Cochlospermum angolense Welw ex Oliv: Phytochemical Profile, Antioxidant Activity, and Therapeutic Prospects
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Sample Collection and Authentication
3.2. Chemicals and Reagents
3.3. Samples Preparation and Extraction
3.4. Evaluation of Antioxidant Activity of the Extracts
3.4.1. 2,2-Diphenyl-1-picrylhydrazil Radical (DPPH•)-Scavenging Activity
3.4.2. Nitric Oxide Radical Assay
3.4.3. Superoxide Radical Assay
3.4.4. Lipid Peroxidation Inhibition Assay
3.5. Profiling of Chemical Compounds by High-Performance Liquid Chromatography with Electrospray Ionization and Tandem Mass Spectrometry Detection (HPLC-ESI-MS-MS)
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huntley, B.J.; Russo, V.; Lages, F.; Ferrand, N. Biodiversity of Angola: Science & Conservation: A Modern Synthesis; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Huntley, B.J. Ecology of Angola; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar]
- Almeida, M.Z.d. Plantas Medicinais Mara Zélia de Almeida, 3rd ed.; EDUFBA (Editora da Universidade Federal da Bahia): Salvador, Brasil, 2011. [Google Scholar]
- Neto, F.G.G.; Almeida, G.S.S.A.; Jesus, N.G.; Fonseca, M.R. Estudo Etnobotânico de plantas medicinais utilizadas pela Comunidade do Sisal no município de Catu, Bahia, Brasil. Rev. Bras. de Plantas Med. 2014, 16, 856–865. [Google Scholar] [CrossRef]
- Amaral, F.M.M.; Ribeiro, M.N.S.; Barbosa-Filho, J.M.; Reis, A.S.; Nascimento, F.R.F.; Macedo, R.O. Plants and chemical constituents with giardicidal activity. Rev. Bras. Farmacogn. 2006, 16, 696–720. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and Antimicrobial Activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. Essential Oils and Their Major Monoterpene Alcohols Alone and in Combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef] [PubMed]
- Haidara, M.; Bourdy, G.; De Tommasi, N.; Braca, A.; Traore, K.; Giani, S.; Sanogo, R. Medicinal plants used in Mali for the treatment of malaria and liver diseases. Nat. Prod. Commun. 2016, 11, 339–352. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Frías-Moreno, M.N.; Parra-Quezada, R.A.; González-Aguilar, G.; Ruíz-Canizales, J.; Molina-Corral, F.J.; Sepulveda, D.R.; Salas-Salazar, N.; Olivas, G.I. Quality, bioactive compounds, antioxidant capacity, and enzymes of raspberries at different maturity stages, effects of organic vs. Conventional fertilization. Foods 2021, 10, 953. [Google Scholar] [CrossRef]
- Canga, I.L.V.; Vita, P.; De Oliveira, A.I.d.F.T.; De Pinho, C.M.L.; González, M.Á.C. Ethnopharmacological study of medicinal plants from the province of Cuanza Norte (Angola). Rev. Context. Saúde 2022, 22, e13336. [Google Scholar] [CrossRef]
- Abourashed, E.A.; Fu, H.W. Hydroxybenzoic acids are significant contributors to the antioxidant effect of borututu bark, Cochlospermum angolensis Welw. ex Oliv. Antioxidants 2017, 6, 9. [Google Scholar] [CrossRef]
- Gebashe, F.; Aremu, A.O.; Van Staden, J.; Gruz, J.; Finnie, J.F. Phytochemical profiles and antioxidant activity of grasses used in South African traditional medicine. Plants 2020, 9, 371. [Google Scholar] [CrossRef]
- De Menezes Filho, A.C.P.; de Sousa, W.C.; Christofoli, M.; de Castro, C.F.S. Perfil químico e atividades antioxidante e antifúngica do óleo essencial da flor de Cochlospermum regium (Mart. ex Schrank.)–Pilg. (Bixaceae). Colloq. Agrar. 2020, 16, 89–101. [Google Scholar] [CrossRef]
- Arunachalam, K.; Damazo, A.S.; Pavan, E.; Oliveira, D.M.; Figueiredo, F.d.F.; Machado, M.T.M.; Balogun, S.O.; Soares, I.M.; Barbosa, R.d.S.; Alvim, T.d.C.; et al. Cochlospermum regium (Mart. ex Schrank) Pilg.: Evaluation of chemical profile, gastroprotective activity and mechanism of action of hydroethanolic extract of its xylopodium in acute and chronic experimental models. J. Ethnopharmacol. 2019, 233, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Ezeja, M.I.; Anaga, A.O. Anti-ulcerogenic activity of the methanol root bark extract of Cochlospermum planchonii (Hook f). Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Gulumian, M.; Yahaya, E.S.; Steenkamp, V. African Herbal Remedies with Antioxidant Activity: A Potential Resource Base for Wound Treatment. Evid. Based Complement. Altern. Med. 2018, 2018, 4089541. [Google Scholar] [CrossRef]
- Aguilar-Guadarrama, A.B.; Rios, M.Y. Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity. Molecules 2018, 23, 1952. [Google Scholar] [CrossRef]
- De Miranda Pedroso, T.F.; Bonamigo, T.R.; da Silva, J.; Vasconcelos, P.; Félix, J.M.; Cardoso, C.A.L.; Souza, R.I.C.; dos Santos, A.C.; Volobuff, C.R.F.; Formagio, A.S.N.; et al. Chemical constituents of Cochlospermum regium (Schrank) Pilg. root and its antioxidant, antidiabetic, antiglycation, and anticholinesterase effects in Wistar rats. Biomed. Pharmacother. 2019, 111, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Thanzami, K.; Malsawmtluangi, C.; Lalhlenmawia, H.; Seelan, T.V.; Palanisamy, S.; Kandasamy, R.; Pachuau, L. Characterization and in vitro antioxidant activity of Albizia stipulata Boiv. gum exudates. Int. J. Biol. Macromol. 2015, 80, 231–239. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Alves, M.J.; Pereira, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profile and antimicrobial activity of different dietary supplements based on Cochlospermum angolensis Welw. Ind. Crops Prod. 2015, 74, 412–416. [Google Scholar] [CrossRef]
- Carvalho, F.; Lahlou, R.A.; Silva, L.R. Phenolic Compounds from Cherries and Berries for Chronic Disease Management and Cardiovascular Risk Reduction. Nutrients 2024, 16, 1597. [Google Scholar] [CrossRef]
- Inácio, M.C.; Paz, T.A.; Bertoni, B.W.; Vieira, M.A.R.; Marques, M.O.M.; Pereira, A.M.S. Histochemical investigation of Cochlospermum regium (Schrank) Pilg. leaves and chemical composition of its essential oil. Nat. Prod. Res. 2014, 28, 727–731. [Google Scholar] [CrossRef]
- Pereira, C.; Barreira, J.C.M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Barros, L.; Ferreira, I.C.F.R. New insights into the effects of formulation type and compositional mixtures on the antioxidant and cytotoxic activities of dietary supplements based-on hepatoprotective plants. Food Funct. 2014, 5, 2052–2060. [Google Scholar] [CrossRef][Green Version]
- Zhen, J.; Guo, Y.; Villani, T.; Carr, S.; Brendler, T.; Mumbengegwi, D.R.; Kong, A.N.T.; Simon, J.E.; Wu, Q. Phytochemical analysis and anti-inflammatory activity of the extracts of the african medicinal plant Ximenia caffra. J. Anal. Methods Chem. 2015, 2015, 948262. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar] [CrossRef]
- Ferrera, T.S.; Heldwein, A.B.; Dos Santos, C.O.; Somavilla, J.C.; Sautter, C.K. Substâncias fenólicas, flavonoides e capacidade antioxidante em erveiras sob diferentes coberturas do solo e sombreamentos. Rev. Bras. Plantas Med. 2016, 18, 588–596. [Google Scholar] [CrossRef]
- Koffi, J.A.; Silué, K.D.; Tano, D.K.; Dable, T.M.; Yavo, W. Evaluation of antiplasmodial activity of extracts from endemic medicinal plants used to treat malaria in Côte d’Ivoire. BioImpacts 2020, 10, 151–157. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Polat, D.Ç.; Şafak, E.K.; Yıldız, G.; Küpeli, A.; Selenkol, E.; Sobarzo-Sánchez, E. Phytochemical Composition and Biological Activities of Arctium minus (Hill) Bernh.: A Potential Candidate as Antioxidant, Enzyme Inhibitor, and Cytotoxic Agent. Antioxidants 2022, 11, 1852. [Google Scholar] [CrossRef]
- Hamrouni, H.; Othman, K.B.; Benmoussa, H.; Idoudi, S.; Najjaa, H.; Neffati, M.; Elfalleh, W. Phenolic Profiling, Antioxidant, and Antibacterial Activities of Selected Medicinal Plants from Tunisia. Chem. Biodivers. 2023, 20, 2–10. [Google Scholar] [CrossRef]
- Lahlou, R.A.; Gonçalves, A.C.; Bounechada, M.; Nunes, A.R.; Soeiro, P.; Alves, G.; Moreno, D.A.; Garcia-Viguera, C.; Raposo, C.; Silvestre, S.; et al. Antioxidant, Phytochemical, and Pharmacological Properties of Algerian Mentha aquatica Extracts. Antioxidants 2024, 13, 1512. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Li, Q.; Dai, H.; Zhou, Q.; Meng, X.; Chen, Z.; Zhang, X.; Zhang, Z.; Liu, Y.; et al. Metabolomic Profiling Using UPLC–MS/MS Revealed Differential Changes in Metabolites of Three Tea Plant Varieties and Their Potential Antioxidant Activities. J. Food Biochem. 2024, 2024, 1–14. [Google Scholar] [CrossRef]
- Lahlou, R.A.; Carvalho, F.; Pereira, M.J.; Lopes, J.; Silva, L.R. Overview of Ethnobotanical–Pharmacological Studies Carried Out on Medicinal Plants from the Serra da Estrela Natural Park: Focus on Their Antidiabetic Potential. Pharmaceutics 2024, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Guo, Z.; Bi, X.; Zhao, Y. Polyphenolic profile as well as anti-oxidant and anti-diabetes effects of extracts from freeze-dried black raspberries. J. Funct. Foods. 2017, 31, 179–187. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- de Oliveira Galvão, F.; da Silva Dantas, F.G.; de Lima Santos, C.R.; Marchioro, S.B.; Cardoso, C.A.L.; Wender, H.; Sangalli, A.; de Almeida-Apolonio, A.A.; de Oliveira, K.M.P. Cochlospermum regium (Schrank) pilger leaf extract inhibit methicillin-resistant Staphylococcus aureus biofilm formation. J. Ethnopharmacol. 2020, 261, 113167. [Google Scholar] [CrossRef]
- Pereira, C.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties, anti-hepatocellular carcinoma activity and hepatotoxicity of artichoke, milk thistle and borututu. Ind. Crops Prod. 2013, 49, 61–65. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Bhat, R. The sorbus spp.—Underutilised plants for foods and nutraceuticals: Review on polyphenolic phytochemicals and antioxidant potential. Antioxidants 2020, 9, 813. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Anumula, M.; Mondi, S.R.; Gottumukkula, K.M. A Review on Cochlospermum gossypium. Int. J. Pharm. Biol. Sci. 2019, 9, 374–377. [Google Scholar]
- Calderaro, A.; Patanè, G.T.; Tellone, E.; Barreca, D.; Ficarra, S.; Misiti, F.; Laganà, G. The Neuroprotective Potentiality of Flavonoids on Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 14835. [Google Scholar] [CrossRef]
- Mahendra, C.; Murali, M.; Manasa, G.; Ponnamma, P.; Abhilash, M.R.; Lakshmeesha, T.R.; Satish, A.; Amruthesh, K.N.; Sudarshana, M.S. Antibacterial and antimitotic potential of bio-fabricated zinc oxide nanoparticles of Cochlospermum religiosum (L.). Microb. Pathog. 2017, 110, 620–629. [Google Scholar] [CrossRef]
- Cornélio Favarin, D.; Robison De Oliveira, J.; Jose Freire De Oliveira, C.; De Paula Rogerio, A. Potential effects of medicinal plants and secondary metabolites on acute lung injury. Biomed Res. Int. 2013, 2013, 17–23. [Google Scholar] [CrossRef]
- De Sá, M.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Han, L.; Sun, B.S. Extraction yields and anti-oxidant activity of proanthocyanidins from different parts of grape pomace: Effect of mechanical treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Apolonio, A.A.; Cupozak-Pinheiro, W.J.; Berres, V.M.; Dantas, F.G.S.; Svidzinski, T.I.E.; Oliveira, K.M.P.; Chang, M.R. Control of Cryptococcus Gattii Biofilms by an Ethanolic Extract of Cochlospermum Regium (Schrank) Pilger Leaves. Sci. World, J. 2018, 2018, 5764187. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, V.V.; dos Santos Ramos, A.A.; da Silva, B.B.; Bauab, M.M. Antimicrobial activity of natural products against Helicobacter pylori: A review. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 54. [Google Scholar] [CrossRef]
- Leme, D.E.M.; Rodrigues, A.B.; De Almeida-Apolonio, A.A.; Dantas, F.G.D.S.; Negri, M.F.N.; Svidzinski, T.I.E.; Mota, J.D.S.; Cardoso, C.A.L.; Oliveira, K.M.P. De In Vitro Control of Uropathogenic Microorganisms with the Ethanolic Extract from the Leaves of Cochlospermum regium (Schrank) Pilger. Evid. Based Complement. Altern. Med. 2017, 2017, 4687154. [Google Scholar] [CrossRef]
- Sousa, C.M.D.M.; Silva, H.R.E.; Vieira, G.M.; Ayres, M.C.C.; Da Costa, C.L.S.; Araújo, D.S.; Cavalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.D.M.; Brandão, M.S.; et al. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim. Nova 2007, 30, 351–355. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Ellagic Acid and Derivatives from Cochlospermum angolensis Welw. Extracts: HPLC–DAD–ESI/MS n Profiling, Quantification and In Vitro Anti-depressant, Anti-cholinesterase and Anti-oxidant Activities. Phytochem. Anal. 2013, 24, 534–540. [Google Scholar] [CrossRef]
- Köksal, E.; Tohma, H.; Kılıç, Ö.; Alan, Y.; Aras, A.; Gülçin, I.; Bursal, E. Assessment of antimicrobial and antioxidant activities of nepeta trachonitica: Analysis of its phenolic compounds using HPLC-MS/MS. Sci. Pharm. 2017, 85, 24. [Google Scholar] [CrossRef]
- Reis Mansur, M.C.P.P.; Leitão, S.G.; Cerqueira-Coutinho, C.; Vermelho, A.B.; Silva, R.S.; Presgrave, O.A.F.; Leitão, Á.A.C.; Leitão, G.G.; Ricci-Júnior, E.; Santos, E.P. In vitro and in vivo evaluation of efficacy and safety of photoprotective formulations containing antioxidant extracts. Braz. J. Pharmacogn. 2016, 26, 251–258. [Google Scholar] [CrossRef]
- Carvalho, R.S.; Carollo, C.A.; de Magalhães, J.C.; Palumbo, J.M.C.; Boaretto, A.G.; Nunes e Sá, I.C.; Ferraz, A.C.; Lima, W.G.; de Siqueira, J.M.; Ferreira, J.M.S. Antibacterial and antifungal activities of phenolic compound-enriched ethyl acetate fraction from Cochlospermum regium (mart. Et. Schr.) Pilger roots: Mechanisms of action and synergism with tannin and gallic acid. S. Afr. J. Bot. 2018, 114, 181–187. [Google Scholar] [CrossRef]
- Tchicaillat-Landou, M.; Petit, J.; Gaiani, C.; Miabangana, E.S.; Kimbonguila, A.; Nzikou, J.M.; Scher, J.; Matos, L. Ethnobotanical study of medicinal plants used by traditional healers for the treatment of oxidative stress-related diseases in the Congo Basin. J. Herb. Med. 2018, 13, 76–90. [Google Scholar] [CrossRef]
- Presber, W.; Hegenscheid, B.; Friedmann-Alvermann, B.; Dorge, S.; Voigt, G.; Hiller, K.; Hils, J.; May, A.; Bothig, B. Antiviral activity of extracts from Cochlospermum angolense Welw. Pharmazie 1987, 42, 707–708. [Google Scholar]
- Presber, W.; Herrmann, D.K.; Hegenscheid, B. The effect of an extract from Cochlospermum angolense (“Burututu”) on Plasmodium berghei in the mouse malaria suppression test. Angew. Parasitol. 1991, 32, 7–9. [Google Scholar] [PubMed]
- Presber, W.; Hegenscheid, B.; Hernandez-Alvarez, H.; Herrmann, D.; Brendel, C. Inhibition of the growth of Plasmodium falciparum and Plasmodium berghei in vitro by an extract of Cochlospermum angolense (Welw.). Acta Trop. 1992, 50, 331–338. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.P.; Da Silva, T.L.T.; De Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Granato, D.; Koot, A.; Schnitzler, E.; van Ruth, S.M. Authentication of geographical origin and crop system of grape juices by phenolic compounds and antioxidant activity using chemometrics. J. Food Sci. 2015, 80, C584–C593. [Google Scholar] [CrossRef] [PubMed]
- Tchacondo, T.; Karou, S.D.; Batawila, K.; Agban, A.; Ouro-Bang’na, K.; Anani, K.T.; Gbeassor, M.; de Souza, C. Herbal remedies and their adverse effects in Tem tribe traditional medicine in Togo. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Çekiç, Ç.; Özgen, M. Comparison of antioxidant capacity and phytochemical properties of wild and cultivated red raspberries (Rubus idaeus L.). J. Food Compos. Anal. 2010, 23, 540–544. [Google Scholar] [CrossRef]
- Chidewe, C.K.; Chirukamare, P.; Nyanga, L.K.; Zvidzai, C.J.; Chitindingu, K. Phytochemical Constituents and the Effect of Processing on Antioxidant Properties of Seeds of an Underutilized Wild Legume B auhinia P etersiana. J. Food Biochem. 2016, 40, 326–334. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Jatau, A.I.; Khalid, G.M.; Alshargi, O.Y. Traditional uses, phytochemistry, and pharmacological activities of Cochlospermum tinctorium A. Rich (Cochlospermaceae): A review. Futur. J. Pharm. Sci. 2021, 7, 20. [Google Scholar] [CrossRef]
- Abubakar, I.B.; Malami, I.; Muhammad, A.; Salihu Shinkafi, T.; Shehu, D.; Maduabuchi Aja, P. A review of the medicinal uses and biological activity of Piliostigma thonningii (Schum). Milne-Redh. RPS Pharm. Pharmacol. Rep. 2024, 3, rqae004. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C.F.R. Analytical Tools Used to Distinguish Chemical Profiles of Plants Widely Consumed as Infusions and Dietary Supplements: Artichoke, Milk Thistle, and Borututu. Food Anal. Methods. 2014, 7, 1604–1611. [Google Scholar] [CrossRef]
- Anaga, A.O.; Oparah, N. Investigation of the methanol root extract of Cochlospermum planchonii for pharmacological activities in vitro and in vivo. Pharm. Biol. 2009, 47, 1027–1034. [Google Scholar] [CrossRef]
- Ahmed, T.S.; Magaji, M.G.; Yaro, A.H.; Musa, A.M.; Adamu, A.K. Aqueous methanol extracts of Cochlospermum tinctorium (A. Rich) possess analgesic and anti-inflammatory activities. J. Young Pharm. 2011, 3, 237–242. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Da Mota, R.V.; Cordenunsi, B.R.; Lajolo, F.M. Physico-chemical characterization and bioactive compounds of blackberry fruits (Rubus sp.) grown in Brazil. Cienc. Tecnol. Aliment. 2008, 28, 702–708. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Sabarathinam, S. Unraveling the therapeutic potential of quercetin and quercetin-3-O-glucuronide in Alzheimer’s disease through network pharmacology, molecular docking, and dynamic simulations. Sci. Rep. 2024, 14, 14852. [Google Scholar] [CrossRef] [PubMed]
- Ez Zoubi, Y.; Bousta, D.; Farah, A. A Phytopharmacological review of a Mediterranean plant: Lavandula stoechas L. Clin. Phytoscience 2020, 6, 9. [Google Scholar] [CrossRef]
- Dankwa, B.; Broni, E.; Enninful, K.S.; Kwofie, S.K.; Wilson, M.D. Consensus docking and MM-PBSA computations identify putative furin protease inhibitors for developing potential therapeutics against COVID-19. Struct. Chem. 2022, 33, 2221–2241. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Lee, J.J.; Kim, I.S.; Kim, Y.; Myung, C.S. Stimulation of glucose uptake and improvement of insulin resistance by aromadendrin. Pharmacology 2011, 88, 266–274. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Tolba, M.F.; Karonen, M. Antioxidant and hepatoprotective activities of flavonoids from Bauhinia hookeri. Rec. Nat. Prod. 2016, 10, 812–817. [Google Scholar]
- Chahal, A.; Saini, A.K.; Chhillar, A.K.; Saini, R.V. Natural antioxidants as defense system against cancer. Asian J. Pharm. Clin. Res. 2018, 11, 38–44. [Google Scholar] [CrossRef]
- Mezghani, I.; Bargougui, K.; Salah, H.B.; Frikha, D.; Zouari, N.; Chaieb, M. LC-ESI-MS characterization, antioxidant and antibacterial properties of various solvent extracts from Tricholaena teneriffae L. S. Afr. J. Bot. 2021, 143, 116–122. [Google Scholar] [CrossRef]
- Manga, H.M.; Brkic, D.; Marie, D.E.P.; Quetin-Leclercq, J. In vivo anti-inflammatory activity of Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg. (Euphorbiaceae). J. Ethnopharmacol. 2004, 92, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, O.S.; Kim, B.Y.; Jeong, S.J. Apigetrin from scutellaria baicalensis georgi inhibits neuroinflammation in BV-2 microglia and exerts neuroprotective effect in HT22 hippocampal cells. J. Med. Food. 2016, 19, 1032–1040. [Google Scholar] [CrossRef]
- Farag, M.A.; Sakna, S.T.; El-Fiky, N.M.; Shabana, M.M.; Wessjohann, L.A. Phytochemical, antioxidant and antidiabetic evaluation of eight Bauhinia L. species from Egypt using UHPLC-PDA-qTOF-MS and chemometrics. Phytochemistry 2015, 119, 41–50. [Google Scholar] [CrossRef]
- Cock, I.E.; Van Vuuren, S.F. A review of the traditional use of southern African medicinal plants for the treatment of fungal skin infections. J. Ethnopharmacol. 2020, 251, 112539. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.; Lee, S.J.; Ham, I.; Whang, W.K. Antioxidant activities of new flavonoids from Cudrania tricuspidata root bark. Arch. Pharm. Res. 2009, 32, 195–200. [Google Scholar] [CrossRef]
- Yang, Q.; Huan, R.; Meng, D.; Qi, J.; Xia, L. Progress in the study of anti-tumor effects and mechanisms of vitexin. Pharmacol. Rep. 2024, 77, 124–134. [Google Scholar] [CrossRef]
- Ranjan, R.; Kishore, K.; Ranjan, R.; Sheikh, T.J.; Jha, A.K.; Ojha, B.K.; Kumar, S.; Kumar, R. Nutraceutical Potential of Vitexin: A Flavone Glycoside. J. Phytopharm. 2023, 12, 44–50. [Google Scholar] [CrossRef]
- Yu, P.R.; Tseng, C.Y.; Hsu, C.C.; Chen, J.H.; Lin, H.H. In vitro and in vivo protective potential of quercetin-3-glucuronide against lipopolysaccharide-induced pulmonary injury through dual activation of nuclear factor-erythroid 2 related factor 2 and autophagy. Arch. Toxicol. 2024, 98, 1415–1436. [Google Scholar] [CrossRef]
- Mottaghi, S.; Abbaszadeh, H. The anticarcinogenic and anticancer effects of the dietary flavonoid, morin: Current status, challenges, and future perspectives. Phyther. Res. 2021, 35, 6843–6861. [Google Scholar] [CrossRef]
- Hong, D.G.; Lee, S.; Kim, J.; Yang, S.; Lee, M.; Ahn, J.; Lee, H.; Chang, S.-C.; Ha, N.-C.; Lee, J. Anti-Inflammatory and Neuroprotective Effects of Morin in an MPTP-Induced Parkinson’s Disease Model. Int. J. Mol. Sci. 2022, 23, 10578. [Google Scholar] [CrossRef] [PubMed]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Lauria, G.; Aiello, F.; Carullo, G.; Curcio, R.; Fiorillo, M.; Campiani, G.; Dolce, V.; Cappello, A.R. Exploiting Glycyrrhiza glabra L. (Licorice) Flavanones: Licoflavanone’s Impact on Breast Cancer Cell Bioenergetics. Int. J. Mol. Sci. 2024, 25, 7907. [Google Scholar] [CrossRef] [PubMed]
- Orallo, F.; Camiña, M.; Álvarez, E.; Basaran, H.; Lugnier, C. Implication of cyclic nucleotide phosphodiesterase inhibition in the vasorelaxant activity of the citrus-fruits flavonoid (±)-naringenin. Planta Med. 2005, 71, 99–107. [Google Scholar] [CrossRef]
- Sánchez-Salgado, J.C.; Estrada-Soto, S.; García-Jiménez, S.; Montes, S.; Gómez-Zamudio, J.; Villalobos-Molina, R. Analysis of flavonoids bioactivity for cholestatic liver disease: Systematic literature search and experimental approaches. Biomolecules 2019, 9, 102. [Google Scholar] [CrossRef]
- Martínez-Rodriguez, L.; Murguía-Hernández, K.; García-Juárez, I.; Uribe-Esquivel, M.; Gómez-Reyes, E. La historia oscura de la rosa amarilla: Un reporte de caso de toxicidad hepática asociado al consumo de Cochlospermum vitifolium como remedio herbolario. Rev. Gastroenterol. México. 2015, 80, 220–222. [Google Scholar] [CrossRef]
- Sanchez-Salgado’a, J.C.; Ortiz-Andradea, R.R.; Aguirre-Crespoa, F.; Vergara-Galiciaa, J.; Leon-Rivera’b, I.S.; Montesc, R.; Villalobos-Molinad, S.E.-S. Cochlospermum 6 word. J. Ethnopharmacol. 2007, 109, 400–405. [Google Scholar][Green Version]
- Assini, J.M.; Mulvihill, E.E.; Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Chhoker, S.S.; Sawyez, C.G.; Drangova, M.; Adams, A.C.; Kharitonenkov, A.; et al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology 2015, 156, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Deng, Z.; Rong, X.; Li, R.; You, Z.; Guo, X.; Cai, C.; Zhao, Y.; Gao, P.; Cao, G.; et al. Naringenin Prevents Oxidative Stress and Inflammation in LPS-Induced Liver Injury through the Regulation of LncRNA-mRNA in Male Mice. Molecules 2022, 28, 198. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kang, M.W.; Roh, J.H.; Choi, S.U.; Zee, O.P. Cytotoxic phenolic compounds from Chionanthus retusus. Arch. Pharm. Res. 2009, 32, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, N.H.; Kim, J.Y.; Park, J.H.; Shin, S.Y.; Kwon, Y.S.; Lee, H.J.; Kim, S.S.; Chun, W. Aromadendrin inhibits lipopolysaccharide-induced nuclear translocation of NF-κB and phosphorylation of JNK in RAW 264.7 macrophage cells. Biomol. Ther. 2013, 21, 216–221. [Google Scholar] [CrossRef]
- De Almeida, S.C.X.; De Lemos, T.L.G.; Silveira, E.R.; Pessoa, O.D.L. Volatile and non-volatile chemical constituents of Cochlospermum vitifolium (willdenow) sprengel. Quim. Nova 2005, 28, 57–60. [Google Scholar] [CrossRef]
- Zołnierczyk, A.K.; Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Woźniak, E.; Anioł, M. Isoxanthohumol—Biologically active hop flavonoid. Fitoterapia 2015, 103, 71–82. [Google Scholar] [CrossRef]
- Pohjanvirta, R.; Nasri, A. The Potent Phytoestrogen 8-Prenylnaringenin: A Friend or a Foe? Int. J. Mol. Sci. 2022, 23, 3168. [Google Scholar] [CrossRef]
- Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic perspectives of 8-prenylnaringenin, a potent phytoestrogen from hops. Molecules 2018, 23, 660. [Google Scholar] [CrossRef]
- Shirani, M.; Raeisi, R.; Heidari-Soureshjani, S.; Asadi-Samani, M.; Luther, T. A review for discovering hepatoprotective herbal drugs with least side effects on kidney. J. Nephropharmacology. 2017, 6, 38–48. [Google Scholar] [CrossRef]
- Campos, J.F.; de Toledo Espindola, P.P.; Torquato, H.F.V.; Vital, W.D.; Justo, G.Z.; Silva, D.B.; Carollo, C.A.; Souza, K.d.P.; Paredes-Gamero, E.J.; dos Santos, E.L. Leaf and root extracts from Campomanesia adamantium (Myrtaceae) promote apoptotic death of leukemic cells via activation of intracellular calcium and caspase-3. Front. Pharmacol. 2017, 8, 466. [Google Scholar] [CrossRef]
- Ballin, N.Z.; Traore, M.; Tinto, H.; Sittie, A.; Mølgaard, P.; Olsen, C.E.; Kharazmi, A.; Christensen, S.B. Antiplasmodial compounds from Cochlospermum tinctorium. J. Nat. Prod. 2002, 65, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, L.R.; Bae, M.S.; Kim, B.M.; Oh, G.S.; Chai, K.Y. A chalcone glycoside from the fruits of Sorbus commixta Hedl. Molecules 2009, 14, 5323–5327. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tielas, C.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Atividades Biológicas e Novas Aplicações das Chalconas. Planta Daninha. 2016, 34, 607–616. [Google Scholar] [CrossRef]
- Soares, S.E. Phenolic acids as antioxidants. Rev. Nutr. 2002, 15, 71–81. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Morais, A.L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Insights on the formulation of herbal beverages with medicinal claims according with their antioxidant properties. Molecules 2013, 18, 2851–2863. [Google Scholar] [CrossRef]
- De Oliveira, C.B.; Comunello, L.N.; Lunardelli, A.; Amaral, R.H.; Pires, M.G.S.; Da Silva, G.L.; Manfredini, V.; Vargas, C.R.; Gnoatto, S.C.B.; De Oliveira, J.R.; et al. Phenolic enriched extract of Baccharis trimera presents anti-inflammatory and antioxidant activities. Molecules 2012, 17, 1113–1123. [Google Scholar] [CrossRef]
- Hayashi, M.A.; Bizerra, F.C.; Ismael, P.; Silva, D. Antimicrobial compounds from natural sources. Front. Microbiol. 2013, 4, 3389. [Google Scholar] [CrossRef]
- Othman, S.N.N.; Lum, P.T.; Sekar, M.; Mazlan, N.A.; Yusri, P.Z.S.; Ghazali, N.F.; Idi, H.M.; Azman, S.; Ismail, M.; Noor, A.A.M. Molecules of Interest—Embelin—A Review. Res. J. Pharm. Technol. 2020, 13, 3485. [Google Scholar] [CrossRef]
- Joshi, R.; Kamat, J.P.; Mukherjee, T. Free radical scavenging reactions and antioxidant activity of embelin: Biochemical and pulse radiolytic studies. Chem. Biol. Interact. 2007, 167, 125–134. [Google Scholar] [CrossRef]
- Chitra, M.; Shyamala Devi, C.S.; Sukumar, E. Antibacterial activity of embelin. Fitoterapia 2003, 74, 401–403. [Google Scholar] [CrossRef]
- Mahendran, S.; Thippeswamy, B.S.; Veerapur, V.P.; Badami, S. Anticonvulsant activity of embelin isolated from Embelia ribes. Phytomedicine 2011, 18, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Danquah, M. Embelin and Its Derivatives: Design, Synthesis, and Potential Delivery Systems for Cancer Therapy. Pharmaceuticals 2022, 15, 1131. [Google Scholar] [CrossRef]
- DeFilipps, R.A.; Krupnick, G.A. The medicinal plants of Myanmar. PhytoKeys 2018, 102, 1–341. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H. Bin Bioactive compounds and bioactivities of ginger (zingiber officinale roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Hsiang, C.Y.; Lo, H.Y.; Huang, H.C.; Li, C.C.; Wu, S.L.; Ho, T.Y. Ginger extract and zingerone ameliorated trinitrobenzene sulphonic acid-induced colitis in mice via modulation of nuclear factor-κB activity and interleukin-1β signalling pathway. Food Chem. 2013, 136, 170–177. [Google Scholar] [CrossRef]
- Promdam, N.; Panichayupakaranant, P. [6]-Gingerol: A narrative review of its beneficial effect on human health. Food Chem. Adv. 2022, 1, 100043. [Google Scholar] [CrossRef]
- Kerboua, M.; Monia, A.A.; Samba, N.; Silva, L.; Raposo, C.; Díez, D.; Rodilla, J.M. Phytochemical Composition of Lichen Parmotrema hypoleucinum (J. Steiner) Hale from Algeria. Molecules 2022, 27, 5229. [Google Scholar] [CrossRef]
- Zahoor, A.; Yang, C.; Yang, Y.; Guo, Y.; Zhang, T.; Jiang, K.; Guo, S.; Deng, G. 6-Gingerol exerts anti-inflammatory effects and protective properties on LTA-induced mastitis. Phytomedicine 2020, 76, 153248. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Imeneo, M.; Luzza, F. Olive tree biophenols in inflammatory bowel disease: When bitter is better. Int. J. Mol. Sci. 2019, 20, 1390. [Google Scholar] [CrossRef]
- Coppa, C.F.S.C.; Rosim, R.E.; De Oliveira, C.A.F.; Da Costa Rodrigues, C.E.; Gonçalves, C.B. Extração de oleuropeína a partir de folhas de oliveira utilizando solvente hidroalcoólico. Braz. J. Food Technol. 2017, 20, e2016169. [Google Scholar] [CrossRef]
- Otero, D.M.; Oliveira, F.M.; Lorini, A.; da Fonseca Antunes, B.; Oliveira, R.M.; Zambiazi, R.C. Oleuropein: Methods for extraction, purifying and applying. Rev. Ceres 2020, 67, 315–329. [Google Scholar] [CrossRef]
- Al Fares, E.; Sanikidze, T.; Kalmakhelidze, S.; Topuria, D.; Mansi, L.; Kitson, S.; Molazadeh, M. The Alleviating Effect of Herniarin Against Ionizing Radiation-Induced Genotoxicity and Cytotoxicity in Human Peripheral Blood Lymphocytes. Curr. Radiopharm. 2022, 15, 141–147. [Google Scholar] [CrossRef]
- Paulsen, E.; Otkjær, A.; Andersen, K.E. The coumarin herniarin as a sensitizer in German chamomile [Chamomilla recutita (L.) Rauschert, Compositae]. Contact Dermat. 2010, 62, 338–342. [Google Scholar] [CrossRef]
- Salehcheh, M.; Safari, O.; Khodayar, M.J.; Mojiri-Forushani, H.; Cheki, M. The protective effect of herniarin on genotoxicity and apoptosis induced by cisplatin in bone marrow cells of rats. Drug Chem. Toxicol. 2022, 45, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Michel, T.; Nde, C.B.M.; Njuh, A.N.; Cisilotto, J.; Ndinteh, D.T.; Clyne, C.; Fernandez, X.; Creczynski-Pasa, T.B.; Njamen, D. Estrogen-like and tissue-selective effects of 7-methoxycoumarin from Ficus umbellata (Moraceae): An in vitro and in vivo study. BMC Complement. Altern. Med. 2017, 17, 383. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Shah, M.H.; Li, T.; Fu, X.; Guo, X.; Liu, R.H. Ethnomedicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. J. Ethnopharmacol. 2015, 162, 333–345. [Google Scholar] [CrossRef] [PubMed]
- De Assis, C.M.; Moreno, P.R.H.; Young, M.C.M.; De Arruda Campos, I.P.; Suffredini, I.B. Isolation and evaluation of the biological activity related to the major alkaloids in Tabernaemontana angulata Mart. ex Müll. Arg., Apocynaceae. Rev. Bras. Farmacogn. 2009, 19, 626–631. [Google Scholar] [CrossRef]
- Tufariello, J.J.; Ali, S.A. Elaeocarpus Alkaloids. The Synthesis of dl-Elaeokanine-A and dl-Elaeokanine-C. Tetrahedron Lett. 1979, 20, 4445–4448. [Google Scholar] [CrossRef]
- Adams, M.; Mahringer, A.; Kunert, O.; Fricker, G.; Efferth, T.; Bauer, R. Cytotoxicity and P-glycoprotein modulating effects of quinolones and indoloquinazolines from the Chinese herb Evodia rutaecarpa. Planta Med. 2007, 73, 1554–1557. [Google Scholar] [CrossRef]
- Hochfellner, C.; Evangelopoulos, D.; Zloh, M.; Wube, A.; Guzman, J.D.; Mchugh, T.D.; Kunert, O.; Bhakta, S.; Bucar, F. Antagonistic effects of indoloquinazoline alkaloids on antimycobacterial activity of evocarpine. J. Appl. Microbiol. 2015, 118, 864–872. [Google Scholar] [CrossRef]
- Rubio, N.; Moreda-Pineiro, A.; Bermejo Barrera, P.; Bermejo, A. Perfil de alcaloides de la hoja de coca en el fluido oral de un mascador de hoja de coca y un bebedor de té de coca. Acta Toxicológica Argent. 2019, 27, 72–80. [Google Scholar]
- Zhang, L.; Li, X.; Li, T.; Xiong, R.; Li, Y.; Yan, D.; Chen, P. Farnesoic acid methyltransferase 6 (BmFAMeT6) interrelates with moltinism of dominant trimolter in silkworm, Bombyx mori. Biologia 2021, 76, 2231–2240. [Google Scholar] [CrossRef]
- Felipe, L.O.; Bicas, J.L. Terpenos, aromas e a química dos compostos naturais. Química Nov. Na Esc. 2017, 39, 120–130. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Y.; Shang, H.; Luo, Y.; Tian, Y. Ganoderic Acid A and Its Amide Derivatives as Potential Anti-Cancer Agents by Regulating the p53-MDM2 Pathway: Synthesis and Biological Evaluation. Molecules 2023, 28, 2374. [Google Scholar] [CrossRef] [PubMed]
- Solon, S.; Carollo, C.A.; Brandão, L.F.G.; De MacEdo, C.D.S.; Klein, A.; Dias-Junior, C.A.; De Siqueira, J.M. Phenolic derivatives and other chemical compounds from. Quim. Nova 2012, 35, 1169–1172. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Yen, G.-C. Ganoderic Acid and Lucidenic Acid (Triterpenoid). Enzymes. 2014, 36, 33–56. [Google Scholar]
- Ban, N.K.; Giang, V.H.; Linh, T.M.; Lien, L.Q.; Ngoc, N.T.; Thao, D.T.; Nam, N.H.; Cuong, N.X.; Van Kiem, P.; Van Minh, C. Two new 11-noriridoids from the aerial parts of Morinda umbellata. Phytochem. Lett. 2013, 6, 267–269. [Google Scholar] [CrossRef]
- Kouda, R.; Yamamoto, K.; Katsuyama, A.; Ichikawa, S.; Yakushiji, F. Concise Synthesis of 11-Noriridoids via Pauson–Khand Reaction. Chem. Pharm. Bull. 2024, 72, c24-00190. [Google Scholar] [CrossRef]
- Dexter, H.R.; Allen, E.; Williams, D.M. A concise stereoselective synthesis of pterosin B. Tetrahedron Lett. 2018, 59, 4323–4325. [Google Scholar] [CrossRef]
- Yahara, Y.; Takemori, H.; Okada, M.; Kosai, A.; Yamashita, A.; Kobayashi, T.; Fujita, K.; Itoh, Y.; Nakamura, M.; Fuchino, H.; et al. Pterosin B prevents chondrocyte hypertrophy and osteoarthritis in mice by inhibiting Sik3. Nat. Commun. 2016, 7, 10959. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Z.; Chen, J.; Liu, B.; Wu, C.; Li, Y.; Xu, J.; Li, P. Lucialdehyde B suppresses proliferation and induces mitochondria-dependent apoptosis in nasopharyngeal carcinoma CNE2 cells. Pharm. Biol. 2023, 61, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Do Silva, A.N.; Uetanabaro, A.P.T.; Lucchese, A.M. Chemical composition and antibacterial activity of essential oils from Myrcia alagoensis (Myrtaceae). Nat. Prod. Commun. 2013, 8, 269–271. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, P.; Li, J.; Wang, Y.; Li, J.; Chen, P. A farnesoic acid O-methyltransferase (FAMeT) from Exopalaemon carinicauda is responsive to Vibrio anguillarum and WSSV challenge. Cell Stress Chaperones 2014, 19, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Holford, K.C.; Edwards, K.A.; Bendena, W.G.; Tobe, S.S.; Wang, Z.; Borst, D.W. Purification and characterization of a mandibular organ protein from the American lobster, Homarus americanus: A putative farnesoic acid O-methyltransferase. Insect Biochem. Mol. Biol. 2004, 34, 785–798. [Google Scholar] [CrossRef]
- Pires-Moreira, M.; Chan-Poot, S.; Aviles-Berzunza, E.; Garcia-Sosa, K.; Godoy-Hernandez, G.; Pena-Rodriguez, L.M. Influence of the Flowering Stage in the Production of Urechitol a in Agrobacterium Rhizogenes-Transformed Plants of Pentalinon Andrieuxii. Bot. Sci. 2024, 102, 438–446. [Google Scholar] [CrossRef]
- Yam-Puc, A.; Chee-González, L.; Escalante-Erosa, F.; Arunachalampillai, A.; Wendt, O.F.; Sterner, O.; Godoy-Hernández, G.; Peña-Rodríguez, L.M. Steroids from the root extract of Pentalinon andrieuxii. Phytochem. Lett. 2012, 5, 45–48. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A.; et al. Loliolide, a new therapeutic option for neurological diseases? In vitro neuroprotective and anti-inflammatory activities of a monoterpenoid lactone isolated from codium tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef]
- Fontaine, B.M.; Nelson, K.; Lyles, J.T.; Jariwala, P.B.; García-Rodriguez, J.M.; Quave, C.L.; Weinert, E.E. Identification of ellagic acid rhamnoside as a bioactive component of a complex botanical extract with anti-biofilm activity. Front. Microbiol. 2017, 8, 496. [Google Scholar] [CrossRef]
- Rwegoshora, F.; Mabiki, F.; Machumi, F.; Chacha, M.; Styrishave, B.; Cornett, C. A New Ellagic Acid Rhamnoside from Synadenium glaucescens and Its Cytotoxicity Evaluation. Chem. Nat. Compd. 2023, 59, 670–674. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, S.U.; Lee, K.R. Gymnopilin K: A new cytotoxic gymnopilin from Gymnopilus spectabilis. J. Antibiot. 2012, 65, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Laufer, H.; Borst, D.; Baker, F.C.; Carrasco, C.; Sinkus, M.; Reuter, C.C.; Tsai, L.W.; Schooley, D.A. Identification of a juvenile hormone-like compound in a crustacean. Science 1987, 235, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Figueiredo, C.S.S.e.S.; Viera de Oliveira, P.; De Silva Saminez, W.F.; Muniz Diniz, R.; Silva Rodrigues, J.F.; Maia da Silva, M.S.; Nascimento da Silva, L.C.; Grigolin Grisotto, M.A. Óleo essencial da Canela (Cinamaldeído) e suas aplicações biológicas. Rev. Investig. Biomed. 2018, 9, 192. [Google Scholar] [CrossRef]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Giovanelli, S.; Cioni, P.L.; Flamini, G.; Pistelli, L. Evaluation of volatile constituents of Cochlospermum angolense. Nat. Prod. Commun. 2012, 7, 629–632. [Google Scholar] [CrossRef]
- Tlhapi, D.; Malebo, N.; Manduna, I.T.; Lautenschläger, T.; Mawunu, M. A Review of Medicinal Plants Used in the Management of Microbial Infections in Angola. Plants 2024, 13, 2991. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Kumar, G.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Mahomoodally, M.F.; Seebaluck-Sandoram, R.; Etienne, O.K.; Zengin, G. An insight into Cochlospermum planchonii extracts obtained by traditional and green extraction methods: Relation between chemical compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 147, 112226. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction—A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Kiendrebeogo, M.; Compaoré, M.; Meda, R.N.T.; Bacher, M.; Koenig, K.; Pacher, T.; Fuehrer, H.P.; Noedl, H.; Willcox, M.; et al. Quality assessment and antiplasmodial activity of West African Cochlospermum species. Phytochemistry 2015, 119, 51–61. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Abudunia, A.M.; Marmouzi, I.; Faouzi, M.E.A.; Ramli, Y.; Taoufik, J.; El Madani, N.; Essassi, E.M.; Salama, A.; Khedid, K.; Ansar, M.; et al. Activité anti-candidose, antibactérienne, cytotoxique et antioxydante des fleurs de Calendula arvensis. J. Mycol. Med. 2017, 27, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Heinze, C.; Dundão, M.D.F.; Neinhuis, C.; Lautenschläger, T. Economic Potential of Selected Native Plants from Cuanza Norte, Northern Angola. Econ. Bot. 2019, 73, 96–111. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant capacity and phenolic contents of some Mediterranean medicinal plants and their potential role in the inhibition of cyclooxygenase-1 and acetylcholinesterase activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Akagi, H.; Matsuo, N.; Suzuki, T. FREE RADICALS AND THE MAIN DIETARY ANTIOXIDANTS. Eisei Kagaku 1989, 35, 123–130. [Google Scholar] [CrossRef]
- Sabir, S.M.; Abbas, S.R.; Shahida, S.; Khan, M.F. In-Vitro antioxidant, anti-lipid peroxidative activities and In-Silico study of Terminalia chebula bioactive compounds. Clin. Phytoscience 2020, 6, 93. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Pombal, S.; Hernández, Y.; Diez, D.; Mondolis, E.; Mero, A.; Morán-Pinzón, J.; Guerrero, E.I.; Rodilla, J.M. Antioxidant Activity of Carvone and Derivatives against Superoxide Ion. Nat. Prod. Commun. 2017, 12, 653–655. [Google Scholar] [CrossRef]
- Tvete Inngjerdingen, K.; Ballo, N.; Zhang, B.-Z.; Malterud, K.E.; Michaelsen, T.E.; Diallo, D.; Smestad Paulsen, B. A comparison of bioactive aqueous extracts and polysaccharide fractions from roots of wild and cultivated Cochlospermum tinctorium A. Rich. Phytochemistry 2013, 93, 136–143. [Google Scholar] [CrossRef]
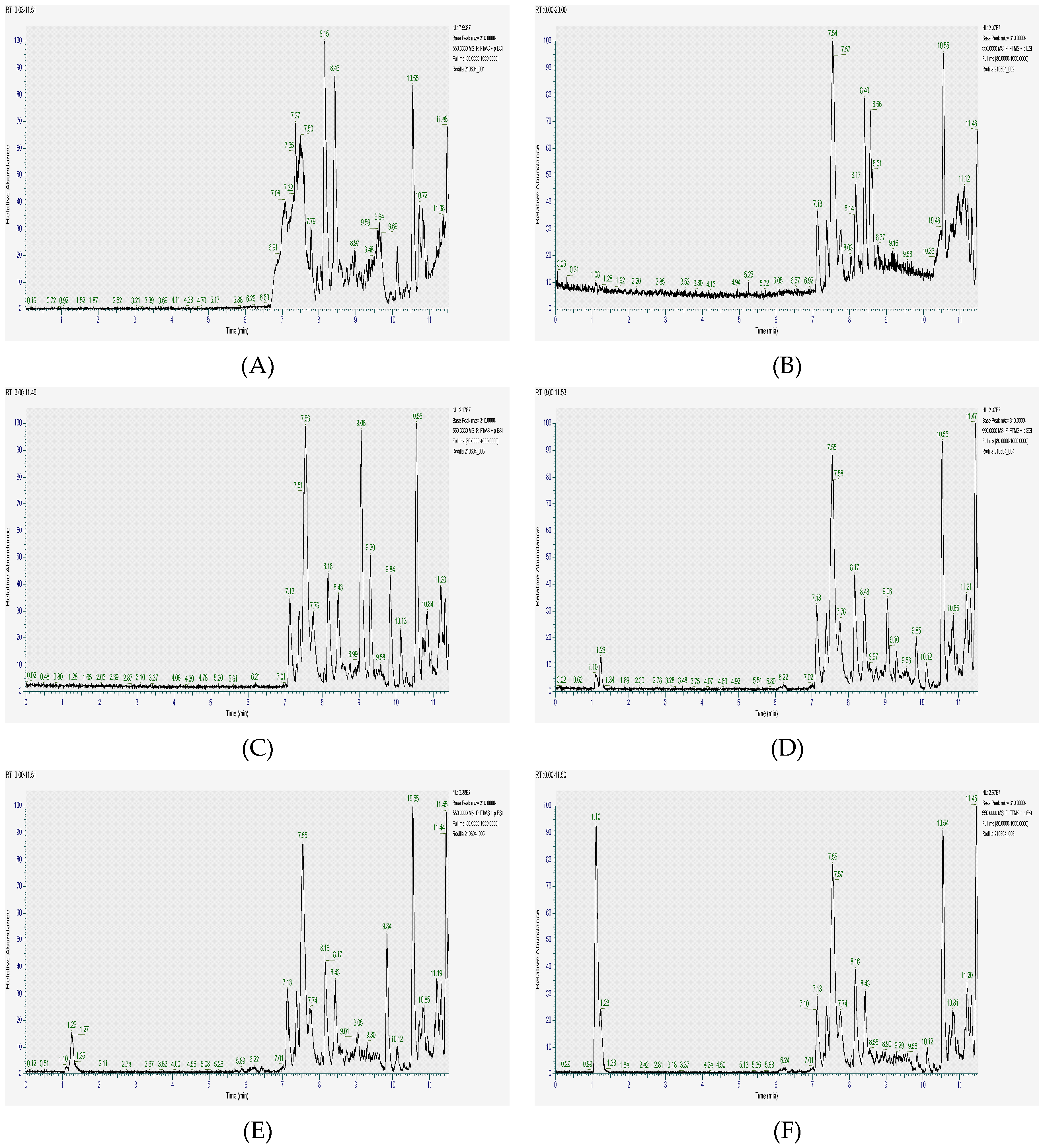

| RT (min) | Compound | [M + H]+ | MW Calc. | Formula | Frags + | Frags + | Frags + | Frags + | Leaves | Barks | Roots | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (acetone) | (ethanol) | (acetone) | (ethanol) | (acetone) | (ethanol) | |||||||||
| 7.73 | (+)-Abscisic acid | 265.1432 | 264.1354 | C15H20O4 | 91.0545 | 105.0701 | 79.0547 | 119.0854 | - | - | - | - | Detected | - |
| 7.95 | Loliolide | 197.1171 | 196.1093 | C11H16O3 | 91.0545 | 65.0392 | 105.0701 | 79.0545 | - | - | - | - | Detected | - |
| 7.97 | Quercitrin | 449.1073 | 448.0995 | C21H20O11 | 329.0648 | 299.0545 | 353.0644 | 413.0858 | - | Detected | - | - | - | - |
| 7.98 | Samandarin | 306.2460 | 305.2382 | C19H31NO2 | 100.1123 | 55.0549 | 127.0755 | 182.1537 | Detected | - | - | - | - | - |
| 8.10 | Astragalin | 449.1072 | 448.0994 | C21H20O11 | 299.0547 | 329.0652 | 353.0649 | 325.0698 | - | Detected | - | - | - | - |
| 8.10 | Eupatoriochromene | 219.1013 | 218.0935 | C13H14O3 | 115.0542 | 128.0620 | 91.0545 | 174.0670 | - | - | Detected | Detected | - | - |
| 8.20 | Vitexin | 433.1125 | 432.1047 | C21H20O10 | 283.0596 | 313.0699 | 121.0284 | 297.0747 | - | Detected | - | - | - | - |
| 8.38 | Apigetrin | 433.1122 | 432.1044 | C21H20O10 | 283.0594 | 313.0699 | 337.0698 | 165.0179 | - | Detected | - | - | - | - |
| 8.40 | Dihydromyricetin | 321.06 | 320.0522 | C15H12O8 | 163.0386 | 219.0284 | 191.0335 | 107.0492 | - | - | - | - | Detected | - |
| 8.47 | Hydroconiferyl Alcohol | 183.1014 | 182.0936 | C10H14O3 | 95.0494 | 51.0236 | 91.0546 | 115.0542 | - | Detected | - | - | - | - |
| 8.47 | Naringenin | 273.0752 | 272.0674 | C15H12O5 | 153.0179 | 91.0544 | 68.9975 | 119.0491 | - | - | Detected | Detected | - | - |
| 8.55 | Quercetin-3′-glucuronide | 479.0813 | 478.0735 | C21H18O13 | 303.0493 | 85.0287 | 153.0180 | 229.0490 | - | Detected | - | - | - | - |
| 8.58 | Zingerone | 195.1014 | 194.0936 | C11H14O3 | 91.0542 | 95.0494 | 79.0547 | 53.0392 | - | - | - | Detected | - | |
| 8.60 | Isoquercetin | 465.1023 | 464.0945 | C21H20O12 | 303.0493 | 85.0288 | 229.0490 | 137.0231 | - | Detected | - | - | - | - |
| 8.62 | (+)-Dihydrokaempferol | 289.0703 | 288.0625 | C15H12O6 | 107.0493 | 153.0179 | 149.0231 | 215.0698 | - | - | Detected | - | - | - |
| 8.62 | Dihydroisorhamnetin | 319.0809 | 318.0731 | C16H14O7 | 137.0596 | 153.0181 | 149.0232 | 122.0364 | - | - | - | - | Detected | - |
| 8.64 | Pterosin B | 219.1377 | 218.1299 | C14H18O2 | 121.0648 | 91.0545 | 107.0493 | 149.0595 | - | - | - | - | Detected | Detected |
| 8.70 | Morin | 303.0495 | 302.0417 | C15H10O7 | 153.0179 | 229.0494 | 137.0233 | 68.9976 | - | Detected | - | - | - | - |
| 8.73 | Quercetin-3-Arabinoside | 435.0916 | 434.0838 | C20H18O11 | 303.0493 | 73.0289 | 153.0179 | 229.0485 | - | Detected | - | - | - | - |
| 8.90 | Evocarpine | 340.2590 | 339.2512 | C23H33NO | 114.0914 | 96.0810 | 69.0704 | 209.1645 | - | - | - | - | Detected | |
| 8.95 | Kaempferol | 287.0545 | 286.0467 | C15H10O6 | 153.0180 | 121.0284 | 68.9976 | 213.0541 | - | Detected | - | - | - | - |
| 8.98 | Cinnamaldehyde | 133.0648 | 132.0570 | C9H8O | 51.0236 | 95.0494 | 115.0541 | 91.0546 | Detected | - | - | - | - | - |
| 8.98 | (+)-Fargesin | 371.1484 | 370.1406 | C21H22O6 | 137.0597 | 156.0567 | 217.0858 | 285.0750 | - | - | Detected | - | - | - |
| 9.00 | Eschweilenol C | 449.0709 | 448.0631 | C20H16O12 | 317.0287 | 285.0024 | 257.0073 | 302.0053 | - | - | - | - | Detected | - |
| 9.01 | Ellagic acid 2-rhamnoside | 449.0708 | 448.0630 | C20H16O12 | 317.0287 | 285.0024 | 257.0075 | 302.0054 | - | - | - | Detected | - | - |
| 9.03 | (−)-8-Prenylnaringenin | 341.1377 | 340.1299 | C20H20O5 | 137.0595 | 248.0821 | 203.0850 | 291.1010 | - | - | Detected | Detected | - | |
| 9.03 | Licoflavanone | 341.138 | 340.1302 | C20H20O5 | 137.0597 | 114.0913 | 203.0850 | 227.0699 | - | - | Detected | - | - | |
| 9.08 | Edpetiline | 592.3893 | 591.3815 | C33H53NO8 | 89.0599 | 133.0858 | 575.3634 | 99.0807 | - | - | Detected | - | - | - |
| 9.13 | Oleuropeinol | 363.1644 | 362.1566 | C16H26O9 | 69,0340 | 87.0444 | 155.0703 | Detected | - | - | - | - | - | |
| 9.16 | 3-O-Methylellagic acid | 317.0287 | 316.0209 | C15H8O8 | 257.0088 | 285.0027 | 201.0181 | 145.0282 | - | - | - | - | Detected | - |
| 9.18 | Kaempferol-3-Glucuronide | 463.0866 | 462.0788 | C21H18O12 | 317.0287 | 285.0026 | 257.0075 | 302.0057 | - | - | - | - | Detected | - |
| 9.30 | Morachalcone A | 341.1378 | 340.1300 | C20H20O5 | 137.0596 | 248.0836 | 291.1011 | 211.0755 | - | - | Detected | - | Detected | - |
| 9.36 | 5-(2-Oxopropyl) hygrine | 198.1486 | 197.1408 | C11H19NO2 | 55.0548 | 91.0544 | 69.0704 | 79.0547 | - | - | - | Detected | - | - |
| 9.52 | Pinobanksin | 273.0753 | 272.0675 | C15H12O5 | 153.0179 | 91.0545 | 119.0491 | - | - | Detected | - | - | - | |
| 9.53 | Dihydrogenistein | 273.0753 | 272.0675 | C15H12O5 | 153.0179 | 91.0545 | 119.0490 | 147.0437 | - | - | - | Detected | - | - |
| 9.82 | Angustin B | 333.0964 | 332.0886 | C17H16O7 | 137.0596 | 167.0336 | 163.0387 | 122.0362 | - | - | - | - | Detected | - |
| 9.83 | Blumeatin B | 333.0964 | 332.0886 | C17H16O7 | 137.0597 | 167.0336 | 122.0365 | 259.0963 | - | - | - | - | - | Detected |
| 9.90 | Elaeokanine C | 212.1641 | 211.1563 | C12H21NO2 | 55.0549 | 69.0704 | 79.0547 | 91.0546 | Detected | - | - | - | Detected | Detected |
| 10.12 | Coumarandione | 149.023 | 148.0152 | C8H4O3 | 65.0392 | 53.0028 | 88.7265 | 111.4721 | Detected | Detected | Detected | Detected | Detected | Detected |
| 10.24 | 7-Methoxycoumarin | 177.0544 | 176.0466 | C10H8O3 | 65.0391 | 121.0286 | 149.0233 | 91.0546 | Detected | Detected | Detected | Detected | Detected | Detected |
| 10.34 | Dichotomocej A | 226.1796 | 225.1718 | C13H23NO2 | 55.0549 | 79.0547 | 67.0547 | 180.1744 | Detected | Detected | Detected | Detected | - | Detected |
| 10.53 | Antiarol | 185.0805 | 184.0727 | C9H12O4 | 68.9976 | 129.0179 | 87.0078 | Detected | - | - | - | - | ||
| 10.54 | Umbellatolide B | 185.0807 | 184.0729 | C9H12O4 | 185.0807 | 184.0729 | 185.0807 | 184.0729 | - | - | - | - | - | Detected |
| 10.55 | Urechitol B | 305.1588 | 304.1510 | C14H24O7 | 129.0182 | 68.9976 | 185.0807 | 139.0023 | Detected | - | - | - | - | - |
| 10.63 | 4-Heptylresorcinol | 209.1533 | 208.1455 | C13H20O2 | 91,0545 | 105.0702 | 79.0546 | 179.1066 | Detected | - | Detected | Detected | Detected | - |
| 10.65 | 5-Heptylresorcinol | 209.1533 | 208.1455 | C13H20O2 | 91.0546 | 55.0548 | 105.0700 | 179.1062 | - | Detected | - | - | - | - |
| 10.79 | Embelin | 295.1900 | 294.1822 | C17H26O4 | 57.0705 | 73.0289 | 101.0236 | 221.1165 | Detected | Detected | - | Detected | Detected | Detected |
| 10.80 | (+)-[6]-Gingerol | 295.1899 | 294.1821 | C17H26O4 | 57.0705 | 73.0289 | 221.1169 | 101.0236 | - | - | Detected | - | - | - |
| 11.09 | Farnesoic acid | 237.1845 | 236.1767 | C15H24O2 | 57.0705 | 121.1011 | 181.1219 | 79.0547 | Detected | - | - | - | - | - |
| 11.18 | 5-O-Methyl embelin | 309.2055 | 308.1977 | C18H28O4 | 57.0705 | 221.1164 | 107.0493 | 165.0546 | - | Detected | - | - | - | Detected |
| 11.35 | Valerenic acid | 235.1689 | 234.1611 | C15H22O2 | 57.0705 | 179.1062 | 107.0494 | 123.0444 | Detected | - | - | Detected | Detected | Detected |
| 11.35 | Drimenin | 235.1690 | 234.1612 | C15H22O2 | 57.0705 | 179.1060 | 91.0547 | 107.0491 | - | - | Detected | - | - | - |
| 11.42 | Kulactone | 453.3355 | 452.3277 | C30H44O3 | 119.0855 | 107.0857 | 145.1010 | 95.0858 | - | - | Detected | - | - | - |
| 11.44 | Lucialdehyde B | 453.3354 | 452.3276 | C30H44O3 | 119.0855 | 107.0856 | 95.0858 | 133.1012 | - | - | - | Detected | - | Detected |
| 11.44 | Ganoderic acid S | 453.3355 | 452.3277 | C30H44O3 | 119.0855 | 145.1010 | 189.1632 | 201.1636 | - | - | - | - | Detected | - |
| 11.86 | Benzaldehyde | 107.0492 | 106.0414 | C7H6O | 51.0236 | 95.0494 | 77.0392 | 105.0451 | Detected | - | Detected | Detected | Detected | Detected |
| 12.54 | 2,5-Dimethyl-3,6-bis(tetradecylamino)-1,4-benzoquinone | 559.5188 | 558.5110 | C36H66N2O2 | 280.2632 | 81.0703 | 95.0858 | 263.2365 | - | - | - | Detected | - | Detected |
| 12.54 | Farnesylacetone | 263.2364 | 262.2286 | C18H30O | 67.0547 | 81.0703 | 95.0858 | 105.0701 | - | - | Detected | Detected | Detected | - |
| DPPH• | NO | O2•− | Lipid Peroxidation | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | Emax (%) | IC50 (μg/mL) | Emax (%) | IC50 (μg/mL) | Emax (%) | IC50 (μg/mL) | Emax (%) | IC50 (μg/mL) |
| Quercetin | 77.9 ± 4.4 | 8.6 ± 0.5 | 74.8 ± 3.5 | 14.3 ± 0.8 | 53.1 ± 0.8 | 102.1 ± 5.2 | - | - |
| Curcumin | 108.7 ± 22.1 | 16.6 ± 1.2 | 78.2 ± 3.2 | 55.0 ± 0.3 | - | - | 97.2 ± 0.3 | 1.5 ± 0.1 |
| L.E.A | 77.8 ± 2.9 | 12.2 ± 0.7 * | 34.8 ± 2.1 *, † | 250.8 ± 12.5 *, † | 45.9 ± 2.7 † | Nd | 93.9 ± 0.8 | 2.5 ± 0.2 * |
| L.E.E | 83.8 ± 0.2 | 11.8 ± 0.6 * | 63.3 ± 1.7 | 44.4 ± 2.3 * | 38.9 ± 1.6 | Nd | 92.8 ± 0.7 | 8.4 ± 0.5 * |
| B.E.A | 77.8 ± 1.1 | 2.6 ± 0.2 *, † | 65.9 ± 2.1 | 24.7 ± 1.3 *, † | 54.6 ± 3.4 | 96.4 ± 4.9 | 95.5 ± 0.1 | 3.8 ± 0.3 * |
| B.E.E | 78.3 ± 0.9 | 2.9 ± 0.2 *, † | 42.9 ± 1.7 * | Nd | 55.8 ± 0.5 | Nd | 93.9 ± 0.5 | 4.9 ± 0.3 * |
| R.E.A | 12.7 ± 3.2 | Nd | 55.5 ± 7.3 | Nd | 37.3 ± 1.3 † | Nd | 94.5 ± 0.5 | 5.9 ± 0.4 * |
| R.E.E | 53.0 ± 6.9 † | 16.4 ± 0.9 * | 53.5 ± 0.5 | 101.1 ± 5.1 * | 41.0 ± 3.7 † | Nd | 66.6 ± 19.2 * | 84.4 ± 4.3 * |
| Plant Material | Initial Mass | H.E g | A.E g | E.E g | % H.E | % A.E | % E.E |
|---|---|---|---|---|---|---|---|
| Leaves | 308.97 | 8.88 | 9.15 | 9.48 | 2.8 | 3.0 | 3.2 |
| Barks | 544.31 | 6.30 | 7.65 | 7.69 | 1.1 | 1.4 | 1.4 |
| Roots | 720.82 | 4.87 | 6.96 | 5.12 | 0.6 | 0.9 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samba, N.; Barrios, A.M.; De León, E.G.; Raposo, C.; Lahlou, R.A.; Curto, J.; Rodilla, J.M.; Roncero, A.M.; Diez, D.; Silva, L. Cochlospermum angolense Welw ex Oliv: Phytochemical Profile, Antioxidant Activity, and Therapeutic Prospects. Molecules 2025, 30, 2768. https://doi.org/10.3390/molecules30132768
Samba N, Barrios AM, De León EG, Raposo C, Lahlou RA, Curto J, Rodilla JM, Roncero AM, Diez D, Silva L. Cochlospermum angolense Welw ex Oliv: Phytochemical Profile, Antioxidant Activity, and Therapeutic Prospects. Molecules. 2025; 30(13):2768. https://doi.org/10.3390/molecules30132768
Chicago/Turabian StyleSamba, Nsevolo, Abdy Morales Barrios, Estela Guerrero De León, Cesar Raposo, Radhia Aitfella Lahlou, Joana Curto, Jesus M. Rodilla, Alejandro M. Roncero, David Diez, and Lúcia Silva. 2025. "Cochlospermum angolense Welw ex Oliv: Phytochemical Profile, Antioxidant Activity, and Therapeutic Prospects" Molecules 30, no. 13: 2768. https://doi.org/10.3390/molecules30132768
APA StyleSamba, N., Barrios, A. M., De León, E. G., Raposo, C., Lahlou, R. A., Curto, J., Rodilla, J. M., Roncero, A. M., Diez, D., & Silva, L. (2025). Cochlospermum angolense Welw ex Oliv: Phytochemical Profile, Antioxidant Activity, and Therapeutic Prospects. Molecules, 30(13), 2768. https://doi.org/10.3390/molecules30132768











