A Novel HDAC6 Inhibitor Enhances the Efficacy of Paclitaxel Against Ovarian Cancer Cells
Abstract
1. Introduction
2. Results
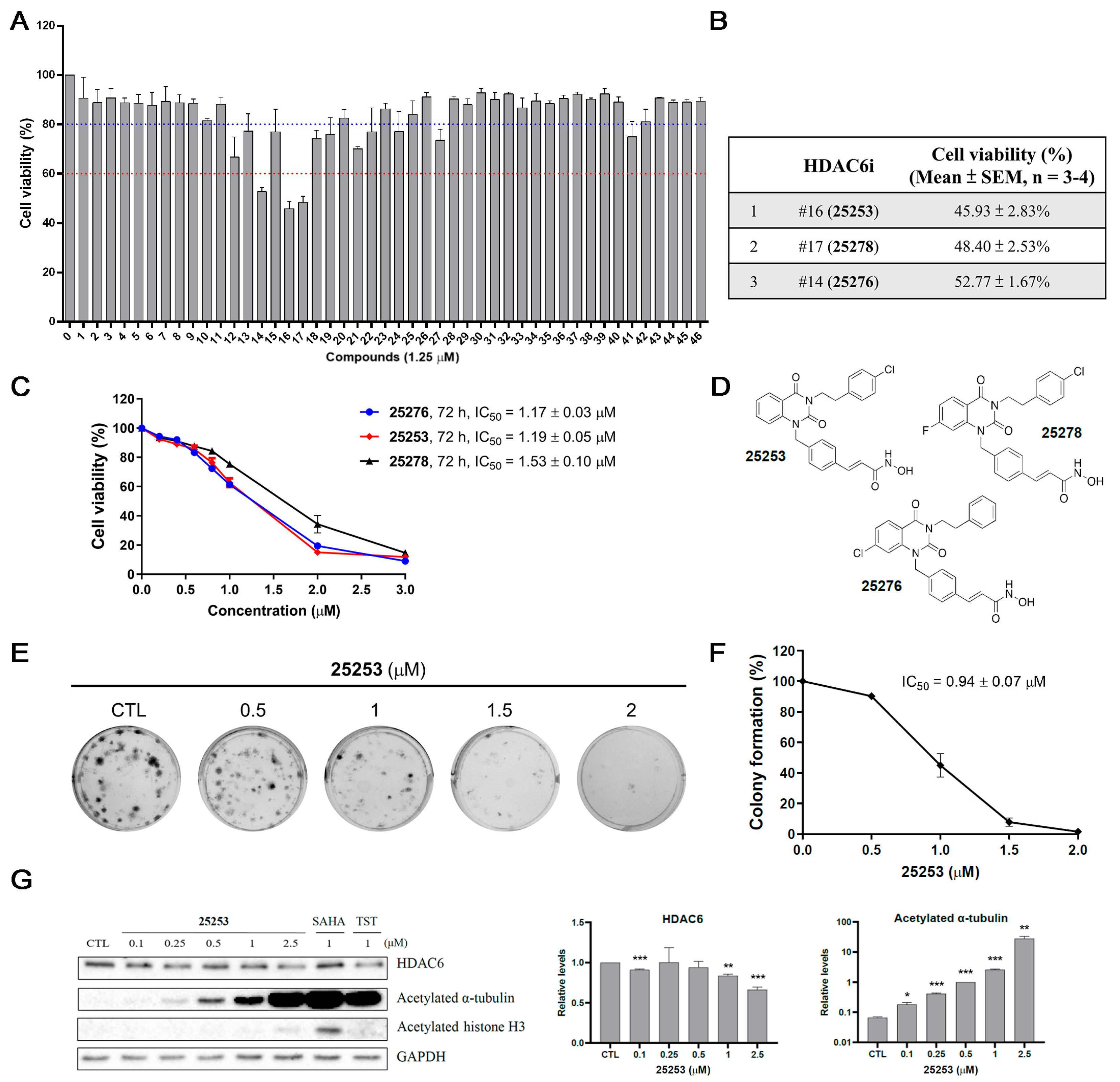
2.1. Determination of Antiproliferative Effect of 46 Potential Selective HDAC6 Inhibitors in ES-2 Ovarian Cancer Cells
2.2. Screening for Anticancer Agents That Can Synergize with HDAC6 Inhibitors Against ES-2 Ovarian Cancer Cells
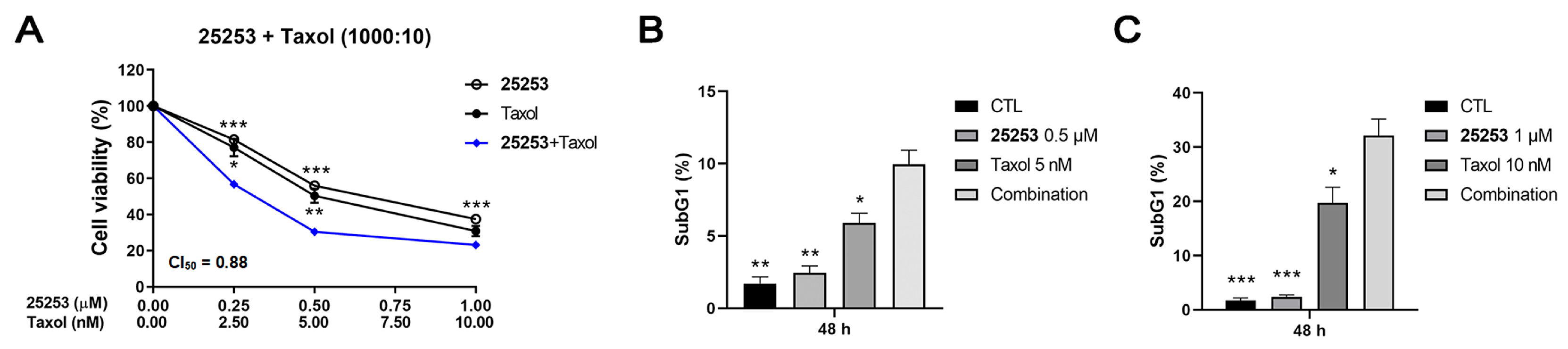
2.3. HDAC6 Inhibitor 25253 Displays the Best Synergistic Effect When Combined with Taxol Against ES-2 Cells
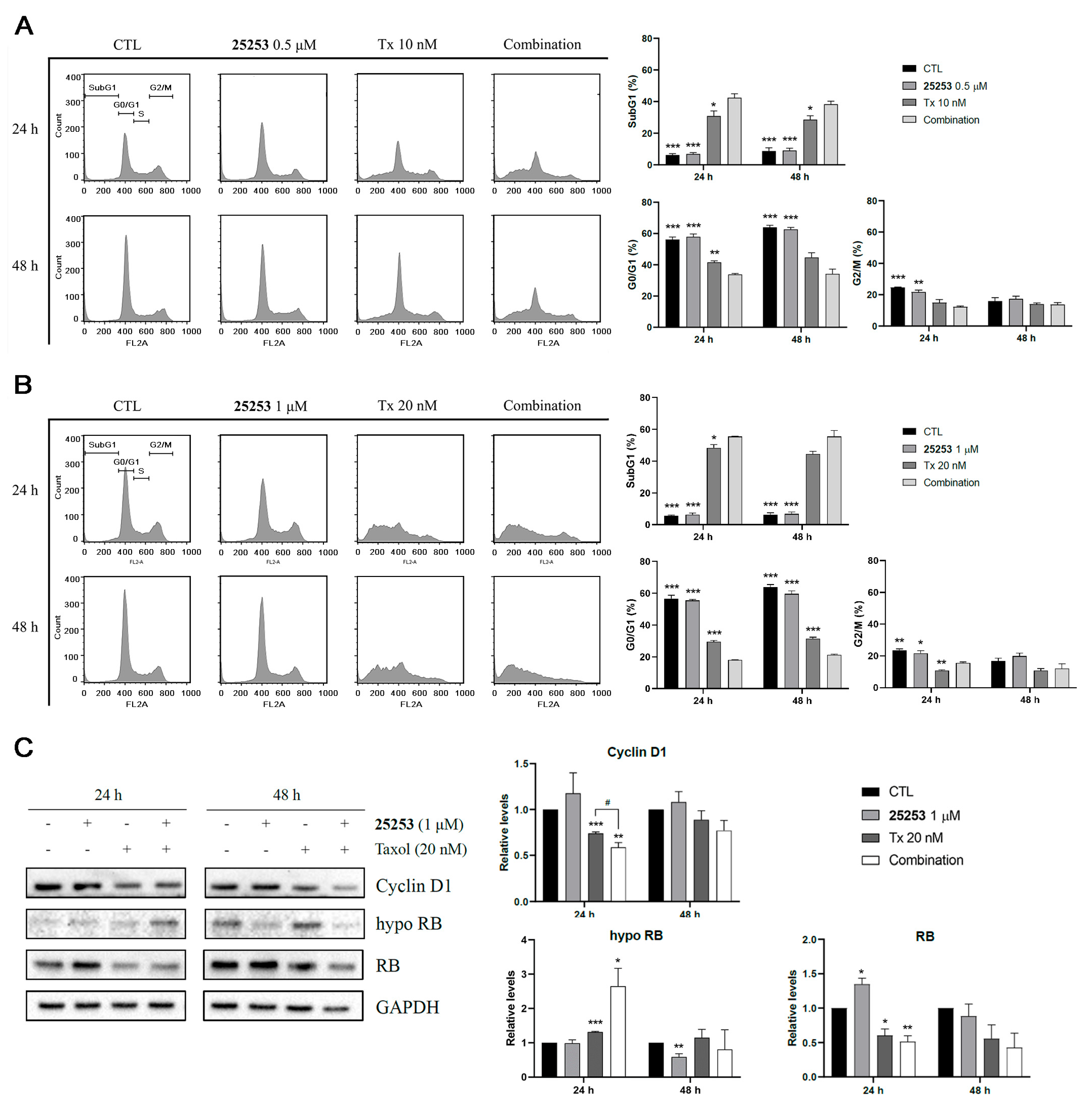
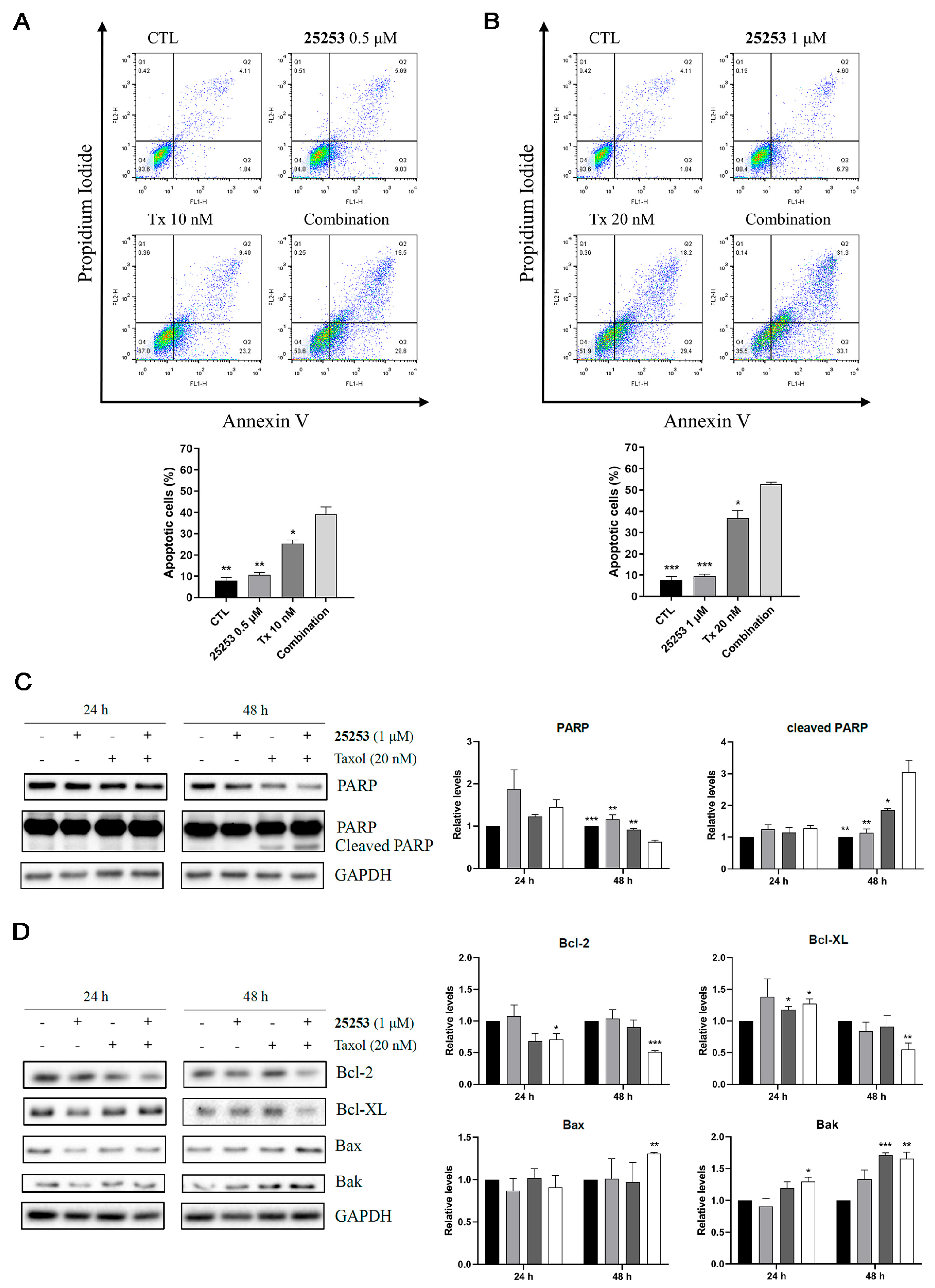
2.4. Effects of the Combination of 25253 and Taxol on Cell Cycle Progression and Apoptosis in ES-2 Cells
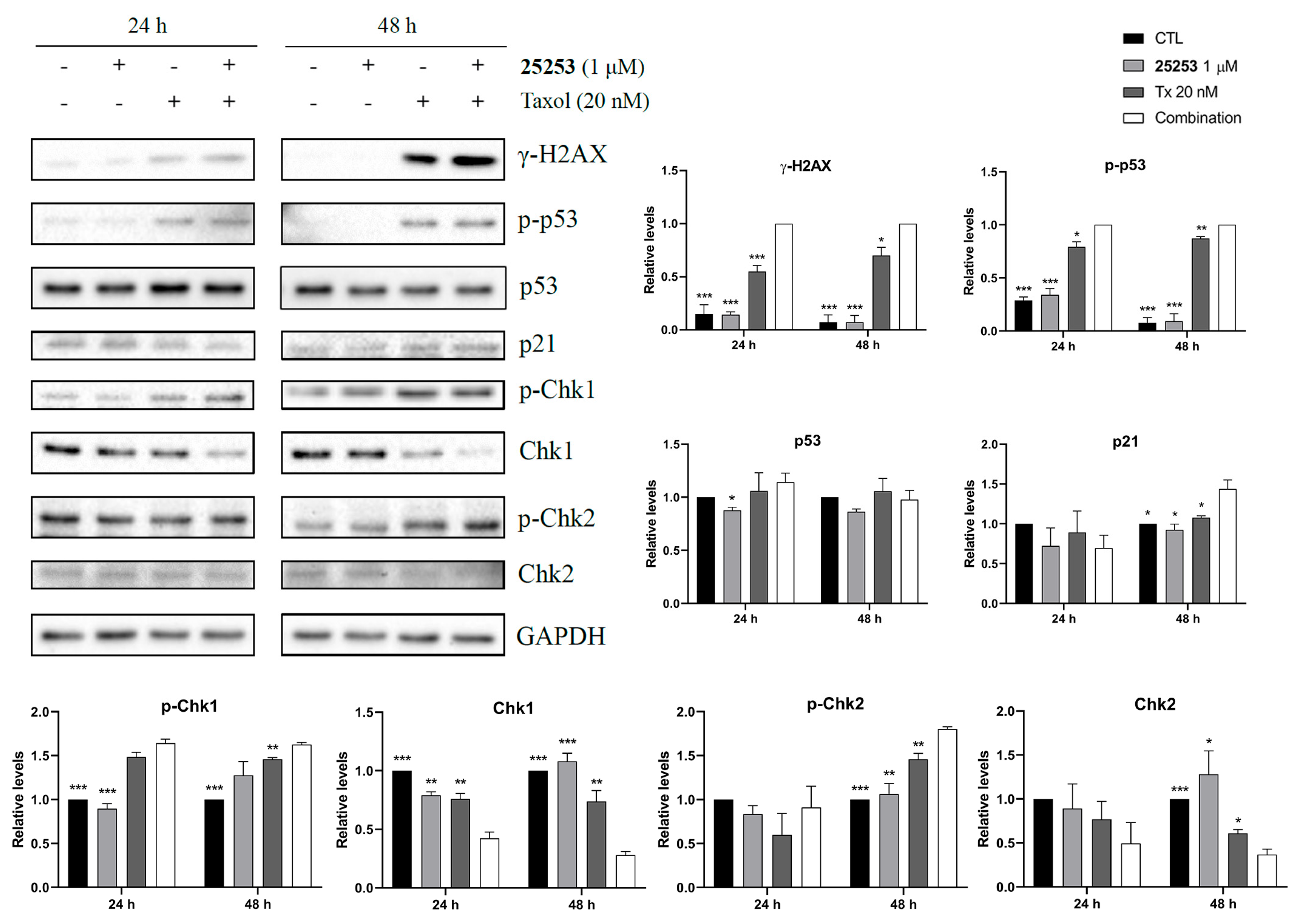
2.5. The Combination of 25253 and Taxol Synergistically Induces DNA Damage in ES-2 Cells
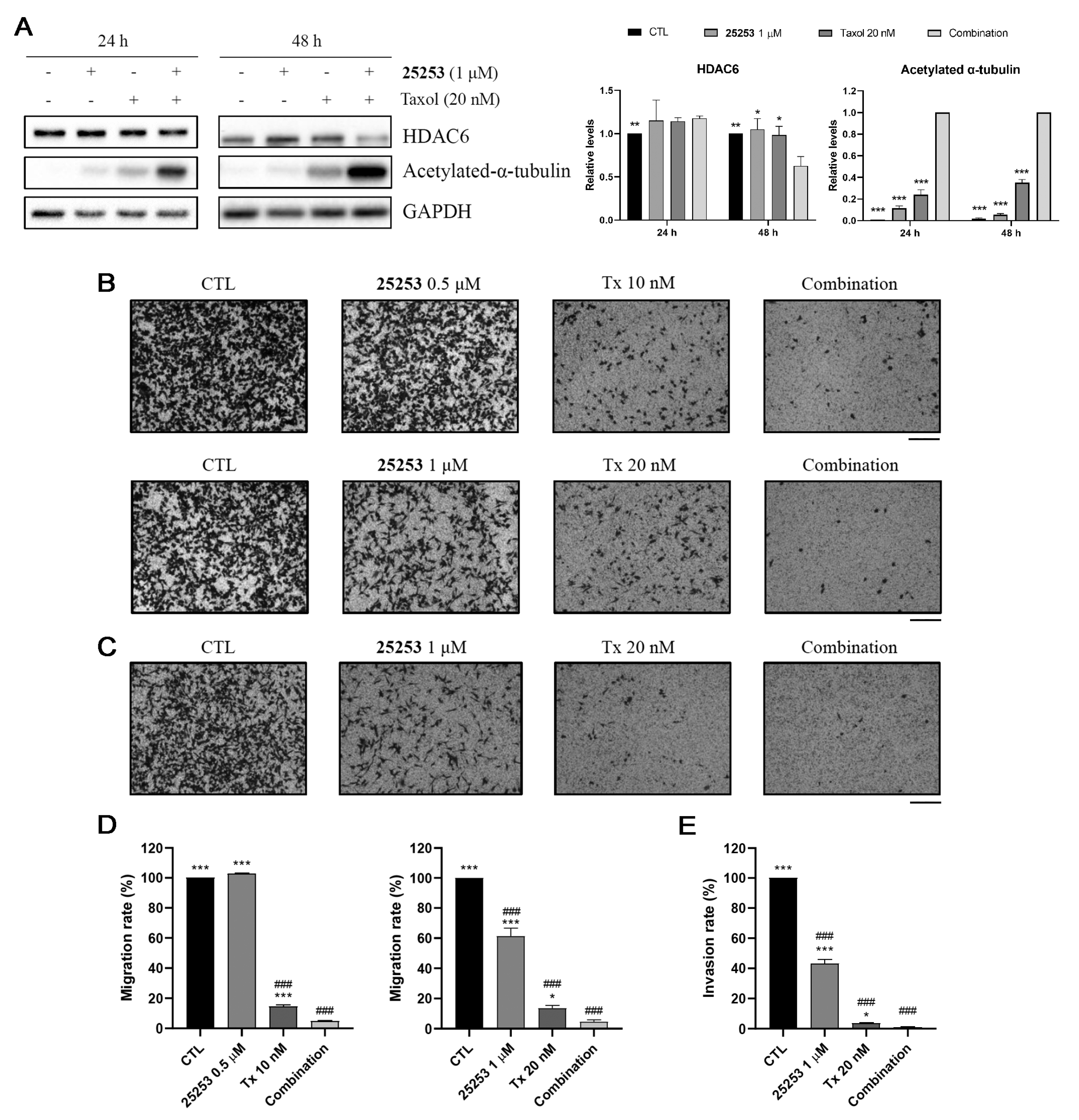
2.6. 25253 Enhances the Inhibitory Effects of Taxol on Cell Migration and Invasion in ES-2 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Lines and Cell Culture
4.3. Cell Viability and Drug Combination Analysis
4.4. Colony Formation Assay
4.5. Western Blot Analysis
4.6. PI Staining and Flow Cytometric Analysis
4.7. Annexin V-FITC/PI Double Analysis
4.8. Transwell Cell Migration and Invasion Assays
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PARP | poly (ADP-ribose) polymerase |
| HDAC | Human histone deacetylase |
| SAHA | Suberoylanilide hydroxamic acid |
| IC50 | 50% inhibitory concentration |
| TST | Tubastatin A |
| SC | Synergy score |
| CI | Combination index |
| CI50 | Combination index at IC50 |
| PI | Propidium iodide |
| Tx | Taxol |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- American Cancer Society. Survival Rates for Ovarian Cancer. Available online: https://www.cancer.org/cancer/types/ovarian-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 1 April 2025).
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and new options in advanced epithelial ovarian cancer treatment. Obstet. Gynecol. 2021, 137, 108–121. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Treating Ovarian Cancer. Available online: https://www.cancer.org/cancer/types/ovarian-cancer/treating.html (accessed on 1 April 2025).
- Pignata, S.; Lorusso, D.; Scambia, G.; Sambataro, D.; Tamberi, S.; Cinieri, S.; Mosconi, A.M.; Orditura, M.; Brandes, A.A.; Arcangeli, V.; et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): A randomised, open-label, phase 2 trial. Lancet Oncol. 2015, 16, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef]
- Li, Y.; Seto, E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Prospect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef]
- Li, T.; Zhang, C.; Hassan, S.; Liu, X.; Song, F.; Chen, K.; Zhang, W.; Yang, J. Histone deacetylase 6 in cancer. J. Hematol. Oncol. 2018, 11, 111. [Google Scholar] [CrossRef]
- Ye, P.C.; Leu, W.J.; Yeh, T.Y.; Hsu, Y.T.; Lin, Y.C.; Wei, Z.Y.; Chen, Y.C.; Chiang, Y.C.; Hsu, J.L.; Chan, S.H.; et al. A novel HDAC6 inhibitor interferes microtubule dynamics and spindle assembly checkpoint and sensitizes cisplatin-induced apoptosis in castration-resistant prostate cancer. Prostate 2024, 84, 605–619. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, H.S.; Soong, Y.K. Paclitaxtel induced cell death: Where the cell cycle and apoptosis come together. Cancer 2000, 88, 2619–2628. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, B.Y.; Lai, W.T.; Wu, S.F.; Guh, J.H.; Cheng, A.L.; Hsu, L.C. The Akt inhibitor MK-2206 enhances the cytotoxicity of paclitaxel (Taxol) and cisplatin in ovarian cancer cells. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 19–31. [Google Scholar] [CrossRef]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022, 50, W739–W743. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Demidenko, Z.N.; Kalurupalle, S.; Hanko, C.; Lim, C.U.; Broude, E.; Blagosklonny, M.V. Mechanism of G1-like arrest by low concentrations of paclitaxel: Next cell cycle p53-dependent arrest with sub G1 DNA content mediated by prolonged mitosis. Oncogene 2008, 27, 4402–4410. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2267–2681. [Google Scholar] [CrossRef]
- Orth, J.D.; Loewer, A.; Lahav, G.; Mitchison, T.J. Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Mol. Biol. Cell 2012, 23, 567–576. [Google Scholar]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Yousef, H.A.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Huang, P.; Almeciga-Pinto, I.; Jarpe, M.; van Duzer, J.H.; Mazitschek, R.; Yang, M.; Jones, S.S.; Quayle, S.N. Selective HDAC inhibition by ACY-241 enhances the activity of paclitaxel in solid tumor models. Oncotarget 2017, 8, 2694–2707. [Google Scholar] [CrossRef]
- Eshun-Wilson, L.; Zhang, R.; Portran, D.; Nachury, M.V.; Toso, D.B.; Löhr, T.; Vendruscolo, M.; Bonomi, M.; Fraser, J.S.; Nogales, E. Effects of α-tubulin acetylation on microtubule structure and stability. Proc. Natl. Acad. Sci. USA 2019, 116, 10366–10371. [Google Scholar] [CrossRef]
- Peng, J.; Xie, F.; Qin, P.; Liu, Y.; Niu, H.; Sun, J.; Xue, H.; Zhao, Q.; Liu, J.; Wu, J. Recent development of selective inhibitors targeting the HDAC6 as anti-cancer drugs: Structure, function and design. Bioorg. Chem. 2023, 138, 106622. [Google Scholar] [CrossRef]
- Yano, M.; Miyazawa, M.; Ogane, N.; Ogasawara, A.; Hasegawa, K.; Narahara, H.; Yasuda, M. Up-regulation of HDAC6 results in poor prognosis and chemoresistance in patients with advanced ovarian high-grade serous carcinoma. Anticancer. Res. 2021, 41, 1647–1654. [Google Scholar] [CrossRef]
- Yoo, J.; Jeon, Y.H.; Lee, D.H.; Kim, G.W.; Lee, S.W.; Kim, S.Y.; Park, J.; Kwon, S.H. HDAC6-selective inhibitors enhance anticancer effects of paclitaxel in ovarian cancer cells. Oncol. Lett. 2021, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Kapali, M.; DeLoia, J.A.; Gallion, H.H. Centrosome abnormalities in ovarian cancer. Int. J. Cancer 2005, 113, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Berns, K.; Caumanns, J.J.; Hijmans, E.M.; Gennissen, A.M.C.; Severson, T.M.; Evers, B.; Wisman, G.B.A.; Meersma, G.J.; Lieftink, C.; Beijerbergen, R.L.; et al. ARID1A mutation sensitizes most ovarian clear cell carcinomas to BET inhibitors. Oncogene 2018, 37, 4611–4625. [Google Scholar] [CrossRef]
- Ikui, A.E.; Yang, C.P.; Matsumoto, T.; Horwitz, S.B. Low concentrations of taxol cause mitotic delay followed by premature dissociation of p55CDC from Mad2 and BubR1 and abrogation of the spindle checkpoint, leading to aneuploidy. Cell Cycle 2005, 4, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.V.; Hicks, M.A.; Nakajima, W.; Richardson, A.C.; Windle, J.J.; Harada, H. Paclitaxel-induced apoptosis is BAK-dependent but BAX and BIM-independent in breast tumor. PLoS ONE 2013, 8, e60685. [Google Scholar] [CrossRef]
- Moufarrij, S.; O’Cearbhaill, R.E. Novel therapeutics in ovarian cancer: Expanding the toolbox. Curr. Oncol. 2023, 31, 97–114. [Google Scholar] [CrossRef]
- Weng, H.C.; Sung, C.J.; Hsu, J.L.; Leu, W.J.; Guh, J.H.; Kung, F.L.; Hsu, L.C. The combination of a novel GLUT1 inhibitor and cisplatin synergistically inhibits breast cancer cell growth by enhancing the DNA damaging effect and modulating the Akt/mTOR and MAPK signaling pathways. Front. Pharmacol. 2022, 13, 879748. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, A.-J.; Hsu, J.-L.; Xiao, Y.-X.; Chern, J.-W.; Guh, J.-H.; Yu, C.-W.; Hsu, L.-C. A Novel HDAC6 Inhibitor Enhances the Efficacy of Paclitaxel Against Ovarian Cancer Cells. Molecules 2025, 30, 2793. https://doi.org/10.3390/molecules30132793
Chi A-J, Hsu J-L, Xiao Y-X, Chern J-W, Guh J-H, Yu C-W, Hsu L-C. A Novel HDAC6 Inhibitor Enhances the Efficacy of Paclitaxel Against Ovarian Cancer Cells. Molecules. 2025; 30(13):2793. https://doi.org/10.3390/molecules30132793
Chicago/Turabian StyleChi, An-Jui, Jui-Ling Hsu, Yun-Xin Xiao, Ji-Wang Chern, Jih-Hwa Guh, Chao-Wu Yu, and Lih-Ching Hsu. 2025. "A Novel HDAC6 Inhibitor Enhances the Efficacy of Paclitaxel Against Ovarian Cancer Cells" Molecules 30, no. 13: 2793. https://doi.org/10.3390/molecules30132793
APA StyleChi, A.-J., Hsu, J.-L., Xiao, Y.-X., Chern, J.-W., Guh, J.-H., Yu, C.-W., & Hsu, L.-C. (2025). A Novel HDAC6 Inhibitor Enhances the Efficacy of Paclitaxel Against Ovarian Cancer Cells. Molecules, 30(13), 2793. https://doi.org/10.3390/molecules30132793






