Estrogenic Effect of Various Plant Extracts on Eel (Anguilla japonica) Hepatocytes
Abstract
1. Introduction
2. Results
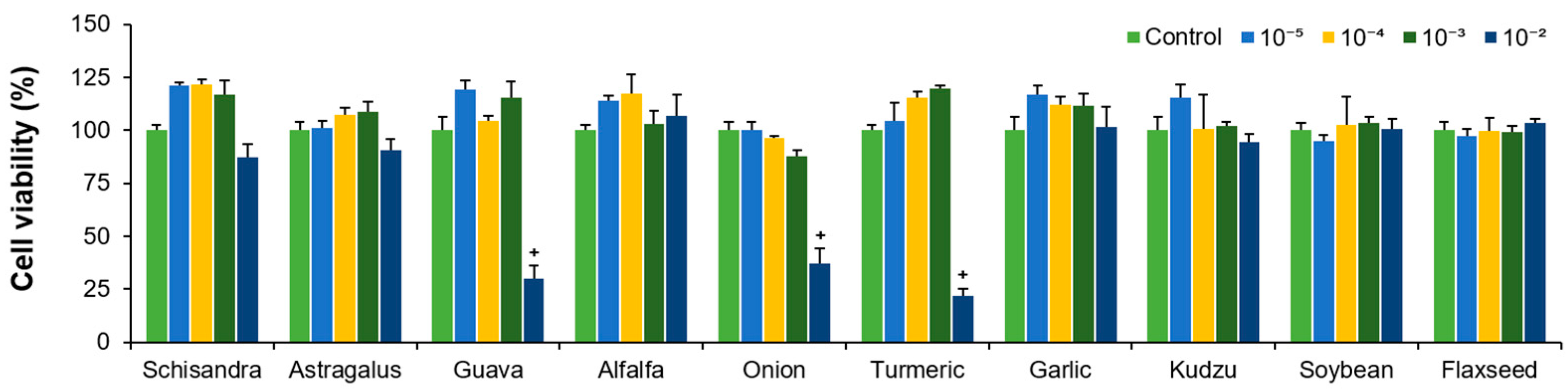
2.1. Analysis of Hepatocyte Viability After Treatment with Plant Extracts
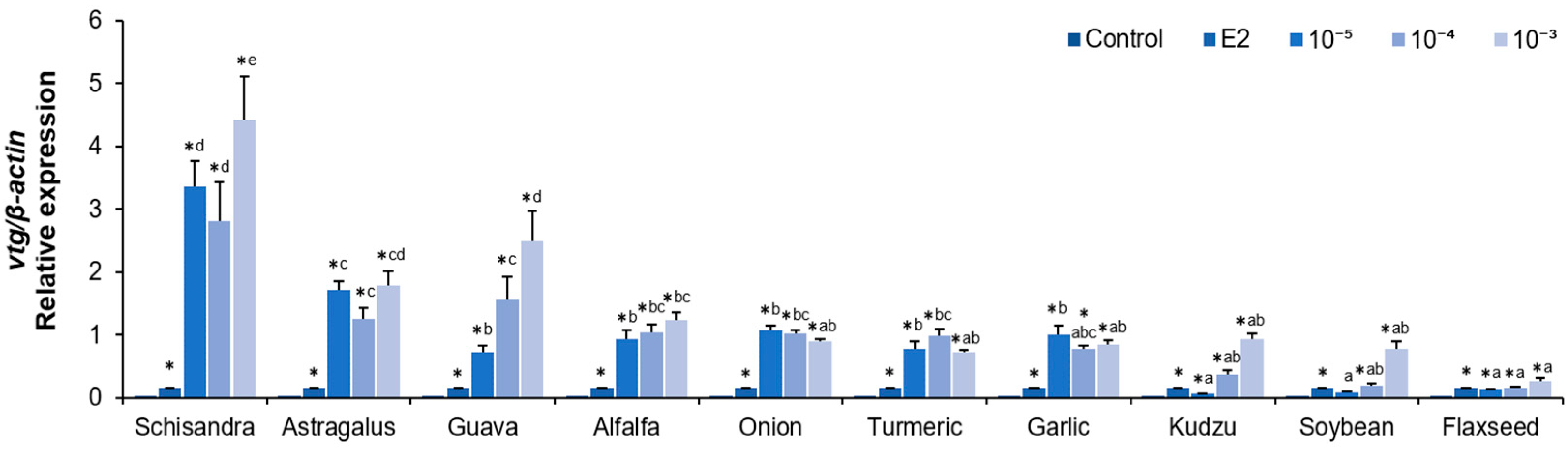
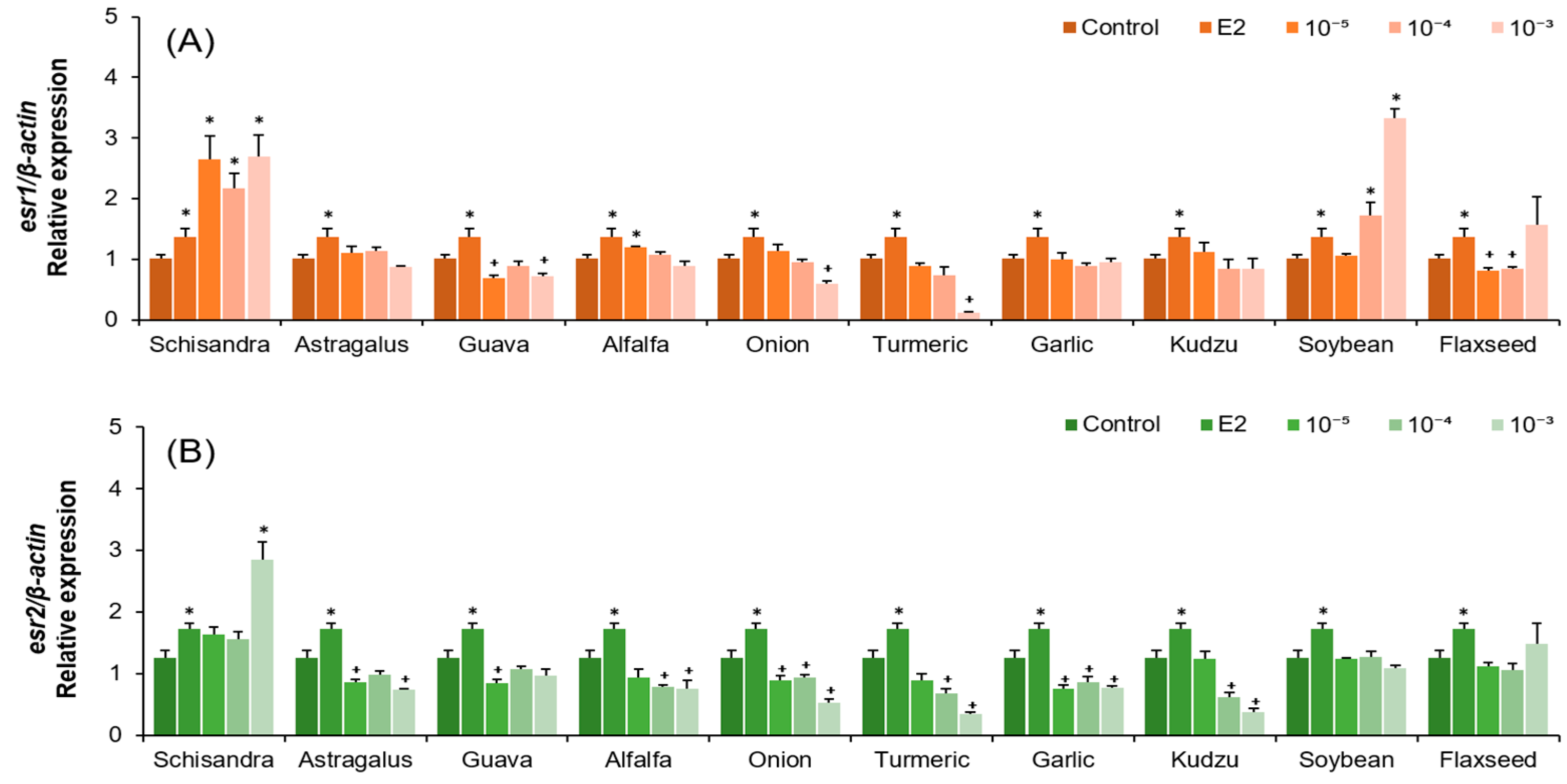
2.2. Analysis of vtg, esr1, and esr2 mRNA Expression in Hepatocytes After Plant Extract Treatments
2.3. HPLC (High-Performance Liquid Chromatography) Analysis of Phytoestrogens in Schisandra and Astragalus Extracts
2.4. Analysis of vtg mRNA Expression in Hepatocytes After Phytoestrogen Treatment
3. Discussion
4. Materials and Methods
4.1. Hepatocytes Culture
4.2. Ultrasonic Extraction and Filtration of Plants
4.3. Hepatocyte Viability Test of Plant Extracts
4.4. Treatment of Hepatocytes with Plant Extracts
4.5. RNA Extraction and cDNA Synthesis
4.6. Quantitative Real-Time PCR
4.7. HPLC Analysis
4.8. Treatment of Hepatocytes with Phytoestrogens
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| E2 | 17β-estradiol |
| esr | Estrogen receptor |
| vtg | Vitellogenin |
| HPLC | High-performance liquid chromatography |
| Sch | Schizandrin |
| Gom | Gomisin |
| Cal | Calycosin |
| For | Formononetin |
References
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.; Bentley, G.E.; Cabrera, R.; Ortega, H.H.; Perfito, N.; Wu, T.J.; Micevych, P. Hormonal regulation of female reproduction. Horm. Metab. Res. 2012, 44, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Lee, A.R. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012, 135, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.; Busza, A.; Allen, L.M.; Kelly, C.; Wolfman, W.; Jacobson, M.; Lega, I.C. Comparing estrogen-based hormonal contraceptives and hormone therapy on bone mineral density in women with premature ovarian insufficiency: A systematic review. Menopause 2022, 29, 351–359. [Google Scholar] [CrossRef]
- Valéra, M.C.; Fontaine, C.; Dupuis, M.; Noirrit-Esclassan, E.; Vinel, A.; Guillaume, M.; Arnal, J.F. Towards optimization of estrogen receptor modulation in medicine. Pharmacol. Ther. 2018, 189, 123–129. [Google Scholar] [CrossRef]
- Palstra, A.P.; Bouwman, L.J.; Jéhannet, P.; Kruijt, L.; Schipper, H.; Blokland, M.H.; Lokman, P.M. Steroid implants for the induction of vitellogenesis in feminized European silver eels (Anguilla anguilla L.). Front. Genet. 2022, 13, 969202. [Google Scholar] [CrossRef]
- Nakada, N.; Nyunoya, H.; Nakamura, M.; Hara, A.; Iguchi, T.; Takada, H. Identification of estrogenic compounds in wastewater effluent. Environ. Toxicol. Chem. 2004, 23, 2807–2815. [Google Scholar] [CrossRef]
- Wojnarowski, K.; Podobiński, P.; Cholewińska, P.; Smoliński, J.; Dorobisz, K. Impact of estrogens present in environment on health and welfare of animals. Animals 2021, 11, 2152. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef]
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef]
- Setchell, K.D.R. Phytoestrogens: The Biochemistry, Physiology, and Implications for Human Health of Soy Isoflavones. In Phytoestrogens in Functional Foods; Yildiz, F., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 3–16. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef] [PubMed]
- Clotfelter, E.D.; Rodriguez, A.C. Behavioral changes in fish exposed to phytoestrogens. Environ. Pollut. 2006, 144, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Mazur, W.M.; Duke, J.A.; Wähälä, K.; Rasku, S.; Adlercreutz, H. Isoflavonoids and lignans in legumes: Nutritional and health aspects in humans. J. Nutr. Biochem. 1998, 9, 193–200. [Google Scholar] [CrossRef]
- Mazur, W. Phytoestrogen content in foods. Baillieres Clin. Endocrinol. Metab. 1998, 12, 729–742. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr. Cancer 2006, 54, 184–201. [Google Scholar] [CrossRef]
- Koo, D.C.; Suh, W.S.; Baek, S.Y.; Shim, S.H. Quantitative determination of lignans from Schizandra chinensis by HPLC. Korean J. Pharmacogn. 2011, 42, 233–239. [Google Scholar]
- Tucak, M.; Čupić, T.; Horvat, D.; Popović, S.; Krizmanić, G.; Ravlić, M. Variation of phytoestrogen content and major agronomic traits in alfalfa (Medicago sativa L.) populations. Agronomy 2020, 10, 87. [Google Scholar] [CrossRef]
- Alvarez, D.V.; Hernández, M.S.; Hernández, V.A.G.; Engleman, E.M.; Nava, A.D. Flavonoids in Psidium guajava L. leaves. Hortic. Int. J. 2021, 5, 38–41. [Google Scholar] [CrossRef]
- Lee, Y.S.; Wee, J.H.; Lee, S.K.; Ji, S.H.; Kim, P.H.; Lee, S.H.; Ma, K.C.; Lee, J.W. Comparison of curcuminoid contents of turmeric (Curcuma longa L.) according to color, drying temperature, and extraction conditions. J. Korean Tea Soc. 2022, 28, 48–55. [Google Scholar] [CrossRef]
- Shin, D.; Hong, S.B.; Geum, J.H.; Ma, J.Y.; Chung, H.S. Effects of Schisandrae Fructus on menopause symptoms in ovariectomized mice. J. Korean Med. 2016, 37, 39–46. [Google Scholar] [CrossRef]
- Echeverria, V.; Echeverria, F.; Barreto, G.E.; Echeverría, J.; Mendoza, C. Estrogenic plants: To prevent neurodegeneration and memory loss and other symptoms in women after menopause. Front. Pharmacol. 2021, 12, 644103. [Google Scholar] [CrossRef]
- Rizzo, L.Y.; Longato, G.B.; Ruiz, A.L.T.G.; Tinti, S.V.; Possenti, A.; Vendramini-Costa, D.B.; Carvalho, J.E. In vitro, in vivo and in silico analysis of the anticancer and estrogen-like activity of guava leaf extracts. Curr. Med. Chem. 2014, 21, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V. The influence of turmeric and curcumin on female reproductive processes. Planta Med. 2022, 88, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Khalilzadeh, S.; Nejatbakhsh, F. Therapeutic effects of garlic (Allium sativum) on female reproductive system: A systematic review. Heliyon 2023, 9, e22555. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Influence of flaxseed (Linum usitatissimum) on female reproduction. Planta Med. 2023, 89, 608–615. [Google Scholar] [CrossRef]
- Navas, J.M.; Segner, H. Vitellogenin synthesis in primary cultures of fish liver cells as endpoint for in vitro screening of the (anti)estrogenic activity of chemical substances. Aquat. Toxicol. 2006, 80, 1–22. [Google Scholar] [CrossRef]
- Sundaray, J.K.; Rather, M.A. Sex Hormones and Their Role in Gonad Development and Reproductive Cycle of Fishes. In Recent Updates in Molecular Endocrinology and Reproductive Physiology of Fish; Kumar, S., Agarwal, D., Eds.; Springer: Singapore, 2021; pp. 1–25. Available online: https://link.springer.com/chapter/10.1007/978-981-15-8369-8_1 (accessed on 2 June 2025).
- Peyon, P.; Baloche, S.; Burzawa-Gérard, E. Potentiating effect of growth hormone on vitellogenin synthesis induced by 17β-estradiol in primary culture of female silver eel (Anguilla anguilla) hepatocytes. Gen. Comp. Endocrinol. 1996, 102, 263–273. [Google Scholar] [CrossRef]
- Latonnelle, K.; Le Menn, F.; Kaushik, S.J.; Bennetau-Pelissero, C. Effects of dietary phytoestrogens in vivo and in vitro in rainbow trout and Siberian sturgeon: Interests and limits of the in vitro studies of interspecies differences. Gen. Comp. Endocrinol. 2002, 126, 39–51. [Google Scholar] [CrossRef]
- Bickley, L.K.; Lange, A.; Winter, M.J.; Tyler, C.R. Evaluation of a carp primary hepatocyte culture system for screening chemicals for oestrogenic activity. Aquat. Toxicol. 2009, 94, 195–203. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Manor, M.L. Effects of phytoestrogens on growth-related and lipogenic genes in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 170, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Latonnelle, K.; Fostier, A.; Le Menn, F.; Bennetau-Pelissero, C. Binding affinities of hepatic nuclear estrogen receptors for phytoestrogens in rainbow trout (Oncorhynchus mykiss) and Siberian sturgeon (Acipenser baeri). Gen. Comp. Endocrinol. 2002, 129, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Addissouky, T.A.; El Sayed, I.E.T.; Ali, M.M.; Alubiady, M.H.S.; Wang, Y. Schisandra chinensis in liver disease: Exploring the mechanisms and therapeutic promise of an ancient Chinese botanical. Arch. Pharmacol. Ther. 2024, 6, 27–33. [Google Scholar] [CrossRef]
- Ahn, S.J.; Kim, H.J.; Lee, A.; Min, S.S.; In, S.; Kim, E. Determination of 12 herbal compounds for estimating the presence of Angelica Gigas Root, Cornus Fruit, Licorice Root, Pueraria Root, and Schisandra Fruit in foods by LC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2020, 37, 1437–1448. [Google Scholar] [CrossRef]
- Im, K.R.; Kim, M.J.; Jung, T.K.; Yoon, K.S. Analysis of isoflavonoid contents in Astragalus membranaceus Bunge cultivated in different areas and at various ages. KSBB J. 2010, 25, 271–276. [Google Scholar]
- Li, S.; Liu, C.; Zhang, Y.; Tsao, R. On-line coupling pressurised liquid extraction with two-dimensional counter current chromatography for isolation of natural acetylcholinesterase inhibitors from Astragalus membranaceus. Phytochem. Anal. 2021, 32, 640–653. [Google Scholar] [CrossRef]
- Iuchi, K.; Arai, Y.; Sasaki, K.; Sato, N.; Yokoyama, C.; Saruwatari, T.; Hisatomi, H. A simple method for isolation and culture of primary hepatocytes from Salvelinus leucomaenis (White-spotted Charr). Cytotechnology 2020, 72, 731–739. [Google Scholar] [CrossRef]
- Lee, M.H.; Lin, C.C. Comparison of techniques for extraction of isoflavones from the root of Radix Puerariae: Ultrasonic and pressurized solvent extractions. Food Chem. 2007, 105, 223–228. [Google Scholar] [CrossRef]
- Blicharski, T.; Oniszczuk, A. Extraction methods for the isolation of isoflavonoids from plant material. Open Chem. 2017, 15, 34–45. [Google Scholar] [CrossRef]
- Oomah, B.D.; Hosseinian, F.S. Phytoestrogens. In Methods of Analysis for Functional Foods and Nutraceuticals, 1st ed.; Wildman, R.E.C., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 1–17. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Hyeon, J.Y.; Hur, S.P.; Kim, B.H.; Byun, J.H.; Kim, E.S.; Lim, B.S.; Kim, S.J. Involvement of estrogen and its receptors in morphological changes in the eyes of the Japanese eel, Anguilla japonica, in the process of artificially-induced maturation. Cells 2019, 8, 310. [Google Scholar] [CrossRef]
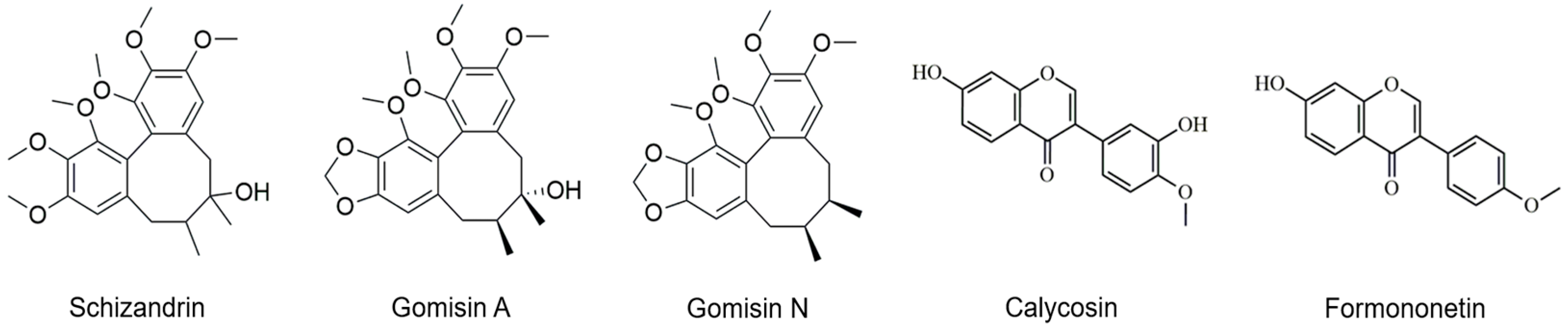

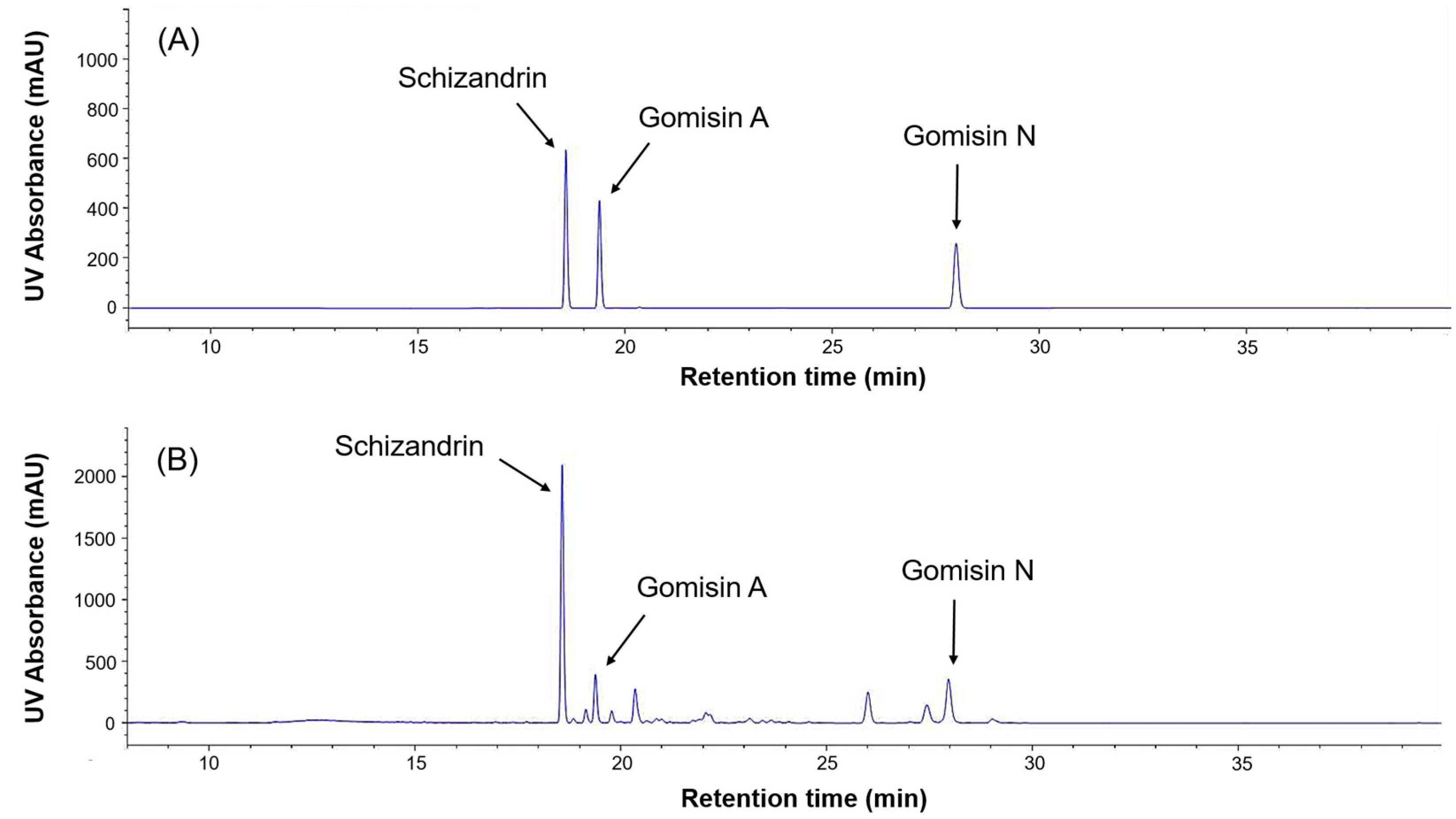
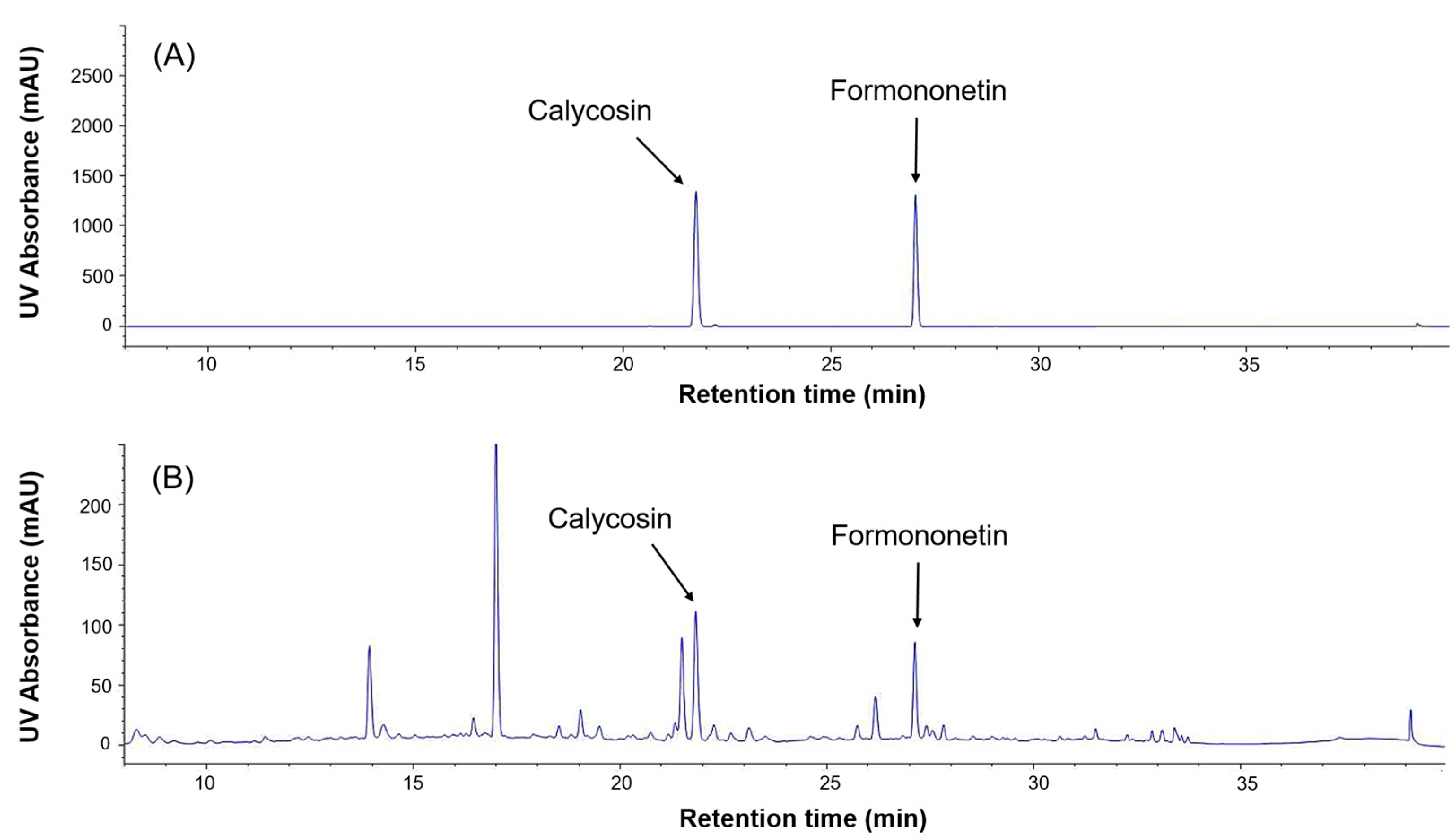

| Compound | Plant Source | RT (Standard, min) | RT (Sample, min) | Content (mg/g Extract) |
|---|---|---|---|---|
| Schizandrin | Schisandra | 18.57 | 18.56 | 5.61 |
| Gomisin A | Schisandra | 19.38 | 19.37 | 1.52 |
| Gomisin N | Schisandra | 28.25 | 27.97 | 2.44 |
| Calycosin | Astragalus | 21.89 | 21.86 | 0.181 |
| Formononetin | Astragalus | 27.13 | 27.13 | 0.068 |
| Gene | Primer Sequence | Product Size | |
|---|---|---|---|
| β-actin | F | GAGACCTTCAACACCCCAG | 95 |
| R | CCAGAGTCCATCACAATACCAG | ||
| vtg | F | GACAGTGTAGTGCAGATGAAG | 117 |
| R | ATAGAGAGACAGCCCATCAC | ||
| esr1 | F | TTTCGACATGATCCTCGCCA | 102 |
| R | GCACCGGAGTTGAGCAGTAT | ||
| esr2 [44] | F | AAGTACACCTGCTGGAGTGC | 220 |
| R | AGGCACACATACTCCTCCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.H.; Ha, J.E.; Kwon, J.Y. Estrogenic Effect of Various Plant Extracts on Eel (Anguilla japonica) Hepatocytes. Molecules 2025, 30, 2781. https://doi.org/10.3390/molecules30132781
Yoon JH, Ha JE, Kwon JY. Estrogenic Effect of Various Plant Extracts on Eel (Anguilla japonica) Hepatocytes. Molecules. 2025; 30(13):2781. https://doi.org/10.3390/molecules30132781
Chicago/Turabian StyleYoon, Jeong Hee, Ji Eun Ha, and Joon Yeong Kwon. 2025. "Estrogenic Effect of Various Plant Extracts on Eel (Anguilla japonica) Hepatocytes" Molecules 30, no. 13: 2781. https://doi.org/10.3390/molecules30132781
APA StyleYoon, J. H., Ha, J. E., & Kwon, J. Y. (2025). Estrogenic Effect of Various Plant Extracts on Eel (Anguilla japonica) Hepatocytes. Molecules, 30(13), 2781. https://doi.org/10.3390/molecules30132781






