Simultaneous Liquid Digestate Treatment and High-Value Microalgal Biomass Production: Influence of Post-Harvest Storage on Biochemical Profiles
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of Raw Liquid Digestate
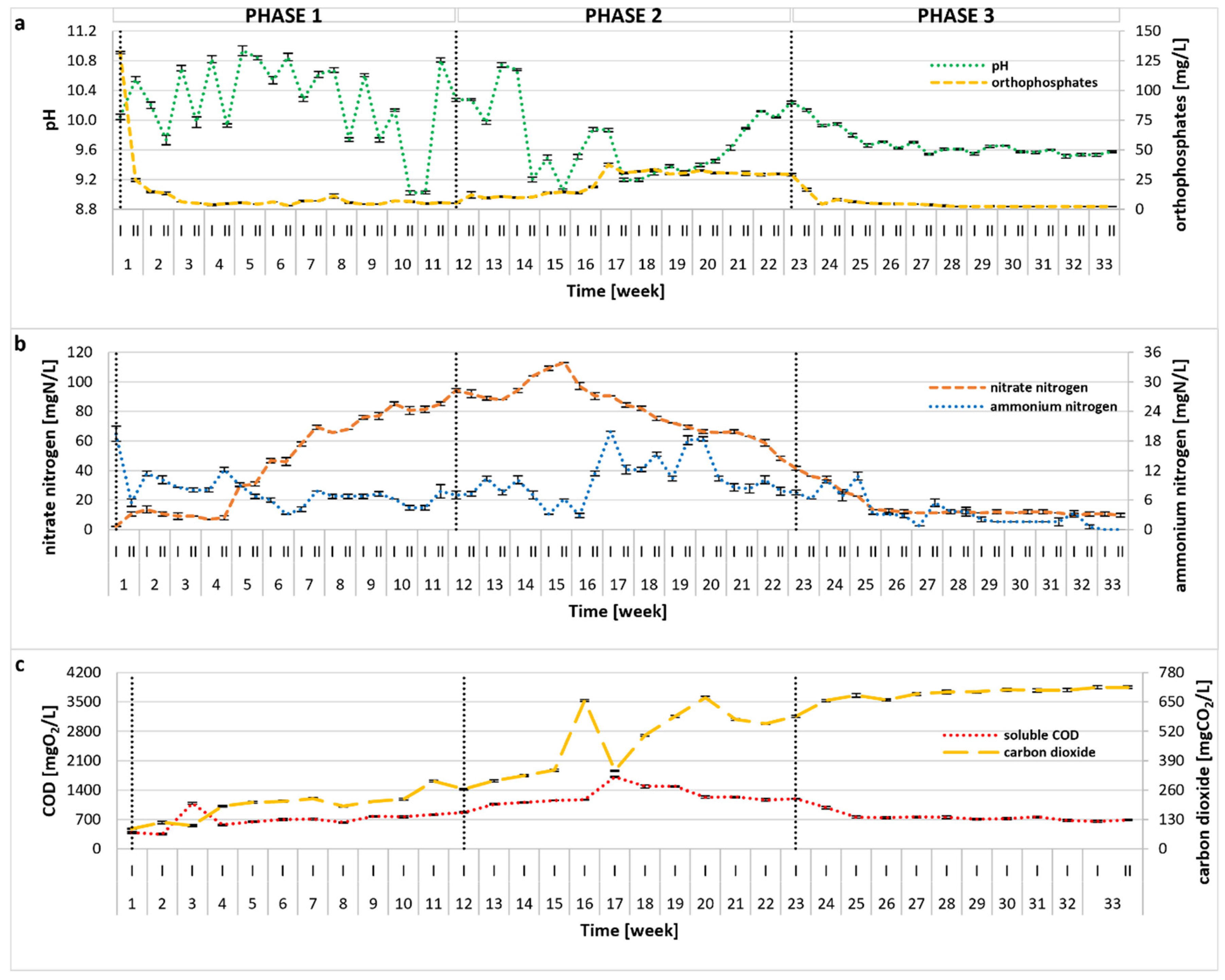
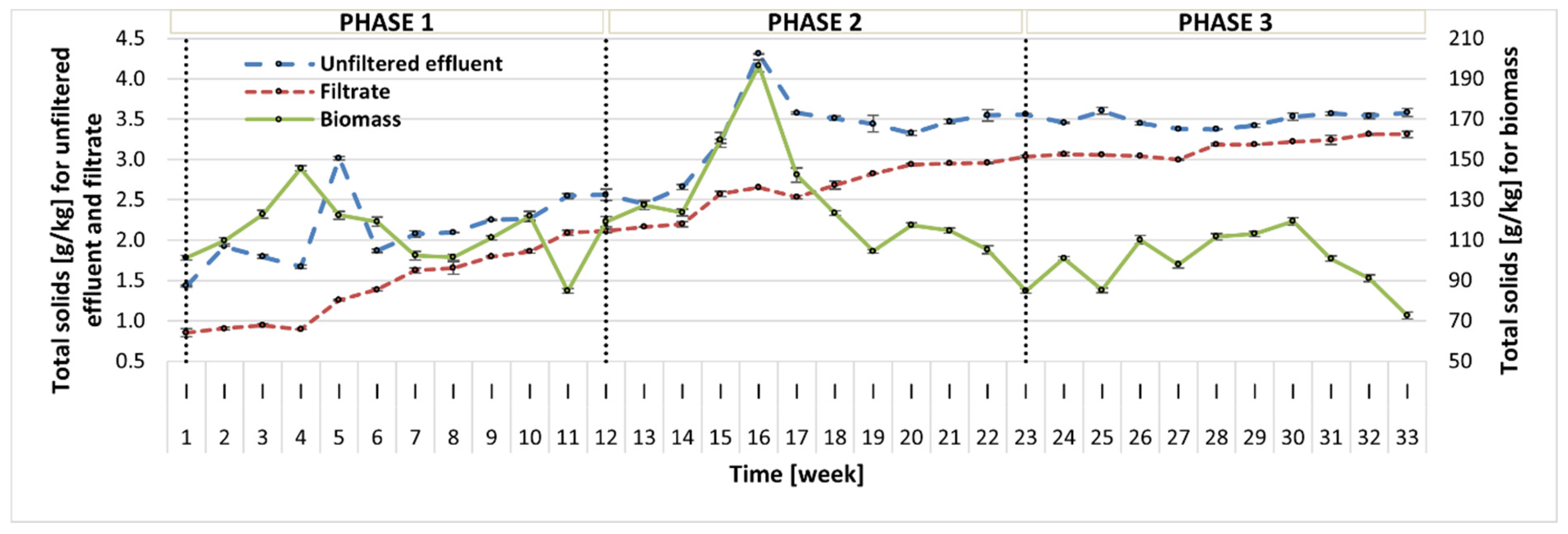
2.2. Photobioreactor Performance
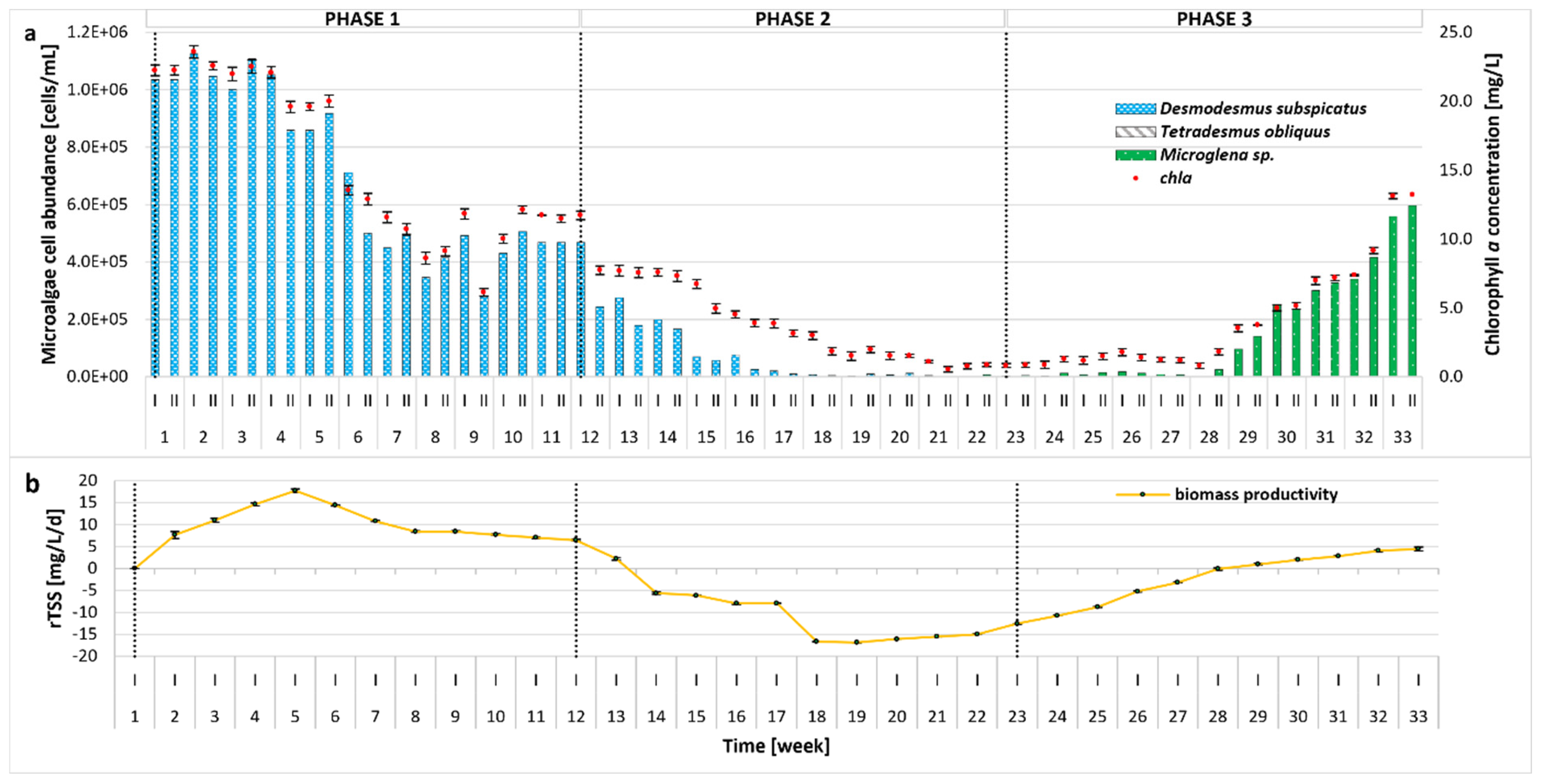
2.3. Microalgal Community and Productivity
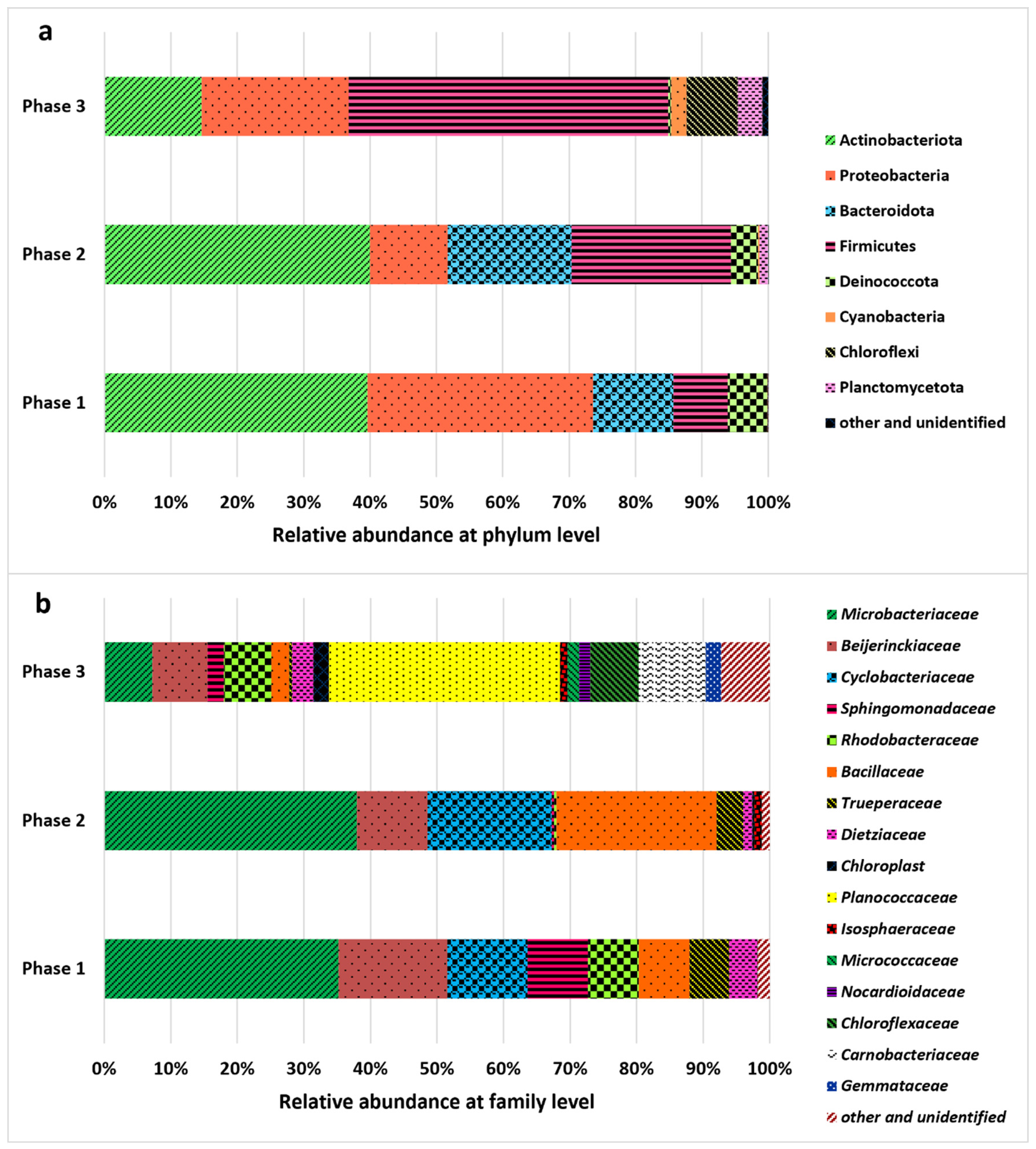
2.4. Bacterial Community
2.5. Elemental Analysis
2.6. Biochemical Characterization of Biomass
2.6.1. Biomass Analysis by Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy
2.6.2. Lipids
2.6.3. Fatty Acids
2.6.4. Proteins and Pigments
3. Materials and Methods
3.1. Liquid Substrate
3.2. Microalgae Inoculum
3.3. Photobioreactor Setup and Experimental Organization
3.4. Analytical Methods
3.4.1. Physicochemical Tests
3.4.2. Microalgae Biomass Parameters
3.4.3. Metagenomic Analysis
3.4.4. Elemental Analysis
3.4.5. Algae Biomass Valorization
ATR-FTIR Spectroscopy
Total Lipids Determination
Separation of Lipid Fractions
Fatty Acid Analysis
Protein Extraction and Quantification
Total Carotenoids and Total Chlorophylls Determination
Pigments Extraction and Quantification
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- van Dijk, M.; Goedegebure, R.; Nap, J.-P. Public Acceptance of Biomass for Bioenergy: The Need for Feedstock Differentiation and Communicating a Waste Utilization Frame. Renew. Sustain. Energy Rev. 2024, 202, 114670. [Google Scholar] [CrossRef]
- Meyer, A.K.P.; Ehimen, E.A.; Holm-Nielsen, J.B. Future European Biogas: Animal Manure, Straw and Grass Potentials for a Sustainable European Biogas Production. Biomass Bioenergy 2018, 111, 154–164. [Google Scholar] [CrossRef]
- Peng, W.; Lü, F.; Hao, L.; Zhang, H.; Shao, L.; He, P. Digestate Management for High-Solid Anaerobic Digestion of Organic Wastes: A Review. Bioresour. Technol. 2020, 297, 122485. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Quattrocelli, P.; Bordiean, A. Effects of Liquid Digestate Treatment on Sustainable Microalgae Biomass Production. BioEnergy Res. 2022, 15, 357–370. [Google Scholar] [CrossRef]
- Kaur, G.; Wong, J.W.C.; Kumar, R.; Patria, R.D.; Bhardwaj, A.; Uisan, K.; Johnravindar, D. Value Addition of Anaerobic Digestate From Biowaste: Thinking Beyond Agriculture. Curr. Sustain. Energy Rep. 2020, 7, 48–55. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic Digestate as a Low-Cost Nutrient Source for Sustainable Microalgae Cultivation: A Way Forward through Waste Valorization Approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef]
- Xia, A.; Murphy, J.D. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Yu, H.; Kim, J.; Lee, C. Nutrient Removal and Microalgal Biomass Production from Different Anaerobic Digestion Effluents with Chlorella Species. Sci. Rep. 2019, 9, 6123. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Ishii, K. Attempts to Alleviate Inhibitory Factors of Anaerobic Digestate for Enhanced Microalgae Cultivation and Nutrients Removal: A Review. J. Environ. Manage 2022, 304, 114266. [Google Scholar] [CrossRef]
- Wang, Q.; Cherones, J.; Higgins, B. Acclimation of an Algal Consortium to Sequester Nutrients from Anaerobic Digestate. Bioresour. Technol. 2021, 342, 125921. [Google Scholar] [CrossRef]
- Massa, M.; Buono, S.; Langellotti, A.L.; Castaldo, L.; Martello, A.; Paduano, A.; Sacchi, R.; Fogliano, V. Evaluation of Anaerobic Digestates from Different Feedstocks as Growth Media for Tetradesmus Obliquus, Botryococcus Braunii, Phaeodactylum Tricornutum and Arthrospira Maxima. N. Biotechnol. 2017, 36, 8–16. [Google Scholar] [CrossRef]
- Uma, V.S.; Usmani, Z.; Sharma, M.; Diwan, D.; Sharma, M.; Guo, M.; Tuohy, M.G.; Makatsoris, C.; Zhao, X.; Thakur, V.K.; et al. Valorisation of Algal Biomass to Value-Added Metabolites: Emerging Trends and Opportunities. Phytochem. Rev. 2023, 22, 1015–1040. [Google Scholar] [CrossRef]
- Chuka-ogwude, D.; Ogbonna, J.; Moheimani, N.R. A Review on Microalgal Culture to Treat Anaerobic Digestate Food Waste Effluent. Algal Res. 2020, 47, 101841. [Google Scholar] [CrossRef]
- Ledda, C.; Schievano, A.; Scaglia, B.; Rossoni, M.; Acién Fernández, F.G.; Adani, F. Integration of Microalgae Production with Anaerobic Digestion of Dairy Cattle Manure: An Overall Mass and Energy Balance of the Process. J. Clean Prod. 2016, 112, 103–112. [Google Scholar] [CrossRef]
- Koutra, E.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Bio-Based Products from Microalgae Cultivated in Digestates. Trends Biotechnol. 2018, 36, 819–833. [Google Scholar] [CrossRef]
- Bauer, L.; Ranglová, K.; Masojídek, J.; Drosg, B.; Meixner, K. Digestate as Sustainable Nutrient Source for Microalgae—Challenges and Prospects. Appl. Sci. 2021, 11, 1056. [Google Scholar] [CrossRef]
- Sobolewska, E.; Borowski, S.; Nowicka-Krawczyk, P.; Jurczak, T. Growth of Microalgae and Cyanobacteria Consortium in a Photobioreactor Treating Liquid Anaerobic Digestate from Vegetable Waste. Sci. Rep. 2023, 13, 22651. [Google Scholar] [CrossRef]
- Sobolewska, E.; Borowski, S.; Nowicka-Krawczyk, P. Effect of Solar and Artificial Lighting on Microalgae Cultivation and Treatment of Liquid Digestate. J. Environ. Manag. 2023, 344, 118445. [Google Scholar] [CrossRef]
- Sakarika, M.; Kornaros, M. Effect of PH on Growth and Lipid Accumulation Kinetics of the Microalga Chlorella Vulgaris Grown Heterotrophically under Sulfur Limitation. Bioresour. Technol. 2016, 219, 694–701. [Google Scholar] [CrossRef]
- Rossi, S.; Mantovani, M.; Marazzi, F.; Bellucci, M.; Casagli, F.; Mezzanotte, V.; Ficara, E. Microalgal Cultivation on Digestate: Process Efficiency and Economics. Chem. Eng. J. 2023, 460, 141753. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J. Algal Production in Wastewater Treatment High Rate Algal Ponds for Potential Biofuel Use. Water Sci. Technol. 2011, 63, 2403–2410. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and Harvesting of Microalgae for Wastewater Treatment, Biofuels, and Bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef]
- Hu, X.; Meneses, Y.E.; Stratton, J.; Wang, B. Acclimation of Consortium of Micro-Algae Help Removal of Organic Pollutants from Meat Processing Wastewater. J. Clean. Prod. 2019, 214, 95–102. [Google Scholar] [CrossRef]
- Shao, H.; Sun, Y.; Jiang, X.; Hu, J.; Guo, C.; Lu, C.; Guo, F.; Sun, C.; Wang, Y.; Dai, C. Towards Biomass Production and Wastewater Treatment by Enhancing the Microalgae-Based Nutrients Recovery from Liquid Digestate in an Innovative Photobioreactor Integrated with Dialysis Bag. J. Environ. Manag. 2022, 317, 115337. [Google Scholar] [CrossRef]
- Tran, N.-A.T.; Seymour, J.R.; Siboni, N.; Evenhuis, C.R.; Tamburic, B. Photosynthetic Carbon Uptake Induces Autoflocculation of the Marine Microalga Nannochloropsis Oculata. Algal Res. 2017, 26, 302–311. [Google Scholar] [CrossRef]
- Praveen, P.; Loh, K.C. Photosynthetic Aeration in Biological Wastewater Treatment Using Immobilized Microalgae-Bacteria Symbiosis. Appl. Microbiol. Biotechnol. 2015, 99, 10345–10354. [Google Scholar] [CrossRef]
- Semmens, M.J. Alternative MBR Configurations: Using Membranes for Gas Transfer. Desalination 2008, 231, 236–242. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Ho, S.-H. Converting Nitrogen and Phosphorus Wastewater into Bioenergy Using Microalgae-Bacteria Consortia: A Critical Review. Bioresour. Technol. 2021, 342, 126056. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Cheng, K.-W.; Chen, F. Investigation of Carbon and Energy Metabolic Mechanism of Mixotrophy in Chromochloris Zofingiensis. Biotechnol. Biofuels 2021, 14, 36. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Alhajeri, N.S.; Osman, A.I.; Rooney, D.W. Cultivation of Microalgae on Liquid Anaerobic Digestate for Depollution, Biofuels and Cosmetics: A Review. Environ. Chem. Lett. 2022, 20, 3631–3656. [Google Scholar] [CrossRef]
- Lee, S.A.; Lee, N.; Oh, H.M.; Ahn, C.Y. Enhanced and Balanced Microalgal Wastewater Treatment (COD, N, and P) by Interval Inoculation of Activated Sludge. J. Microbiol. Biotechnol. 2019, 29, 1434–1443. [Google Scholar] [CrossRef]
- Xu, S.; Selvam, A.; Karthikeyan, O.P.; Wong, J.W.C. Responses of Microbial Community and Acidogenic Intermediates to Different Water Regimes in a Hybrid Solid Anaerobic Digestion System Treating Food Waste. Bioresour. Technol. 2014, 168, 49–58. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Y.; Zhao, G.; Zhang, H. Nutrient Removal and Biogas Upgrading by Integrating Freshwater Algae Cultivation with Piggery Anaerobic Digestate Liquid Treatment. Appl. Microbiol. Biotechnol. 2015, 99, 6493–6501. [Google Scholar] [CrossRef]
- Pizzera, A.; Scaglione, D.; Bellucci, M.; Marazzi, F.; Mezzanotte, V.; Parati, K.; Ficara, E. Digestate Treatment with Algae-Bacteria Consortia: A Field Pilot-Scale Experimentation in a Sub-Optimal Climate Area. Bioresour. Technol. 2019, 274, 232–243. [Google Scholar] [CrossRef]
- Deng, X.-Y.; Gao, K.; Zhang, R.-C.; Addy, M.; Lu, Q.; Ren, H.-Y.; Chen, P.; Liu, Y.-H.; Ruan, R. Growing Chlorella Vulgaris on Thermophilic Anaerobic Digestion Swine Manure for Nutrient Removal and Biomass Production. Bioresour. Technol. 2017, 243, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Sayedin, F.; Kermanshahi-pour, A.; He, Q.S.; Tibbetts, S.M.; Lalonde, C.G.E.; Brar, S.K. Microalgae Cultivation in Thin Stillage Anaerobic Digestate for Nutrient Recovery and Bioproduct Production. Algal Res. 2020, 47, 101867. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Chen, P.; Min, M.; Chen, Y.; Zhu, J.; Ruan, R.R. Anaerobic Digested Dairy Manure as a Nutrient Supplement for Cultivation of Oil-Rich Green Microalgae Chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Kusmayadi, A.; Leong, Y.K.; Lu, P.-H.; Huang, C.-Y.; Yen, H.-W.; Chang, J.-S. Simultaneous Nutrients Removal and Bio-Compounds Production by Cultivating Chlorella Sorokiniana SU-1 with Unsterilized Anaerobic Digestate of Dairy Wastewater. Algal Res. 2022, 68, 102896. [Google Scholar] [CrossRef]
- Cheng, J.; Ye, Q.; Xu, J.; Yang, Z.; Zhou, J.; Cen, K. Improving Pollutants Removal by Microalgae Chlorella PY-ZU1 with 15% CO2 from Undiluted Anaerobic Digestion Effluent of Food Wastes with Ozonation Pretreatment. Bioresour. Technol. 2016, 216, 273–279. [Google Scholar] [CrossRef]
- Cai, T.; Ge, X.; Park, S.Y.; Li, Y. Comparison of Synechocystis Sp. PCC6803 and Nannochloropsis Salina for Lipid Production Using Artificial Seawater and Nutrients from Anaerobic Digestion Effluent. Bioresour. Technol. 2013, 144, 255–260. [Google Scholar] [CrossRef]
- Abdur Razzak, S.; Bahar, K.; Islam, K.M.O.; Haniffa, A.K.; Faruque, M.O.; Hossain, S.M.Z.; Hossain, M.M. Microalgae Cultivation in Photobioreactors: Sustainable Solutions for a Greener Future. Green. Chem. Eng. 2024, 5, 418–439. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Science: Amsterdam, The Netherlands, 2005; ISBN 9780120884261. [Google Scholar]
- Wang, S.; Cao, M.; Wang, B.; Deng, R.; Gao, Y.; Liu, P. Optimization of Growth Requirements and Scale-up Cultivation of Freshwater Algae Desmodesmus Armatus Using Response Surface Methodology. Aquac. Res. 2019, 50, 3313–3325. [Google Scholar] [CrossRef]
- Ambat, I.; Bec, S.; Peltomaa, E.; Srivastava, V.; Ojala, A.; Sillanpää, M. A Synergic Approach for Nutrient Recovery and Biodiesel Production by the Cultivation of Microalga Species in the Fertilizer Plant Wastewater. Sci. Rep. 2019, 9, 19073. [Google Scholar] [CrossRef]
- Ji, F.; Wang, Y.K.; Li, G.; Zhou, Y.G.; Dong, R.J. Isolation of Microalgae with Growth Restriction and Nutrient Removal from Alkaline Wastewater. Int. J. Agric. Biol. Eng. 2015, 8, 62–68. [Google Scholar] [CrossRef]
- Sarfraz, R.; Taneez, M.; Sardar, S.; Danish, L.; Hameed, A. Evaluation of Desmodesmus Subspicatus for the Treatment of Wastewater. Int. J. Environ. Anal. Chem. 2023, 103, 3575–3586. [Google Scholar] [CrossRef]
- Chaïb, S.; Pistevos, J.C.A.; Bertrand, C.; Bonnard, I. Allelopathy and Allelochemicals from Microalgae: An Innovative Source for Bio-Herbicidal Compounds and Biocontrol Research. Algal Res. 2021, 54, 102213. [Google Scholar] [CrossRef]
- Casanova, L.M.; Macrae, A.; de Souza, J.E.; Neves Junior, A.; Vermelho, A.B. The Potential of Allelochemicals from Microalgae for Biopesticides. Plants 2023, 12, 1896. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Wiśniewska, K.; Konarzewska, Z.; Cieszyńska, A.; Barreiro Felpeto, A.; Lewandowska, A.U.; Latała, A. The Current State of Knowledge on Taxonomy, Modulating Factors, Ecological Roles, and Mode of Action of Phytoplankton Allelochemicals. Sci. Total Environ. 2021, 773, 145681. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T.L. Interference by Pigment in the Estimation of Microalgal Biomass Concentration by Optical Density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef]
- Eddiwan, K.; Dahril, T.; Efawani, E. Relationship Between Pigment Concentration and Dry Weight in Determining Microalgae Abundance in Artificial Water Samples. Int. J. Res. Sci. Innov. 2023, X, 118–127. [Google Scholar] [CrossRef]
- Ayre, J.M.; Moheimani, N.R.; Borowitzka, M.A. Growth of Microalgae on Undiluted Anaerobic Digestate of Piggery Effluent with High Ammonium Concentrations. Algal Res. 2017, 24, 218–226. [Google Scholar] [CrossRef]
- Halfhide, T.; Åkerstrøm, A.; Lekang, O.I.; Gislerød, H.R.; Ergas, S.J. Production of Algal Biomass, Chlorophyll, Starch and Lipids Using Aquaculture Wastewater under Axenic and Non-Axenic Conditions. Algal Res. 2014, 6, 152–159. [Google Scholar] [CrossRef]
- Muñoz, R.; Guieysse, B. Algal–Bacterial Processes for the Treatment of Hazardous Contaminants: A Review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Synergistic Cooperation between Wastewater-Born Algae and Activated Sludge for Wastewater Treatment: Influence of Algae and Sludge Inoculation Ratios. Bioresour. Technol. 2012, 105, 67–73. [Google Scholar] [CrossRef]
- Luo, Y.; Yao, J.; Wang, X.; Zheng, M.; Guo, D.; Chen, Y. Efficient Municipal Wastewater Treatment by Oxidation Ditch Process at Low Temperature: Bacterial Community Structure in Activated Sludge. Sci. Total Environ. 2020, 703, 135031. [Google Scholar] [CrossRef] [PubMed]
- García, D.; Alcántara, C.; Blanco, S.; Pérez, R.; Bolado, S.; Muñoz, R. Enhanced Carbon, Nitrogen and Phosphorus Removal from Domestic Wastewater in a Novel Anoxic-Aerobic Photobioreactor Coupled with Biogas Upgrading. Chem. Eng. J. 2017, 313, 424–434. [Google Scholar] [CrossRef]
- Zhou, T.; Xiang, Y.; Liu, S.; Ma, H.; Shao, Z.; He, Q.; Chai, H. Microbial Community Dynamics and Metagenomics Reveal the Potential Role of Unconventional Functional Microorganisms in Nitrogen and Phosphorus Removal Biofilm System. Sci. Total Environ. 2023, 905, 167194. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Huang, R.; Li, T.; Pan, D.; Shao, S.; Wu, X. Effect of Antibiotics on the Performance of Moving Bed Biofilm Reactor for Simultaneous Removal of Nitrogen, Phosphorus and Copper(II) from Aquaculture Wastewater. Ecotoxicol. Environ. Saf. 2023, 266, 115590. [Google Scholar] [CrossRef]
- Tomasek, A.; Staley, C.; Wang, P.; Kaiser, T.; Lurndahl, N.; Kozarek, J.L.; Hondzo, M.; Sadowsky, M.J. Increased Denitrification Rates Associated with Shifts in Prokaryotic Community Composition Caused by Varying Hydrologic Connectivity. Front. Microbiol. 2017, 8, 2304. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Azarbaijani, R.; Parsa Yeganeh, L.; Angelidaki, I.; Nizami, A.-S.; Bhat, R.; Dashora, K.; Vijay, V.K.; Aghbashlo, M.; et al. Pretreatment of Lignocelluloses for Enhanced Biogas Production: A Review on Influencing Mechanisms and the Importance of Microbial Diversity. Renew. Sustain. Energy Rev. 2021, 135, 110173. [Google Scholar] [CrossRef]
- Mandic-Mulec, I.; Stefanic, P.; van Elsas, J.D. Ecology of Bacillaceae. Microbiol. Spectr. 2015, 3, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Elreedy, A.; Fujii, M.; Ni, S.-Q.; Tawfik, A.; Elsamadony, M. Fatigue of Anammox Consortia under Long-Term 1,4-Dioxane Exposure and Recovery Potential: N-Kinetics and Microbial Dynamics. J. Hazard. Mater. 2021, 414, 125533. [Google Scholar] [CrossRef] [PubMed]
- Eusébio, A.; Neves, A.; Marques, I.P. Structure of Microbial Communities When Complementary Effluents Are Anaerobically Digested. Appl. Sci. 2021, 11, 1293. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97. [Google Scholar] [CrossRef]
- Smiley, R.; Jackson, H. Organic Chemistry. In Chemistry and the Chemical Industry; CRC Press: Boca Raton, FL, USA, 2002; pp. 35–72. [Google Scholar]
- Ganeson, Y.; Paramasivam, P.; Palanisamy, K.M.; Govindan, N.; Maniam, G.P. LCMS and FTIR Profiling of Microalga chlorella Sp. for Cosmetics and Skin Care Applications. Clean. Water 2024, 2, 100028. [Google Scholar] [CrossRef]
- Xia, L.; Li, H.; Song, S. Cell Surface Characterization of Some Oleaginous Green Algae. J. Appl. Phycol. 2016, 28, 391–407. [Google Scholar] [CrossRef]
- Bartošová, A.; Blinová, L.; Gerulová, K. Characterisation Of Polysacharides And Lipids From Selected Green Algae Species By FTIR-ATR Spectroscopy. Res. Pap. Fac. Mater. Sci. Technol. Slovak. Univ. Technol. 2015, 23, 97–102. [Google Scholar] [CrossRef]
- Sukhikh, S.; Prosekov, A.; Ivanova, S.; Maslennikov, P.; Andreeva, A.; Budenkova, E.; Kashirskikh, E.; Tcibulnikova, A.; Zemliakova, E.; Samusev, I.; et al. Identification of Metabolites with Antibacterial Activities by Analyzing the FTIR Spectra of Microalgae. Life 2022, 12, 1395. [Google Scholar] [CrossRef]
- Duygu, D.; Udoh, A.; Ozer, T.; Akbulut, A.; Erkaya, I.; Yildiz, K.; Guler, D. Fourier Transform Infrared (FTIR) Spectroscopy for Identification of Chlorella Vulgaris Beijerinck 1890 and Scenedesmus Obliquus (Turpin) Kützing 1833. Afr. J. Biotechnol. 2012, 11, 3817–3824. [Google Scholar] [CrossRef]
- Bataller, B.G.; Capareda, S.C. A Rapid and Non-Destructive Method for Quantifying Biomolecules in Spirulina Platensis via Fourier Transform Infrared–Attenuated Total Reflectance Spectroscopy. Algal Res. 2018, 32, 341–352. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.; Zhao, C.; Huang, F.; Deng, L.; Wang, W. Establishment of Stable Microalgal-Bacterial Consortium in Liquid Digestate for Nutrient Removal and Biomass Accumulation. Bioresour. Technol. 2018, 268, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-B.; Yang, L.-B.; Zhang, W.-W.; Zhao, X.-C. Lipids Production and Nutrients Recycling by Microalgae Mixotrophic Culture in Anaerobic Digestate of Sludge Using Wasted Organics as Carbon Source. Bioresour. Technol. 2020, 297, 122379. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Liu, F.; Zhu, S.; Feng, P.; Wang, Z.; Yuan, Z.; Shang, C.; Chen, H. Performance of a Microalgal-Bacterial Consortium System for the Treatment of Dairy-Derived Liquid Digestate and Biomass Production. Bioresour. Technol. 2020, 306, 123101. [Google Scholar] [CrossRef]
- Arif, M.; Bai, Y.; Usman, M.; Jalalah, M.; Harraz, F.A.; Al-Assiri, M.S.; Li, X.; Salama, E.-S.; Zhang, C. Highest Accumulated Microalgal Lipids (Polar and Non-Polar) for Biodiesel Production with Advanced Wastewater Treatment: Role of Lipidomics. Bioresour. Technol. 2020, 298, 122299. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Biodiesel from Microalgae: A Critical Evaluation from Laboratory to Large Scale Production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, H.; Zuo, M.; Zuo, L.; Wang, X.; Song, P.; Guo, S.; Shen, B. Pyrolysis Mechanism of Isolated Microalgal Composition and Their Potential as Liquid Biofuels: Neutral Lipids, Phospholipids, Glycolipids, and Sterols. Energy Convers. Manag. X 2025, 26, 100960. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Natural Surfactants Used in Cosmetics: Glycolipids. Int. J. Cosmet. Sci. 2009, 31, 255–261. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive Compounds in Microalgae and Their Potential Health Benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Razzak, S.A.; Lucky, R.A.; Hossain, M.M.; DeLasa, H. Valorization of Microalgae Biomass to Biofuel Production: A Review. Energy Nexus 2022, 7, 100139. [Google Scholar] [CrossRef]
- Manning, S.R. Microalgal Lipids: Biochemistry and Biotechnology. Curr. Opin. Biotechnol. 2022, 74, 1–7. [Google Scholar] [CrossRef]
- Rebolloso Fuentes, M. Biomass Nutrient Profiles of the Microalga Porphyridium Cruentum. Food Chem. 2000, 70, 345–353. [Google Scholar] [CrossRef]
- Stansell, G.R.; Gray, V.M.; Sym, S.D. Microalgal Fatty Acid Composition: Implications for Biodiesel Quality. J. Appl. Phycol. 2012, 24, 791–801. [Google Scholar] [CrossRef]
- Khethiwe, E.; Clever, K.; Jerekias, G. Effects of Fatty Acids Composition on Fuel Properties of Jatropha Curcas Biodiesel. Smart Grid Renew. Energy 2020, 11, 165–180. [Google Scholar] [CrossRef]
- Sainger, M.; Jaiwal, A.; Sainger, P.A.; Chaudhary, D.; Jaiwal, R.; Jaiwal, P.K. Advances in Genetic Improvement of Camelina Sativa for Biofuel and Industrial Bio-Products. Renew. Sustain. Energy Rev. 2017, 68, 623–637. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Supakorn, P.; Chonlada, Y.; Anchalee, S. Pigment Production under Cold Stress in the Green Microalga Chlamydomonas Reinhardtii. Agriculture 2021, 11, 564. [Google Scholar] [CrossRef]
- Gouveia, L.; Empis, J. Relative Stabilities of Microalgal Carotenoids in Microalgal Extracts, Biomass and Fish Feed: Effect of Storage Conditions. Innov. Food Sci. Emerg. Technol. 2003, 4, 227–233. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lee, D.-J.; Chang, J.-S. Lutein Production from Biomass: Marigold Flowers versus Microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Sobolewska, E.; Borowski, S.; Nowicka-Krawczyk, P.; Banach, K. Treatment of Liquid Digestate by Green Algal Isolates from Artificial Eutrophic Pond. Molecules 2022, 27, 6856. [Google Scholar] [CrossRef]
- Sobolewska, E.; Borowski, S.; Nowicka-Krawczyk, P. Cultivation of Microalgae in Liquid Digestate to Remove Nutrients and Organic Contaminants. BioEnergy Res. 2024, 17, 1843–1855. [Google Scholar] [CrossRef]
- Molina Grima, E.; Fernández, F.G.A.; García Camacho, F.; Chisti, Y. Photobioreactors: Light Regime, Mass Transfer, and Scaleup. J. Biotechnol. 1999, 70, 231–247. [Google Scholar] [CrossRef]
- Saccardo, A.; Bezzo, F.; Sforza, E. Microalgae Growth in Ultra-Thin Steady-State Continuous Photobioreactors: Assessing Self-Shading Effects. Front. Bioeng. Biotechnol. 2022, 10, 977429. [Google Scholar] [CrossRef]
- Baird, R.B.; Rice, E.W.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Min, M.; Wang, L.; Li, Y.; Mohr, M.J.; Hu, B.; Zhou, W.; Chen, P.; Ruan, R. Cultivating Chlorella sp. in a Pilot-Scale Photobioreactor Using Centrate Wastewater for Microalgae Biomass Production and Wastewater Nutrient Removal. Appl. Biochem. Biotechnol. 2011, 165, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Menges, F. Spectragryph-Optical Spectroscopy Software. 2022. Available online: https://www.effemm2.de/spectragryph/ (accessed on 12 February 2024).
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid Extraction by Methyl-Terf-Butyl Ether for High-Throughput Lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Lipid Analysis: Isolation, Separation, Identification and Lipidomic Analysis; Oily Press Lipid Library Series; Woodhead Publishing Ltd.: Sawston, UK, 2010; ISBN 9780857097866. [Google Scholar]
- Assunção, M.F.G.; Varejão, J.M.T.B.; Santos, L.M.A. Nutritional Characterization of the Microalga Ruttnera Lamellosa Compared to Porphyridium Purpureum. Algal Res. 2017, 26, 8–14. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An Evaluation of Methods for Extraction and Quantification of Protein from Marine Macro- and Microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Stengel, D.; Connan, S. Natural Products From Marine Algae, Methods and Protocols; Humana: New York, NY, USA, 2015; ISBN 978-1-4939-2683-1. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. ISBN 0076-6879. [Google Scholar]
- Bauer, A.; Minceva, M. Direct Extraction of Astaxanthin from the Microalgae: Haematococcus Pluvialis Using Liquid-Liquid Chromatography. RSC Adv. 2019, 9, 22779–22789. [Google Scholar] [CrossRef]
- Mendes, C.R.; Cartaxana, P.; Brotas, V. HPLC Determination of Phytoplankton and Microphytobenthos Pigments: Comparing Resolution and Sensitivity of a C18 and a C8 Method. Limnol. Oceanogr. Methods 2007, 5, 363–370. [Google Scholar] [CrossRef]
- Van Heukelem, L.; Thomas, C.S. Computer-Assisted High-Performance Liquid Chromatography Method Development with Applications to the Isolation and Analysis of Phytoplankton Pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef] [PubMed]

| Indicator | Unit | Average Value ± SD |
|---|---|---|
| pH | - | 6.50 ± 0.03 |
| Total solids, TS | g/kg | 2.88 ± 0.04 |
| Volatile solids, VS | g/kg | 1.34 ± 0.03 |
| Volatile solids, VS | % TS | 46.54 ± 1.67 |
| Total suspended solids, TSS | mg/L | 7.08 ± 0.67 |
| Turbidity | FAU | 108.42 ± 2.47 |
| Apparent color | mg/L Pt-Co | 409.67 ± 4.10 |
| True color | mg/L Pt-Co | 368.92 ± 3.15 |
| Total chemical oxygen demand, tCOD | mgO2/L | 7414.17 ± 39.65 |
| Soluble chemical oxygen demand, sCOD | mgO2/L | 6411.67 ± 62.50 |
| Total volatile fatty acids, tVFAs | mg/L | 3100.00 ± 76.99 |
| Ammonium nitrogen, NH4+ | mg/L | 121.67 ± 6.56 |
| Nitrates, NO3− | mg/L | 1061.67 ± 43.66 |
| Nitrites, NO2− | mg/L | 10.58 ± 1.09 |
| Total nitrogen, TN ** | mgN/L | 337.58 ± 10.51 |
| Orthophosphates, PO43− | mg/L | 491.67 ± 26.23 |
| Sulfates, SO42− | mg/L | 0.00 ± 0.00 |
| Sulfides, S2− | μg/L | 0.00 ± 0.00 |
| Chlorides, Cl− | mg/L | 282.50 ± 7.54 |
| Iron, Fe | mg/L | 9.34 ± 0.16 |
| Copper, Cu | mg/L | 7.06 ± 0.16 |
| Zinc, Zn | mg/L | 14.08 ± 0.79 |
| Aluminum, Al3+ | mg/L | 0.64 ± 0.05 |
| Wavenumber (cm−1) | Chemical Bound | BM1 | BM2 | References |
|---|---|---|---|---|
| 436–445 | Aryl disulfides (S-S stretch) | ++++ | ++++ | [65] |
| 524–536 | Benzene group (C–Br stretch) | ++++ | +++ | [66] |
| 867–869 | Bending of the C-H, uronic acid | + | + | [67] |
| 1019–1021 | v(C-O-C) of polysaccharides | +++++ | +++++ | [68] |
| 1237–1238 | Phosphate-containing compounds (P = O) stretching of phosphodiesters | + | + | [69] |
| 1307 | Deformation vibrations of CH3 groups | + | nd | [70] |
| 1397–1402 | Carboxylic acid vs(C-O) of COO- groups of carboxylates | ++ | ++ | [69] |
| 1451 | Scissoring vibrations of CH2 groups | ++ | sh | [70] |
| 1537 | Protein amide II band mainly δ(N-H) bending and v(C-N) stretching | +++ | +++ | [71] |
| 1637–1638 | Amide (C-N) and carbonyl (C=O) in protein | +++ | +++ | [69] |
| 2851 | CH3-methyl | +++ | +++ | [71] |
| 2919 | CH2-methyl | +++ | +++ | [71] |
| 3276 | O-H bounds | ++++ | +++ | [72] |
| Individual FAs | Formula | BM1 [% of Total FAs] | BM2 [% of Total FAs] | ||||
|---|---|---|---|---|---|---|---|
| NLs | GLs | PLs | NLs | GLs | PLs | ||
| SFA | |||||||
| Capric acid | (C10:0) | 2.17 ± 0.15 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Undecylic acid | (C11:0) | 1.47 ± 0.24 | 3.99 ± 0.88 | 2.20 ± 0.37 | n.d. | 8.83 ± 0.82 | n.d. |
| Tridecylic acid | (C13:0) | 0.51 ± 0.27 | 1.66 ± 0.18 | 0.91 ± 0.07 | n.d. | 3.47 ± 0.32 | n.d. |
| Myristic acid | (C14:0) | n.d. | 1.27 ± 0.11 | 1.00 ± 0.04 | n.d. | n.d. | n.d. |
| Pentadecylic acid | (C15:0) | 1.09 ± 0.47 | 0.94 ± 0.16 | n.d. | n.d. | n.d. | n.d. |
| Palmitic acid | (C16:0) | 28.17 ± 1.35 | 40.79 ± 0.91 | 53.68 ± 0.56 | 44.38 ± 0.78 | 43.75 ± 1.04 | 55.13 ± 0.69 |
| Margaric acid | (C17:0) | 1.06 ± 0.60 | n.d. | 1.65 ± 0.16 | n.d. | n.d. | n.d. |
| Stearic acid | (C18:0) | 18.51 ± 0.88 | 26.65 ± 1.14 | 16.85 ± 1.39 | 30.97 ± 0.58 | 30.16 ± 1.66 | 20.76 ± 0.49 |
| Arachidic acid | (C20:0) | 3.23 ± 0.16 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Heneicosylic acid | (C21:0) | 0.53 ± 0.32 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Behenic acid | (C22:0) | 1.91 ± 0.08 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lignoceric acid | (C24:0) | 1.37 ± 0.09 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total | 60.02 ± 4.61 | 75.30 ± 3.38 | 76.30 ± 2.59 | 75.35 ± 1.36 | 86.21 ± 3.84 | 75.89 ± 1.18 | |
| MUFA | |||||||
| Myristoleic acid | (C14:1) | 0.80 ± 0.18 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Palmitoleic acid | (C16:1) | 1.77 ± 0.08 | 2.18 ± 0.23 | 3.12 ± 0.28 | n.d. | n.d. | 4.70 ± 0.10 |
| Heptadecanoic acid | (C17:1) | 1.55 ± 0.13 | 1.58 ± 0.26 | 1.29 ± 0.13 | n.d. | n.d. | n.d. |
| Elaidic acid | (C18:1w9 trans) | 1.37 ± 0.72 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Oleic acid | (18:1w9 cis) | 8.93 ± 0.65 | 6.09 ± 0.48 | 6.23 ± 0.35 | 9.06 ± 2.38 | 3.85 ± 0.78 | 7.82 ± 0.06 |
| Gondoic acid | (C20:1w9) | 11.22 ± 0.89 | 7.23 ± 0.88 | 7.58 ± 0.91 | 6.52 ± 0.60 | 5.73 ± 0.45 | 7.87 ± 0.56 |
| Total | 25.64 ± 2.65 | 17.08 ± 1.85 | 18.22 ± 1.67 | 15.58 ± 2.98 | 9.58 ± 1.23 | 20.39 ± 0.72 | |
| PUFA | |||||||
| Linolelaidic acid | (C18:2w6 trans) | 2.33 ± 0.14 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Linoleic acid | (C18:2w6 cis) | 9.02 ± 0.64 | 5.37 ± 0.55 | 4.54 ± 0.30 | 4.64 ± 0.63 | 2.32 ± 0.15 | 3.73 ± 0.32 |
| Gamma-linolenic acid | (C18:3w6) | 1.44 ± 0.27 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Eicosadienoic acid | (C20:2) | 1.23 ± 0.08 | 1.14 ± 0.15 | 0.94 ± 0.23 | n.d. | n.d. | n.d. |
| Eicosatrienoic acid | (C20:3w3) | 1.09 ± 0.12 | n.d. | n.d. | 4.43 ± 1.82 | n.d. | n.d. |
| Total | 15.11 ± 1.25 | 6.51 ± 0.70 | 5.48 ± 0.53 | 9.07 ± 2.45 | 2.32 ± 0.15 | 3.73 ± 0.32 | |
| SFA + PUFA | |||||||
| Tricosylic acid + Arachidonate acid | (C23:0) + (C20:4w6) | n.d. | 1.11 ± 0.32 | n.d. | n.d. | 1.89 ± 0.31 | n.d. |
| Total | n.d. | 1.11 ± 0.32 | n.d. | n.d. | 1.89 ± 0.31 | n.d. | |
| Pigment | Unit | Mean Value ± Standard Deviation | |
|---|---|---|---|
| BM1 | BM2 | ||
| Neoxanthin | %total pigments | 5.10 ± 0.82 | 3.99 ± 0.31 |
| Violaxanthin | 10.08 ± 1.62 | 9.69 ± 0.51 | |
| Lutein | 21.73 ± 1.65 | 19.81 ± 0.73 | |
| chla | 30.52 ± 2.07 | 39.48 ± 0.12 | |
| chlb | 21.71 ± 1.47 | 22.41 ± 0.83 | |
| β-carotene | 10.88 ± 1.76 | 4.63 ± 0.18 | |
| chla | μg/mL | 129.74 ± 6.83 | 120.30 ± 3.13 |
| chlb | 46.66 ± 11.72 | 86.66 ± 12.25 | |
| chla + chlb | 178.34 ± 6.71 | 209.28 ± 9.25 | |
| Carotenoids | 52.66 ± 6.33 | 36.38 ± 6.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolewska, E.; Komar, M.; Borowski, S.; Nowicka-Krawczyk, P.; Portugal, A.; Mesquita, N.; Assunção, M.F.G.; Aksoy, B.; Cotas, J.; Pereira, L. Simultaneous Liquid Digestate Treatment and High-Value Microalgal Biomass Production: Influence of Post-Harvest Storage on Biochemical Profiles. Molecules 2025, 30, 2778. https://doi.org/10.3390/molecules30132778
Sobolewska E, Komar M, Borowski S, Nowicka-Krawczyk P, Portugal A, Mesquita N, Assunção MFG, Aksoy B, Cotas J, Pereira L. Simultaneous Liquid Digestate Treatment and High-Value Microalgal Biomass Production: Influence of Post-Harvest Storage on Biochemical Profiles. Molecules. 2025; 30(13):2778. https://doi.org/10.3390/molecules30132778
Chicago/Turabian StyleSobolewska, Ewelina, Michał Komar, Sebastian Borowski, Paulina Nowicka-Krawczyk, António Portugal, Nuno Mesquita, Mariana F. G. Assunção, Berk Aksoy, João Cotas, and Leonel Pereira. 2025. "Simultaneous Liquid Digestate Treatment and High-Value Microalgal Biomass Production: Influence of Post-Harvest Storage on Biochemical Profiles" Molecules 30, no. 13: 2778. https://doi.org/10.3390/molecules30132778
APA StyleSobolewska, E., Komar, M., Borowski, S., Nowicka-Krawczyk, P., Portugal, A., Mesquita, N., Assunção, M. F. G., Aksoy, B., Cotas, J., & Pereira, L. (2025). Simultaneous Liquid Digestate Treatment and High-Value Microalgal Biomass Production: Influence of Post-Harvest Storage on Biochemical Profiles. Molecules, 30(13), 2778. https://doi.org/10.3390/molecules30132778










