An Overview of α-Pyrones as Phytotoxins Produced by Plant Pathogen Fungi
Abstract
1. Introduction
2. α-Pyrones
2.1. α-Pyrones Produced from Fungi Pathogenic for Agrarian Plants
2.2. α-Pyrones Produced from Fungi Pathogenic for Forest Plants
| Compound | Fungal Producer | Other Biological Activities | Ref. |
|---|---|---|---|
| Hydroxypestalopyrone (51) | Pestalotiopsis micropspora | Not reported | [63] |
| Pestaloside (52) | ″ | ″ | ″ |
| Diplopyrone (53) | Diplodia mutila | ″ | [64] |
| Diplopyrone B(54) | Diplodia corticola | Antifungal | [66] |
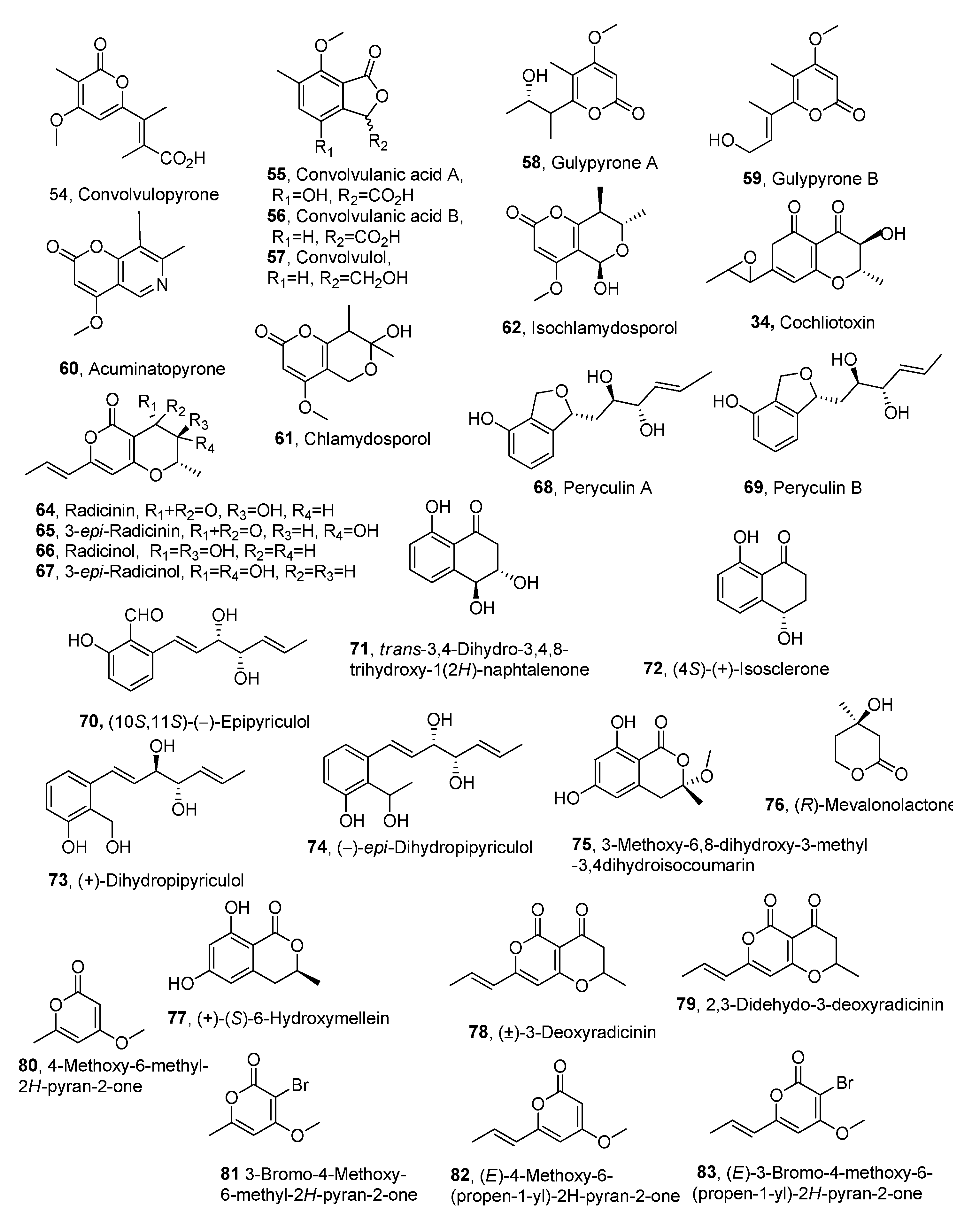
2.3. α-Pyrones Produced from Fungi Pathogenic for Weed and Parasitic Plants
| Compound | Fungal Producer | Other Biological Activities | Ref. |
|---|---|---|---|
| Convolvulopyrone (54) | Phornopsis corzvolvulus | Not reported | [67] |
| Convolvulanic acid A (55) | ″ | ″ | ″ |
| Convolvulanic acid B (56) | ″ | ″ | ″ |
| Convolvulol (57) | ″ | ″ | ″ |
| Gulypyrones A (58) | Diaporthe gulyae | ″ | [70] |
| Gulypyrones B (59) | ″ | ″ | ″ |
| Acuminatopyrone (60) | Fusarium tricinctum | [73] | |
| Chlamydosporol (61) | ″ | ″ | ″ |
| Isochlamydosporol (62) | ″ | ″ | ″ |
| Cochliotoxin (63) | Cochliobolus australiensis | ″ | [88] |
| Radicinin (64) | Cochliobolus australiensis ″ Curvularia clavata | ″Antifungal Bacteriocide | [78,88] [79] [80] |
| 3-epi-Radicinin (65) | Cochliobolus australiensis | Not reported | [88] |
| Radicinol (66) | ″ | ″ | ″ |
| 3-epi-Radicinol (67) | ″ | ″ | ″ |
| Peryculin A (68) | Perycularia grisea | ″ | [81] |
| Peryculin A (69) | ″ | ″ | ″ |
| (10S,11S)-(-)-Epipyriculol (70) | ″ | ″ | ″ |
| trans-3,4-Dihydro-3,4,8- trihydroxy-1(2H)-naphtalenone (71) | ″ | ″ | ″ |
| (4S)-(+)-Isosclerone (72) | ″ | ″ | ″ |
| (+)-Dihydropipyriculol (73) | ″ | ″ | [82] |
| (-)-epi-Dihydropipyriculol (74) | ″ | ″ | ″ |
| 3-Methoxy-6,8-dihydroxy-3-methyl -3,4dihydroisocoumarin (75) | ″ | ″ | ″ |
| (R)-Mevalonolactone (76) | ″ | ″ | ″ |
| (+)-(S)-6-Hydroxymellein (77) | ″ | ″ | ″ |
| (±)-3-Deoxyradicinin (78) | Anticancer | [87] | |
| 2,3-Dehydro-3-deoxyradicinin (79) | ″ | Not reported | ″ |
| 4-Methoxy-6-methyl- 2H-pyran-2-one (80) | ″ | ″ | ″ |
| 3-Bromo-4-Methoxy- 6-methyl-2H-pyran-2-one (81) | ″ | ″ | ″ |
| (E)-4-Methoxy-6-(propen-1-yl) -2H-pyran-2-one (82) | ″ | ″ | ″ |
| (E)-3-Bromo-4-methoxy-6- (propen-1-yl)-2H-pyran-2-one (83) | ″ | ″ | ″ |
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Belennkii, L.I.; Evdokimenkova, Y.B. The literature of heterocyclic chemistry, part XII 2010–2011. Adv. Heterocycl. Chem. 2014, 111, 147–274. [Google Scholar]
- Streitwieser, A.; Heathcock, C.H. Introduction to Organic Chemistry, 3rd ed.; Macmillan: New York, NY, USA, 1985; pp. 1038–1040. [Google Scholar]
- Dickinson, J.M. Microbial pyran-2-ones and dihydropyran-2-ones. Nat. Prod. Rep. 1993, 10, 71–98. [Google Scholar] [CrossRef] [PubMed]
- McGlacken, G.P.; Fairlamb, I.J. 2-Pyrone natural products and mimetics: Isolation, characterisation and biological activity. Nat. Prod. Rep. 2005, 22, 369–385. [Google Scholar] [CrossRef]
- Lee, J.S. Recent advances in the synthesis of 2-pyrones. Mar. Drugs 2015, 13, 1581–1620. [Google Scholar] [CrossRef]
- Schäberle, T.F. Biosynthesis of α-pyrones. Beilstein J. Org. Chem. 2016, 12, 571–588. [Google Scholar] [CrossRef]
- Azizian, M.; Gheshlaghi, S.; Danesh, A.; Forouzanfar, F.; Shakeri, A. α-Pyrones: Natural occurrence, chemistry, and biological approaches—An Update. Rev. Bras. Farmacogn. 2024, 34, 1201–1217. [Google Scholar] [CrossRef]
- Bhat, Z.S.; Rather, M.A.; Maqbool, M.; Lah, H.U.; Yousuf, S.K.; Ahmad, Z. α-Pyrones: Small molecules with versatile structural diversity reflected in multiple pharmacological activities-an update. Biomed. Pharmacother. 2017, 91, 265–277. [Google Scholar] [CrossRef]
- Evidente, A.; Cimmino, A.; Andolfi, A. The effect of stereochemistry on the biological activity of natural phytotoxins, fungicides, insecticides and herbicides. Chirality 2013, 25, 59–78. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef]
- Al-Khdhairawi, A.A.Q.; Cordell, G.A.; Thomas, N.F.; Nagojappa, N.B.S.; Weber, J.F.F. Natural diterpene pyrones: Chemistry and biology. Org. Biomol. Chem. 2019, 17, 8943–8957. [Google Scholar] [CrossRef] [PubMed]
- Entwistle, A.R. Root diseases. In Onions and Allied Crops; Brewster, J.L., Rabinowitch, H.D., Eds.; CRC Press: Boca Rato, FL, USA, 1990; Volume III, p. 284. [Google Scholar]
- Niwa, M.; Ogiso, S.; Endo, T.; Furukawa, H.; Yamamura, S. Isolation and structure of citreopyrone, a metabolite of Penicillium citreo-viride Biourge. Tetrahedron Lett. 1980, 21, 4481–4482. [Google Scholar] [CrossRef]
- Sato, H.; Konoma, K.; Sakamura, S.; Furusaki, A.; Matsumoto, T.; Matsuzaki, T. X-ray crystal structure of pyrenocine A, a phytotoxin from Pyrenochaeta terrestris. Agric. Biol. Chem. 1981, 45, 795–797. [Google Scholar] [CrossRef]
- Sparace, S.A.; Mudd, J.B.; Burke, B.A.; Aasen, A.J. Pyrenocine C, a phytotoxin-related metabolite produced by onion pink root fungus, Pyrenochaeta terrestris. Phytochemistry 1984, 23, 2693–2694. [Google Scholar] [CrossRef]
- Sato, H.; Konoma, K.; Sakamura, S. Three new phytotoxins produced by Pyrenochaeta terrestris: Pyrenochaetic acids A, B and C. Agric. Biol. Chem. 1981, 45, 1675–1679. [Google Scholar] [CrossRef]
- Sparace, S.A.; Reeleder, R.D.; Khanizadeh, S. Antibiotic activity of the pyrenocines. Can. J. Microbiol. 1987, 33, 327–330. [Google Scholar] [CrossRef]
- Ichihara, A.; Oikawa, H. Biosynthesis of phytotoxins from Alternaria solani. Biosci. Biotechnol. Biochem. 1997, 61, 12–18. [Google Scholar] [CrossRef]
- Tabuchi, H.; Ichihara, A. Structures and stereochemistries of new compounds related to alternaric acid. J. Chem. Soc. Perkin Trans. 1 1994, 125–133. [Google Scholar] [CrossRef]
- Ichihara, A.; Tazaki, H.; Sakamura, S. Solanapyrones A, B and C, phytotoxic metabolites from the fungus Alternaria solani. Tetrahedron Lett. 1983, 24, 5373–5376. [Google Scholar] [CrossRef]
- Oikawa, H.; Yokota, T.; Ichihara, A.; Sakamura, S. Structure and absolute configuration of solanapyrone D: A new clue to the occurrence of biological Diels–Alder reaction J. Chem. Soc. Chem. Commun. 1989, 17, 1284–1285. [Google Scholar] [CrossRef]
- Oikawa, H.; Yokota, T.; Sakano, C.; Suzuki, Y.; Naya, A.; Ichihara, A. Solanapyrones, phytotoxins produced by Alternaria solani: Biosynthesis and isolation of minor components. Biosci. Biotechnol. Biochem. 1998, 62, 2016–2022. [Google Scholar] [CrossRef]
- Wang, X.Z.; Luo, X.H.; Xiao, J.; Zhai, M.M.; Yuan, Y.; Zhu, Y.; Crews, P.; Yuan, C.S.; Wu, Q.X. Pyrone derivatives from the endophytic fungus Alternaria tenuissima SP-07 of Chinese herbal medicine Salvia przewalskii. Fitoterapia 2014, 99, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Rodeva, R.; Andolfi, A.; Stoyanova, Z.; Perrone, C.; Motta, A. Phytotoxic polyketides produced by Phomopsis foeniculi, a strain isolated from diseased Bulgarian fennel Eur. J. Plant Pathol. 2011, 130, 173–182. [Google Scholar] [CrossRef]
- Mishra, P.D.; Verekar, S.A.; Deshmukh, S.K.; Joshi, K.S.; Fiebig, H.H.; Kelter, G. Altersolanol A: A selective cytotoxic anthraquinone from a Phomopsis sp. Lett. Appl. Microbiol. 2015, 60, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, J.; Li, F.; Zhou, D.; Xu, L.; Li, C. Identification and antifungal activity of metabolites from the mangrove fungus Phoma sp. L28. Chem. Nat. Comp. 2017, 53, 237–240. [Google Scholar] [CrossRef]
- Evidente, A.; Lanzetta, R.; Abouzeid, M.A.; Corsaro, M.M.; Mugnai, L.; Surico, G. Foeniculoxin, a new phytotoxic geranylhydroquinone from Phomopsis foeniculi. Tetrahedron 1994, 50, 10371–10378. [Google Scholar] [CrossRef]
- Shirota, O.; Pathak, V.; Faiz Hossain, C.; Sekita, S.; Takatori, K.; Satake, M. Structural elucidation of trichotetronines: Polyketides possessing a bicyclo [2.2.2]octane skeleton with a tetronic acid moiety isolated from Trichoderma sp. J. Chem. Soc. Perkin Trans. 1 1997, 2961–2964. [Google Scholar] [CrossRef]
- Abe, N.; Murata, T.; Hirota, A. Novel DPPH radical scavengers, bisorbicillinol and demethyltrichodimerol, from a fungus. Biosci. Biotechnol. Biochem. 1998, 62, 661–666. [Google Scholar] [CrossRef]
- He, T.; Li, X.; del Carmen Flores-Vallejo, R.; Radu, A.M.; van Dijl, J.M.; Haslinger, K. The endophytic fungus Cosmosporella sp. VM-42 from Vinca minor is a source of bioactive compounds with potent activity against drug-resistant bacteria. Curr. Res. Microb. Sci. 2025, 8, 100390. [Google Scholar] [CrossRef]
- Abrell, L.M.; Cheng, X.C.; Crew, P. New nectriapyrones by salt water cultures of a fungus separated from an indo-pacific sponge. Tetrahedron Lett. 1994, 35, 9159–9160. [Google Scholar] [CrossRef]
- Evidente, A.; Zonno, M.C.; Andolfi, A.; Troise, C.; Cimmino, A.; Vurro, M. Phytotoxic α-pyrones produced by Pestalotiopsis guepinii, the causal agent of hazelnut twig blight. J. Antibiot. 2012, 65, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Sun, W.; Mao, Z.; Xu, D.; Wang, X.; Lu, S.; Daowan, L.; Yang Liu, Y.; Ligang Zhou, L.; Zhang, G. Main ustilaginoidins and their distribution in rice false smut balls. Toxins 2015, 7, 4023–4034. [Google Scholar] [CrossRef]
- Kawai, K.; Hisada, K.; Mori, S.; Nozawa, Y.; Koyama, K.; Natori, S. The impairing effect of chaetochromin A and related mycotoxins on mitochondrial respiration. Proc. Jpn. Assoc. Mycotoxicol. 1991, 33, 31–35. [Google Scholar] [CrossRef]
- Masi, M.; Cimmino, A.; Boari, A.; Zonno, M.C.; Górecki, M.; Pescitelli, G.; Tuzi, A.; Vurro, M.; Evidente, A. Colletopyrandione, a new phytotoxic tetrasubstituted indolylidenepyran-2, 4-dione, and colletochlorins G and H, new tetrasubstituted chroman-and isochroman-3, 5-diols isolated from Colletotrichum higginsianum. Tetrahedron 2017, 73, 6644–6650. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Wei, H.; Solomon, P.S.; Vuong, D.; Lacey, E.; Stubbs, K.A.; Pigott, A.M.; Chooi, Y.H. Chemical ecogenomics-guided discovery of phytotoxic α-pyrones from the fungal wheat pathogen Parastagonospora nodorum. Org. Lett. 2018, 20, 6148–6152. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Gu, G.; Dang, P.; Zhang, X.; Wang, W.; Dai, J.; Liu, Y.; Lai, D.; Zhou, L. Sorbicillinoids from the fungus Ustilaginoidea virens and their phytotoxic, cytotoxic, and antimicrobial activities. Front. Chem. 2019, 7, 435. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Reveglia, P.; Baaijens-Billones, R.; Górecki, M.; Pescitelli, G.; Savocchia, S.; Evidente, A. Phytotoxic metabolites from three Neofusicoccum species causal agents of Botryosphaeria dieback in Australia, luteopyroxin, neoanthraquinone, and luteoxepinone, a disubstituted furo-α-pyrone, a hexasubstituted anthraquinone, and a trisubstituted oxepi-2-one from Neofusicoccum luteum. J. Nat. Prod. 2020, 83, 453–460. [Google Scholar]
- Reveglia, P.; Masi, M.; Evidente, A. Melleins—Intriguing natural compounds. Biomolecules 2020, 10, 772. [Google Scholar] [CrossRef]
- Zhai, M.-M.; Qi, F.-M.; Li, J.; Jiang, C.-X.; Hou, Y.; Shi, Y.-P.; Di, D.-L.; Zhang, J.-W.; Wu, Q.-X. Isolation of secondary metabolites from the soil-derived fungus Clonostachys rosea YRS-06, a biological control agent, and evaluation of antibacterial activity. J. Agric. Food Chem. 2016, 64, 2298–2306. [Google Scholar] [CrossRef]
- Masi, M.; Bashiri, S.; Cimmino, A.; Bahmani, Z.; Abdollahzadeh, J.; Evidente, A. Phytotoxins produced by two Biscogniauxia rosacearum strains, causal agents of grapevine trunk diseases, and charcoal canker of oak trees in Iran. Toxins 2021, 13, 812. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Schmey, T.; Tominello-Ramirez, C.S.; Brune, C.; Stam, R. Alternaria diseases on potato and tomato. Mol. Plant Pathol. 2024, 25, e13435. [Google Scholar] [CrossRef]
- Furuichi, N.; Nishimura, S.; Langsdorf, G. Effect of alternaric acid, a toxin of Alternaria solani, on the hypersensitive response of potato to Phytophthora infestans. Jpn. J. Phytopathol. 1992, 58, 1–7. [Google Scholar] [CrossRef]
- Tabuchi, H.; Hamamoto, T.; Miki, S.; Tejima, T.; Ichihara, A. Total synthesis and stereochemistry of alternaric acid. J. Org. Chem. 1994, 59, 4749–4759. [Google Scholar] [CrossRef]
- Alam, S.S.; Bilton, J.N.; Slawin, A.M.; Williams, D.J.; Sheppard, R.N.; Strange, R.N. Chickpea blight: Production of the phytotoxins solanapyrones A and C by Ascochyta rabiei. Phytochemistry 1989, 28, 2627–2630. [Google Scholar] [CrossRef]
- Nene, Y.L. A review of Ascochyta blight of chickpea. Trop. Pest Manag. 1982, 28, 61–70. [Google Scholar] [CrossRef]
- Ichihara, A.; Oikawa, H.; Hayashi, K.; Sakamura, S. Structures of betaenones A and B, novel phytotoxins from Phoma betae Fr. J. Am. Chem Soc. 1983, 105, 2907–290830. [Google Scholar] [CrossRef]
- Barash, I.; Manulis, S.; Kashman, Y.; Springer, J.P.; Chen, M.H.; Clardy, J.; Strobel, G.A. Crystallization and X-ray analysis of stemphyloxin I, a phytotoxin from Stemphylium botryosum. Science 1983, 220, 1065–1066. [Google Scholar] [CrossRef]
- Kim, W.; Park, C.M.; Park, J.J.; Akamatsu, H.O.; Peever, T.L.; Xian, M.; Gang, D.R.; Wandermark, G.; Chen, W. Functional analyses of the Diels-Alderase gene sol5 of Ascochyta rabiei and Alternaria solani indicate that the solanapyrone phytotoxins are not required for pathogenicity. Mol. Plant-Microbe Interact. 2015, 28, 482–496. [Google Scholar] [CrossRef]
- Kimura, Y.; Hamasaki, T.; Nakajima, H. Streochemistry and biological activities of LL-P880g, a pestalotin analogue, produced by Penicillium citro-viride. Agric. Biol. Chem. 1986, 50, 1649–1650. [Google Scholar]
- Choi, E.M.; Hwang, J.K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 2004, 75, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, M.M.; De Castro, C.; Evidente, A.; Lanzetta, R.; Molinaro, A.; Mugnai, L.; Parrilli, M.; Surico, G. Chemical structure of two phytotoxic exopolysaccharides produced by Phomopsis foeniculi. Carbohydr. Res. 1998, 308, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubbaiah, P.; van Dyke, C.G.; Chilton, W.S. Phytotoxins produced by Pestalotiopsys oenotherae, a pathogen of evening primrose. Phytochemistry 1991, 30, 1471–1474. [Google Scholar] [CrossRef]
- Turkann, M. Phytotoxins produced by Pestalotiopsis guepinii, the causal agent of halznut twig blight. Phytopathol. Mediterr. 2011, 50, 154–158. [Google Scholar]
- Damm, U.; O’connell, R.J.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum destructivum species complex-hemibiotrophic pathogens of forage and field crops. Stud. Mycol. 2014, 79, 49–84. [Google Scholar] [CrossRef]
- Cimmino, A.; Mathieu, V.; Masi, M.; Baroncelli, R.; Boari, A.; Pescitelli, G.; Vurro, M.; Evidente, A. Higginsianins A and B, two diterpenoid α-pyrones produced by Colletotrichum higginsianum, with in vitro cytostatic activity. J. Nat. Prod. 2016, 79, 116–125. [Google Scholar] [CrossRef]
- Trifonov, L.S.; Hilpert, H.; Floersheim, P.; Dreiding, A.S.; Rast, D.M.; Skrivanova, R.; Hoesch, L. Bisvertinols: A new group of dimeric vertinoids from Verticillium intertextum. Tetrahedron 1986, 42, 3157–3179. [Google Scholar] [CrossRef]
- Andrade, R.; Ayer, W.A.; Trifonov, L.S. The metabolites of Trichoderma longibrachiatum. III. Two new tetronic acids: 5-hydroxyvertinolide and bislongiquinolide. Aust. J. Chem. 1997, 50, 255–258. [Google Scholar] [CrossRef]
- Abe, N.; Yamamoto, K.; Hirota, A. Novel fungal metabolites, demethylsorbicillin and oxosorbicillinol, isolated from Trichoderma sp. USF2690. Biosci. Biotechnol. Biochem. 2000, 64, 620–622. [Google Scholar] [CrossRef]
- Chen, K.; Song, K.; Hao, X.; Wang, C.; Zhang, L.; Yue, Q.; Xu, Y. Higginsianin F, one skeletal rearrangement diterpenoid α-pyridone with phytotoxic activity isolated from Colletotrichum higginsianum. Phytochemistry 2025, 235, 114475. [Google Scholar] [CrossRef]
- Evidente, A.; Maddau, L.; Spanu, E.; Franceschini, A.; Lazzaroni, S.; Motta, A. Diplopyrone, a new phytotoxic tetrahydropyranpyran-2-one produced by Diplodia mutila, a fungus pathogen of cork oak. J. Nat. Prod. 2003, 66, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, E.; Maddau, L.; Spanu, E.; Evidente, A.; Rosini, C. Assignment of the absolute configuration of (+)-diplopyrone, the main phytotoxin produced by Diplodia mutila, the pathogen of the cork oak decline, by a nonempirical analysis of its chiroptical properties. J. Org. Chem. 2005, 70, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Fusè, M.; Mazzeo, G.; Longhi, G.; Abbate, S.; Masi, M.; Evidente, A.; Puzzarini, C.; Barone, V. Unbiased determination of absolute configurations by vis-à-vis comparison of experimental and simulated spectra: The challenging case of diplopyrone. J. Phys. Chem. B 2019, 123, 9230–9237. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Cimmino, A.; D’Amico, W.; Scanu, B.; Evidente, M.; Tuzi, A.; Evidente, A. Bioactive secondary metabolites produced by the oak pathogen Diplodia corticola. J. Agric. Food Chem. 2016, 64, 217–225. [Google Scholar] [CrossRef]
- Tsantrizos, Y.S.; Ogilvie, K.K.; Watson, A.K. Phytotoxic metabolites of Phomopsis convolvulus, a host-specific pathogen of field bindweed. Can. J. Chem. 1992, 70, 2276–2284. [Google Scholar] [CrossRef]
- Alex, J.F. Canada. In Biology and Ecology of Weeds, 1st ed.; Holzner, W., Nurnata, M., Junk, W., Eds.; The Hague, Springer Sciences: Dordrecht, The Netherlands, 1982; pp. 309–332. [Google Scholar]
- Rosenthal, S.; Andres, L.; Huffaker, C. Field bindweed in California. Cal. Agric. 1983, 37, 18–22. [Google Scholar]
- Andolfi, A.; Boari, A.; Evidente, M.; Cimmino, A.; Vurro, M.; Ash, G.; Evidente, A. Gulypyrones A and B and phomentrioloxins B and C produced by Diaporthe gulyae, a potential mycoherbicide for saffron thistle (Carthamus lanatus). J. Nat. Prod. 2015, 78, 623–629. [Google Scholar] [CrossRef]
- Briese, D.T. Weed status of twelve thistle species in New South Wales. Plant Protect. Quaterly 1988, 3, 135–141. [Google Scholar]
- Sindel, B. Glyphosate resistance discovered in annual ryegrass. Resist. Pest Manag. 1996, 8, 5–6. [Google Scholar]
- Masi, M.; Meyer, S.; Pescitelli, G.; Cimmino, A.; Clement, S.; Peacock, B.; Evidente, A. Phytotoxic activity against Bromus tectorum for secondary metabolites of a seed-pathogenic Fusarium strain belonging to the F. tricinctum species complex. Nat. Prod. Res. 2017, 31, 2768–2777. [Google Scholar] [CrossRef]
- Santoro, E.; Mazzeo, G.; Marsico, G.; Masi, M.; Longhi, G.; Superchi, S.; Evidente, A.; Abbate, S. Assignment through chiroptical methods of the absolute configuration of fungal dihydropyranpyran-4-5-diones phytotoxins, potential herbicides for buffelgrass (Cenchrus ciliaris) biocontrol. Molecules 2019, 24, 3022. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Ishida, T.; Otsuka, Y.; Hamasaki, T.; Ichinoe, M. Phytotoxins and related metabolites produced by Bipolaris coicis, the pathogen of job’s tears. Phytochemistry 1997, 45, 41–45. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Vitti, C.; De Girolamo, A.; Visconti, A.; Logrieco, A.; Panizzi, F.P.J. Radicinols and radicinin phytotoxins produced by Alternaria radicina on carrots. J. Agric. Food Chem. 2004, 52, 3655–3660. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakuno, E.; Ishihara, A.; Tamura, J.I.; Nakajima, H. Conversions of deoxyradicinin to radicinin and of radicinin to 3-epi-radicinin in the phytopathogenic fungus Bipolaris coicis. Phytochemistry 2012, 75, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Kong, L.C.; Jiang, D.H.; Yin, C.P.; Cai, Q.M.; Chen, Q.; Zheng, J.Y. Phytotoxic and antifungal metabolites from Curvularia sp. FH01 isolated from the gut of Atractomorpha sinensis. Bioresour. Technol. 2011, 102, 3575–3577. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, T.J.; Rolshausen, P.E.; Roper, M.C.; Reader, J.M.; Steinhaus, M.J.; Rapicavoli, J.; Vosburg, D.A.; Maloney, K.N. Radicinin from Cochliobolus sp. inhibits Xylella fastidiosa, the causal agent of Pierce’s Disease of grapevine. Phytochemistry 2015, 116, 130–137. [Google Scholar] [CrossRef]
- Vujčić, M.; Jankov, R.M. Microbiologic transformation of progesterone by Curvularia clavata Jain. Steroids 1990, 55, 17–21. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Górecki, M.; Mandoli, A.; Di Bari, L.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Clement, S.; Evidente, A. Pyriculins A and B, two monosubstituted hex-4-ene-2,3-diols and other phytotoxic metabolites produced by Pyricularia grisea isolated from buffelgrass (Cenchrus ciliaris). Chirality 2017, 29, 726–736. [Google Scholar] [CrossRef]
- Masi, M.; Santoro, E.; Clement, S.; Meyer, S.; Scafato, P.; Superchi, S.; Evidente, A. Further secondary metabolites produced by the fungus Pyricularia grisea isolated from buffelgrass (Cenchrus ciliaris). Chirality 2020, 32, 1234–1242. [Google Scholar] [CrossRef]
- Masi, M.; Freda, F.; Sangermano, F.; Calabrò, V.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Radicinin, a fungal phytotoxin as a target-specific bioherbicide for invasive buffelgrass (Cenchrus ciliaris) control. Molecules 2019, 24, 1086. [Google Scholar] [CrossRef]
- Masi, M.; Freda, F.; Clement, S.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Phytotoxic activity and structure–activity relationships of radicinin derivatives against the invasive weed buffelgrass (Cenchrus ciliaris). Molecules 2019, 24, 2793. [Google Scholar] [CrossRef] [PubMed]
- Marsico, G.; Ciccone, M.S.; Masi, M.; Freda, F.; Cristofaro, M.; Evidente, A.; Superchi, S.; Scafato, P. Synthesis and herbicidal activity against buffelgrass (Cenchrus ciliaris) of (±)-3-deoxyradicinin. Molecules 2019, 24, 3193. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.J.; Cao, F.; Hu, L.S.; Xu, C.X.; Zhu, Y.A.; Chen, X.; Mao, X.M. Complex interplay and catalytic versatility of tailoring enzymes for efficient and selective biosynthesis of fungal mycotoxins. J. Agric. Food Chem. 2022, 71, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, V.; Superchi, S.; Masi, M.; Scafato, P.; Kornienko, A.; Evidente, A. In vitro effects of fungal phytotoxins on cancer cell viability: First insight into structure activity relationship of a potent metabolite of Cochliobolus australiensis radicinin. Toxins 2022, 14, 517. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Clement, S.; Cimmino, A.; Cristofaro, M.; Evidente, A. Cochliotoxin, a dihydropyranopyran-4, 5-dione, and its analogues produced by Cochliobolus australiensis display phytotoxic activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 1241–1247. [Google Scholar] [CrossRef]
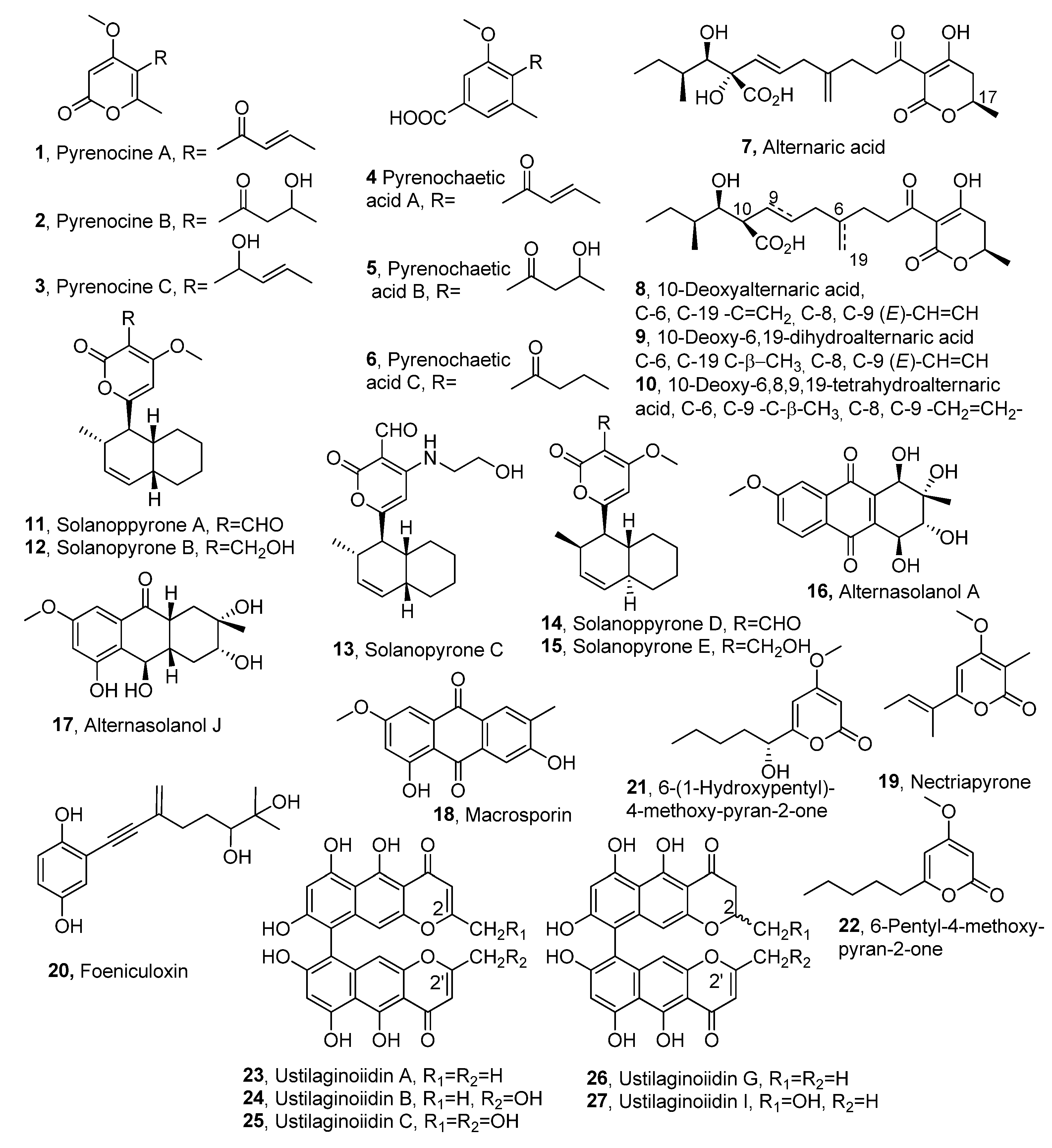
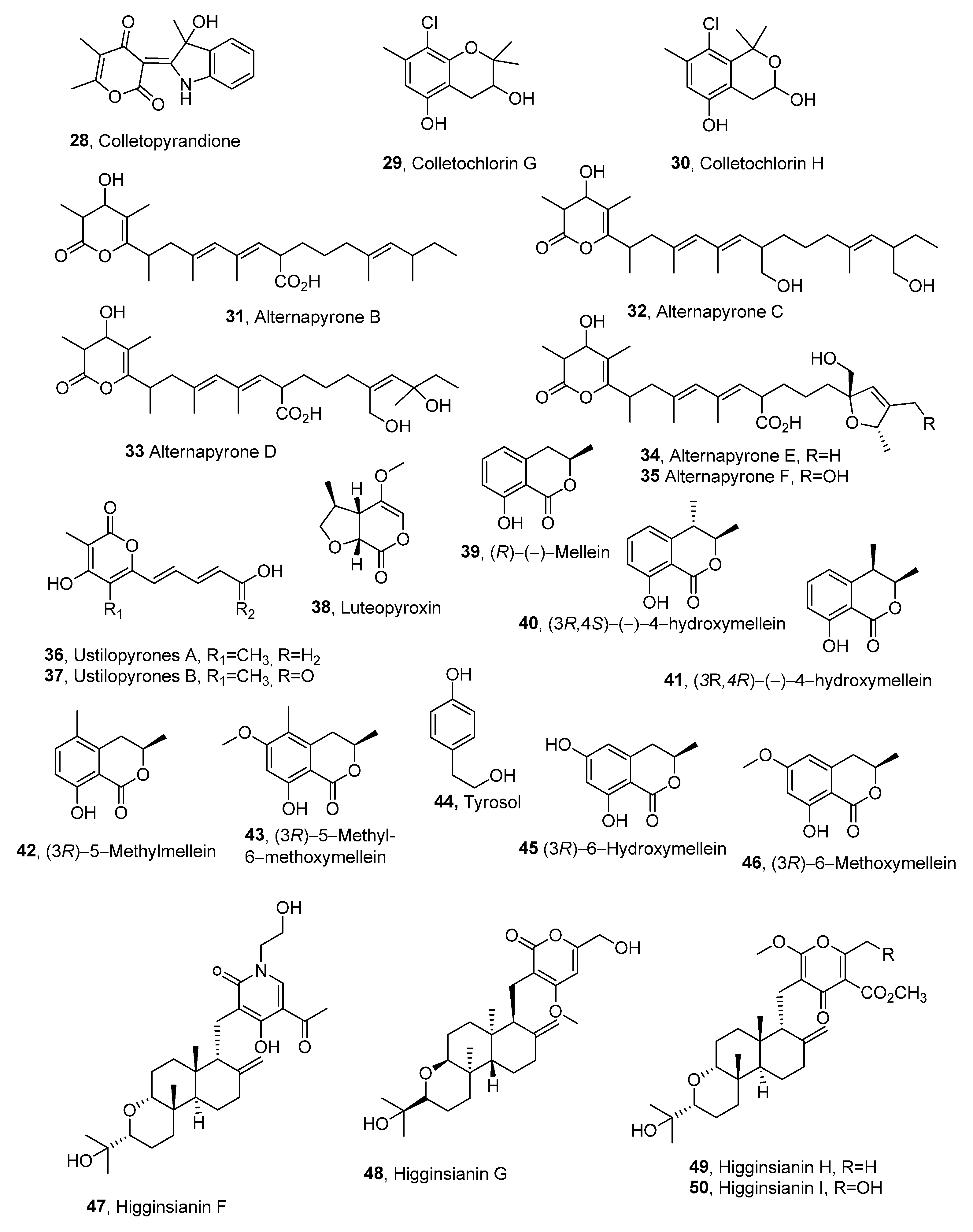

| Compound | Fungal Producer | Other Biological Activities | Ref. |
|---|---|---|---|
| Pyrenocine A (1) | Pyrenoclzaeta terrestris | Antibiotic activity | [13,15] [18] |
| Pyrenocine B (2) | ″ | Weak antibiotic activity | ″ |
| Pyrenocine C (3) | ″ | ″ | ″ |
| Pyrenochaetic acid A (4) | ″ | Not reported | [17] |
| Pyrenochaetic acid B (5) | ″ | ″ | ″ |
| Pyrenochaetic acid C (6) | ″ | ″ | ″ |
| Alternaric acid (7) | Alternaria solani | [19] | |
| 10-Deoxyalternaric acid (8) | ″ | ″ | [20] |
| 10-Deoxy-6,19-dihydro alternaric acid (9) | ″ | ″ | ″ |
| 10-Deoxy-6,8,9,19-tetrahydro alternaric acid (10) | ″ | ″ | ″ |
| Solanapyrones A (11) | A. solani Ascochyta rabiei | ″ Antibiotic activity | [21,22,23] [24] |
| Solanapyrones B (12) | ″ | Not reported | [21,22,23]″ |
| Solanapyrones C (13) | ″ | ″ | ″ |
| Solanapyrones D (14) | A. solani | ″ | [21,22] |
| Solanapyrones E (15) | ″ | ″ | ″ |
| Alternasolanol A (16) | Diaporthe angelicae Phomopis sp. | ″ Cytotoxic activity | [25] [26] |
| Alternasolanol J (17) | D. angelica | Not reported | [25] |
| Macrosporin (18) | ″ Phoma sp. | ″ Antifungal activity | ″ [27] |
| Nectriapyrone (=Pestalopyrone, 19) | D. angelica Pestalotiopsis oenotherae Pestalotiopsis micropspora Biscogniauxia rosacearum Cosmosporella sp. | Not Reported ″ ″ ″ Antibiotic | [25] [28] [29] [30] [31] |
| Foeniculoxin (20) | Diaporthe angelicae | Not reported | [32] |
| 6-(1-Hydroxypentyl)- 4-methoxy-pyran-2-one (21) | Pestalotiopsis guepinii Cosmosporella sp. | ″ Antibiotic | [33] [31] |
| 6-Pentyl-4-methoxy- pyran-2-one (22) | P. guepenii Cosmosporella sp. | Not reported″ Antibiotic | [33] [31] |
| Ustilaginoiidin A, R1=R2=H (23) | Villosiclava virens Chaetomium spp. | Not reported Cytotoxic activity | [34] [35] |
| Ustilaginoiidin B, R1=H, R2=OH (24) | ″ Villosiclava virens | Not reported | [34] |
| Ustilaginoidin C, R1=R2=OH (25) | ″ | ″ | ″ |
| Ustilaginoidin G, R1=R2=H (26) | ″ | ″ | ″ |
| Ustilaginoidin I, R1=H, R2=OH (27) | ″ | ″ | ″ |
| Colletopyrandione (28) | Colletotrichum higginsianum | [36] | |
| Colletochlorin G (29) | ″ | ″ | ″ |
| Colletochlorin H (30) | ″ | ″ | ″ |
| Alternapyrones B (31) | Parastagonospora nodorum | [37] | |
| Alternapyrones C (32) | ″ | ″ | ″ |
| Alternapyrones D (33) | ″ | ″ | ″ |
| Alternapyrones E (34) | ″ | ″ | ″ |
| Alternapyrones F (35) | ″ | ″ | ″ |
| Ustilopyrones A (36) | Ustilaginoidea virens | ″ | [38] |
| Ustilopyrones B (37) | ″ | ″ | ″ |
| Luteopyroxin (38) | Neofusicoccum luteum | ″ | [39] |
| (R)-(−)-Mellein (39) | Neofusicoccum luteum Neofusicoccum parvum Neofusicoccum australe | ″ Antibacterial, insecticidal, and fungicidal activity | [39] [40] |
| (3R,4S)-(−)-4-Hydroxymellein (40) | Neofusicoccum luteum Neofusicoccum parvum | Not reported Antibacterial activity | [39] [40] |
| (3R,4R)-(−)-4-Hydroxymellein (41) | Neofusicoccum luteum Neofusicoccum parvum | Not reported Antibacterial activity | [39] Antibacterial activity |
| (3R)-5-Methylmellein (42) | Biscogniauxia rosacearum | Not reported | [41] |
| (3R)-5-Methyl-6-methoxymellein (43) | ″ | ″ Antibacterial activity | ″ [40] |
| Tyrosol (44) | ″ | Not reported Antiatherogenic, cardioprotective, anticancer, neuroprotective, and endocrine effects | [42] [43] |
| (3R)-6-Hydroxymellein (45) | ″ | Not reported | [42] |
| (3R)-6-Methoxymellein (46) | ″ | ″ | ″ |
| Higginsianin F (47) | Colletotrichum higginsianum | [43] | |
| Higginsianin G (48) | ″ | ″ | ″ |
| Higginsianin H (48) | ″ | ″ | ″ |
| Higginsianin I (50) | ″ | ″ | ″ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evidente, A. An Overview of α-Pyrones as Phytotoxins Produced by Plant Pathogen Fungi. Molecules 2025, 30, 2813. https://doi.org/10.3390/molecules30132813
Evidente A. An Overview of α-Pyrones as Phytotoxins Produced by Plant Pathogen Fungi. Molecules. 2025; 30(13):2813. https://doi.org/10.3390/molecules30132813
Chicago/Turabian StyleEvidente, Antonio. 2025. "An Overview of α-Pyrones as Phytotoxins Produced by Plant Pathogen Fungi" Molecules 30, no. 13: 2813. https://doi.org/10.3390/molecules30132813
APA StyleEvidente, A. (2025). An Overview of α-Pyrones as Phytotoxins Produced by Plant Pathogen Fungi. Molecules, 30(13), 2813. https://doi.org/10.3390/molecules30132813






