Synthesis and In Vitro Pharmacological Evaluation of 5,8-Dideaza Analogs of Methotrexate
Abstract
1. Introduction
2. Results and Discussion
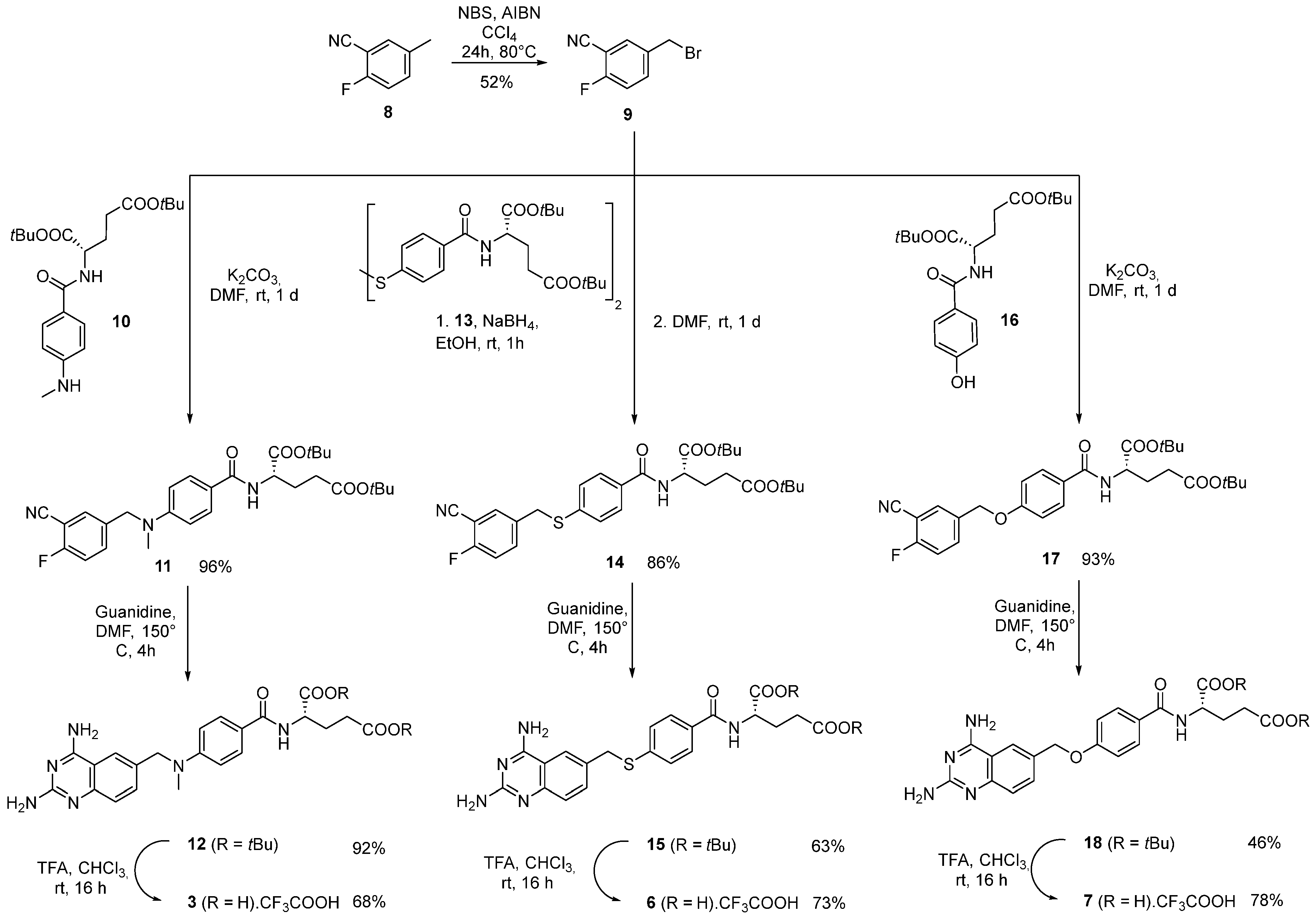
2.1. Synthesis
2.2. In Vitro Cytotoxicity and Enzymatic Inhibition Activity
2.3. In Vitro Metabolism
3. Materials and Methods
3.1. Synthesis
3.1.1. Compound 9: 5-(Bromomethyl)-2-fluorobenzonitrile
3.1.2. Compound 11: Di-tert-butyl (4-((3-cyano-4-fluorobenzyl)(methyl)amino)benzoyl)-L-glutamate
3.1.3. Compound 12: Di-tert-butyl (4-(((2,4-diaminoquinazolin-6-yl)methyl)(methyl)amino)benzoyl)-L-glutamate
3.1.4. Compound 3: (4-(((2,4-Diaminoquinazolin-6-yl)methyl)(methyl)amino)benzoyl)-L-glutamic Acid TFA Salt
3.1.5. Compound 14: Di-tert-butyl (4-((3-cyano-4-fluorobenzyl)thio)benzoyl)-L-glutamate
3.1.6. Compound 15: Di-tert-butyl (4-(((2,4-diaminoquinazolin-6-yl)methyl)thio)benzoyl)-L-glutamate
3.1.7. Compound 6: (4-(((2,4-Diaminoquinazolin-6-yl)methyl)thio)benzoyl)-L-glutamic Acid TFA Salt
3.1.8. Compound 17: Di-tert-butyl (4-((3-cyano-4-fluorobenzyl)oxy)benzoyl)-L-glutamate
3.1.9. Compound 18: Di-tert-butyl (4-((2,4-diaminoquinazolin-6-yl)methoxy)benzoyl)-L-glutamate
3.1.10. Compound 7: (4-((2,4-Diaminoquinazolin-6-yl)methoxy)benzoyl)-L-glutamic Acid TFA Salt
3.2. Cytotoxicity Experiments on A549 Human Cancer Cells
3.3. DHFR Inhibition Assay
3.4. In Vitro Metabolism
3.4.1. Monitoring of Hepatic Metabolism
3.4.2. Aldehyde Oxidase Assay
3.4.3. Human Liver Cytosol Assay
3.4.4. Human Pooled S9 Fractions Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnhart, K.; Coutifaris, C.; Esposito, M. The pharmacology of methotrexate. Expert Opin. Pharmacother. 2001, 2, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Kutkowska, J.; Obmińska-Mrukowicz, B.; Rapak, A. Methotrexate induces high level of apoptosis in canine lymphoma/leukemia cell lines. Res. Vet. Sci. 2017, 114, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N. The mechanism of action of methotrexate. Rheum. Dis. Clin. N. Am. 1997, 23, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.T.R.; Zhang, Z.; McCourt, L.; Dwyer, M.; Benkovic, S.J.; Hammes, G.G. Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics. Proc. Natl. Acad. Sci. USA 2002, 99, 13481–13486. [Google Scholar] [CrossRef]
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Moore, P.K. Pharmacology, 5th ed.; Churchill Livingstone: Edinburgh, UK, 2003. [Google Scholar]
- Carmona-Martínez, V.; Ruiz-Alcaraz, A.J.; Vera, M.; Guirado, A.; Martínez-Esparza, M.; García-Peñarrubia, P. Therapeutic potential of pteridine derivatives: A comprehensive review. Med. Res. Rev. 2019, 39, 461–516. [Google Scholar] [CrossRef]
- Kovalev, I.S.; Zyryanov, G.V.; Santra, S.; Majee, A.; Varaksin, M.V.; Charushin, V.N. Folic Acid Antimetabolites (Antifolates): A Brief Review on Synthetic Strategies and Application Opportunities. Molecules 2022, 27, 6229. [Google Scholar] [CrossRef]
- Raimondi, M.V.; Randazzo, O.; La Franca, M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef]
- Kisliuk, R.L. Deaza Analogs of Folic Acid as Antitumor Agents. Curr. Pharm. Des. 2003, 9, 2615–2625. [Google Scholar] [CrossRef]
- Breithaupt, H.; Küenzlen, E. Pharmacokinetics of Methotrexate and 7-Hydroxymethotrexate Following Infusions of High-Dose Methotrexate. Cancer Treat. 1982, 66, 1733–1741. [Google Scholar]
- Moriyasu, A.; Sugihara, K.; Nakatani, K.; Ohta, S.; Kitamura, S. In vivo-In vitro Relationship of Methotrexate 7-Hydroxylation by Aldehyde Oxidase in Four Different Strain Rats. Drug Metab. Pharmacokinet. 2006, 21, 485–491. [Google Scholar] [CrossRef]
- Chabner, B.A.; Allegra, C.J.; Curt, G.A.; Clendeninn, N.J.; Baram, J.; Koizumi, S.; Drake, J.C.; Jolivet, J. Polyglutamation of Methotrexate Is Methotrexate a Prodrug? J. Clin. Investig. 1985, 76, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Smeland, E.; Fuskevåg, O.M.; Nymann, K.; Svendesn, J.S.; Olsen, R.; Lindal, S.; Bremnes, R.M.; Aarbakke, J. High-dose 7-hydromethotrexate: Acute toxicity and lethality in a rat model. J. Cancer Chemother. Pharmacol. 1996, 37, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.A.; Stoller, R.G.; Chabner, B.A.; Johns, D.G. 7-Hydroxymethotrexate as a Urinary Metabolite in Human Subjects and Rhesus Monkeys Receiving High Dose Methotrexate. J. Clin. Investig. 1976, 58, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Hynes, J.B.; Harmon, S.J.; Floyd, G.G.; Farrington, M.; Hart, L.D.; Gale, G.R.; Washtien, W.L.; Susten, S.S.; Freisheim, J.H. Chemistry and antitumor evaluation of selected classical 2,4-diaminoquinazoline analogs of folic acid. J. Med. Chem. 1985, 28, 209–215. [Google Scholar] [CrossRef]
- Graffner-Nordberg, M.; Marelius, J.; Ohlsson, S.; Persson, Å.; Swedberg, G.; Andersson, P.; Andersson, S.E.; Åqvist, J.; Hallberg, A. Computational Predictions of Binding Affinities to Dihydrofolate Reductase: Synthesis and Biological Evaluation of Methotrexate Analogues. J. Med. Chem. 2000, 43, 3852–3861. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Piper, J.R.; Elliott, R.D.; Temple Jr, C.; Roberts, E.C.; Shealy, Y.F. Analogues of methotrexate. J. Med. Chem. 1979, 22, 862–868. [Google Scholar] [CrossRef]
- Sirotnak, F.M.; Chello, P.L.; Moccio, D.M.; Piper, J.R.; Montgomery, J.A.; Parham, J.C. Analog specific aberrancies in antifolate inhibition of L1210 cell dihydrofolate reductase. Biochem. Pharmacol. 1980, 29, 3293–3298. [Google Scholar] [CrossRef]
- Nair, M.G.; Bridges, T.W.; Henkel, T.J.; Kisliuk, R.L.; Gaumont, Y.; Sirotnak, F.M. Folate analogs altered in the C9-N10 bridge region. 18. Synthesis and antitumor evaluation of 11-oxahomoaminopterin and related compounds. J. Med. Chem. 1981, 24, 1068–1073. [Google Scholar] [CrossRef]
- Espinosa, A.; Rascol, E.; Abellán-Flos, M.; Skarbek, C.; Lieben, P.; Bannerman, E.; Diez-Martinez, A.; Pethe, S.; Benoit, P.; Nélieu, S.; et al. Re-designing environmentally persistent pharmaceutical pollutant through programmed inactivation: The case of methotrexate. Chemosphere 2022, 306, 135616. [Google Scholar] [CrossRef]
- Davoll, J.; Johnson, A.M. Quinazoline analogues of folic acid. J. Chem. Soc. C Org. 1970, 8, 997–1002. [Google Scholar] [CrossRef]
- Milicevic Sephton, S.; Vetterli, P.T.; Pedani, V.; Cermak, S.; Chiotellis, A.; Roscales, S.; Müller Herde, A.; Schibli, R.; Auberson, Y.P.; Ametamey, S.M. Synthesis and Biological Evaluation of Quinoxaline Derivatives for PET Imaging of the NMDA Receptor. Helv. Chim. Acta 2017, 100, e1700204. [Google Scholar] [CrossRef]
- Betts, H.M.; Milicevic Sephton, S.; Tong, C.; Awais, R.O.; Hill, P.J.; Perkins, A.C.; Aigbirhio, F.I. Synthesis, in Vitro Evaluation, and Radiolabeling of Fluorinated Puromycin Analogues: Potential Candidates for PET Imaging of Protein Synthesis. J. Med. Chem. 2016, 59, 9422–9430. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Creighton, C.; Cunningham, M.; Finn, J.; Hilgers, M.; Jung, M.; Aguirre Kohnen, L.; Lam, T.; Li, X.; Stidham, M.; et al. Dihydrofolate Reductase Inhibitors. WO2011153310A1, 8 December 2011. [Google Scholar]
- Yoon, S.-A.; Choi, J.R.; Kim, J.-O.; Shin, J.-Y.; Zhang, X.; Kang, J.-H. Influence of Reduced Folate Carrier and Dihydrofolate Reductase Genes on Methotrexate-Induced Cytotoxicity. Cancer Res. Treat. 2010, 42, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, A.; Akbari, H.; Ghaderpoori, M.; Jeihooni, A.K.; Adibzadeh, A. Catalytic ozonation process using a MgO nano-catalyst to degrade methotrexate from aqueous solutions and cytotoxicity studies in human lung epithelial cells (A549) after treatment. RSC Adv. 2019, 9, 8204–8214. [Google Scholar] [CrossRef]
- Coutinho, A.J.; Lima, S.; Afonso, C.; Reis, S. Mucoadhesive and pH responsive fucoidan-chitosan nanoparticles for the oral delivery of methotrexate. Int. J. Biol. Macromol. 2020, 158, 180–188. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, C.; Shi, X.; Guo, R.; Zhang, K.; Gu, J.; Li, L.; Li, S.; Zheng, Q.; Cui, M.; et al. Targeting dihydrofolate reductase: Design, synthesis and biological evaluation of novel 6-substituted pyrrolo[2,3-d]pyrimidines as nonclassical antifolates and as potential antitumor agents. Eur. J. Med. Chem. 2019, 178, 329–340. [Google Scholar] [CrossRef]
- Walling, J. From methotrexate to pemetrexed and beyond. A review of the pharmacodynamic and clinical properties of antifolates. Investig. New Drugs 2006, 24, 37–77. [Google Scholar] [CrossRef]
- Wang, Y.; Mi, T.; Li, Y.; Kan, W.; Xu, G.; Li, J.; Zhou, Y.; Li, J.; Jiang, X. Design, synthesis and biological evaluation of thioether-containing lenalidomide and pomalidomide derivatives with anti-multiple myeloma activity. Eur. J. Med. Chem. 2021, 209, 112912. [Google Scholar] [CrossRef]


| Compounds | A549 IC50 (µM) ± SD | DHFR IC50 (nM) ± SD |
|---|---|---|
| Methotrexate (1) | 0.12 ± 0.02 | 30 ± 3 |
| 5,8-Dideaza-methotrexate (3) | 0.04 ± 0.01 | 7 ± 2 |
| 6 | 0.36 ± 0.04 | 13 ± 4 |
| 7 | 0.12 ± 0.02 | 30 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abellán-Flos, M.; Skarbek, C.; Gibbons, D.J.; Rascol, E.; García, A.; Labruère, R. Synthesis and In Vitro Pharmacological Evaluation of 5,8-Dideaza Analogs of Methotrexate. Molecules 2025, 30, 2772. https://doi.org/10.3390/molecules30132772
Abellán-Flos M, Skarbek C, Gibbons DJ, Rascol E, García A, Labruère R. Synthesis and In Vitro Pharmacological Evaluation of 5,8-Dideaza Analogs of Methotrexate. Molecules. 2025; 30(13):2772. https://doi.org/10.3390/molecules30132772
Chicago/Turabian StyleAbellán-Flos, Marta, Charles Skarbek, Dáire J. Gibbons, Estelle Rascol, Ainhoa García, and Raphaël Labruère. 2025. "Synthesis and In Vitro Pharmacological Evaluation of 5,8-Dideaza Analogs of Methotrexate" Molecules 30, no. 13: 2772. https://doi.org/10.3390/molecules30132772
APA StyleAbellán-Flos, M., Skarbek, C., Gibbons, D. J., Rascol, E., García, A., & Labruère, R. (2025). Synthesis and In Vitro Pharmacological Evaluation of 5,8-Dideaza Analogs of Methotrexate. Molecules, 30(13), 2772. https://doi.org/10.3390/molecules30132772







