.; Killestein, J.; Popescu, V.; Rijkers, E.; Vrenken, H.; Lütjohann, D.; Barkhof, F.; Polman, C.; Teunissen, C. Oxysterols and cholesterol precursors correlate to magnetic resonance imaging measures of neurodegeneration in multiple sclerosis. Mult. Scler. J. 2014, 20, 412–417. [Google Scholar] [CrossRef]
- Teunissen, C.; Floris, S.; Sonke, M.; Dijkstra, C.; De Vries, H.; Lütjohann, D. 24S-hydroxycholesterol in relation to disease manifestations of acute experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2007, 85, 1499–1505. [Google Scholar] [CrossRef]
- Leoni, V.; Lütjohann, D.; Masterman, T. Levels of 7-oxocholesterol in cerebrospinal fluid are more than one thousand times lower than reported in multiple sclerosis. J. Lipid Res. 2005, 46, 191–195. [Google Scholar] [CrossRef]
- Leoni, V. On the Possible Use of Oxysterols for the Diagnosis and Evaluation of Patients with Neurological and Neurodegenerative Diseases; Karolinska Institutet: Stockholm, Sweden, 2005. [Google Scholar]
- Vejux, A.; Namsi, A.; Nury, T.; Moreau, T.; Lizard, G. Biomarkers of amyotrophic lateral sclerosis: Current status and interest of oxysterols and phytosterols. Front. Mol. Neurosci. 2018, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Noh, M.-Y.; Kim, H.; Cheon, S.-Y.; Lee, K.M.; Lee, J.; Cha, E.; Park, K.S.; Lee, K.-W.; Sung, J.-J.; et al. 25-Hydroxycholesterol is involved in the pathogenesis of amyotrophic lateral sclerosis. Oncotarget 2017, 8, 11855. [Google Scholar] [CrossRef] [PubMed]
- Zakyrjanova, G.F.; Tsentsevitsky, A.N.; Kuznetsova, E.A.; Petrov, A.M. Immune-related oxysterol modulates neuromuscular transmission via non-genomic liver X receptor-dependent mechanism. Free Radic. Biol. Med. 2021, 174, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Khalik, J.; Yutuc, E.; Crick, P.J.; Gustafsson, J.-Å.; Warner, M.; Roman, G.; Talbot, K.; Gray, E.; Griffiths, W.J.; Turner, M.R.; et al. Defective cholesterol metabolism in amyotrophic lateral sclerosis. J. Lipid Res. 2017, 58, 267–278. [Google Scholar] [CrossRef]
- Wuolikainen, A.; Acimovic, J.; Lövgren-Sandblom, A.; Parini, P.; Andersen, P.M.; Björkhem, I. Cholesterol, oxysterol, triglyceride, and coenzyme Q homeostasis in ALS. Evidence against the hypothesis that elevated 27-hydroxycholesterol is a pathogenic factor. PLoS ONE 2014, 9, e113619. [Google Scholar] [CrossRef]
- Cappa, M.; Todisco, T.; Bizzarri, C. X-linked adrenoleukodystrophy and primary adrenal insufficiency. Front. Endocrinol. 2023, 14, 1309053. [Google Scholar] [CrossRef]
- Vigne, S.; Pot, C. Implication of oxysterols and phytosterols in aging and human diseases. In Implication of Oxysterols and Phytosterols in Aging and Human Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 231–260. [Google Scholar]
- Nury, T.; Yammine, A.; Menetrier, F.; Zarrouk, A.; Vejux, A.; Lizard, G. 7-Ketocholesterol-and 7β-hydroxycholesterol-induced peroxisomal disorders in glial, microglial and neuronal cells: Potential role in neurodegeneration: 7-ketocholesterol and 7β-hydroxycholesterol-induced peroxisomal disorders and neurodegeneration. In Peroxisome Biology: Experimental Models, Peroxisomal Disorders and Neurological Diseases; Springer: Berlin/Heidelberg, Germany, 2020; pp. 31–41. [Google Scholar]
- Jha, S.; Srivastava, S.Y.; Brickey, W.J.; Iocca, H.; Toews, A.; Morrison, J.P.; Chen, V.S.; Gris, D.; Matsushima, G.K.; Ting, J.P.-Y. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J. Neurosci. 2010, 30, 15811–15820. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Jin Hur, H.; Cho, H.-J.; Hwang, I.; Pyo Kang, Y.; Im, I.; Lee, H.; Lee, E.; Yang, W.; et al. 25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome. Nat. Commun. 2016, 7, 13129. [Google Scholar] [CrossRef]
- Siniscalco, D.; Schultz, S.; Brigida, A.L.; Antonucci, N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals 2018, 11, 56. [Google Scholar] [CrossRef]
- Grayaa, S.; Zerbinati, C.; Messedi, M.; HadjKacem, I.; Chtourou, M.; Touhemi, D.B.; Naifar, M.; Ayadi, H.; Ayedi, F.; Iuliano, L. Plasma oxysterol profiling in children reveals 24-hydroxycholesterol as a potential marker for Autism Spectrum Disorders. Biochimie 2018, 153, 80–85. [Google Scholar] [CrossRef]
- Menteşe Babayiğit, T.; Gümüş-Akay, G.; Uytun, M.Ç.; Doğan, Ö.; Serdar, M.A.; Efendi, G.Y.; Erman, A.G.; Yürümez, E.; Öztop, D.B. Investigation of Liver X Receptor Gene Variants and Oxysterol Dysregulation in Autism Spectrum Disorder. Children 2024, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Babayiğit, T.M.; Uytun, M.Ç.; Doğan, Ö.; Akay, G.G.; Serdar, M.A.; Efendi, G.Y.; Yürümez, E.; Öztop, D.B. Oxysterol Metabolism Balance as a Candidate Biomarker in Autism Spectrum Disorder. J. Clin. Pract. Res. 2024, 46, 290–297. [Google Scholar] [CrossRef]
- Olivier, E.; Rat, P. Role of Oxysterols in Ocular Degeneration Mechanisms and Involvement of P2X7 Receptor. In Implication of Oxysterols and Phytosterols in Aging and Human Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 277–292. [Google Scholar]
- Vrensen, G.F. Early cortical lens opacities: A short overview. Acta Ophthalmol. 2009, 87, 602–610. [Google Scholar] [CrossRef]
- Cenedella, R.J. Cholesterol and cataracts. Surv. Ophthalmol. 1996, 40, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Girao, H.; Mota, M.C.; Ramalho, J.; Pereira, P. Cholesterol oxides accumulate in human cataracts. Exp. Eye Res. 1998, 66, 645–652. [Google Scholar] [CrossRef]
- Vejux, A.; Samadi, M.; Lizard, G. Contribution of cholesterol and oxysterols in the physiopathology of cataract: Implication for the development of pharmacological treatments. J. Ophthalmol. 2011, 2011, 471947. [Google Scholar] [CrossRef]
- Malvitte, L.; Montange, T.; Joffre, C.; Vejux, A.; Maiza, C.; Bron, A.; Creuzot-Garcher, C.; Lizard, G. Analogies between atherosclerosis and age-related maculopathy: Expected roles of oxysterols. J. Fr. D’ophtalmologie 2006, 29, 570–578. [Google Scholar] [CrossRef]
- Pariente, A.; Peláez, R.; Pérez-Sala, Á.; Larráyoz, I.M. Inflammatory and cell death mechanisms induced by 7-ketocholesterol in the retina. Implications for age-related macular degeneration. Exp. Eye Res. 2019, 187, 107746. [Google Scholar] [CrossRef]
- Moreira, E.F.; Larrayoz, I.M.; Lee, J.W.; Rodríguez, I.R. 7-Ketocholesterol is present in lipid deposits in the primate retina: Potential implication in the induction of VEGF and CNV formation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 523–532. [Google Scholar] [CrossRef]
- Indaram, M.; Ma, W.; Zhao, L.; Fariss, R.N.; Rodriguez, I.R.; Wong, W.T. 7-Ketocholesterol increases retinal microglial migration, activation and angiogenicity: A potential pathogenic mechanism underlying age-related macular degeneration. Sci. Rep. 2015, 5, 9144. [Google Scholar] [CrossRef]
- Javitt, N.B.; Javitt, J.C. The retinal oxysterol pathway: A unifying hypothesis for the cause of age-related macular degeneration. Curr. Opin. Ophthalmol. 2009, 20, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Fourgeux, C.; Dugas, B.; Richard, F.; Björkhem, I.; Acar, N.; Bron, A.M.; Korobelnik, J.-F.; Leveziel, N.; Zerbib, J.; Puche, N.; et al. Single nucleotide polymorphism in the cholesterol-24S-hydroxylase (CYP46A1) gene and its association with CFH and LOC387715 gene polymorphisms in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7026–7033. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Yang, J.; Tang, F.; Wei, Z.; Chao, Y.; Li, N.; Li, H.; Wu, S.; Dong, X. New function of cholesterol oxidation products involved in osteoporosis pathogenesis. Int. J. Mol. Sci. 2022, 23, 2020. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yuan, L.; Xu, S.; Wang, K.; Zhang, T. Cholestane-3β, 5α, 6β-triol inhibits osteoblastic differentiation and promotes apoptosis of rat bone marrow stromal cells. J. Cell. Biochem. 2005, 96, 198–208. [Google Scholar] [CrossRef]
- DuSell, C.D.; Nelson, E.R.; Wang, X.; Abdo, J.; Mödder, U.I.; Umetani, M.; Gesty-Palmer, D.; Javitt, N.B.; Khosla, S.; McDonnell, D.P. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology 2010, 151, 3675–3685. [Google Scholar] [CrossRef]
- Nelson, E.R.; Wardell, S.E.; McDonnell, D.P. The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: Implications for the treatment and prevention of osteoporosis. Bone 2013, 53, 42–50. [Google Scholar] [CrossRef]
- Nelson, E.R.; DuSell, C.D.; Wang, X.; Howe, M.K.; Evans, G.; Michalek, R.D.; Umetani, M.; Rathmell, J.C.; Khosla, S.; Gesty-Palmer, D.; et al. The oxysterol, 27-hydroxycholesterol, links cholesterol metabolism to bone homeostasis through its actions on the estrogen and liver X receptors. Endocrinology 2011, 152, 4691–4705. [Google Scholar] [CrossRef]
- Aghaloo, T.L.; Amantea, C.M.; Cowan, C.M.; Richardson, J.A.; Wu, B.M.; Parhami, F.; Tetradis, S. Oxysterols enhance osteoblast differentiation in vitro and bone healing in vivo. J. Orthop. Res. 2007, 25, 1488–1497. [Google Scholar] [CrossRef]
- Kha, H.T.; Basseri, B.; Shouhed, D.; Richardson, J.; Tetradis, S.; Hahn, T.J.; Parhami, F. Oxysterols regulate differentiation of mesenchymal stem cells: Pro-bone and anti-fat. J. Bone Miner. Res. 2004, 19, 830–840. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, E.; Han, S.; Kang, K.L.; Heo, J.S. Evaluating the oxysterol combination of 22 (S)-hydroxycholesterol and 20 (S)-hydroxycholesterol in periodontal regeneration using periodontal ligament stem cells and alveolar bone healing models. Stem Cell Res. Ther. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- He, S.; Nelson, E.R. 27-Hydroxycholesterol, an endogenous selective estrogen receptor modulator. Maturitas 2017, 104, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Feldman, D.; Stefanick, M.L.; McDonnell, D.P.; Thompson, B.M.; McDonald, J.G.; Lee, J.S. 27-hydroxycholesterol, an endogenous SERM, and risk of fracture in postmenopausal women: A nested case-cohort study in the Women’s Health Initiative. J. Bone Miner. Res. 2019, 34, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Chen, C.; O, K.; Kim, W.K. 25-Hydroxycholesterol inhibits adipogenic differentiation of C3H10T1/2 pluripotent stromal cells. Int. J. Mol. Sci. 2020, 21, 412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, H.; Yang, Y.; Liu, L.; Luo, D.; Yu, D.; Chen, T. Iron-lead mixed exposure causes bone damage in mice: A multi-omics analysis. Ecotoxicol. Environ. Saf. 2025, 292, 117967. [Google Scholar] [CrossRef]
- Priyadarsini, N.; Nanda, P.; Devi, S.; Mohapatra, S. Sarcopenia: An age-related multifactorial disorder. Curr. Aging Sci. 2022, 15, 209–217. [Google Scholar] [CrossRef]
- Chhetri, J.K.; de Souto Barreto, P.; Fougère, B.; Rolland, Y.; Vellas, B.; Cesari, M. Chronic inflammation and sarcopenia: A regenerative cell therapy perspective. Exp. Gerontol. 2018, 103, 115–123. [Google Scholar] [CrossRef]
- Luo, J.; Liu, W.; Feng, F.; Chen, L. Apelin/APJ system: A novel therapeutic target for locomotor system diseases. Eur. J. Pharmacol. 2021, 906, 174286. [Google Scholar] [CrossRef]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradere, J.-P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- Ghzaiel, I.; Zarrouk, A.; Pires, V.; de Barros, J.-P.P.; Hammami, S.; Ksila, M.; Hammami, M.; Ghrairi, T.; Jouanny, P.; Vejux, A.; et al. 7β-Hydroxycholesterol and 7-ketocholesterol: New oxidative stress biomarkers of sarcopenia inducing cytotoxic effects on myoblasts and myotubes. J. Steroid Biochem. Mol. Biol. 2023, 232, 106345. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, T.; Liu, H.; Li, Z.; Peng, L.; Wang, C.; Wang, T. Inflammaging: The ground for sarcopenia? Exp. Gerontol. 2022, 168, 111931. [Google Scholar] [CrossRef]
- Jang, I.-Y.; Lee, S.; Kim, J.H.; Lee, E.; Lee, J.Y.; Park, S.J.; Kim, D.A.; Hamrick, M.W.; Park, J.H.; Kim, B.-J. Lack of association between circulating apelin level and frailty-related functional parameters in older adults: A cross-sectional study. BMC Geriatr. 2020, 20, 420. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xue, J.; Xu, L.; Cheng, L.; Zhang, Y.; Wang, X. Muscle-specific PGC-1α modulates mitochondrial oxidative stress in aged sarcopenia through regulating Nrf2. Exp. Gerontol. 2024, 193, 112468. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, F.A.; Levy, D.; Reichert, C.O.; Cunha-Neto, E.; Kalil, J.; Bydlowski, S.P. Effects of oxysterols on immune cells and related diseases. Cells 2022, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Rossin, D.; Dias, I.; Solej, M.; Milic, I.; Pitt, A.R.; Iaia, N.; Scoppapietra, L.; Devitt, A.; Nano, M.; Degiuli, M.; et al. Increased production of 27-hydroxycholesterol in human colorectal cancer advanced stage: Possible contribution to cancer cell survival and infiltration. Free Radic. Biol. Med. 2019, 136, 35–44. [Google Scholar] [CrossRef]
- Biasi, F.; Mascia, C.; Astegiano, M.; Chiarpotto, E.; Nano, M.; Vizio, B.; Leonarduzzi, G.; Poli, G. Pro-oxidant and proapoptotic effects of cholesterol oxidation products on human colonic epithelial cells: A potential mechanism of inflammatory bowel disease progression. Free Radic. Biol. Med. 2009, 47, 1731–1741. [Google Scholar] [CrossRef]
- Chalubinski, M.; Zemanek, K.; Skowron, W.; Wojdan, K.; Gorzelak, P.; Broncel, M. The effect of 7-ketocholesterol and 25-hydroxycholesterol on the integrity of the human aortic endothelial and intestinal epithelial barriers. Inflamm. Res. 2013, 62, 1015–1023. [Google Scholar] [CrossRef]
- Liu, C.; Yang, X.V.; Wu, J.; Kuei, C.; Mani, N.S.; Zhang, L.; Yu, J.; Sutton, S.W.; Qin, N.; Banie, H.; et al. Oxysterols direct B-cell migration through EBI2. Nature 2011, 475, 519–523. [Google Scholar] [CrossRef]
- Raselli, T.; Wyss, A.; Gonzalez Alvarado, M.; Weder, B.; Mamie, C.; Spalinger, M.R.; Van Haaften, W.T.; Dijkstra, G.; Sailer, A.W.; Imenez Silva, P.; et al. The oxysterol synthesising enzyme CH25H contributes to the development of intestinal fibrosis. J. Crohn’s Colitis 2019, 13, 1186–1200. [Google Scholar] [CrossRef]
- Trindade, B.C.; Ceglia, S.; Berthelette, A.; Raso, F.; Howley, K.; Muppidi, J.R.; Reboldi, A. The cholesterol metabolite 25-hydroxycholesterol restrains the transcriptional regulator SREBP2 and limits intestinal IgA plasma cell differentiation. Immunity 2021, 54, 2273–2287.e2276. [Google Scholar] [CrossRef]
- Emgård, J.; Kammoun, H.; García-Cassani, B.; Chesné, J.; Parigi, S.M.; Jacob, J.-M.; Cheng, H.-W.; Evren, E.; Das, S.; Czarnewski, P.; et al. Oxysterol sensing through the receptor GPR183 promotes the lymphoid-tissue-inducing function of innate lymphoid cells and colonic inflammation. Immunity 2018, 48, 120–132.e8. [Google Scholar] [CrossRef]
- Foo, C.X.; Fessler, M.B.; Ronacher, K. Oxysterols in Infectious Diseases. In Implication of Oxysterols and Phytosterols in Aging and Human Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 125–147. [Google Scholar]
- Ngo, M.D.; Bartlett, S.; Bielefeldt-Ohmann, H.; Foo, C.X.; Sinha, R.; Arachchige, B.J.; Reed, S.; Mandrup-Poulsen, T.; Rosenkilde, M.M.; Ronacher, K. A blunted GPR183/oxysterol axis during dysglycemia results in delayed recruitment of macrophages to the lung during mycobacterium tuberculosis infection. J. Infect. Dis. 2022, 225, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, A.C.; Castro, E.; Tocheny, C.E.; Assmann, M.; Schwarz, B.; Bohrnsen, E.; Makiya, M.A.; Legrand, F.; Hilligan, K.L.; Baker, P.J.; et al. Rapid GPR183-mediated recruitment of eosinophils to the lung after Mycobacterium tuberculosis infection. Cell Rep. 2022, 40, 111144. [Google Scholar] [CrossRef] [PubMed]
- Varaksa, T.; Bukhdruker, S.; Grabovec, I.; Marin, E.; Kavaleuski, A.; Gusach, A.; Kovalev, K.; Maslov, I.; Luginina, A.; Zabelskii, D.; et al. Metabolic fate of human immunoactive sterols in Mycobacterium tuberculosis. J. Mol. Biol. 2021, 433, 166763. [Google Scholar] [CrossRef] [PubMed]
- Lathe, R.; Sapronova, A.; Kotelevtsev, Y. Atherosclerosis and Alzheimer-diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr. 2014, 14, 36. [Google Scholar] [CrossRef]
- Bartlett, S.; Gemiarto, A.T.; Ngo, M.D.; Sajiir, H.; Hailu, S.; Sinha, R.; Foo, C.X.; Kleynhans, L.; Tshivhula, H.; Webber, T.; et al. GPR183 regulates interferons, autophagy, and bacterial growth during Mycobacterium tuberculosis infection and is associated with TB disease severity. Front. Immunol. 2020, 11, 601534. [Google Scholar] [CrossRef]
- Foo, C.X.; Bartlett, S.; Chew, K.Y.; Ngo, M.D.; Bielefeldt-Ohmann, H.; Arachchige, B.J.; Matthews, B.; Reed, S.; Wang, R.; Smith, C.; et al. GPR183 antagonism reduces macrophage infiltration in influenza and SARS-CoV-2 infection. Eur. Respir. J. 2023, 61, 2201306. [Google Scholar] [CrossRef]
- Conlon, T.M.; Yildirim, A.Ö. Oxysterol metabolism dictates macrophage influx during SARS-CoV-2 infection. Eur. Respir. J. 2023, 61, 2202417. [Google Scholar] [CrossRef]
- Zu, S.; Deng, Y.-Q.; Zhou, C.; Li, J.; Li, L.; Chen, Q.; Li, X.-F.; Zhao, H.; Gold, S.; He, J.; et al. 25-Hydroxycholesterol is a potent SARS-CoV-2 inhibitor. Cell Res. 2020, 30, 1043–1045. [Google Scholar] [CrossRef]
- Asano, T.; Wakabayashi, T.; Kondo, Y.; Okada, K.; Yamamuro, D.; Koga, Y.; Oka, K.; Sakurai, M.; Sawayama, N.; Takahashi, M.; et al. Serum 25-hydroxycholesterol levels are increased in patients with coronavirus disease 2019. J. Clin. Lipidol. 2023, 17, 78–86. [Google Scholar] [CrossRef]
- Aksu, N.; Samadi, A.; Yalçınkaya, A.; Çetin, T.; Eser, B.; Lay, İ.; Öziş, T.N.; Öztaş, Y.; Sabuncuoğlu, S. Evaluation of oxysterol levels of patients with silicosis by LC–MS/MS method. Mol. Cell. Biochem. 2020, 467, 117–125. [Google Scholar] [CrossRef]
- Fischer, B.M.; Voynow, J.A.; Ghio, A.J. COPD: Balancing oxidants and antioxidants. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Sugiura, H.; Koarai, A.; Ichikawa, T.; Minakata, Y.; Matsunaga, K.; Nakanishi, M.; Hirano, T.; Akamatsu, K.; Yanagisawa, S.; et al. Increase of 27-hydroxycholesterol in the airways of patients with COPD: Possible role of 27-hydroxycholesterol in tissue fibrosis. Chest 2012, 142, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Sugiura, H.; Togo, S.; Koarai, A.; Abe, K.; Yamada, M.; Ichikawa, T.; Kikuchi, T.; Numakura, T.; Onodera, K.; et al. 27-Hydroxycholesterol accelerates cellular senescence in human lung resident cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L1028–L1041. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Conlon, T.M.; Sarker, R.S.; Taşdemir, D.; Smirnova, N.F.; Srivastava, B.; Verleden, S.E.; Güneş, G.; Wu, X.; Prehn, C.; et al. Cholesterol metabolism promotes B-cell positioning during immune pathogenesis of chronic obstructive pulmonary disease. EMBO Mol. Med. 2018, 10, e8349. [Google Scholar] [CrossRef]
- Sugiura, H.; Koarai, A.; Ichikawa, T.; Minakata, Y.; Matsunaga, K.; Hirano, T.; Akamatsu, K.; Yanagisawa, S.; Furusawa, M.; Uno, Y.; et al. Increased 25-hydroxycholesterol concentrations in the lungs of patients with chronic obstructive pulmonary disease. Respirology 2012, 17, 533–540. [Google Scholar] [CrossRef]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute lung injury: A clinical and molecular review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef]
- Bottemanne, P.; Paquot, A.; Ameraoui, H.; Guillemot-Legris, O.; Alhouayek, M.; Muccioli, G.G. 25-Hydroxycholesterol metabolism is altered by lung inflammation, and its local administration modulates lung inflammation in mice. FASEB J. 2021, 35, e21514. [Google Scholar] [CrossRef]
- Zanjani, B.N.; Samadi, A.; Isikhan, S.Y.; Lay, I.; Beyaz, S.; Gelincik, A.; Buyukozturk, S.; Arda, N. Plasma levels of oxysterols 7-ketocholesterol and cholestane-3β, 5α, 6β-triol in patients with allergic asthma. J. Asthma 2023, 60, 288–297. [Google Scholar] [CrossRef]
- Shen, Z.-J.; Hu, J.; Kashi, V.P.; Kelly, E.A.; Denlinger, L.C.; Lutchman, K.; McDonald, J.G.; Jarjour, N.N.; Malter, J.S. Epstein-Barr virus–induced gene 2 mediates allergen-induced leukocyte migration into airways. Am. J. Respir. Crit. Care Med. 2017, 195, 1576–1585. [Google Scholar] [CrossRef]
- Konuşma, D. Anormal GM2 gangliosit birikiminin hücre metabolizmasındaki etkisi: Erken başlangıçlı Tay-Sachs hastalığının fare modeli. Acta Medica 2017, 48, 1–29. [Google Scholar]
- Emmi, G.; Bettiol, A.; Hatemi, G.; Prisco, D. Behçet’s syndrome. Lancet 2024, 403, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Messedi, M.; Frigui, M.; Mahfoudh, K.B.; Feki, H.; Mahfoudh, S.T.B.; Mnif, J.; Bahloul, Z.; Ayadi, F. Intima-media thickness of carotid artery in patients with Behçet’s disease. Arch. Med. Res. 2011, 42, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Acar, O.; Sarac, G.A.; Rota, D.D.; Aksoy, H. Evaluation of pro-atherogenic lipid profile and high atherohenic indexes in patients with Behçet’s disease: A case–control study. J. Cosmet. Dermatol. 2023, 22, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Messedi, M.; Guidara, W.; Grayaa, S.; Khrouf, W.; Snoussi, M.; Bahloul, Z.; Bonnefont-Rousselot, D.; Lamari, F.; Ayadi, F. Selected plasma oxysterols as a potential multi-marker biosignature panel for Behçet’s disease. J. Steroid Biochem. Mol. Biol. 2022, 221, 106122. [Google Scholar] [CrossRef]
- Dang, E.V.; McDonald, J.G.; Russell, D.W.; Cyster, J.G. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell 2017, 171, 1057–1071.e11. [Google Scholar] [CrossRef]
- Chekaoui, A.; Lahmar, K.; Belguendouz, H.; Mazari, F.; Terahi, M.; Hakem, D.; Youinou, P.; Touil-Boukoffa, C. Increased IL-1β levels are associated with an imbalance of “oxidant/antioxidant” status during Behçet’s disease. Eur. Cytokine Netw. 2018, 29, 95–102. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Gamba, P.; Gargiulo, S.; Biasi, F.; Poli, G. Inflammation-related gene expression by lipid oxidation-derived products in the progression of atherosclerosis. Free Radic. Biol. Med. 2012, 52, 19–34. [Google Scholar] [CrossRef]
- Wang, F.; Stappenbeck, F.; Tang, L.-Y.; Zhang, Y.E.; Hui, S.T.; Lusis, A.J.; Parhami, F. Oxy210, a semi-synthetic oxysterol, exerts anti-inflammatory effects in macrophages via inhibition of toll-like receptor (TLR) 4 and TLR2 signaling and modulation of macrophage polarization. Int. J. Mol. Sci. 2022, 23, 5478. [Google Scholar] [CrossRef]
- Aye, I.L.; Waddell, B.J.; Mark, P.J.; Keelan, J.A. Oxysterols exert proinflammatory effects in placental trophoblasts via TLR4-dependent, cholesterol-sensitive activation of NF-κB. MHR Basic Sci. Reprod. Med. 2012, 18, 341–353. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gamba, P.; Testa, G.; Rossin, D.; Biasi, F.; Poli, G.; Leonarduzzi, G. Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell 2015, 14, 569–581. [Google Scholar] [CrossRef]
- Hedayat, M.; Netea, M.G.; Rezaei, N. Targeting of Toll-like receptors: A decade of progress in combating infectious diseases. Lancet Infect. Dis. 2011, 11, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Moghimpour Bijani, F.; Vallejo, J.G.; Rezaei, N. Toll-like receptor signaling pathways in cardiovascular diseases: Challenges and opportunities. Int. Rev. Immunol. 2012, 31, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Chávez-Sánchez, L.; Madrid-Miller, A.; Chávez-Rueda, K.; Legorreta-Haquet, M.; Tesoro-Cruz, E.; Blanco-Favela, F. Activation of TLR2 and TLR4 by minimally modified low-density lipoprotein in human macrophages and monocytes triggers the inflammatory response. Hum. Immunol. 2010, 71, 737–744. [Google Scholar] [CrossRef]
- de Freitas, F.A.; Levy, D.; Zarrouk, A.; Lizard, G.; Bydlowski, S.P. Impact of oxysterols on cell death, proliferation, and differentiation induction: Current status. Cells 2021, 10, 2301. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.; Mangelsdorf, D. Liver X receptors and cholesterol homoeostasis: Spotlight on the adrenal gland. Biochem. Soc. Trans. 2006, 34, 1110–1113. [Google Scholar] [CrossRef]
- Lu, R.; Ito, J.; Iwamoto, N.; Nishimaki-Mogami, T.; Yokoyama, S. FGF-1 induces expression of LXRα and production of 25-hydroxycholesterol to upregulate the apoE gene in rat astrocytes. J. Lipid Res. 2009, 50, 1156–1164. [Google Scholar] [CrossRef]
- Willinger, T. Oxysterols in intestinal immunity and inflammation. J. Intern. Med. 2019, 285, 367–380. [Google Scholar] [CrossRef]
- Guillemot-Legris, O.; Mutemberezi, V.; Muccioli, G.G. Oxysterols in metabolic syndrome: From bystander molecules to bioactive lipids. Trends Mol. Med. 2016, 22, 594–614. [Google Scholar] [CrossRef]
- Ito, A.; Hong, C.; Rong, X.; Zhu, X.; Tarling, E.J.; Hedde, P.N.; Gratton, E.; Parks, J.; Tontonoz, P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. elife 2015, 4, e08009. [Google Scholar] [CrossRef]
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcer, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D.; et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 2008, 134, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Morello, F.; Saglio, E.; Noghero, A.; Schiavone, D.; Williams, T.A.; Verhovez, A.; Bussolino, F.; Veglio, F.; Mulatero, P. LXR-activating oxysterols induce the expression of inflammatory markers in endothelial cells through LXR-independent mechanisms. Atherosclerosis 2009, 207, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic acid metabolism and kidney inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Lahoua, Z.; Vial, H.; Michel, F.; De Paulet, A.C.; Astruc, M. Oxysterol activation of arachidonic acid release and protaglindin E2 biosynthesis in NRK 49F cells is partially dependent on protein kinase activity. Cell. Signal. 1991, 3, 559–567. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamaguchi, T.; Ishihara, N.; Nakamura, S.; Tanaka, S.; Oka, R.; Imamura, H.; Sato, Y.; Ban, N.; Kawana, H.; et al. 7-Ketocholesterol induces ROS-mediated mRNA expression of 12-lipoxygenase, cyclooxygenase-2 and pro-inflammatory cytokines in human mesangial cells: Potential role in diabetic nephropathy. Prostaglandins Other Lipid Mediat. 2018, 134, 16–23. [Google Scholar] [CrossRef]
- Panini, S.R.; Yang, L.; Rusinol, A.E.; Sinensky, M.S.; Bonventre, J.V.; Leslie, C.C. Arachidonate metabolism and the signaling pathway of induction of apoptosis by oxidized LDL/oxysterol. J. Lipid Res. 2001, 42, 1678–1686. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Coletto, L.A.; Sciorati, C.; Bozzolo, E.P.; Manunta, P.; Rovere-Querini, P.; Manfredi, A.A. Ion channels and transporters in inflammation: Special focus on TRP channels and TRPC6. Cells 2018, 7, 70. [Google Scholar] [CrossRef]
- Reinmuth, L.; Hsiao, C.-C.; Hamann, J.; Rosenkilde, M.; Mackrill, J. Multiple targets for oxysterols in their regulation of the immune system. Cells 2021, 10, 2078. [Google Scholar] [CrossRef]
- Mackrill, J.J. Oxysterols and calcium signal transduction. Chem. Phys. Lipids 2011, 164, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, K.; Nakamura, Y. Activation mechanisms and diverse functions of mammalian phospholipase C. Biomolecules 2023, 13, 915. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.E.; Yu, Y.-R.A.; He, S.; Wardell, S.E.; Chang, C.-Y.; Kwon, S.; Pillai, R.V.; McDowell, H.B.; Thompson, J.W.; Dubois, L.G.; et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 2017, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Bezzerri, V.; d’Adamo, P.; Rimessi, A.; Lanzara, C.; Crovella, S.; Nicolis, E.; Tamanini, A.; Athanasakis, E.; Tebon, M.; Bisoffi, G.; et al. Phospholipase C-β3 is a key modulator of IL-8 expression in cystic fibrosis bronchial epithelial cells. J. Immunol. 2011, 186, 4946–4958. [Google Scholar] [CrossRef]
- Rutkowska, A.; Preuss, I.; Gessier, F.; Sailer, A.W.; Dev, K.K. EBI2 regulates intracellular signaling and migration in human astrocyte. Glia 2015, 63, 341–351. [Google Scholar] [CrossRef]
- Zhang, C.; Mo, M.; Ding, W.; Liu, W.; Yan, D.; Deng, J.; Luo, X.; Liu, J. High-mobility group box 1 (HMGB1) impaired cardiac excitation–contraction coupling by enhancing the sarcoplasmic reticulum (SR) Ca2+ leak through TLR4–ROS signaling in cardiomyocytes. J. Mol. Cell. Cardiol. 2014, 74, 260–273. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Jiang, X.; Lv, J.; Li, Y.; Ye, H.; Liu, W.; Wang, G.; Zhang, C.; Zheng, N.; et al. Toll-like receptor 4–induced ryanodine receptor 2 oxidation and sarcoplasmic reticulum Ca2+ leakage promote cardiac contractile dysfunction in sepsis. J. Biol. Chem. 2018, 293, 794–807. [Google Scholar] [CrossRef]
- Matsuo, I.; Kawamura, N.; Ohnuki, Y.; Suita, K.; Ishikawa, M.; Matsubara, T.; Mototani, Y.; Ito, A.; Hayakawa, Y.; Nariyama, M.; et al. Role of TLR4 signaling on Porphyromonas gingivalis LPS-induced cardiac dysfunction in mice. PLoS ONE 2022, 17, e0258823. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gamba, P.; Testa, G.; Sottero, B.; Maina, M.; Guina, T.; Biasi, F.; Poli, G.; Leonarduzzi, G. Molecular signaling involved in oxysterol-induced β1-integrin over-expression in human macrophages. Int. J. Mol. Sci. 2012, 13, 14278–14293. [Google Scholar] [CrossRef]
- Hammoud, Y.; Rice, T.; Mackrill, J.J. Oxysterols modulate calcium signalling in the A7r5 aortic smooth muscle cell-line. Biochimie 2013, 95, 568–577. [Google Scholar] [CrossRef]
- Smyth, J.T.; Hwang, S.Y.; Tomita, T.; DeHaven, W.I.; Mercer, J.C.; Putney, J.W. Activation and regulation of store-operated calcium entry. J. Cell. Mol. Med. 2010, 14, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Pulli, I.; Lassila, T.; Pan, G.; Yan, D.; Olkkonen, V.M.; Törnquist, K. Oxysterol-binding protein related-proteins (ORPs) 5 and 8 regulate calcium signaling at specific cell compartments. Cell Calcium 2018, 72, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhu, B.; Cao, X.; Li, S.; Li, D.; Zhou, H.; Olkkonen, V.M.; Zhong, W.; Xu, J.; Yan, D. OSBP-Related Protein 5L Maintains Intracellular IP3/Ca2+ Signaling and Proliferation in T Cells by Facilitating PIP2 Hydrolysis. J. Immunol. 2020, 204, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Cao, X.; Liu, B.; Li, C.; Li, D.; Zheng, J.; Lai, C.; Olkkonen, V.M.; Zhong, W.; Yan, D. OSBP-related protein 4L promotes phospholipase Cβ3 translocation from the nucleus to the plasma membrane in Jurkat T-cells. J. Biol. Chem. 2018, 293, 17430–17441. [Google Scholar] [CrossRef]
- Cao, X.; Chen, J.; Li, D.; Xie, P.; Xu, M.; Lin, W.; Li, S.; Pan, G.; Tang, Y.; Xu, J.; et al. ORP4L couples IP3 to ITPR1 in control of endoplasmic reticulum calcium release. FASEB J. 2019, 33, 13852–13865. [Google Scholar] [CrossRef]
- Kuznetsova, E.A.; Fedorov, N.S.; Zakyrjanova, G.F.; Malomouzh, A.I.; Petrov, A.M. 25-Hydroxycholesterol as a negative regulator of diaphragm muscle contractions via estrogen receptor and Ca2+-dependent pathway. Histochem. Cell Biol. 2025, 163, 42. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, S.H.; Das, A.; Yang, S.-N.; Jung, K.H.; Kim, M.K.; Berggren, P.-O.; Lee, Y.; Chai, J.C.; Kim, H.J.; et al. TLR3-/4-priming differentially promotes Ca2+ signaling and cytokine expression and Ca2+-dependently augments cytokine release in hMSCs. Sci. Rep. 2016, 6, 23103. [Google Scholar] [CrossRef]
- Mizuma, A.; Kim, J.Y.; Kacimi, R.; Stauderman, K.; Dunn, M.; Hebbar, S.; Yenari, M.A. Microglial calcium release-activated calcium channel inhibition improves outcome from experimental traumatic brain injury and microglia-induced neuronal death. J. Neurotrauma 2019, 36, 996–1007. [Google Scholar] [CrossRef]
- Xia, L.; Wang, X.; Yao, W.; Wang, M.; Zhu, J. Lipopolysaccharide increases exosomes secretion from endothelial progenitor cells by toll-like receptor 4 dependent mechanism. Biol. Cell 2022, 114, 127–137. [Google Scholar] [CrossRef]
- Birla, H.; Xia, J.; Gao, X.; Zhao, H.; Wang, F.; Patel, S.; Amponsah, A.; Bekker, A.; Tao, Y.-X.; Hu, H. Toll-like receptor 4 activation enhances Orai1-mediated calcium signal promoting cytokine production in spinal astrocytes. Cell Calcium 2022, 105, 102619. [Google Scholar] [CrossRef]
- Alqinyah, M.; Alhamed, A.S.; Alnefaie, H.O.; Algahtani, M.M.; Badr, A.M.; Albogami, A.M.; Mohany, M.; Alassmrry, Y.A.; Alghaith, A.F.; Alhamami, H.N.; et al. Targeting store-operated calcium entry regulates the inflammation-induced proliferation and migration of breast cancer cells. Biomedicines 2023, 11, 1637. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, S.J.; Nguyen, H.S.; Jeong, S.-W. Store-operated calcium entry in the satellite glial cells of rat sympathetic ganglia. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2024, 28, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Kountz, T.S.; Biyasheva, A.; Schleimer, R.P.; Prakriya, M. Extracellular Nucleotides and Histamine Suppress TLR3-and RIG-I–Mediated Release of Antiviral IFNs from Human Airway Epithelial Cells. J. Immunol. 2022, 208, 2390–2402. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Cardinali, C.; Morelli, M.B.; Santoni, M.; Nabissi, M.; Amantini, C. Danger-and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J. Neuroinflamm. 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Boonen, B.; Alpizar, Y.A.; Meseguer, V.M.; Talavera, K. TRP channels as sensors of bacterial endotoxins. Toxins 2018, 10, 326. [Google Scholar] [CrossRef]
- Berthier, A.; Lemaire-Ewing, S.; Prunet, C.; Monier, S.; Athias, A.; Bessede, G.; Pais de Barros, J.; Laubriet, A.; Gambert, P.; Lizard, G.; et al. Involvement of a calcium-dependent dephosphorylation of BAD associated with the localization of Trpc-1 within lipid rafts in 7-ketocholesterol-induced THP-1 cell apoptosis. Cell Death Differ. 2004, 11, 897–905. [Google Scholar] [CrossRef]
- Ingueneau, C.; Huynh-Do, U.; Marcheix, B.; Athias, A.; Gambert, P.; Nègre-Salvayre, A.; Salvayre, R.; Vindis, C. TRPC1 is regulated by caveolin-1 and is involved in oxidized LDL-induced apoptosis of vascular smooth muscle cells. J. Cell. Mol. Med. 2009, 13, 1620–1631. [Google Scholar] [CrossRef]
- Franchi, L.; Kanneganti, T.-D.; Dubyak, G.R.; Nunez, G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J. Biol. Chem. 2007, 282, 18810–18818. [Google Scholar] [CrossRef]
- Kälvegren, H.; Skoglund, C.; Helldahl, C.; Lerm, M.; Grenegård, M.; Bengtsson, T. Toll-like receptor 2 stimulation of platelets is mediated by purinergic P2X1-dependent Ca2+ mobilisation, cyclooxygenase and purinergic P2Y1 and P2Y12 receptor activation. Thromb. Haemost. 2010, 103, 398–407. [Google Scholar]
- Olivier, E.; Dutot, M.; Regazzetti, A.; Leguillier, T.; Dargère, D.; Auzeil, N.; Laprévote, O.; Rat, P. P2X7-pannexin-1 and amyloid β-induced oxysterol input in human retinal cell: Role in age-related macular degeneration? Biochimie 2016, 127, 70–78. [Google Scholar] [CrossRef]
- Olivier, E.; Dutot, M.; Regazzetti, A.; Laprévote, O.; Rat, P. 25-Hydroxycholesterol induces both P2X7-dependent pyroptosis and caspase-dependent apoptosis in human skin model: New insights into degenerative pathways. Chem. Phys. Lipids 2017, 207, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ohya, S.; Kito, H. Ca2+-activated K+ channel KCa3.1 as a therapeutic target for immune disorders. Biol. Pharm. Bull. 2018, 41, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Bezine, M.; Maatoug, S.; Khalifa, R.B.; Debbabi, M.; Zarrouk, A.; Wang, Y.; Griffiths, W.J.; Nury, T.; Samadi, M.; Vejux, A.; et al. Modulation of Kv3. 1b potassium channel level and intracellular potassium concentration in 158N murine oligodendrocytes and BV-2 murine microglial cells treated with 7-ketocholesterol, 24S-hydroxycholesterol or tetracosanoic acid (C24: 0). Biochimie 2018, 153, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Tajima, N.; Xiaoyan, L.; Taniguchi, M.; Kato, N. 24S-hydroxycholesterol alters activity of large-conductance Ca2+-dependent K+ (slo1 BK) channel through intercalation into plasma membrane. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 1525–1535. [Google Scholar] [CrossRef]
- Cosme, D.; Soares-da-Silva, P.; Magro, F. Effect of Toll-like receptor-2,-4,-5,-7, and NOD2stimulation on potassium channel conductance in intestinal epithelial cells (vol 323, pg G410, 2022). Am. J. Physiol.-Gastrointest. LIVER Physiol. 2023, 324, G89. [Google Scholar]
- Neekhra, A.; Tran, J.; Esfahani, P.R.; Schneider, K.; Pham, K.; Sharma, A.; Chwa, M.; Luthra, S.; Gramajo, A.L.; Mansoor, S. Memantine, simvastatin, and epicatechin inhibit 7-ketocholesterol-induced apoptosis in retinal pigment epithelial cells but not neurosensory retinal cells in vitro. J. Ophthalmic Vis. Res. 2020, 15, 470. [Google Scholar] [CrossRef]
- Lizard, G.; Miguet, C.; Besséde, G.; Monier, S.; Gueldry, S.; Neel, D.; Gambert, P. Impairment with various antioxidants of the loss of mitochondrial transmembrane potential and of the cytosolic release of cytochrome c occuring during 7-ketocholesterol-induced apoptosis. Free Radic. Biol. Med. 2000, 28, 743–753. [Google Scholar] [CrossRef]
- Miguet-Alfonsi, C.; Prunet, C.; Monier, S.; Bessède, G.; Lemaire-Ewing, S.; Berthier, A.; Ménétrier, F.; Néel, D.; Gambert, P.; Lizard, G. Analysis of oxidative processes and of myelin figures formation before and after the loss of mitochondrial transmembrane potential during 7β-hydroxycholesterol and 7-ketocholesterol-induced apoptosis: Comparison with various pro-apoptotic chemicals. Biochem. Pharmacol. 2002, 64, 527–541. [Google Scholar] [CrossRef]
- Lordan, S.; O’Neill, C.; O’Brien, N.M. Effects of apigenin, lycopene and astaxanthin on 7β-hydroxycholesterol-induced apoptosis and Akt phosphorylation in U937 cells. Br. J. Nutr. 2008, 100, 287–296. [Google Scholar] [CrossRef][Green Version]
- Royer, M.-C.; Lemaire-Ewing, S.; Desrumaux, C.; Monier, S.; de Barros, J.-P.P.; Athias, A.; Néel, D.; Lagrost, L. 7-ketocholesterol incorporation into sphingolipid/cholesterol-enriched (lipid raft) domains is impaired by vitamin E: A specific role for α-tocopherol with consequences on cell death. J. Biol. Chem. 2009, 284, 15826–15834. [Google Scholar] [CrossRef]
- Nury, T.; Zarrouk, A.; Vejux, A.; Doria, M.; Riedinger, J.M.; Delage-Mourroux, R.; Lizard, G. Induction of oxiapoptophagy, a mixed mode of cell death associated with oxidative stress, apoptosis and autophagy, on 7-ketocholesterol-treated 158N murine oligodendrocytes: Impairment by α-tocopherol. Biochem. Biophys. Res. Commun. 2014, 446, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Badreddine, A.; Zarrouk, A.; Karym, E.M.; Debbabi, M.; Nury, T.; Meddeb, W.; Sghaier, R.; Bezine, M.; Vejux, A.; Martine, L.; et al. Argan oil-mediated attenuation of organelle dysfunction, oxidative stress and cell death induced by 7-ketocholesterol in murine oligodendrocytes 158N. Int. J. Mol. Sci. 2017, 18, 2220. [Google Scholar] [CrossRef] [PubMed]
- Yammine, A.; Zarrouk, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Mackrill, J.J.; Greige-Gerges, H.; Auezova, L.; et al. Prevention by dietary polyphenols (resveratrol, quercetin, apigenin) against 7-ketocholesterol-induced oxiapoptophagy in neuronal N2a cells: Potential interest for the treatment of neurodegenerative and age-related diseases. Cells 2020, 9, 2346. [Google Scholar] [CrossRef]
- Nury, T.; Ghzaiel, I.; Hichami, A.; Caccia, C.; Leoni, V.; Pires, V.; Atanasov, A.G.; Zarrouk, A.; Lizard, G.; Vejux, A. Lipid droplets dependent or independent cytoprotective activities of unsaturated fatty acids, Lorenzo’s oil and sulfo-N-succinimidyl oleate on 7-ketocholesterol-induced oxidative stress, organelle dysfunction and cell death on 158N and ARPE-19 cells: Cell targets and benefits of sulfo-N-succinimidyl oleate. Curr. Res. Biotechnol. 2024, 7, 100195. [Google Scholar]
- Yammine, A.; Ghzaiel, I.; Pires, V.; Zarrouk, A.; Kharoubi, O.; Greige-Gerges, H.; Auezova, L.; Lizard, G.; Vejux, A. Cytoprotective effects of α-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, oleic acid and α-tocopherol on 7-ketocholesterol–Induced oxiapoptophagy: Major roles of PI3-K/PDK-1/Akt signaling pathway and glutathione peroxidase activity in cell rescue. Curr. Res. Toxicol. 2024, 6, 100153. [Google Scholar]
- Palozza, P.; Barone, E.; Mancuso, C.; Picci, N. The protective role of carotenoids against 7-keto-cholesterol formation in solution. Mol. Cell. Biochem. 2008, 309, 61–68. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.; Catalano, A.; Boninsegna, A.; Böhm, V.; Fröhlich, K.; Mele, M.C.; Monego, G.; Ranelletti, F.O. Lycopene prevents 7-ketocholesterol-induced oxidative stress, cell cycle arrest and apoptosis in human macrophages. J. Nutr. Biochem. 2010, 21, 34–46. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.; Catalano, A.; Monego, G.; Barini, A.; Mele, M.C.; Parrone, N.; Trombino, S.; Picci, N.; Ranelletti, F.O. Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in human macrophages: Inhibition of NF-κB nuclear binding and increase in PPARγ expression. J. Nutr. Biochem. 2011, 22, 259–268. [Google Scholar] [CrossRef]
- Krishnan, M.; Kumaresan, M.; Ravi, S.; Martin, L.C.; Duraisamy, P.; Munusamy, A.; Ramar, M. 7-ketocholesterol enhances BACE1-amyloid precursor protein cleavage and amyloidogenic peptide generation targeted by natural molecules. arXiv 2024. [Google Scholar] [CrossRef]
- Badreddine, A.; Zarrouk, A.; Nury, T.; El Kharrassi, Y.; Nasser, B.; Malki, M.C.; Lizard, G.; Samadi, M. An expeditious synthesis of spinasterol and schottenol, two phytosterols present in argan oil and in cactus pear seed oil, and evaluation of their biological activities on cells of the central nervous system. Steroids 2015, 99, 119–124. [Google Scholar] [CrossRef]
- Ryan, L.; O’Callaghan, Y.C.; O’Brien, N.M. Comparison of the apoptotic processes induced by the oxysterols 7β-hydroxycholesterol and cholesterol-5β, 6β-epoxide. Cell Biol. Toxicol. 2004, 20, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Leonarduzzi, G.; Vizio, B.; Sottero, B.; Verde, V.; Gamba, P.; Mascia, C.; Chiarpotto, E.; Poli, G.; Biasi, F. Early involvement of ROS overproduction in apoptosis induced by 7-ketocholesterol. Antioxid. Redox Signal. 2006, 8, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Mascia, C.; Maina, M.; Chiarpotto, E.; Leonarduzzi, G.; Poli, G.; Biasi, F. Proinflammatory effect of cholesterol and its oxidation products on CaCo-2 human enterocyte-like cells: Effective protection by epigallocatechin-3-gallate. Free Radic. Biol. Med. 2010, 49, 2049–2057. [Google Scholar] [CrossRef]
- Yamagata, K.; Tanaka, N.; Suzuki, K. Epigallocatechin 3-gallate inhibits 7-ketocholesterol-induced monocyte–endothelial cell adhesion. Microvasc. Res. 2013, 88, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Dugas, B.; Charbonnier, S.; Baarine, M.; Ragot, K.; Delmas, D.; Ménétrier, F.; Lherminier, J.; Malvitte, L.; Khalfaoui, T.; Bron, A.; et al. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: Cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur. J. Nutr. 2010, 49, 435–446. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Segoni, L.; D’Arcangelo, D.; Rossi, S.; Facchiano, F.; Saso, L.; Businaro, R.; Iuliano, L.; Riganò, R. Resveratrol counteracts inflammation in human M1 and M2 macrophages upon challenge with 7-oxo-cholesterol: Potential therapeutic implications in atherosclerosis. Oxidative Med. Cell. Longev. 2014, 2014, 257543. [Google Scholar] [CrossRef]
- Deiana, M.; Corona, G.; Incani, A.; Loru, D.; Rosa, A.; Atzeri, A.; Melis, M.P.; Dessì, M.A. Protective effect of simple phenols from extravirgin olive oil against lipid peroxidation in intestinal Caco-2 cells. Food Chem. Toxicol. 2010, 48, 3008–3016. [Google Scholar] [CrossRef]
- Atzeri, A.; Lucas, R.; Incani, A.; Peñalver, P.; Zafra-Gómez, A.; Melis, M.P.; Pizzala, R.; Morales, J.C.; Deiana, M. Hydroxytyrosol and tyrosol sulfate metabolites protect against the oxidized cholesterol pro-oxidant effect in Caco-2 human enterocyte-like cells. Food Funct. 2016, 7, 337–346. [Google Scholar] [CrossRef]
- Kim, A.; Nam, Y.J.; Lee, C.S. Taxifolin reduces the cholesterol oxidation product-induced neuronal apoptosis by suppressing the Akt and NF-κB activation-mediated cell death. Brain Res. Bull. 2017, 134, 63–71. [Google Scholar] [CrossRef]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Testa, G.; Sottero, B.; Poli, G.; Zeppa, G.; Biasi, F. A dietary mixture of oxysterols induces in vitro intestinal inflammation through TLR2/4 activation: The protective effect of cocoa bean shells. Antioxidants 2019, 8, 151. [Google Scholar] [CrossRef]
- Iaia, N.; Rossin, D.; Sottero, B.; Venezia, I.; Poli, G.; Biasi, F. Efficacy of theobromine in preventing intestinal CaCo-2 cell damage induced by oxysterols. Arch. Biochem. Biophys. 2020, 694, 108591. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Guina, T.; Maina, M.; Cabboi, B.; Deiana, M.; Tuberoso, C.I.; Calfapietra, S.; Chiarpotto, E.; Sottero, B.; Gamba, P.; et al. Phenolic compounds present in Sardinian wine extracts protect against the production of inflammatory cytokines induced by oxysterols in CaCo-2 human enterocyte-like cells. Biochem. Pharmacol. 2013, 86, 138–145. [Google Scholar] [CrossRef]
- Guina, T.; Deiana, M.; Calfapietra, S.; Cabboi, B.; Maina, M.; Tuberoso, C.I.; Leonarduzzi, G.; Gamba, P.; Gargiulo, S.; Testa, G.; et al. The role of p38 MAPK in the induction of intestinal inflammation by dietary oxysterols: Modulation by wine phenolics. Food Funct. 2015, 6, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Deiana, M.; Spencer, J.P.; Corona, G. Olive Oil Phenolics Prevent Oxysterol-Induced Proinflammatory Cytokine Secretion and Reactive Oxygen Species Production in Human Peripheral Blood Mononuclear Cells, Through Modulation of p38 and JNK Pathways. Mol. Nutr. Food Res. 2017, 61, 1700283. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Incani, A.; Serreli, G.; Porru, L.; Melis, M.P.; Tuberoso, C.I.; Rossin, D.; Biasi, F.; Deiana, M. Olive oil polyphenols reduce oxysterols-induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018, 17, 348–354. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Phytochemical indicaxanthin suppresses 7-ketocholesterol-induced THP-1 cell apoptosis by preventing cytosolic Ca2+ increase and oxidative stress. Br. J. Nutr. 2013, 110, 230–240. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Livrea, M.A. Dietary indicaxanthin from cactus pear (Opuntia ficus-indica L. Mill) fruit prevents eryptosis induced by oxysterols in a hypercholesterolaemia-relevant proportion and adhesion of human erythrocytes to endothelial cell layers. Br. J. Nutr. 2015, 114, 368–375. [Google Scholar] [CrossRef]
- Shen, L.; Sun, Z.; Chu, S.; Cai, Z.; Nie, P.; Wu, C.; Yuan, R.; Hu, L.; He, B. Xuezhikang, an extract from red yeast rice, attenuates vulnerable plaque progression by suppressing endoplasmic reticulum stress-mediated apoptosis and inflammation. PLoS ONE 2017, 12, e0188841. [Google Scholar] [CrossRef]
- Brahmi, F.; Nury, T.; Debbabi, M.; Hadj-Ahmed, S.; Zarrouk, A.; Prost, M.; Madani, K.; Boulekbache-Makhlouf, L.; Lizard, G. Evaluation of antioxidant, anti-inflammatory and cytoprotective properties of ethanolic mint extracts from algeria on 7-ketocholesterol-treated murine RAW 264.7 macrophages. Antioxidants 2018, 7, 184. [Google Scholar] [CrossRef]
- Kuo, X.; Herr, D.R.; Ong, W.-Y. Anti-inflammatory and cytoprotective effect of Clinacanthus nutans leaf but not stem extracts on 7-ketocholesterol induced brain endothelial cell injury. Neuromol. Med. 2021, 23, 176–183. [Google Scholar] [CrossRef]
- Zarrouk, A.; Smach, M.A.; Hafsa, J.; Sghaier, R.; Hammami, M.; Charfeddine, B. Effects of carpobrotus edulis extract on oxidative stress and 158n oligodendrocyte death. Biomed. Environ. Sci. 2019, 32, 291–299. [Google Scholar] [PubMed]
- Han, J.-M.; Li, H.; Cho, M.-H.; Baek, S.-H.; Lee, C.-H.; Park, H.-Y.; Jeong, T.-S. Soy-leaf extract exerts atheroprotective effects via modulation of Krüppel-like factor 2 and adhesion molecules. Int. J. Mol. Sci. 2017, 18, 373. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Nowicka, G.; Sygitowicz, G.; Jarosz, M. Anthocyanin-rich Aronox extract from Aroniamelanocarpa E protects against 7β-hydroxycholesterol-induced apoptosis of endothelial cells. Ann. Nutr. Metab. 2009, 53, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Duraisamy, P.; Krishnan, M.; Martin, L.C.; Manikandan, B.; Ramar, M. Sitosterol-rich Digera muricata against 7-ketocholesterol and lipopolysaccharide-mediated atherogenic responses by modulating NF-ΚB/iNOS signalling pathway in macrophages. 3 Biotech 2023, 13, 331. [Google Scholar] [CrossRef]
- Debbabi, M.; Zarrouk, A.; Bezine, M.; Meddeb, W.; Nury, T.; Badreddine, A.; Sghaier, R.; Bretillon, L.; Guyot, S.; Samadi, M.; et al. Comparison of the effects of major fatty acids present in the Mediterranean diet (oleic acid, docosahexaenoic acid) and in hydrogenated oils (elaidic acid) on 7-ketocholesterol-induced oxiapoptophagy in microglial BV-2 cells. Chem. Phys. Lipids 2017, 207, 151–170. [Google Scholar] [CrossRef]
- Meddeb, W.; Rezig, L.; Zarrouk, A.; Nury, T.; Vejux, A.; Prost, M.; Bretillon, L.; Mejri, M.; Lizard, G. Cytoprotective activities of milk thistle seed oil used in traditional Tunisian medicine on 7-ketocholesterol and 24S-hydroxycholesterol-induced toxicity on 158N murine oligodendrocytes. Antioxidants 2018, 7, 95. [Google Scholar] [CrossRef]
- Zarrouk, A.; Salem, Y.B.; Hafsa, J.; Sghaier, R.; Charfeddine, B.; Limem, K.; Hammami, M.; Majdoub, H. 7β-hydroxycholesterol-induced cell death, oxidative stress, and fatty acid metabolism dysfunctions attenuated with sea urchin egg oil. Biochimie 2018, 153, 210–219. [Google Scholar] [CrossRef]
- Maaloul, S.; Ghzaiel, I.; Mahmoudi, M.; Mighri, H.; Pires, V.; Vejux, A.; Martine, L.; de Barros, J.-P.P.; Prost-Camus, E.; Boughalleb, F.; et al. Characterization of Silybum marianum and Silybum eburneum seed oils: Phytochemical profiles and antioxidant properties supporting important nutritional interests. PLoS ONE 2024, 19, e0304021. [Google Scholar] [CrossRef]
- Zarrouk, A.; Nury, T.; Samadi, M.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.M.; Lizard, G.; Mackrill, J.J. Effects of cholesterol oxides on cell death induction and calcium increase in human neuronal cells (SK-N-BE) and evaluation of the protective effects of docosahexaenoic acid (DHA; C22: 6 n-3). Steroids 2015, 99, 238–247. [Google Scholar] [CrossRef]
- Yammine, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Greige-Gerges, H.; Auezova, L.; Lizard, G. Prevention of 7-ketocholesterol-induced overproduction of reactive oxygen species, mitochondrial dysfunction and cell death with major nutrients (polyphenols, ω3 and ω9 unsaturated fatty acids) of the Mediterranean diet on N2a neuronal cells. Molecules 2020, 25, 2296. [Google Scholar] [CrossRef]
- Huang, J.-D.; Amaral, J.; Lee, J.W.; Larrayoz, I.M.; Rodriguez, I.R. Sterculic acid antagonizes 7-ketocholesterol-mediated inflammation and inhibits choroidal neovascularization. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Monier, S.; Samadi, M.; Prunet, C.; Denance, M.; Laubriet, A.; Athias, A.; Berthier, A.; Steinmetz, E.; Jürgens, G.; Nègre-Salvayre, A.; et al. Impairment of the cytotoxic and oxidative activities of 7β-hydroxycholesterol and 7-ketocholesterol by esterification with oleate. Biochem. Biophys. Res. Commun. 2003, 303, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, L.; Bhat, O.M.; Lohner, H.; Li, P.-L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: Antioxidant action of butyrate. Redox Biol. 2018, 16, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Arnal-Levron, M.; Chen, Y.; Greimel, P.; Calevro, F.; Gaget, K.; Riols, F.; Batut, A.; Bertrand-Michel, J.; Hullin-Matsuda, F.; Olkkonen, V.M.; et al. Bis (monoacylglycero) phosphate regulates oxysterol binding protein-related protein 11 dependent sterol trafficking. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 1247–1257. [Google Scholar] [CrossRef]
- Mathieu, J.M.; Wang, F.; Segatori, L.; Alvarez, P.J. Increased resistance to oxysterol cytotoxicity in fibroblasts transfected with a lysosomally targeted Chromobacterium oxidase. Biotechnol. Bioeng. 2012, 109, 2409–2415. [Google Scholar] [CrossRef]
- Ghosh, S.; Khare, S. Biodegradation of 7-Ketocholesterol by Rhodococcus erythropolis MTCC 3951: Process optimization and enzymatic insights. Chem. Phys. Lipids 2017, 207, 253–259. [Google Scholar] [CrossRef]
- Ghosh, S.; Khare, S. Biodegradation of cytotoxic 7-Ketocholesterol by Pseudomonas aeruginosa PseA. Bioresour. Technol. 2016, 213, 44–49. [Google Scholar] [CrossRef]
- Casula, E.; Pisano, M.B.; Serreli, G.; Zodio, S.; Melis, M.P.; Corona, G.; Costabile, A.; Cosentino, S.; Deiana, M. Probiotic lactobacilli attenuate oxysterols-induced alteration of intestinal epithelial cell monolayer permeability: Focus on tight junction modulation. Food Chem. Toxicol. 2023, 172, 113558. [Google Scholar] [CrossRef]
- Perveen, I.; Sehar, S.; Naz, I.; Raza, M.A.; Jahangir, A. Biodegradation of 7-ketocholestrol (7-KC) by Thermobifidafusca IP1. Int. J. Biosci. 2016, 8, 83–93. [Google Scholar]
- Sato, Y.; Ishihara, N.; Nagayama, D.; Saiki, A.; Tatsuno, I. 7-ketocholesterol induces apoptosis of MC3T3-E1 cells associated with reactive oxygen species generation, endoplasmic reticulum stress and caspase-3/7 dependent pathway. Mol. Genet. Metab. Rep. 2017, 10, 56–60. [Google Scholar] [CrossRef]
- Koh, S.S.; Ooi, S.C.-Y.; Lui, N.M.-Y.; Qiong, C.; Ho, L.T.-Y.; Cheah, I.K.-M.; Halliwell, B.; Herr, D.R.; Ong, W.-Y. Effect of ergothioneine on 7-ketocholesterol-induced endothelial injury. Neuromol. Med. 2021, 23, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Nury, T.; Karym, E.-M.; Vejux, A.; Sghaier, R.; Gondcaille, C.; Andreoletti, P.; Trompier, D.; Savary, S.; Cherkaoui-Malki, M.; et al. Attenuation of 7-ketocholesterol-induced overproduction of reactive oxygen species, apoptosis, and autophagy by dimethyl fumarate on 158 N murine oligodendrocytes. J. Steroid Biochem. Mol. Biol. 2017, 169, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sghaier, R.; Zarrouk, A.; Nury, T.; Badreddine, I.; O’Brien, N.; Mackrill, J.J.; Vejux, A.; Samadi, M.; Nasser, B.; Caccia, C.; et al. Biotin attenuation of oxidative stress, mitochondrial dysfunction, lipid metabolism alteration and 7β-hydroxycholesterol-induced cell death in 158N murine oligodendrocytes. Free Radic. Res. 2019, 53, 535–561. [Google Scholar] [CrossRef] [PubMed]
- Ksila, M.; Ghzaiel, I.; Sassi, K.; Zarrouk, A.; Leoni, V.; Poli, G.; Rezig, L.; Pires, V.; Meziane, S.; Atanasov, A.G.; et al. Therapeutic Applications of Oxysterols and Derivatives in Age-Related Diseases, Infectious and Inflammatory Diseases, and Cancers. Adv. Exp. Med. Biol. 2024, 1440, 379–400. [Google Scholar] [CrossRef]
- Laskar, A.; Yuan, X.-M.; Li, W. Dimethyl sulfoxide prevents 7β-hydroxycholesterol-induced apoptosis by preserving lysosomes and mitochondria. J. Cardiovasc. Pharmacol. 2010, 56, 263–267. [Google Scholar] [CrossRef]
- Gorzelak-Pabis, P.; Broncel, M.; Wojdan, K.; Gajewski, A.; Chalubinski, M.; Gawrysiak, M.; Wozniak, E. Rivaroxaban protects from the oxysterol-induced damage and inflammatory activation of the vascular endothelium. Tissue Barriers 2021, 9, 1956284. [Google Scholar] [CrossRef]
- Naito, Y.; Shimozawa, M.; Manabe, H.; Nakabe, N.; Katada, K.; Kokura, S.; Yoshida, N.; Ichikawa, H.; Kon, T.; Yoshikawa, T. Azelnidipine, a new calcium channel blocker, inhibits endothelial inflammatory response by reducing intracellular levels of reactive oxygen species. Eur. J. Pharmacol. 2006, 546, 11–18. [Google Scholar] [CrossRef]
- Shen, L.; Sun, Z.; Nie, P.; Yuan, R.; Cai, Z.; Wu, C.; Hu, L.; Jin, S.; Zhou, H.; Zhang, X.; et al. Sulindac-derived retinoid X receptor-α modulator attenuates atherosclerotic plaque progression and destabilization in ApoE−/− mice. Br. J. Pharmacol. 2019, 176, 2559–2572. [Google Scholar] [CrossRef]
- Wang, Y.; Sanyal, A.J.; Hylemon, P.; Ren, S. Discovery of A Novel Regulator, 3β-Sulfate-5-Cholestenoic Acid, of Lipid Metabolism and Inflammation. Am. J. Physiol.-Endocrinol. Metab. 2025, 328, E543–E554. [Google Scholar] [CrossRef]
- Ghzaiel, I.; Zarrouk, A.; Essadek, S.; Martine, L.; Hammouda, S.; Yammine, A.; Ksila, M.; Nury, T.; Meddeb, W.; Joutey, M.T.; et al. Protective effects of milk thistle (Sylibum marianum) seed oil and α-tocopherol against 7β-hydroxycholesterol-induced peroxisomal alterations in murine C2C12 myoblasts: Nutritional insights associated with the concept of pexotherapy. Steroids 2022, 183, 109032. [Google Scholar] [CrossRef]
- Nury, T.; Yammine, A.; Ghzaiel, I.; Sassi, K.; Zarrouk, A.; Brahmi, F.; Samadi, M.; Rup-Jacques, S.; Vervandier-Fasseur, D.; de Barros, J.P.; et al. Attenuation of 7-ketocholesterol-and 7β-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: Potential for the prevention of age-related diseases. Ageing Res. Rev. 2021, 68, 101324. [Google Scholar] [CrossRef] [PubMed]
- Susanti, I.; Pratiwi, R.; Rosandi, Y.; Hasanah, A.N. Separation methods of phenolic compounds from plant extract as antioxidant agents candidate. Plants 2024, 13, 965. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.-Y. Production of betalains in plant cell and organ cultures: A review. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 158, 28. [Google Scholar] [CrossRef]
- Rezig, L.; Ghzaiel, I.; Ksila, M.; Yammine, A.; Nury, T.; Zarrouk, A.; Samadi, M.; Chouaibi, M.; Vejux, A.; Lizard, G. Cytoprotective activities of representative nutrients from the Mediterranean diet and of Mediterranean oils against 7-ketocholesterol-and 7β-hydroxycholesterol-induced cytotoxicity: Application to age-related diseases and civilization diseases. Steroids 2022, 187, 109093. [Google Scholar] [CrossRef] [PubMed]
- Badreddine, A.; Zarrouk, A.; Meddeb, W.; Nury, T.; Rezig, L.; Debbabi, M.; Bessam, F.Z.; Brahmi, F.; Vejux, A.; Mejri, M.; et al. Antioxidant and neuroprotective properties of Mediterranean oils: Argan oil, olive oil, and milk thistle seed oil. In Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–154. [Google Scholar]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The potential impact of probiotics on human health: An update on their health-promoting properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef]
- Schloendorn, J. Progress Towards Medical Bioremediation by Enzymatic Transformation of 7-Ketocholesterol and the Pyridinium Bisretinoid A2E. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2009. [Google Scholar]
- Oconnor, M.; Clemens, D. Cyclodextrin dimers for the remediation of atherosclerotic plaque. Atherosclerosis 2022, 355, 94. [Google Scholar] [CrossRef]
- Bhargava, P.; Dinh, D.; Teramayi, F.; Silberg, A.; Petler, N.; Anderson, A.M.; Clemens, D.M.; O’Connor, M.S. Selective Removal of 7KC by a Novel Atherosclerosis Therapeutic Candidate Reverts Foam Cells to a Macrophage-like Phenotype. Atherosclerosis 2025, 119217. [Google Scholar] [CrossRef]
- Laskar, A.; Miah, S.; Andersson, R.G.; Li, W. Prevention of 7β-hydroxycholesterol-induced cell death by mangafodipir is mediated through lysosomal and mitochondrial pathways. Eur. J. Pharmacol. 2010, 640, 124–128. [Google Scholar] [CrossRef]
- Ryan, L.; O’Callaghan, Y.C.; O’Brien, N.M. The role of the mitochondria in apoptosis induced by 7β-hydroxycholesterol and cholesterol-5β, 6β-epoxide. Br. J. Nutr. 2005, 94, 519–525. [Google Scholar] [CrossRef]
- Kopp, W. Aging and “Age-Related” Diseases-What Is the Relation? Aging Dis. 2024, 16, 1316–1346. [Google Scholar] [CrossRef]
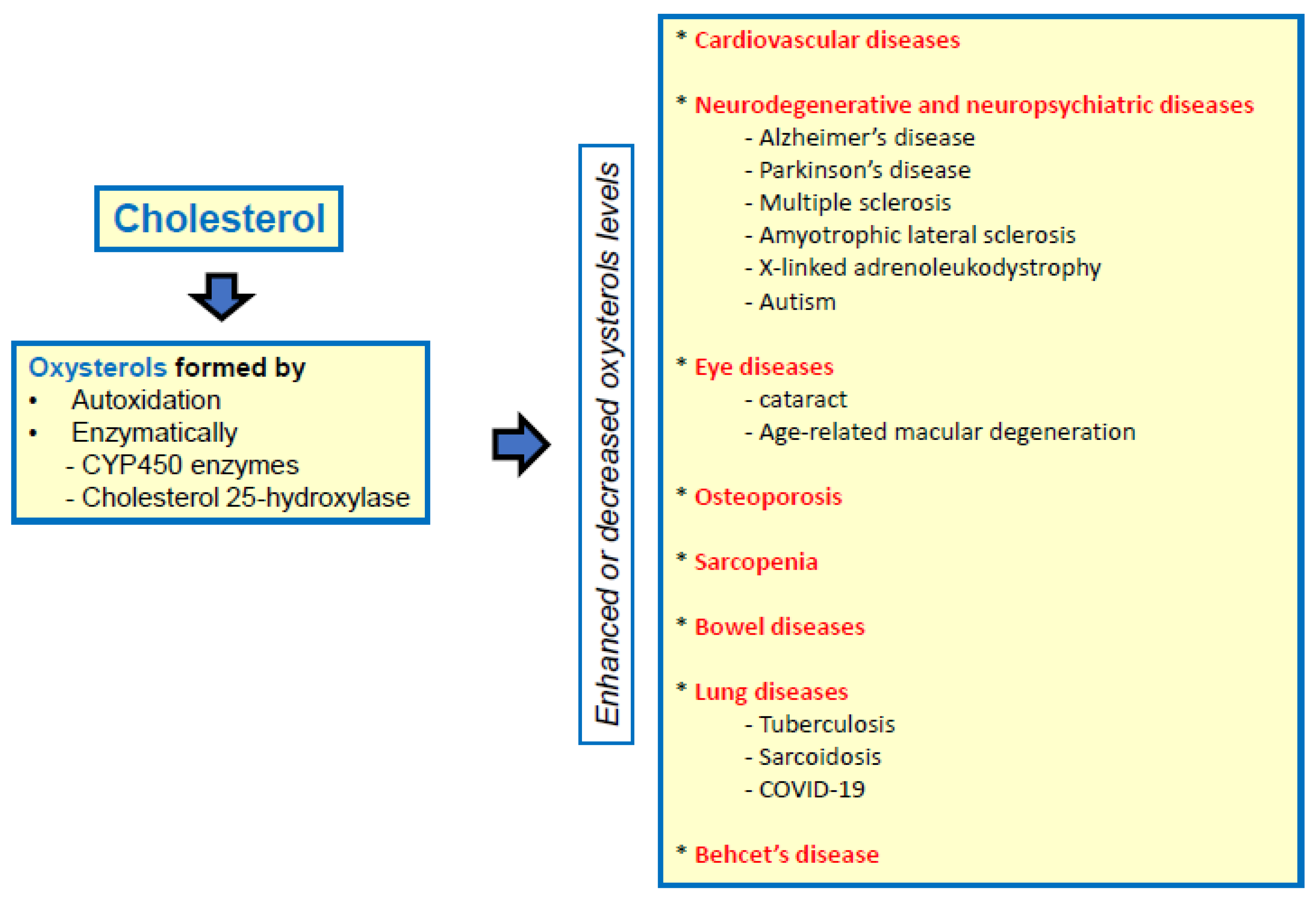
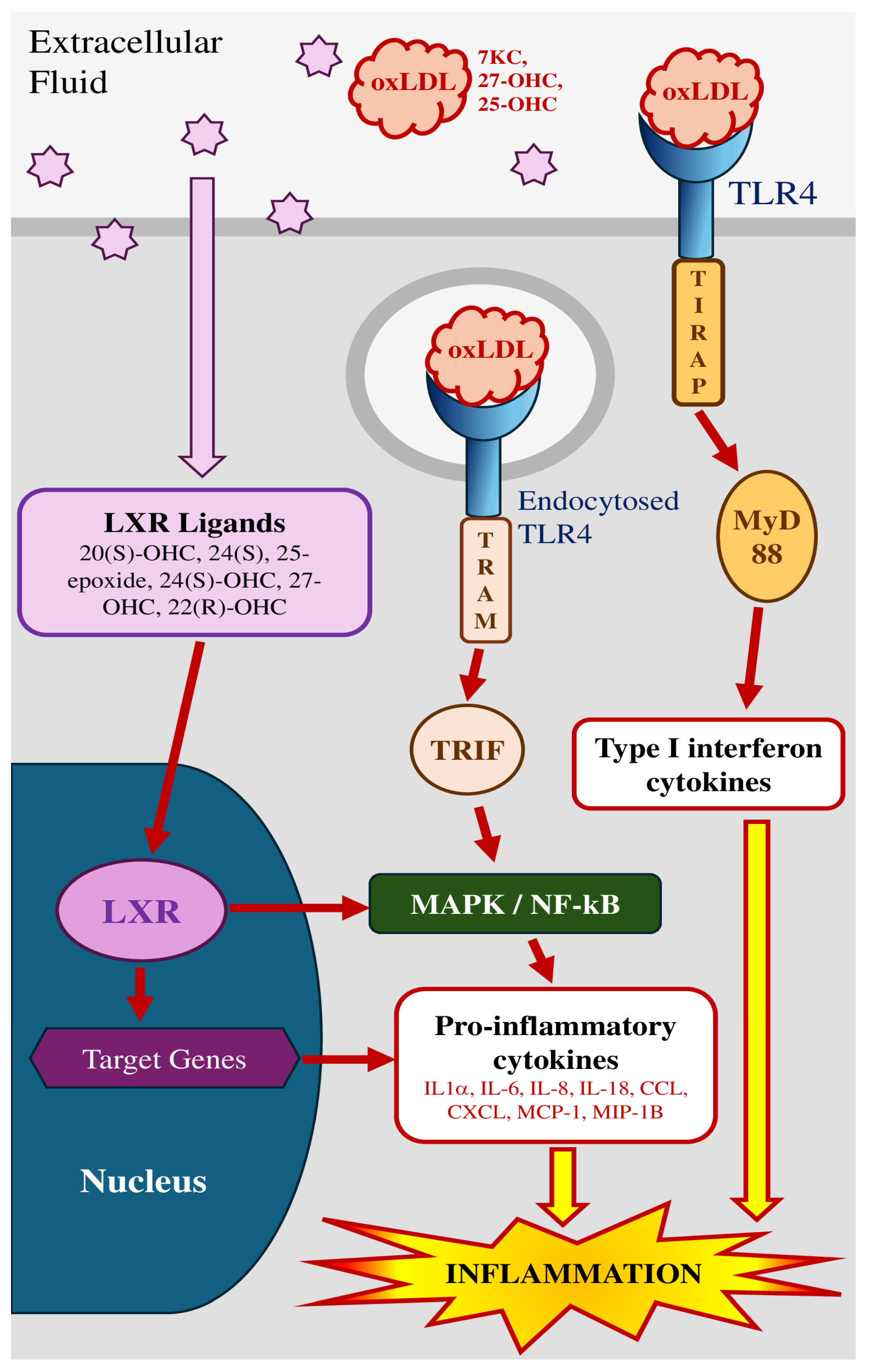
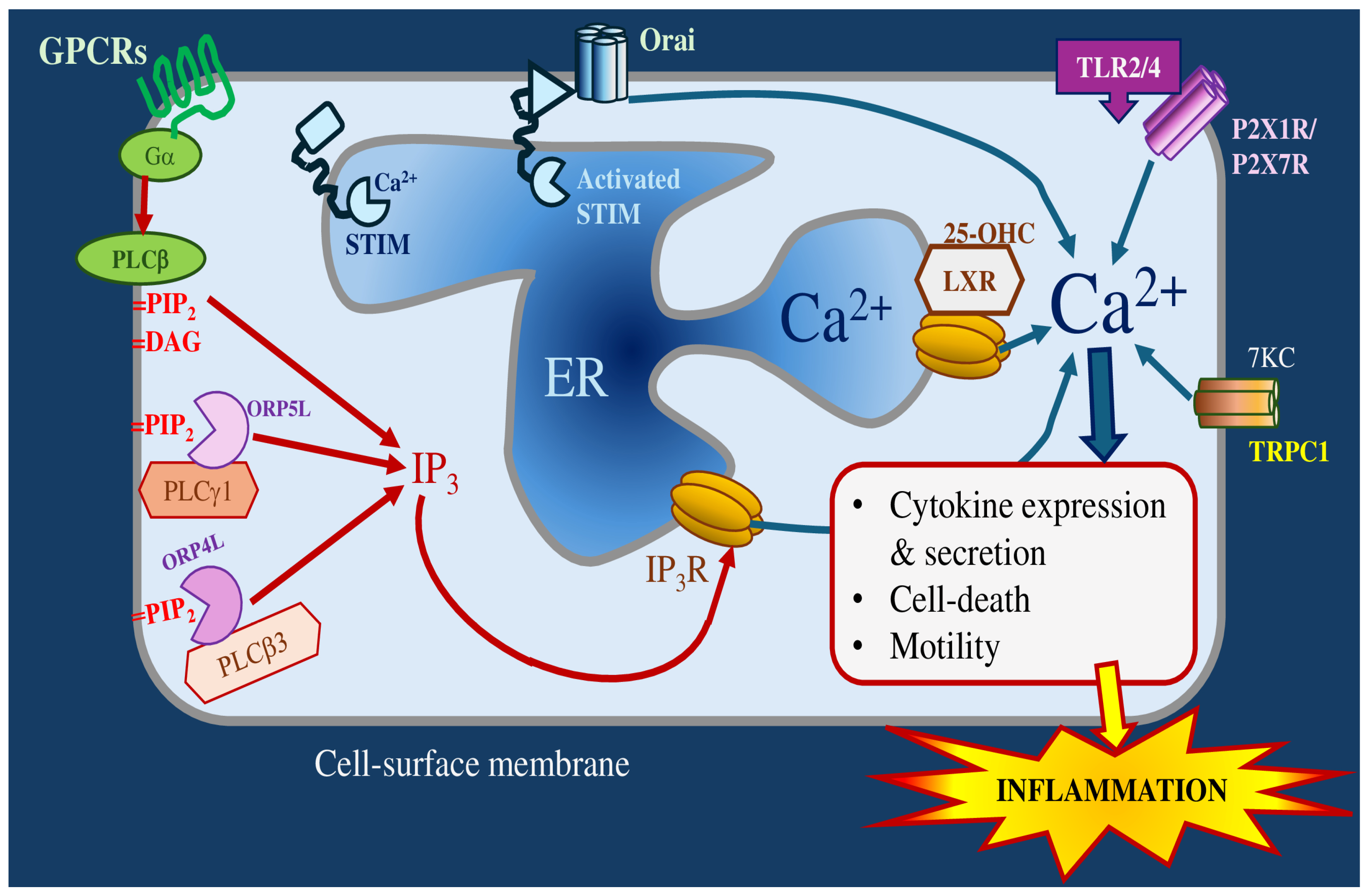
| Compounds Family | Nature Compounds (Concentration) | Used Cells | Oxysterols (Concentration) | References |
|---|---|---|---|---|
| Natural compounds | ||||
| Tocopherols | *α-tocopherol (100 mM) | Human pro-monocytic cells (U937) | 7KC (40 µg/mL) | [233] |
| *α-tocopherol (100 mM) | U937 | 7β-OHC and 7KC (200, 50, and 100 mM) | [234] | |
| *α-tocopherol (10 μM) | U937 | 7β-OH (30 mM) | [235] | |
| *α, γ-tocopherols (100 μM) | Rat aortic smooth muscle cells (A7r5) | 7KC (20 μg/mL) | [236] | |
| *α-tocopherol (400 μM) | Murine oligodendrocytes cells (158 N) | 7KC (12.5, 25, 50, and 100 μM) | [237] | |
| *α-tocopherol (400 μM) | 158 N | 7KC, 7β-OHC and 24S-OHC | [88] | |
| *α, γ-tocopherols (400 μM) | Murine microglial cells (BV-2) | 7KC (50 μM) | [57] | |
| *α-tocopherol (400 μM) | 158 N | 7KC (25–50 μM) | [238] | |
| *α-tocopherol (400 μM) | Murine neuronal cells (N2a) | 7KC (50 µM) | [239] | |
| *α-tocopherol (400 μM) | 158 N and human retinal epithelial cells (ARPE-19) | 7KC (50 μM, 100 μM) | [240] | |
| *α-tocopherol (400 μM | N2a | 7KC (50 μM) | [241] | |
| Terpenes | *β-carotene and oxygenated carotenoids lutein, canthaxanthin, astaxanthin (0.25–1 mM), and Lycopene (0.5–2 μM) | human THP-1 macrophages | 7KC (4–25 μM) and 25-OHC (2–4 μM) | [242,243,244] |
| *Lycopene and astaxanthin (0.1, 0.5 and 1 mM) | U937 | 7β-OHC (30 mM) | [235] | |
| *Bornyl acetate and menthol (50–1000 μg/mL) | SH-SY5Y, a human neuroblastoma cell line | 7KC (50–100 μg/mL) | [245] | |
| *Spinasterol and schottenol (5, 10, 20, and 40 μM). | 158 N, C6 rat glioma cells, and SK-N-BE human neuronal cells | 7 KC (25–50 µM) | [246] | |
| Polyphenols | *Resveratrol (10 µM)) | U937 | 7β-OH and cholesterol-5β, 6β-epoxide (30 μM) | [247] |
| *Epicatechin (5–10 μM) | Murine Monocyte/Macrophage (J774A,1) | 7KC (20 μM) | [248] | |
| Apigenin (0.5, 2, 5, 10, 50 mM) | U937 | 7β-OH (30 mM) | [235] | |
| *Epigallocatechin-3-gallate (1 μM) | human colonic epithelial cells (CaCo-2) | Oxysterol mixture (30 μM) | [249] | |
| *Epigallocatechin-3- gallate (30–50 μM) | Human angiosarcoma cells (ISO-HAS) | 7KC (50 μM) | [250] | |
| *Resveratrol (1.5–30 μM) | N2a, human monocytes, ARPE-19 | 7KC (15–150 μM) | [251,252] | |
| *Resveratrol, apigenin and quercetin (1.5–25 µM) | N2a | 7KC (50 μM) | [239] | |
| *Hydroxytyrosol and tyrosol (2.5–10 μM) | CaCo-2 | 7KC (1875 μM) | [253,254] | |
| *Homovanillic alcohol (5–25 μM) | CaCo-2 | 7KC (25–50) | [253,255] | |
| *Taxifoline (dihydroquercetin) (15 μM) | Rat pheochromocytoma cells (PC12); SH-SY5Y | 7KC (125 μM) | ||
| *Cocoa bean shell extracts with different polyphenol content (10 μg/mL, 25 μg/mL or 50 μg/mL) | Differentiated CaCo-2 | dietary oxysterol mixture (Oxysterol mixture; 60 μM). | [256] | |
| *Theobromine (10 μM) | CaCo-2 | Mixture of dietary oxysterols (60 μM) | [257] | |
| *Polyphenols extracted from wine (25 μg/mL) or caffeic acid, gallic acid, catechin and epicatechin (10 μM), quercetin (1 μM) | Differentiated CaCo-2- | Oxysterols-mixture (30 and 60 μM) | [258] | |
| *Quercetin or quercetin loaded into nanoparticles (5 µM) | SH-SY5Y | 24-OHC, 27-OHC, and 7β-OHC (5 µM) | [52] | |
| *Phenolic compounds extracted from wines (25 μg/mL) caffeic acid, gallic acid acid, (+)-catechin, and (−)-epicatechin (10 μM), quercetin (1 μM) | Differentiated CaCo-2 | Oxysterols-mixture (60 μM) | [259] | |
| *Olive oil phenolics: hydroxytyrosol, tyrosol, and homovanillic alcohol (0.25, 0.5, 1 μM) | Peripheral blood mononuclear cells (PBMCs) | Oxysterols mixture (20 μM) | [260] | |
| *Olive oil polyphenols (1–25 μg/mL) | CaCo-2 | Oxysterols mixture (60 μM) | [261] | |
| Betalains | *Indicaxanthin (1–5 μM) | THP-1 andhuman erythrocytes | 7KC (7–16 μM) | [262,263] |
| Plant extracts | *Red yeast rice extract (100 μg/mL) | RAW 264.7 | 7KC (70 μM) | [264] |
| *Ethanolic mint leaf extract (25–400 μg/mL) | RAW 264.7 | 7KC (20 μM) | [265] | |
| *Clinacanthus nutans (Lindau) extract (100 μg/mL) | hCMEC/D3 human brain endothelial cells | 7KC (30 μM) | [266] | |
| *Carpobrotus edulis extract (20–200 µg/mL) | 158 N | 7β-OHC (20 μg/mL) | [267] | |
| *Soy-leaf ethanolic extract (100 μg/mL) | Human umbilical vein endothelial cells (HUVECs) | 7 KC (20 µg/mL) | [268] | |
| *Anthocyanin-rich extract from Aronia melanocarpa E (50 μg/mL) | HUVECs | 7β-OH (20 μg/mL). | [269] | |
| *Digera muricata extract (25, 50, 100, 200, 400, 600, 800 and 1000 µg/mL) | IC-21 macrophage cell line | 7KC (2, 4, 6, 8, 10 µg/mL) | [270] | |
| Edible oils | *Olive oil (1/1000) | 158 N | 7 KC (50 μM) | [57,271] |
| *Argan and olive oils (1/1000, 1/1000) | 158 N | 7 KC (50 μM) | [238] | |
| *Milk thistle seed oil (3/1000) | 158 N | 7 KC (50 μM) | [272] | |
| *Sea urchin egg oil (10–600 μg/mL) | 158 N | 7β-OHC (50 μM) | [273] | |
| *Silybum seeds oil (60 mg/mL) | THP-1 | 7KC and 7β-OHC (25–50 µM) | [274] | |
| Fatty acids | *Docosahexaenoic acid (50 μM) | 158 N | 7KC, 7β-OHC and 24S-OHC (25–50 µM) | [88] |
| *Docosahexaenoic acid (50 μM) | SK-N-BE | 7KC, 7α and 7β-OHC, 6α and 6β–OHC, 4 α and 4β-OHC 24S-OHC and 27-OHC (50–100 μM) | [275] | |
| *Oleic acid (200 μM) | BV-2 | 7KC (50 μM) | [57] | |
| *Oleic acid and elaidic acid (50, 100, 200, 300, 600 μM), docosahexaenoic acid (12, 25, 50 and 100 μM) | BV-2 | 7KC (25–50 μM) | [271] | |
| *α-linolenic, eicosapentaenoic and docosahexaenoic acids (50 μM) | N2a | 7 KC (50 μM) | [276] | |
| *Docosahexaenoic acid (50 μM) | BV-2, 158 N | 7 KC (25–50 μM) | [88] | |
| *Sterculic acid (1 μM) | ARPE-19 | 7KC (12 μM) | [277] | |
| Hydroxycholesteryl-3-oleate (50 μ M), 7-ketocholesteryl-3-oleate (100 μM) | U937 | 7 β-OHC (50 μM), 7KC (100 μM) | [278] | |
| *Acetate, propionate and butyrate | HUVECs | 7KC | [279] | |
| *Bis(monoacylglycero)phosphate | RAW264.7 | 7KC | [280] | |
| *α-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, erucic acid, and oleic acid (100 μM), Lorenzo’s oil (a mixture of oleic and erucic acid, 4:1) | 158 N and ARPE-19 cells | 7KC (50 μM and 100 μM) | [240] | |
| *α-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, oleic acid (1.5 to 200 μM) | N2a | 7KC (50 μM) | [241] | |
| Probiotics and microbial enzymes | - Cholesterol oxydase (Chromobacterium DS-1) - bacteria Rhodococcus erythropolis MTCC 3951 | Human fibroblasts | 7KC (25–50 μM) | [281,282] |
| Pseudomonas aeruginosa PseA | Bacterial culture | 7KC (1 g/L) | [283] | |
| Lactiplantibacillus plantarum and Lacticaseibacillus casei (70 μL/mL) | CaCo-2 | Oxysterols-mixture (100 μM) | [284] | |
| Thermobifidafusca IP1 | Bacterial culture | 7KC (0.0025 M) | [285] | |
| Synthetic molecules | ||||
| *N-acetyl-l-cysteine (10–15 mM) | U937, murine osteoblats (MC3T3- E1) | KC (100 μM) | [233,286] | |
| *Aminothiols glutathione (10–15 mM) | U937 | 7KC (100 μM) | [233] | |
| *Ergothioneine (0.1–1 mM) | Human brain endothelial cells (hCMEC/D3) | 7KC (30 μM) | [287] | |
| *Dimethyl fumarate/Monomethylfumarate (25–50 μM) | PC12 | 7KC (100 μM) | [232,288] | |
| *Biotin (10 and 100 nM) | 158 N | 7β-OHC (50 µM) | [289] | |
| *UDP-003 (25, 50, 100, and 150 μM) | RAW 264.7 | 7KC (10 μM, 40 μM) | [290] | |
| *Sulfo-N-succinimidyl oleate (25–50 µM) | 158 N and ARPE-19 cells | 7KC | [240] | |
| *Memantine (1 μM, 1 mM) | 158 N | 7KC (25–50 μM) | [232] | |
| *Simvastatine (0.01 μM, 0.05 μM) | ARPE-19 | 7KC (75 μM) | [232] | |
| *Mangafodipir (15 μM to 800 μM) | U-937 | 7β-OHC (25 μM) | [291] | |
| *Rivaroxaban (100 and 500 ng/mL) | HUVECs | 25-OHC (10 μg/mL) | [292] | |
| *Trolox (10 mM) ebselen (2 mM/L) | U937 | 7β-OH and cholesterol-5β, 6β-epoxide (30 µM) | [247] | |
| *Azelnidipine (0.1 μM) | U937 | 7KC (20 μM) | [293] | |
| *K-80003 (20 μM) | RAW264.7 Bone marrow cells | 7KC (40 μM) | [294] | |
| *3β-Sulfate-5-4 cholestenoic acid | Primary human hepatocytes, Huh-7, and HepG-2 cells | Cholestenoic acid, 25-OHC, 27-OHC (20 μM) | [295] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brahmi, F.; Mackrill, J.J.; Ghzaiel, I.; Rezig, L.; Benkhalifa, R.; Zarrouk, A.; Jouanny, P.; Vejux, A.; Lizard, G. Oxysterol-Induced Inflammation in Human Diseases: Strategies for Treatment with Natural Compounds and Synthetic Molecules. Molecules 2025, 30, 2883. https://doi.org/10.3390/molecules30132883
Brahmi F, Mackrill JJ, Ghzaiel I, Rezig L, Benkhalifa R, Zarrouk A, Jouanny P, Vejux A, Lizard G. Oxysterol-Induced Inflammation in Human Diseases: Strategies for Treatment with Natural Compounds and Synthetic Molecules. Molecules. 2025; 30(13):2883. https://doi.org/10.3390/molecules30132883
Chicago/Turabian StyleBrahmi, Fatiha, John J. Mackrill, Imen Ghzaiel, Leila Rezig, Rym Benkhalifa, Amira Zarrouk, Pierre Jouanny, Anne Vejux, and Gérard Lizard. 2025. "Oxysterol-Induced Inflammation in Human Diseases: Strategies for Treatment with Natural Compounds and Synthetic Molecules" Molecules 30, no. 13: 2883. https://doi.org/10.3390/molecules30132883
APA StyleBrahmi, F., Mackrill, J. J., Ghzaiel, I., Rezig, L., Benkhalifa, R., Zarrouk, A., Jouanny, P., Vejux, A., & Lizard, G. (2025). Oxysterol-Induced Inflammation in Human Diseases: Strategies for Treatment with Natural Compounds and Synthetic Molecules. Molecules, 30(13), 2883. https://doi.org/10.3390/molecules30132883










