Antihyperglycemic Activity of Alchemilla viridiflora Herb Methanol Extract in Streptozotocin-Induced Diabetic Male Rats
Abstract
1. Introduction
2. Results
2.1. Main Compound Isolated from Alchemilla viridiflora Methanol Extract
2.2. Quantitative LC-MS Analyses of Alchemilla viridiflora Methanol Extracts
2.3. In Vitro Inhibition of Enzymes
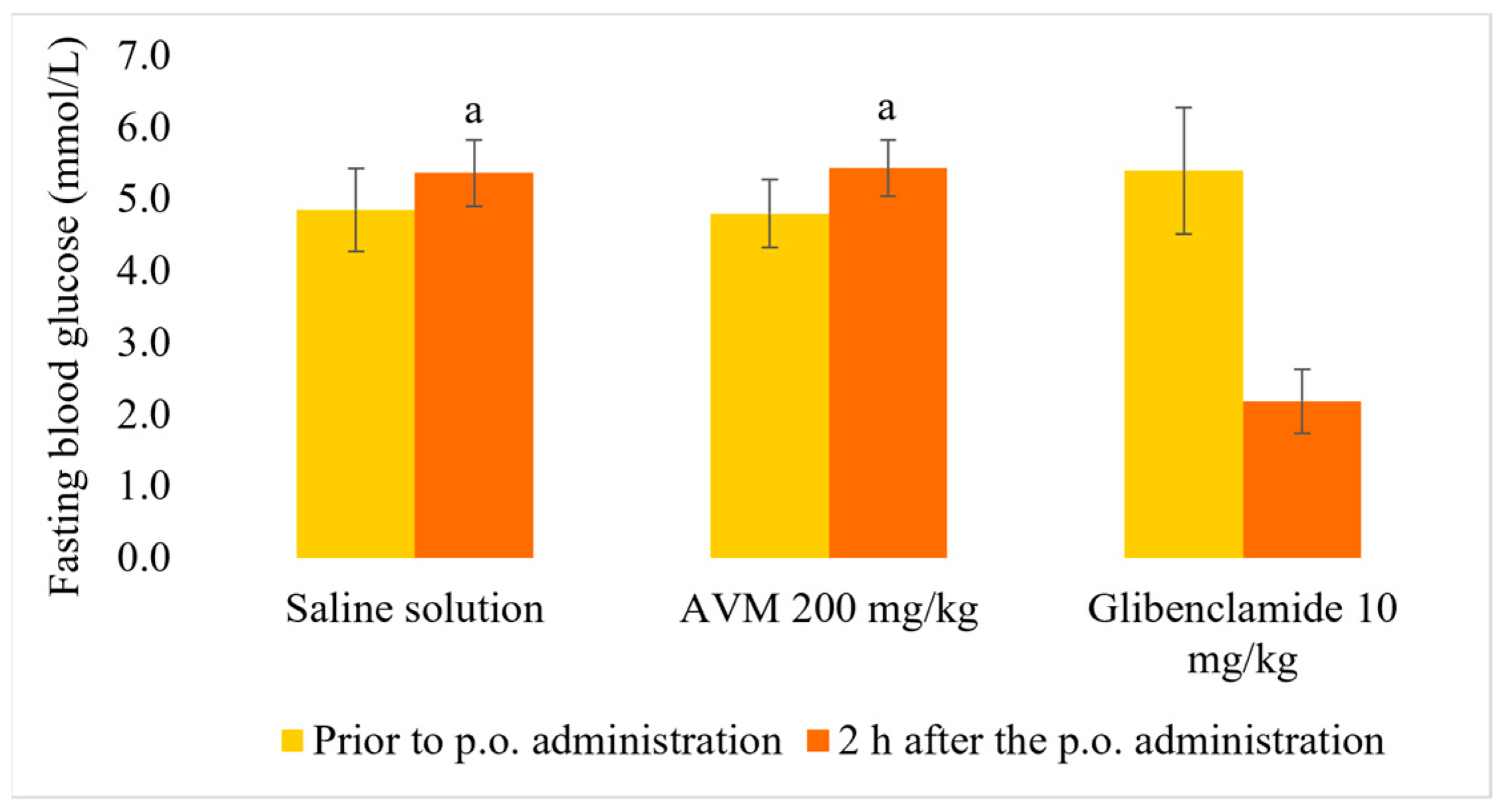
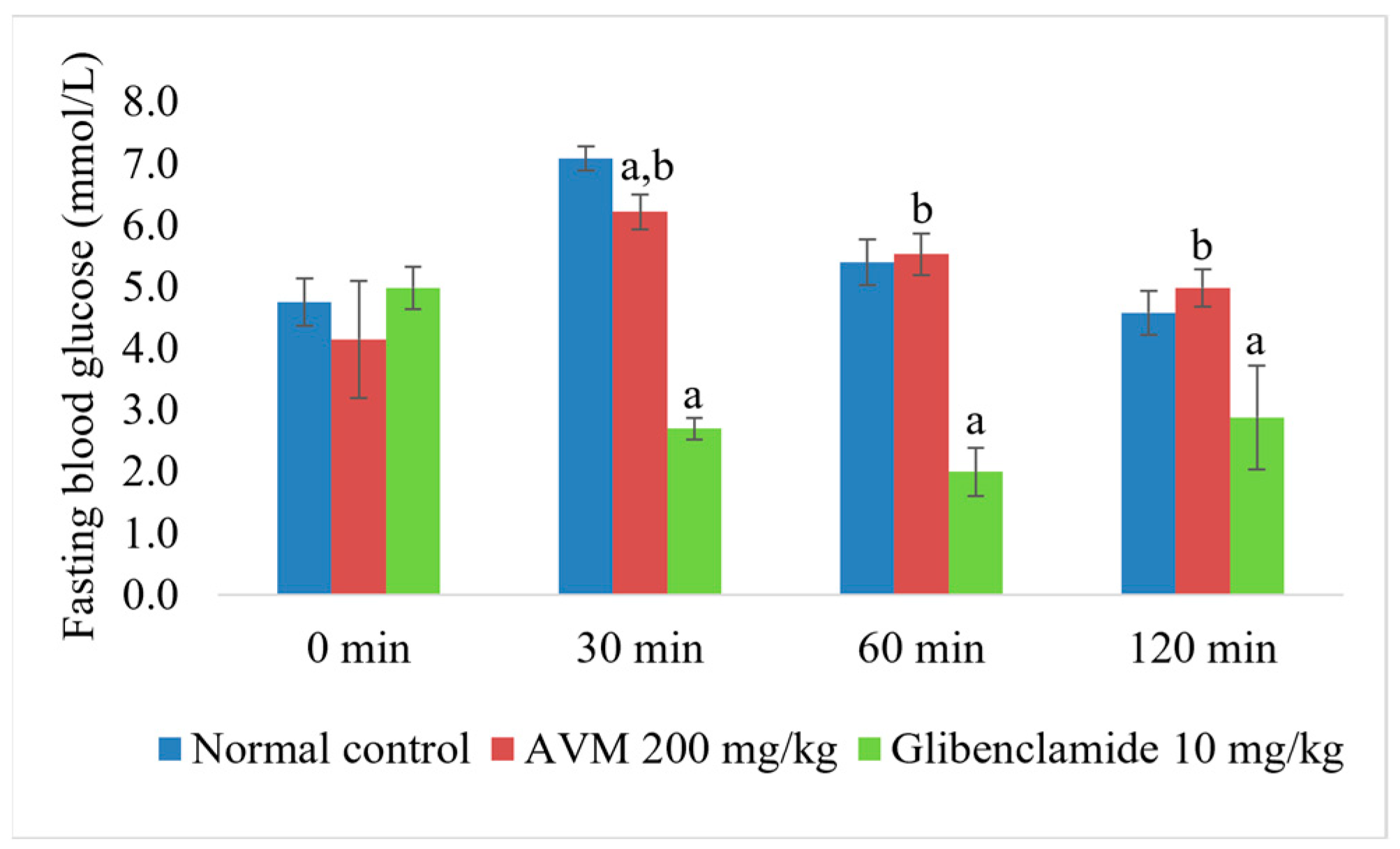
2.4. Normoglycemic and Oral Glucose Tolerance Test
2.5. In Vivo Hypoglycemic Effect of AVM
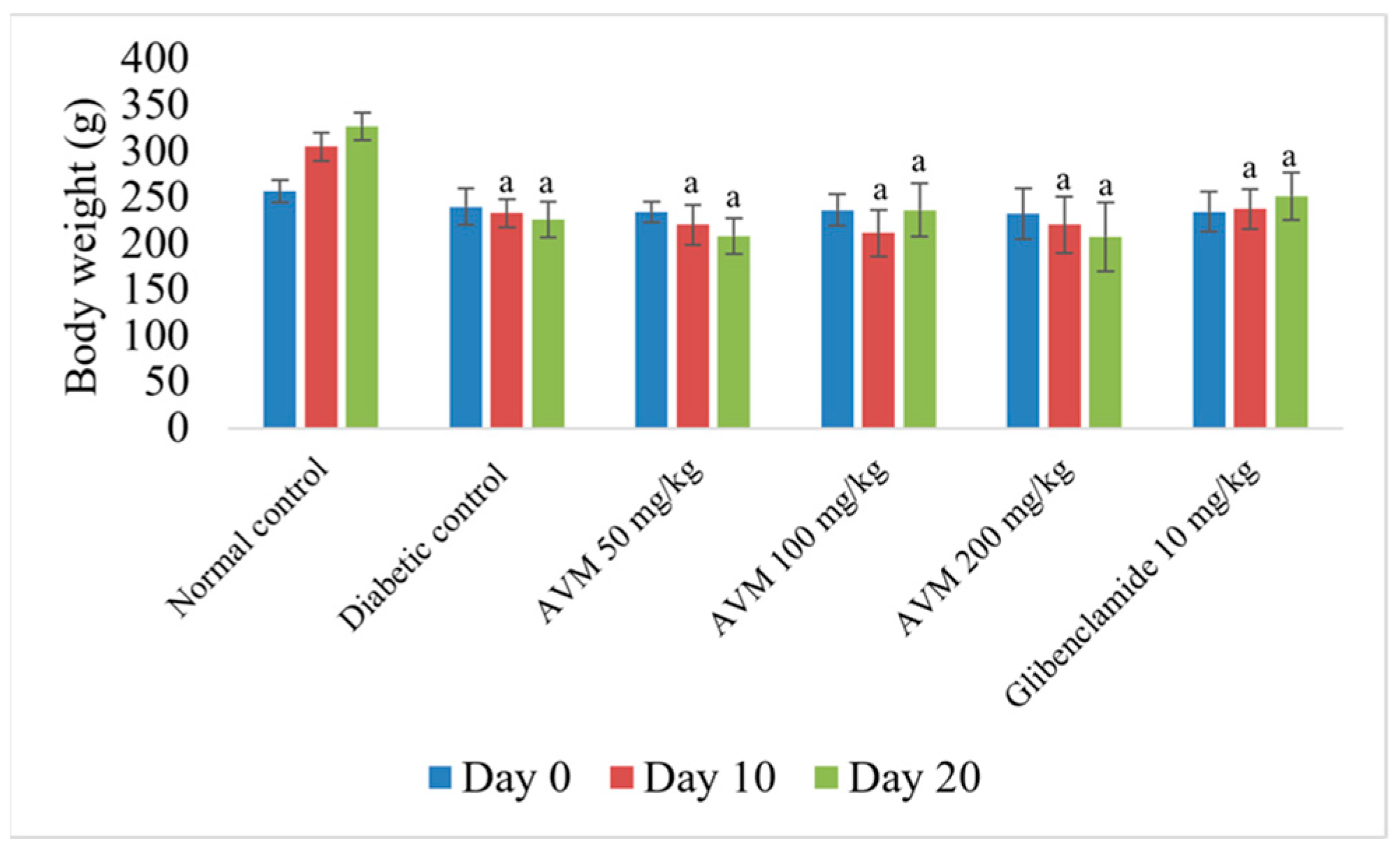
2.6. Effect of AVM on Body Weight
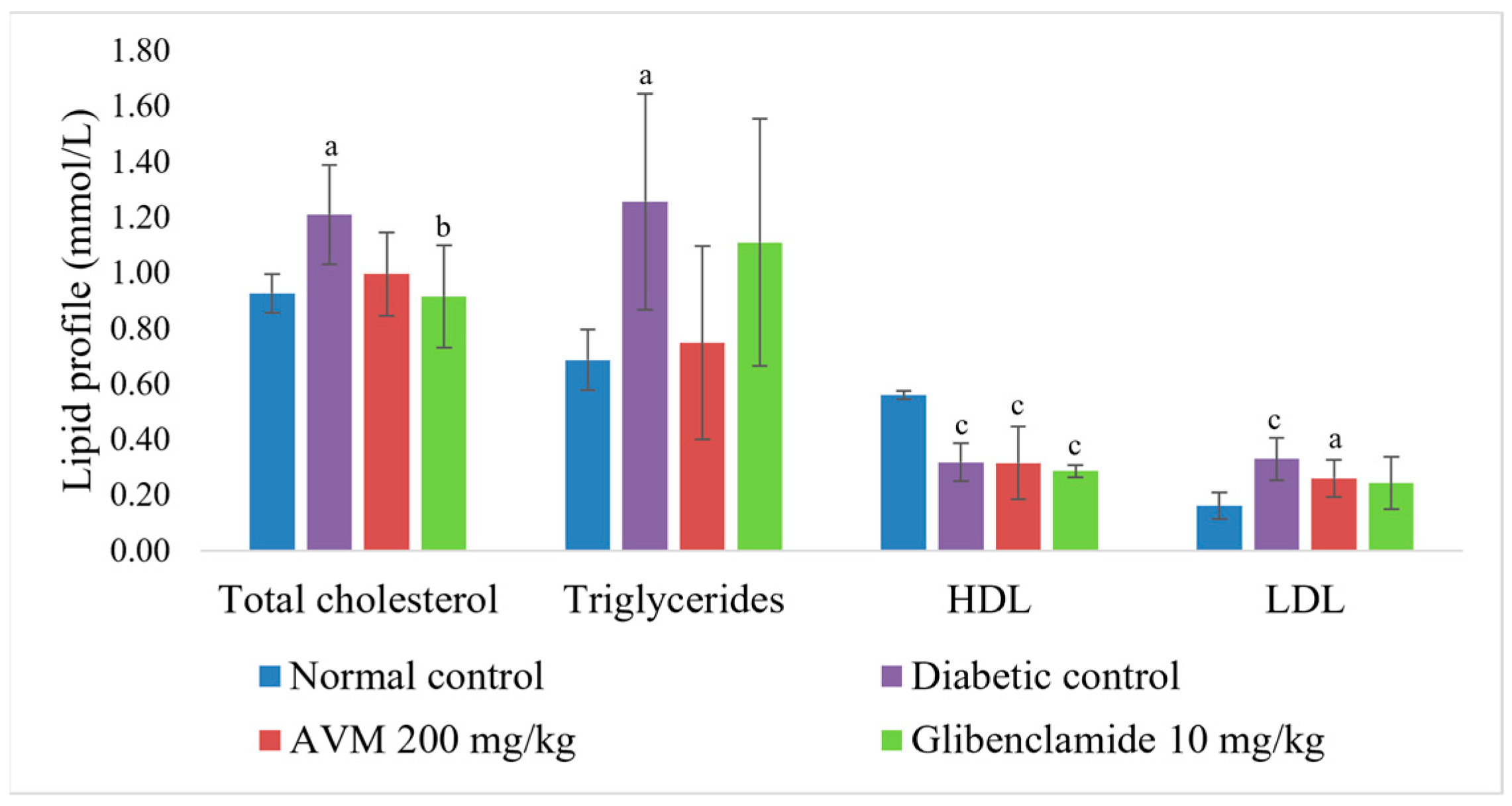
2.7. Effect of AVM on Biochemical Parameters
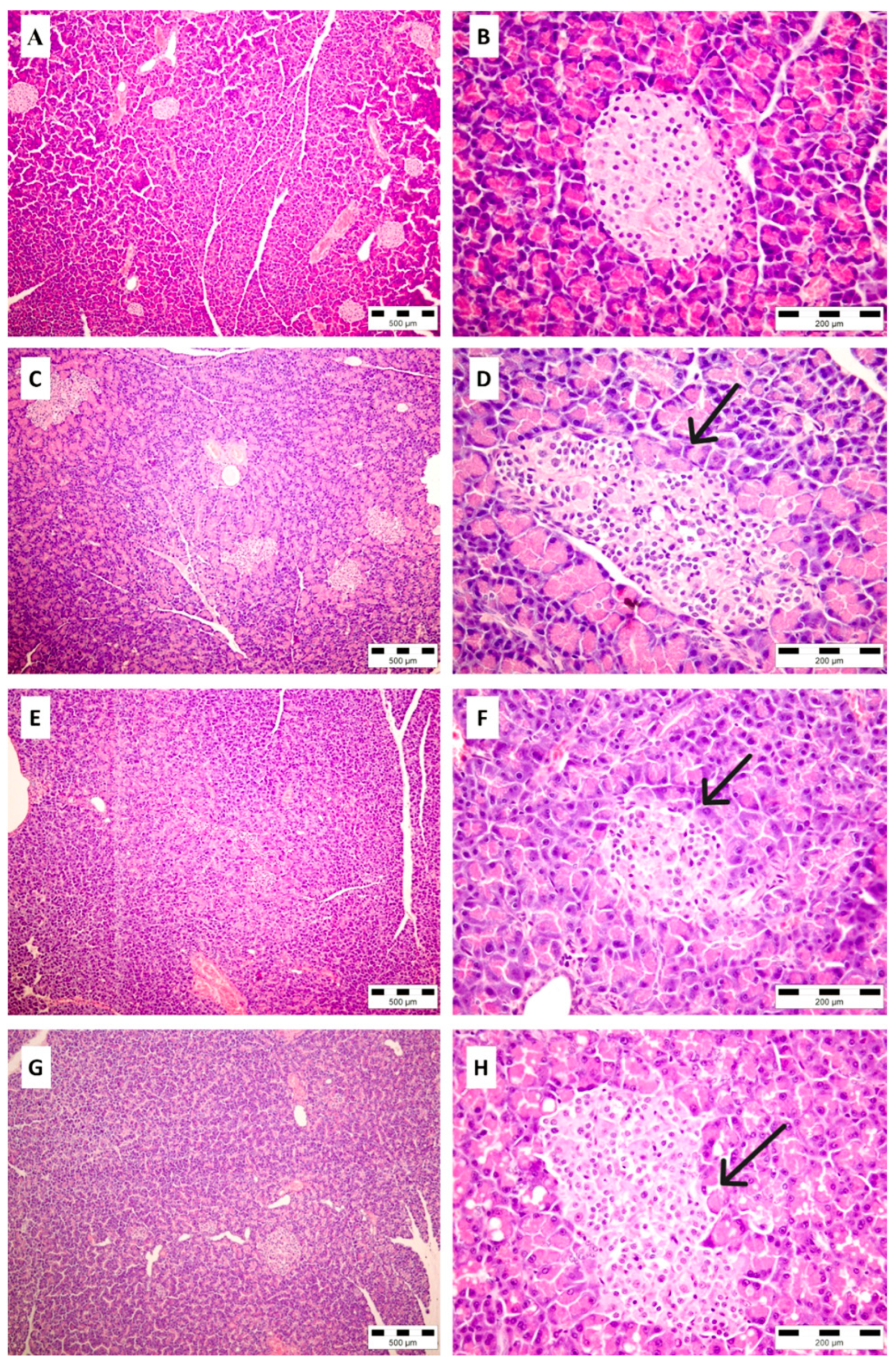
2.8. Histopathological Analysis of Pancreatic Tissue
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction Procedure
4.3. Isolation and Identification of Quercetin-3,4′-Dimethyl Ether-7-O-Glucuronide from Alchemilla viridiflora Methanol Extract
4.4. LC-MS Analysis of Alchemilla viridiflora Methanol Extract
4.5. α-Amylase Inhibitory Assay
4.6. α-Glucosidase Inhibitory Assay
4.7. Pancreatic Lipase Inhibitory Assay
4.8. Experimental Animals
4.9. Normoglycemic Study and Oral Glucose Tolerance Test in Rats
- Group 1: rats administered 1 mL of saline solution, per os (p.o.) once;
- Group 2: rats administered AVM in dose 200 mg/kg b.w., p.o. once;
- Group 3: rats administered glibenclamide, 10 mg/kg b.w., p.o. once.
4.10. Hypoglycemic Effect of AVM in Streptozotocin-Induced DM
- Group 1: normal control, rats administered saline solution (1 mL/kg b.w./day);
- Group 2: diabetic control, rats administered saline solution (1 mL/kg b.w./day);
- Group 3: diabetic rats administered AVM 50 mg/kg b.w./day;
- Group 4: diabetic rats administered AVM 100 mg/kg b.w./day;
- Group 5: diabetic rats administered AVM 200 mg/kg b.w./day;
- Group 6: positive control, rats administered glibenclamide, 10 mg/kg b.w./day.
4.11. Body Weight and Biochemical Estimation
4.12. Histological Examination of the Pancreas
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| AVM | Alchemilla viridiflora methanol extract |
| STZ | Streptozotocin |
| IC50 | Concentrations of extract that leads to 50% of inhibition |
| b.w. | Body weight |
| WHO | World Health Organization |
| CH | Cholesterol |
| TG | Triglycerides |
| HDL | High-density lipoproteins |
| LDL | Low-density lipoproteins |
| LC-MS | Liquid chromatography–mass spectrometry |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| DNSA | 3,5-dinitrosalicylic acid |
| DMSO | Dimethyl sulfoxide |
| IU | International unit |
| PNP-G | p-nitrophenyl-α-D-glucopyranoside |
| p-NPB | p-nitrophenyl butyrate |
| OGTT | Oral glucose tolerance test |
| p.o. | Per os |
| i.p. | Intraperitoneal |
| H&E | Hematoxylin and eosin |
| ANOVA | One-factor analysis of variance |
| GA | Gallic acid |
| GLUT4 | Glucose transporter type 4 |
| (PI3K)/(AKT) | Phosphoinositide 3-kinase/protein kinase B |
References
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes mellitus: Insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Roglić, G. WHO Global report on diabetes: A summary. Int. J. Noncommun. Dis. 2016, 1, 3–8. [Google Scholar] [CrossRef]
- Bhowmik, B.; Siddiquee, T.; Mujumder, A.; Afsana, F.; Ahmed, T.; Mdala, I.A.; Do V. Moreira, N.C.; Khan, A.K.A.; Hussain, A.; Holmboe-Ottesen, G.; et al. Serum lipid profile and its association with diabetes and prediabetes in a rural Bangladeshi population. Int. J. Environ. Res. Public. Health 2018, 15, 1944. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Grigsby, J.; Mbemi, A.; Nelson, D.; Mildort, B.; Latinwo, L.; Tchounwou, P.B. The management of diabetes mellitus using medicinal plants and vitamins. Int. J. Mol. Sci. 2023, 24, 9085. [Google Scholar] [CrossRef]
- Governa, P.; Baini, G.; Borgonetti, V.; Cettolin, G.; Giachetti, D.; Magnano, A.R.; Miraldi, E.; Biagi, M. Phytotherapy in the management of diabetes: A review. Molecules 2018, 23, 105. [Google Scholar] [CrossRef]
- Venkateswaran, M.R.; Vadivel, T.E.; Jayabal, S.; Murugesan, S.; Rajasekaran, S.; Periyasamy, S. A review on network pharmacology based phytotherapy in treating diabetes- An environmental perspective. Environ. Res. 2021, 202, 111656. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2017, 8, 361–376. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Lee, T.H.; Chang, C.L.; Huang, Y.T.; Yang, W.C. Anti-hyperglycemic effects and mechanism of Bidens pilosa water extract. J. Ethnopharmacol. 2009, 122, 379–383. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary polyphenols, mediterranean diet, prediabetes, and type 2 diabetes: A narrative review of the evidence. Oxid. Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- El-Abhar, H.S.; Schaalan, M.F. Phytotherapy in diabetes: Review on potential mechanistic perspectives. World J. Diabetes 2014, 5, 176–197. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Akbulut, S.; Bayramoglu, M.M. Reflections of socio-economic and demographic structure of urban and rural on the use of medicinal and aromatic plants: The sample of Trabzon Province. Stud. Ethno-Med. 2014, 8, 89–100. [Google Scholar] [CrossRef]
- Kanak, S.; Krzemińska, B.; Celiński, R.; Bakalczuk, M.; Dos Santos Szewczyk, K. Phenolic composition and antioxidant activity of Alchemilla species. Plants 2022, 11, 2709. [Google Scholar] [CrossRef]
- Tadić, V.; Krgović, N.; Žugić, A. Lady’s mantle (Alchemilla vulgaris L., Rosaceae): A review of traditional uses, phytochemical profile, and biological properties. Lek. Sirovine 2020, 40, 66–74. [Google Scholar] [CrossRef]
- Samah, S.; Abdullah, K.; Ream, N. Phytochemical screening of Alchemilla vulgaris, Sophora japonica, Crataegus azarolus, and their inhibitory activity on lipase and α-amylase. Int. J. Appl. Sci. Res. 2018, 6, 1–21. [Google Scholar]
- Renda, G.; Ozel, A.; Barut, B.; Korkmaz, B.; Soral, M.; Kandemir, U.; Liptaj, T. Bioassay guided isolation of active compounds from Alchemilla barbatiflora Juz. Rec. Nat. Prod. 2018, 12, 76–85. [Google Scholar] [CrossRef]
- Uzunkaya, Ç.; Gökkaya, İ.; Akkaya, D.; Soral, M.; Seyhan, G.; Barut, B.; Yılmaz, M.A.; Renda, G. Phytochemical analysis and assessment of antioxidant and enzyme inhibitory activity of Alchemilla pseudocartalinica Juz. Chem. Biodivers. 2025, 22, e202401217. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Jelača, S.; Zengin, G.; Mimica-Dukić, N.; Berežni, S.; Miljić, M.; Stevanović, Z.D. Alchemilla vulgaris agg. (Lady’s mantle) from central Balkan: Antioxidant, anticancer and enzyme inhibition properties. RSC Adv. 2019, 9, 37474–37483. [Google Scholar] [CrossRef] [PubMed]
- Özbilgin, S.; Özbek, H.; Kirmizi, N.İ.; Öz, B.E.; Kurtul, E.; Özrenk, B.C.; Işcan, G.S.; Acikara, Ö.B. Evaluation of the antidiabetic activity of Alchemilla persica Rothm. in mice with diabetes induced by alloxan. Turk. J. Pharm. Sci. 2019, 16, 261–264. [Google Scholar] [CrossRef]
- Ozbek, H.; Acikara, O.B.; Keskin, I.; Kirmizi, N.I.; Ozbilgin, S.; Oz, B.E.; Kurtul, E.; Ozrenk, B.C.; Tekin, M.; Saltan, G. Evaluation of hepatoprotective and antidiabetic activity of Alchemilla mollis. Biomed. Pharmacother. 2017, 86, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Swanston-Flatt, S.K.; Day, C.; Bailey, C.J.; Flatt, P.R. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia 1990, 33, 462–464. [Google Scholar] [CrossRef]
- Radović, J.; Suručić, R.; Niketić, M.; Kundaković-Vasović, T. Alchemilla viridiflora Rothm.: The potent natural inhibitor of angiotensin I-converting enzyme. Mol. Cell. Biochem. 2022, 477, 1893–1903. [Google Scholar] [CrossRef]
- Suručić, R.; Radović Selgrad, J.; Kundaković-Vasović, T.; Lazović, B.; Travar, M.; Suručić, L.; Škrbić, R. In silico and in vitro studies of Alchemilla viridiflora Rothm—Polyphenols’ potential for inhibition of SARS-CoV-2 internalization. Molecules 2022, 27, 5174. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, K.A.; Day, A.J.; Needs, P.W.; Sly, W.S.; O’Brien, N.M.; Williamson, G. Flavonoid glucuronides are substrates for human liver beta-glucuronidase. FEBS Lett. 2001, 503, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Okuka, N.; Ivanović, N.D.; Milinković, N.; Polovina, S.; Sumarac-Dumanović, M.; Minić, R.; Djordjević, B.; Veličković, K. Probiotic supplementation improves hematological indices and morphology of red blood cells and platelets in obese women: A double-blind, controlled pilot study. Metabolites 2025, 15, 310. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Afshar, F.H.; Maggi, F.; Ferrari, S.; Peron, G.; Dall’Acqua, S. Secondary metabolites of Alchemilla persica growing in Iran (East Azarbaijan). Nat. Prod. Commun. 2015, 10, 1705–1708. [Google Scholar] [CrossRef]
- Kurtul, E.; Eryilmaz, M.; Sarialtin, S.Y.; Tekin, M.; Acikara, O.B.; Coban, T. Bioactivities of Alchemilla mollis, Alchemilla persica and their active constituents. Brazilian J. Pharm. Sci. 2022, 58, 10. [Google Scholar] [CrossRef]
- Wolniak, M.; Tomczyk, M.; Gudej, J.; Wawer, I. Structural studies of methyl brevifolincarboxylate in solid state by means of NMR spectroscopy and DFT calculations. J. Mol. Struct. 2006, 825, 26–31. [Google Scholar] [CrossRef]
- Tian, J.; Xie, Y.; Zhao, Y.; Li, C.; Zhao, S. Spectroscopy characterization of the interaction between brevifolin carboxylic acid and bovine serum albumin. Luminescence 2011, 26, 296–304. [Google Scholar] [CrossRef]
- Jelača, S.; Dajić-Stevanović, Z.; Vuković, N.; Kolašinac, S.; Trendafilova, A.; Nedialkov, P.; Stanković, M.; Tanić, N.; Tanić, N.T.; Acović, A.; et al. Beyond traditional use of Alchemilla vulgaris: Genoprotective and antitumor activity in vitro. Molecules 2022, 27, 8113. [Google Scholar] [CrossRef]
- Jamous, R.M.; Abu-Zaitoun, S.Y.; Akkawi, R.J.; Ali-Shtayeh, M.S. Antiobesity and antioxidant potentials of selected Palestinian medicinal plants. J. Evid.-Based Complement. Altern. Med. 2018, 2018, 8426752. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Sobrevilla, J.; Boone-Villa, V.D.; Aguilar, C.N.; Román-Ramos, R.; Vega-Avila, E.; Campos-Sepúlveda, E.; Alar-cón-Aguilaret, F. Effect of varying dose and administration of streptozotocin on blood sugar in male CD1 mice. Proc. West. Pharmacol. Soc. 2011, 54, 5–9. [Google Scholar]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Swami, S.; Sztalryd, C.; Kraemer, F.B. Effects of streptozotocin-induced diabetes on low density lipoprotein receptor ex-pression in rat adipose tissue. J. Lipid Res. 1996, 37, 229–236. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.M.; Sode, M.; Gozalov, A.; Ashina, M. The vascular effect of glibenclamide: A systematic review. Cephalalgia Rep. 2019, 2, 1–13. [Google Scholar] [CrossRef]
- Mladenova, S.G.; Vasileva, L.V.; Savova, M.S.; Marchev, A.S.; Tews, D.; Wabitsch, M.; Ferrante, C.; Orlando, G.; Georgiev, M.I. Anti-adipogenic effect of Alchemilla monticola is mediated via PI3K/AKT signaling inhibition in human adipocytes. Front. Pharmacol. 2021, 12, 707507. [Google Scholar] [CrossRef]
- Krivokuća, M.; Niketić, M.; Milenković, M.; Golić, N.; Masia, C.; Scaltrito, M.M.; Sisto, F.; Kundaković, T. Anti-helicobacter pylori activity of four Alchemilla species (Rosaceae). Nat. Prod. Commun. 2015, 10, 1369–1371. [Google Scholar] [CrossRef] [PubMed]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.M.A.K.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as antidiabetic and anti-inflammatory agents: A review on structural activity relationship-based studies and meta-analysis. Int. J. Mol. Sci. 2022, 23, 12605. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Wang, T.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg. Med. Chem. 2003, 11, 5317–5323. [Google Scholar] [CrossRef]
- Matsuda, H.; Kogami, Y.; Nakamura, S.; Sugiyama, T.; Ueno, T.; Yoshikawa, M. Structural requirements of flavonoids for the adipogenesis of 3T3-L1 cells. Bioorg. Med. Chem. 2011, 19, 2835–2841. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Kayano, S.; Matsumura, Y.; Konishi, Y.; Tanaka, Y.; Kikuzaki, H. Antimutagenic and α-glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem. 2011, 125, 471–475. [Google Scholar] [CrossRef]
- Sieniawska, E. Activities of tannins—From in vitro studies to clinical trials. Nat. Prod. Commun. 2015, 10, 1877–1884. [Google Scholar] [CrossRef]
- Kumari, M.; Jain, S. Tannins: An antinutrient with positive effect to manage diabetes. Res. J. Recent Sci. 2012, 1, 70–73. [Google Scholar]
- Kato, C.G.; Gonçalves, G.A.; Peralta, R.A.; Seixas, F.A.V.; de Sá-Nakanishi, A.B.; Bracht, L.; Comar, J.F.; Bracht, A.; Peralta, R.M. Inhibition of α-amylases by condensed and hydrolysable tannins: Focus on kinetics and hypoglycemic actions. Enzyme Res. 2017, 2017, 5724902. [Google Scholar] [CrossRef] [PubMed]
- Ochir, S.; Nishizawa, M.; Park, B.J.; Ishii, K.; Kanazawa, T.; Funaki, M.; Yamagishi, T. Inhibitory effects of Rosa gallica on the digestive enzymes. J. Nat. Med. 2010, 64, 275–280. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Chirikova, N.K.; Olennikov, D.N. Agrimoniin, an active ellagitannin from Comarum palustre herb with anti-α-glucosidase and antidiabetic potential in streptozotocin-induced diabetic rats. Molecules 2017, 22, 73. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.M. Alchemilla, L. In Flora Europaea; Tutin, T., Heywood, V., Burges, N., Moore, D., Valentine, D., Walters, S., Eds.; Cambridge University Press: Cambridge, UK, 1981; pp. 48–64. [Google Scholar]
- Radović, J.; Leković, A.; Tačić, A.; Dodevska, M.; Stanojković, T.; Marinković, T.; Jelić, Č.; Kundaković-Vasović, T. Black trumpet, Craterellus cornucopioides (L.) Pers.: Culinary mushroom with angiotensin converting enzyme inhibitory and cytotoxic activity. Pol. J. Food Nutr. Sci. 2022, 72, 171–181. [Google Scholar] [CrossRef]
- Wickramaratne, M.N.; Punchihewa, J.C.; Wickramaratne, D.B.M. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef]
- Grozdanić, N.; Djuričić, I.; Kosanić, M.; Zdunić, G.; Šavikin, K.; Etahiri, S.; Assobhei, O.; Benba, J.; Petović, S.; Matić, I.Z.; et al. Fucus spiralis extract and fractions: Anticancer and pharmacological potentials. J. BUON 2020, 25, 1219–1229. [Google Scholar]
- Qiu, X.L.; Zhang, Q.F. Chemical profile and pancreatic lipase inhibitory activity of Sinobambusa tootsik (Sieb.) Makino leaves. Peer J. 2019, 7, e7765. [Google Scholar] [CrossRef]
- Jaradat, N.; Zaid, A.N.; Hussein, F.; Zaqzouq, M.; Aljammal, H.; Ayesh, O. Anti-lipase potential of the organic and aqueous extracts of ten traditional edible and medicinal plants in Palestine; a comparison study with orlistat. Medicines 2017, 4, 89. [Google Scholar] [CrossRef]
- Belgacem, A.; Gdara, N.B.; Khemiri, I.; Bitri, L. Exploration of hypoglycemic effect of an extract from leaves of a plant from Tunisian pharmacopeia: Artemisia campestris (Asteraceae). Afr. Health Sci. 2019, 19, 2846–2853. [Google Scholar] [CrossRef]
- Husain, G.M.; Singh, P.N.; Singh, R.K.; Kumar, V. Antidiabetic activity of standardized extract of Quassia amara in nicotinamide-streptozotocin-induced diabetic rats. Phytother. Res. 2011, 25, 1806–1812. [Google Scholar] [CrossRef]
- Gajdosík, A.; Gajdosíková, A.; Stefek, M.; Navarová, J.; Hozová, R. Streptozotocin-induced experimental diabetes in male Wistar rats. Gen. Physiol. Biophys. 1999, 18, 54–62. [Google Scholar] [PubMed]
| Compound | Content | Expressed As |
|---|---|---|
| HHDP-hexoside | 0.89 ± 0.02 a | Gallic acid |
| Pedunculagin isomer 1 | 1.15 ± 0.02 | Gallic acid |
| Pedunculagin isomer 2 | 1.19 ± 0.02 | Gallic acid |
| Galloyl-HHDP-glucose | 1.79 ± 0.03 | Gallic acid |
| Brevifolin carboxylic acid | 8.58 ± 0.05 | Ellagic acid |
| Tellimagrandin 1 isomer 1 | 0.24 ± 0.02 | Gallic acid |
| Sanguiin H-10 isomer 1 | 0.83 ± 0.09 | Gallic acid |
| Tellimagrandin 1 isomer 2 | 0.22 ± 0.03 | Gallic acid |
| Galloyl-bis-HHDP-glucose | 3.16 ± 0.01 | Gallic acid |
| Sanguiin H-10 isomer 2 | 1.06 ± 0.01 | Gallic acid |
| Tellimagrandin 2 | 1.03 ± 0.01 | Gallic acid |
| Miquelianin | 3.58 ± 0.02 | Miquelianin |
| Ellagic acid | 4.91 ± 0.01 | Ellagic acid |
| Agrimoniin | 8.43 ± 0.02 | Gallic acid |
| Quercetin-3-O-pentoside | 0.34 ± 0.14 | Quercetin |
| Quercetin-methyl ether glucuronide | <LOQ | Quercetin |
| Quercetin-3,4′-dimethyl ether-7-O-glucuronide | 8.76 ± 0.05 | Isolated compound |
| Group | Fasting Blood Glucose Level (mmol/L) | ||
|---|---|---|---|
| Day | |||
| 0 | 10 | 20 | |
| Normal control group | 4.9 ± 0.6 | 5.0 ± 0.4 | 5.9 ± 0.3 |
| Diabetic control group | 23.8 ± 6.0 a | 25.4 ± 3.2 a c | 27.6 ± 4.1 a c |
| Diabetic + AVM 50 mg/kg | 20.7 ± 3.4 a | 31.4 ± 4.6 a d e | 23.3 ± 8.3 a |
| Diabetic + AVM 100 mg/kg | 21.4 ± 2.5 a | 30.9 ± 3.8 a d e | 15.7 ± 9.8 b |
| Diabetic + AVM 200 mg/kg | 24.8 ± 4.5 a | 16.8 ± 6.7 a b | 15.3 ± 3.4 b |
| Diabetic + glibenclamide 10 mg/kg | 26.1 ± 5.2 a | 15.9 ± 3.7 a b | 14.4 ± 6.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radović Selgrad, J.S.; Ušjak, D.J.; Milenković, M.T.; Milinković, N.L.; Janković, R.M.; Jevtić, J.B.; Mileski, K.S.; Niketić, M.S.; Kundaković-Vasović, T.D. Antihyperglycemic Activity of Alchemilla viridiflora Herb Methanol Extract in Streptozotocin-Induced Diabetic Male Rats. Molecules 2025, 30, 2819. https://doi.org/10.3390/molecules30132819
Radović Selgrad JS, Ušjak DJ, Milenković MT, Milinković NL, Janković RM, Jevtić JB, Mileski KS, Niketić MS, Kundaković-Vasović TD. Antihyperglycemic Activity of Alchemilla viridiflora Herb Methanol Extract in Streptozotocin-Induced Diabetic Male Rats. Molecules. 2025; 30(13):2819. https://doi.org/10.3390/molecules30132819
Chicago/Turabian StyleRadović Selgrad, Jelena S., Dušan J. Ušjak, Marina T. Milenković, Neda Lj. Milinković, Radmila M. Janković, Jovan B. Jevtić, Ksenija S. Mileski, Marjan S. Niketić, and Tatjana D. Kundaković-Vasović. 2025. "Antihyperglycemic Activity of Alchemilla viridiflora Herb Methanol Extract in Streptozotocin-Induced Diabetic Male Rats" Molecules 30, no. 13: 2819. https://doi.org/10.3390/molecules30132819
APA StyleRadović Selgrad, J. S., Ušjak, D. J., Milenković, M. T., Milinković, N. L., Janković, R. M., Jevtić, J. B., Mileski, K. S., Niketić, M. S., & Kundaković-Vasović, T. D. (2025). Antihyperglycemic Activity of Alchemilla viridiflora Herb Methanol Extract in Streptozotocin-Induced Diabetic Male Rats. Molecules, 30(13), 2819. https://doi.org/10.3390/molecules30132819







