Abstract
The phytochemical investigation and evaluation of the antioxidant activity of the leaves, bark, and roots of Cochlospermum angolense Welw ex Oliv—a valued plant that is widely used in traditional Angolan medicine—hold significant importance. Compounds were extracted from the aforementioned plant using acetone and ethanol and identified by HPLC-ESI-MSn. Both extracts demonstrated notable abilities to scavenge 2,2-diphenyl-1-picrylhydrazyl, nitric oxide, and superoxide radicals, as well as to inhibit lipid peroxidation. A HPLC analysis revealed a diverse array of bioactive compounds, including flavonoids, phenols, alkaloids, quinones, and terpenes, which help neutralize free radicals and protect cells against oxidative stress, thereby contributing to the prevention of various diseases. Moreover, the acetone and ethanol extracts proved to be excellent sources of antioxidants. For the first time, the present study identified new compounds never reported in this species, such as (+)-abscisic acid, angustine B, pinobanksin, dihydrogenistein, (−)-8-prenylnaringenin, isoquercetin, samandarine, dihydromyricetin, and eupatoriocromene, in the leaves, bark, and roots, marking a significant advance in the chemical characterization of C. angolense. These findings enhance our understanding of the bioactive phytochemicals and antioxidant properties of C. angolense and open new avenues for future therapeutic and pharmacological research, further supporting its traditional use in Angolan medicine.
1. Introduction
Biodiversity is fundamental to human livelihoods and overall well-being [1,2]. Throughout history, people have maintained a close relationship with their natural environment, relying on its diverse flora for food, shelter, and medicine [3,4]. The enduring allure of nature lies in its potential to yield chemotherapeutic agents, with plant-derived natural compounds continuing to serve as vital sources of new leads in drug discovery [5,6,7]. Plants have consistently met essential human needs, not only by providing shelter, clothing, food, flavors, and fragrances, but also forming the basis of traditional medicine, from which many modern drugs have emerged [8]. Consequently, pharmacological and phytochemical research is critical for both uncovering new therapeutic agents and preserving medicinal plants as part of our cultural and scientific heritage. Medicinal plants rich in bioactive compounds are highly valued for their ability to reduce the risk of chronic diseases and promote overall health [9].
Angola is home to a rich diversity of medicinal plants whose full potential remains largely untapped [10]. The country’s varied flora represents a significant opportunity for discovering new secondary metabolites with therapeutic value, particularly given the longstanding traditional use of these plants. Known for their antioxidant properties, many of these species can help reduce disease incidence by neutralizing free radicals, thereby protecting cells and potentially preventing oxidative stress-related disorders [2,11]. The importance of these medicinal plants lies in their chemical constituents, which exhibit a range of biological activities and are broadly classified into primary and secondary metabolites [12,13]. These metabolites have garnered considerable attention for their potential as pharmaceutical agents, food additives, and valuable chemicals for human use. The bioactive compounds extracted from medicinal plants are rich in antioxidants, which are beneficial for developing treatments for various diseases [14,15]. Antioxidants prevent the oxidation process, thereby inhibiting the formation of free radicals that can damage healthy cells and contribute to chronic conditions such as cardiovascular diseases, diabetes, and cancer. In addition, these compounds play a crucial role in wound healing by promoting the repair of damaged skin. Several studies have suggested that a significant portion of the therapeutic value of plant extracts can be attributed to their antioxidant activity [16].
Members of the Cochlospermum genus are renowned for their richness in secondary metabolites, notably phenolic compounds, flavonoids, lignans, carotenoids, and sterols, which underpin their extensive medicinal applications [17,18]. The phytochemicals found in these plants, including those in the leaves, bark, and roots of Cochlospermum angolense Welw ex Oliv, exhibit potent antioxidant properties—an essential feature, given that many diseases are associated with free radicals produced during normal physiological processes and counteracted by plant-derived antioxidants [19,20]. Phenolic compounds in particular have been extensively studied for their health benefits. Characterized by multiple hydroxyl groups on aromatic rings, these secondary metabolites contribute to antioxidant, antimicrobial, and anti-inflammatory activities [21]. Despite the high antioxidant content typical of these species, few studies have directly compared the pharmacological and phytochemical properties of acetone and ethanol extracts from the roots, leaves, and bark of C. angolense. This Angolan plant is regarded as phytotherapeutic and is widely used by rural populations to both cure and prevent various ailments. For instance, beverages or infusions made from the roots and bark are traditionally used to combat intestinal diseases, parasitic infections, malaria, diabetes, and hepatitis, while the leaves are employed in baths to treat fevers, especially in children [20,22,23].
This research offers valuable insights into the traditional use of plants by local communities, highlighting their potential for both medicinal applications and biodiversity conservation. The study aimed to evaluate the antioxidant capacity of acetone and ethanol extracts obtained from the leaves, bark, and roots of C. angolense. Antioxidants are vital because they neutralize free radicals, which, if left unchecked, can cause cellular damage and contribute to the development of chronic diseases.
To thoroughly assess the antioxidant potential of C. angolense, our research utilized two complementary approaches. First, the antioxidant activity of the extracts was determined through a series of assays that measured the ability of the compounds to scavenge harmful free radicals. Second, the phytochemical composition of the extracts was meticulously analyzed using High-Performance Liquid Chromatography coupled with Electrospray Ionization and Tandem Mass Spectrometry (HPLC-ESI-MSn). This advanced analytical technique enabled the precise identification and characterization of a broad range of bioactive compounds, including flavonoids, phenolic acids, and other secondary metabolites known for their health-promoting properties. Detailed phytochemical profiling not only confirmed the presence of compounds associated with potent antioxidant activity but also provided a scientific basis for the traditional medicinal use of C. angolense. By documenting the specific bioactive constituents, the study paves the way for further pharmacological research and the potential development of novel therapeutic agents. Moreover, these findings underscore the importance of preserving local biodiversity and traditional knowledge, which are invaluable resources for sustainable healthcare innovations and conservation efforts.
2. Results and Discussion
For this research project, High-Performance Liquid Chromatography coupled with Electrospray Ionization and Tandem Mass Spectrometry (HPLC-ESI-MSn) was employed to comprehensively identify the phytochemical compounds present in the acetone and ethanol extracts obtained from the leaves, bark, and roots of the medicinal plant C. angolense. The primary goal was to correlate these compounds with their potential antioxidant properties [24]. The extraction process was carried out using standardized protocols to ensure reproducibility and a high yield of metabolites. Phytochemicals were identified based on their molecular formulas, retention times, and fragmentation patterns and were subsequently classified into distinct organic classes. The distribution of these compounds across the different plant parts was meticulously recorded in Supplementary File, allowing for a detailed comparative analysis and providing insights into structure–activity relationships.
The analysis resulted in the successful identification of 520 phytochemicals across the samples. Among these, 61 bioactive compounds were specifically associated with the antioxidant activity of C. angolense (Supplementary File). These key compounds primarily belong to the classes of flavonoids, phenolics, alkaloids, terpenoids, quinones, and tannins. Detailed spectral analysis and observed fragmentation patterns provided insights into the structural features of these molecules, which are known to scavenge free radicals and inhibit lipid peroxidation. Numerous studies have reported that these classes of compounds exhibit a range of biological activities—including antioxidant, antimicrobial, anti-inflammatory, antiplasmodial, and anticancer effects—in various medicinal plants [18,20,25,26]. For example, the alkaloids identified in this study have been shown to exert antiplasmodial activity by inhibiting protein synthesis in Plasmodium falciparum [27]. In addition to these major groups, comprehensive phytochemical profiling also revealed secondary metabolites such as aldehydes, coumarins, peptides, and esters, which may act synergistically to enhance the overall antioxidant effect. The precise quantification and structural elucidation achieved with HPLC-ESI-MSn underscore the robustness and reliability of this analytical method. Overall, the wide spectrum of antioxidant phytochemicals identified in C. angolense highlights their critical role in neutralizing free radicals and mitigating oxidative stress [11,28]. The discovery of the phytochemicals listed in Table 1 provides a solid scientific basis for the traditional medicinal use of C. angolense and substantiates the need for further pharmacological research, as well as the development of new therapeutic agents (Table 1).

Table 1.
Phytochemical characterization of bioactive constituents present in acetone and ethanol extracts obtained from leaves, bark, and roots of C. angolense.
After a detailed analysis of the bioactive phytochemicals present in the leaves, bark, and roots of the medicinal plant C. angolense, a diverse range of compounds with potential therapeutic and pharmacological applications was identified. These compounds, comprehensively listed in Supplementary File, belong to several organic classes, including flavonoids, phenols, alkaloids, sesquiterpenoids, quinones, and aldehydes—each known for its unique bioactive properties.
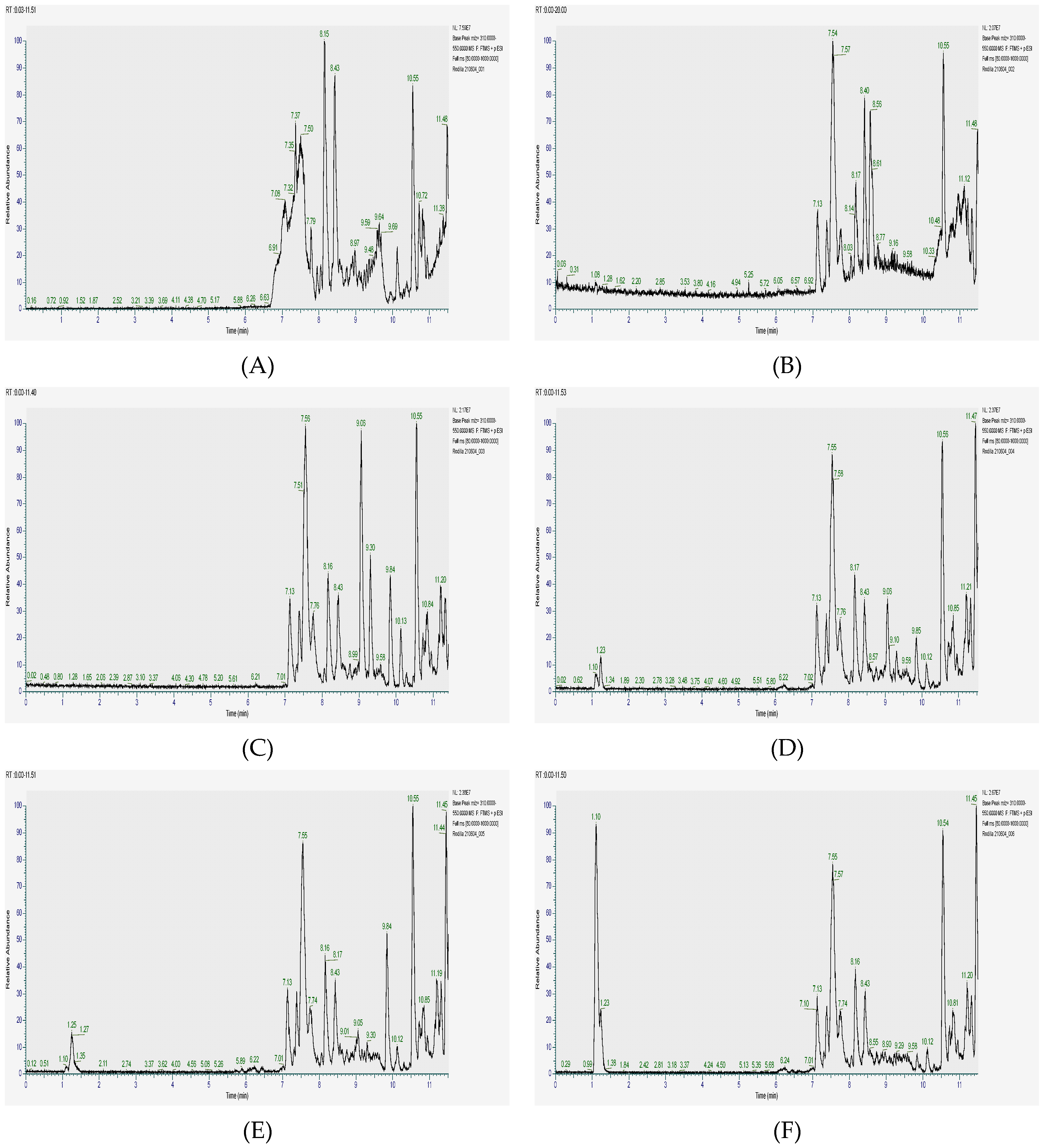
The extraction was performed using both acetone and ethanol, and the resulting extracts were analyzed using HPLC-ESI-MSn. The chromatograms obtained (Figure 1) not only provided a visual representation of complex phytochemical profiles but also enabled the precise identification of individual compounds based on their molecular formulas, retention times, and fragmentation patterns. The data revealed that the highest concentrations of flavonoids and phenols were consistently found across all three plant parts, suggesting that these metabolites play a significant role in the overall antioxidant and therapeutic potential of C. angolense.
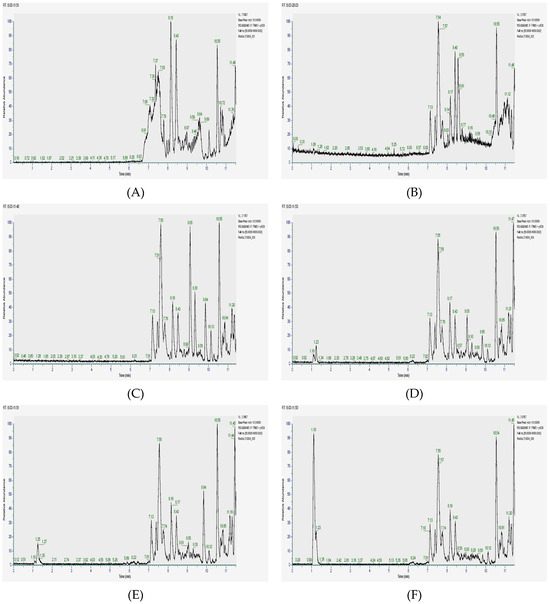
Figure 1.
HPLC-ESI-MSn Chromatograms of Cochlospermum angolense extracts. (A) Acetone extract of the leaves; (B) Ethanolic extract of leaves; (C) Acetone extract of barks; (D) Ethanolic extract of barks; (E) Acetone extract of roots; (F) Ethanolic extract of roots.
A closer comparison of the different plant parts indicated that the leaves are particularly enriched in flavonoids and phenolic compounds, which are well known for their free radical scavenging and anti-inflammatory activities. In contrast, bark exhibited a broader diversity of alkaloids and quinones, compounds that have been associated with antimicrobial and anticancer effects. The roots, on the other hand, displayed a unique chemical profile characterized by a significant presence of sesquiterpenoids combined with phenols, hinting at specialized roles in traditional remedies and potential synergistic effects in pharmacological applications. These findings not only align with previous studies on related species within the same family [29,30] but also underscore the importance of exploring each plant part separately to fully understand its distinct phytochemical contributions. The detailed profiling achieved in this study provided a consistent result for the traditional medicinal use of C. angolense and opens new avenues for further research into its potential as a source of novel therapeutic agents.
All extracts of Cochlospermum angolense Welw ex Oliv exhibited a remarkable diversity of phytochemical compounds endowed with potent antioxidant activities, as demonstrated by multiple in vitro assays [29,31]. The extracts contained high concentrations of bioactive compounds, notably flavonoids, phenols, terpenes, and alkaloids. In-depth analysis revealed that the flavonoid fraction was particularly abundant, with specific compounds such as quercitrin, astragalin, vitexin, apigetrin, quercetin-3′-glucuronide, isoquercetin, morin, quercetin-3-arabinoside, kaempferol, (+)-dihydrokaempferol, naringenin, pinobanksin, angustine B, licochalcone, morachalcone, and (−)-8-prenylnaringenin being identified [21,32,33]. These flavonoids not only exhibit significant elimination of free radical abilities but also modulate cellular signaling pathways, contributing to anti-inflammatory, antimicrobial, and anticancer effects by interfering with key mechanisms involved in cell proliferation and apoptosis [9,34]. In addition to flavonoids, the phenolic compounds present in the extracts are known for their efficiency in donating hydrogen atoms or electrons to neutralize free radicals, thereby stabilizing radical intermediates and preventing oxidative stress [18,35]. Terpenes and alkaloids further enrich the bioactive profile, offering complementary anti-inflammatory and antimicrobial properties. The synergistic interactions among these diverse classes of compounds likely amplify the overall antioxidant potential of C. angolense, enhancing its efficacy in protecting cells from oxidative damage. Recent studies have also suggested that these phytochemicals can influence gene expression related to oxidative stress responses and apoptotic pathways, thereby playing a role in the prevention of chronic diseases such as cardiovascular disorders, neurodegenerative conditions, and cancer [18,20,36,37].
Among the phenolic phytochemicals identified in C. angolense, several compounds exhibit notable antioxidant activity [38,39]. These include 4-heptylresorcinol, antiarol, embelin, zingerone, 5-heptylresorcinol, 3-O-methylellagic acid, oleuropeinol (also known as oleuropein), and (+)-[6]-gingerol. These phenolic compounds are known for their ability to neutralize reactive oxygen species (ROS), thereby mitigating oxidative stress at the cellular level and contributing to the prevention of chronic and degenerative diseases.
In addition to phenolics, several alkaloids identified in this study—including samandarine, evocarpine, hygrine, and elaeocanine C—have demonstrated antioxidant potential along with other biological activities, such as antimicrobial, anti-inflammatory, and antiplasmodial effects [40]. These findings are in line with previous research on the leaves and stem bark of C. gossypium, which also confirmed the presence of bioactive alkaloid phytochemicals with a wide range of pharmacological properties [40]. Moreover, the acetone and ethanol extracts of C. angolense revealed the presence of carboxylic acids, such as 4-pyrimidinecarboxylic acid, 3-hydroxysebacic acid, and 3,5-dihydroxydecanoic acid, as well as terpenoids like farnesoic acid and valerenic acid. These compounds are well-documented for their antioxidant effects, particularly their ability to interrupt oxidative chain reactions by stabilizing radical intermediates and protecting lipids, proteins, and nucleic acids from oxidative damage [41].
Notably, the highest concentrations of flavonoids and phenols were consistently found in the leaves, bark, and roots of C. angolense, reinforcing the idea that all parts of the plant contribute significantly to its antioxidant capacity. These results are consistent with findings from studies on C. religiosum, a closely related species, which also demonstrated high levels of phenolic and flavonoid compounds in multiple plant organs [42]. Altogether, these findings validate the traditional use of C. angolense and provide a strong scientific foundation for its potential therapeutic applications in managing oxidative stress-related conditions.
In this study, the antioxidant capacity of C. angolense samples was evaluated using several assays, including DPPH•, nitric oxide (NO•), superoxide (•O2−), and lipid peroxidation assays, with the detailed results presented in Table 2. The tested extracts, comprising an acetone extract of leaves (L.E.A), ethanolic extract of leaves (L.E.E), acetone extract of bark (B.E.A), ethanolic extract of bark (B.E.E), acetone extract of roots (R.E.A), and ethanolic extract of roots (R.E.E), revealed significant differences in terms of antioxidant activity. The extraction solvent notably influenced both the phytochemical profile and the antioxidant properties of the extracts, as demonstrated by the differences observed in Table 2.

Table 2.
Antioxidant activities of acetonic and ethanolic extracts of leaves, barks, and roots of Cochlospermum angolense Welw ex Oliv. The results are expressed in terms of maximum inhibition effect (Emax, %) and IC50 (μg/mL) values. Emax data are presented as mean ± SD for n = 3. * p < 0.05 vs. Quercetin and † p < 0.05 vs. Curcumin.
Our findings indicated that significant antiradical components are present in the leaves, bark, and roots of C. angolense, which contribute to neutralizing free radicals and protecting cells from oxidative damage, thereby playing a potential role in the prevention of various diseases (Table 2). In this investigation, we employed multiple assays to comprehensively evaluate the antioxidant capacity of the extracts. Extensive literature has described the mechanisms of action and the relationships between phytochemical activity—including that of flavonoids, phenols, tannins, alkaloids, and terpenes—and antioxidant potential [34,43,44]. Antioxidant activity was categorized as high (>70% inhibition), intermediate (40–70% inhibition), or low (<40% inhibition.
The results in Table 2 are expressed in terms of maximum inhibition (Emax, %) and IC50 (mg/mL) values for each assay. Certain IC50 values were not automatically determined by the GraphPad Prism 10 (version 10.4.2) due to limitations in dose–response curve fitting. In cases where data were not determined (nd), the reason was that the concentration range tested did not reach 50% inhibition of the effect, indicating that the extract did not show effective antiradical activity within the range evaluated.
Notably, the leaf extracts (from both ethanol and acetone extractions) exhibited high Emax values in the DPPH assay, comparable to those of reference antioxidants such as quercetin and curcumin, indicating strong free radical scavenging activity. Although the bark and root extracts also demonstrated significant antioxidant activity, their Emax values were slightly lower than those of the leaf extracts. In the nitric oxide assay, the ethanolic leaf extract showed considerably higher antioxidant activity (Emax of 63.3%) compared to the acetone extract (Emax of 34.8%), while the bark and root extracts displayed moderate activity (Table 2). In the superoxide (O2•−) assay, the bark extracts from both solvents exhibited particularly high Emax values, indicating an excellent capacity to neutralize superoxide radicals, although the leaf and root extracts were somewhat less effective in this assay (Table 2).
Finally, in the lipid peroxidation assay, all extracts demonstrated significant antioxidant activity, with the leaf extracts performing exceptionally well and showing Emax values close to those of the reference antioxidants. Overall, these results confirm that the leaves, bark, and roots of C. angolense are rich sources of antioxidants that can counteract oxidative stress and may contribute to the prevention of oxidative stress-related diseases.
The results indicate that both the acetone and ethanol extracts of the leaves, bark, and roots of C. angolense exhibit antioxidant activities comparable to those of standard antioxidants such as quercetin and curcumin. This finding is supported by literature that also emphasizes the effectiveness of ethanolic and acetonic extracts in neutralizing free radicals [45,46,47,48].
The antioxidant activity observed reflects the presence of a diverse array of bioactive phytochemicals in these extracts. Predominantly, polyphenols—including phenols and flavonoids—were abundant, along with other classes such as alkaloids, quinones, terpenes, and aldehydes. These compounds are believed to play a crucial role in mitigating oxidative stress, thereby providing a consistent result for their health-promoting properties [31]. Several studies on C. angolense have consistently identified flavonoids and phenols as the predominant bioactive components [20,49]. For instance, research on the aqueous extract of both fresh and dried bark of C. angolense confirmed high levels of flavonoids and phenols with strong antioxidant activity, which supports the results obtained from the leaves, bark, and roots in our study [20].
Moreover, the chemical composition and corresponding antioxidant activities of the extracts are influenced by variations in the bioactive phytochemical content inherent to medicinal plants [38,50,51]. Natural antioxidants in plants are known to inhibit free radical chain reactions by interfering with both the initiation and propagation stages, ultimately leading to chain termination and slowing down the overall oxidation process [21,52,53].
Additional studies have revealed that aqueous extracts of the roots of C. angolense contain bioactive substances with antiviral properties and the ability to inhibit the growth of Plasmodium falciparum and Plasmodium berghei in vitro [54,55,56]. The identification of various bioactive phytochemicals—including flavonoids, phenols, terpenes, and alkaloids—in our study aligns with these findings and supports previous reports. Preliminary investigations on the aqueous extracts of the roots and bark have also confirmed high concentrations of antioxidant compounds, directly correlating with their performance in the antioxidant assays [11].
The diversity of phytochemical compounds found in the leaves, bark, and roots of C. angolense underscores the significance of this plant as a source of various bioactive substances. The chemical composition of its polyphenolic compounds can vary compared to other species in the same family and is influenced by factors such as climatic conditions, harvest season, soil quality, and the processing and analytical methods used for identification. These variables contribute to the wide range of values reported in different studies [57,58,59].
Differences in the bioactive phytochemicals identified in our results and those reported in the literature can also be attributed to variations in extraction methods, as well as the geographic regions and seasons of harvest. According to [58,60], one of the main challenges in assessing the antioxidant activity of biological materials is the choice of analytical method, as each assay tends to target a specific aspect of antioxidant activity. Nonetheless, the four assays we performed yielded consistent results across the evaluated extracts, further confirming that C. angolense is a rich source of antioxidants.
Some studies have reported superior antioxidant activity in methanolic, hydromethanolic, and infusion extracts of C. angolense collected in southern Angola [39,49,60]. It is suggested that environmental factors—such as climatic conditions, soil types, and the use of relatively more polar solvents—can enhance antioxidant activity. In general, factors that influence the content of secondary metabolites in medicinal plants indicate a positive correlation between solar radiation intensity and the production of phenolic compounds, including flavonoids, tannins, and anthocyanins. However, the specific climatic conditions, time of year, or regions under which these correlations hold true are not always clearly defined, making comprehensive comparisons challenging [26].
The antioxidant activities of the leaves, bark, and roots, as demonstrated by our assays, align with the variations reported in the literature and reflect the broad diversity of bioactive compounds identified in this study [24,61,62]. The high antioxidant capacity observed in our tests correlates positively with the presence of phytochemicals such as flavonoids, phenols, alkaloids, and terpenes. Several studies have documented the relationship between antioxidant activity and polyphenolic content [40].
Moreover, numerous studies have employed various organic solvents for the extraction of compounds from different parts of medicinal plants in the Cochlospermaceae family and other medicinal plant families. Many of these findings support our results [11,60,63,64,65]. For instance, the methanolic extract of the root of C. planchonii has been shown to contain bioactive compounds with potent pharmacological activities in rats and low toxicity, primarily attributed to flavonoids and phenols—compounds also identified in our study [66].
The phytochemical characterization of extracts from the leaves, bark, and roots—as well as the fractions of the hydroethanolic and ethyl acetate extracts—from C. regium (Schrank) confirmed the presence of flavonoids and phenols, both of which are known for their antioxidant and antimicrobial activities. These findings further validated the flavonoid and phenol profiles identified in our study [36,52]. For instance, research on the leaves and stem bark of C. gossypium has documented numerous bioactive constituents, including flavonoids and phenolic compounds with notable antibacterial and antioxidant properties, and has specifically reported the antioxidant activity of the methanolic leaf extract [40]. Additionally, the biological activities of aqueous methanol extracts from the leaves, roots, and root bark of C. tinctorium—where flavonoids are also present—have been well documented [67].
Considering the diverse array of bioactive phytochemicals reported in previous research on various species within the Cochlospermaceae family, it is evident that C. angolense is a rich source of compounds with distinct pharmacological properties. The different bioactive polyphenolic compounds, particularly flavonoids and phenols, have attracted significant interest due to their potential benefits for human health. The chemistry and biology of these compounds are critical to understanding their biological effects [68,69].
Among the flavonoids most reported in the literature on extracts from different parts of the plant are querctrin, naringenin, astragalin, vitexin, apigetrin, pinobanksin, quercetin-3′-glucuronide, isoquercetin, morin, quercetin-3-arabinoside, dihydroisorhamnetin, kaempferol, and (+)-dihydrokaempferol. Notably, dihydrogenistein (found in the bark and roots), (−)-8-prenylnaringenin (present in the leaves and roots), isoquercetin (detected in the bark), and pinobanksin (identified in the leaves and roots) are reported for the first time in C. angolense. Isoquercetin is a glycosylated form of quercetin, the main flavonoid present in the human diet, widely studied for its therapeutic properties. Its main biological activities include antioxidant, anti-inflammatory, antiviral, antitumor, and cardioprotective [70]. Pinobanksin is a flavonol, belonging to the flavonoid class, recognized for its biological properties, including antioxidant, antimicrobial, and anti-inflammatory activities. This compound is commonly found in Lavandula stoechas [30,71]. It has been reported that Pinobanksin, one of the propolis compounds, is also found in some plant species. Propolis compounds have shown inhibitory effects against the ACE2, TMPRSS2, and PAK1 signaling pathways of SARS-CoV-2 in studies. Similarly, isoquercetin is structurally similar to quercitrin, which is currently in clinical trials as a potential drug for COVID-19 [72].
Quercetin, a well-known flavonol, has a longstanding history in folk medicine for treating colds, flu, and rheumatic conditions, and as a diuretic. It is a potent antioxidant with significant biological, pharmacological, and medicinal properties, including the inhibition of human platelet aggregation in vitro [34,64,73,74,75,76]. Additionally, quercetin exhibits potential anticancer effects by inducing cellular differentiation and, in its glycosidic form, inhibiting protein tyrosine kinase [69,75]. Quercitrin, a glycoside of quercetin, has been reported in the methanolic extract of Alchornea cordifolia leaves, where it demonstrated anti-inflammatory properties [77].
Similarly, apigetrin (apigenin-7-glucoside) is a glycosidic flavonoid with notable antioxidant potential. It is found in several medicinal plants, such as Tricholaena teneriffae [76], chamomile [78], and Scutellaria baicalensis [79]. Moreover, the ethanolic extract of Bauhinia hookeri has revealed that flavonoids like kaempferol and quercetin possess multiple biological effects, including robust antioxidant activity [57,74,79,80]. These compounds help prevent oxidative stress by directly scavenging free radicals, chelating metals, and inducing antioxidant enzyme expression.
In addition, quercetin, as identified in Piliostigma thonningii (Schum), has been documented to possess not only strong antioxidant activity but also a wide range of pharmacological properties, including anticancer, antiviral, anti-obesity, antifungal, antibacterial, and antidiabetic effects [64]. In a separate study, the 70% methanolic extract of the leaves and roots of Ximenia caffra from Western and Eastern Sub-Saharan Africa led to the identification of quercetin, further confirming its potent antioxidant capacity [24,81].
Data obtained from the hydroethanolic extract of C. regium (Mart. ex Schrank) revealed the presence of both morin and kaempferol [14]. Kaempferol, a flavonol and a derivative of flavonoids, has demonstrated excellent free radical scavenging abilities in DPPH assays and effectively inhibits lipid peroxidation in human low-density lipoproteins as measured by the TBARS assay [82]. Widely found in plant-based foods such as kale, beans, tea, spinach, and broccoli, kaempferol is recognized for its powerful antioxidant properties as well as its anti-inflammatory, anticancer, and cardioprotective effects [74]. Moreover, kaempferol has shown hepatoprotective properties in models of liver injury induced by CCl4, various drugs, and alcohol, suggesting its potential as a therapeutic agent for patients with atherosclerotic disease [17]. Additionally, flavonols such as rutin, morin, myricetin, and kaempferol have been reported to exert antisecretory and cytoprotective effects in animal models of gastric ulcer, as well as anti-Helicobacter pylori, anti-ulcer, antioxidant, and anti-inflammatory activities [14].
Vitexin (apigenin-8-c-beta-d-glucopyranoside) is another important flavonoid derivative—a glycoside of apigenin—that exhibits a broad spectrum of biological activities, including antioxidant, antimicrobial, anti-inflammatory, neuroprotective, anticancer, antiviral, antihypertensive, antiasthmatic, and antiepileptic effects [83]. This phytochemical is found in several plants, such as passionflower (Passiflora), bamboo (Phyllostachys nigra), pearl millet (Pennisetum glaucum), and hawthorn (Crataegus) [84]. Notably, vitexin has been shown to offer protective effects in the intestines by modulating macrophage polarization in azoxymethane-treated mice [85].
Quercetin-3-glucuronide, a flavonoid present in wine, fruits, and medicinal herbs, is recognized as the main circulating metabolite in human plasma following the consumption of quercetin-rich foods. It exhibits a range of beneficial activities, including antioxidant, anti-inflammatory, antiviral, anticancer, and neuroprotective effects [85]. Given these promising attributes, quercetin-3-glucuronide is considered a potent phytochemical for disease prevention and health promotion. Indeed, both quercetin and its glucuronide derivative rank among the most powerful antioxidants available from natural sources [70].
Morin (2′,3,4′,5,7-pentahydroxyflavone) is a flavonol derived from the flavonoid family, commonly found in fruits and vegetables such as onions, apples, and tea [86,87]. This phytochemical offers promising health benefits, including antioxidant, anti-inflammatory, and anticancer activities [20]. Research has shown that morin can protect against oxidative stress and reduce inflammation, which may be beneficial in preventing and managing various chronic diseases [14].
Licochalcone, also known as 3′-prenylnaringenin, is a flavanone derivative found in Glycyrrhiza glabra. It exhibits significant antioxidant, antimicrobial, and anticancer properties [88]. Notably, licochalcone has been observed to inhibit the energy metabolism of breast cancer cells, thereby reducing tumor proliferation, invasiveness, and stemness [89].
Naringenin, primarily found in citrus fruits and rosemary [90], is recognized as a bioactive compound with strong antioxidant properties and multiple health benefits. This phytochemical has been identified in methanolic extracts of the leaves, root bark, and wood of C. vitifolium [91,92], as well as in ethanolic extracts of the root bark, wood, flowers, and stems of C. gillivraei [17]. Previous studies indicate that naringenin can help prevent oxidative stress and inflammation in liver injuries, act as a potent lipid-lowering agent, and protect against obesity and glucose intolerance in rat models [93,94]. Moreover, it has been shown to reduce blood pressure in rats, suggesting that naringenin may help safeguard liver function by mitigating oxidative and inflammatory damage associated with liver injuries [91,95].
(+)-Dihydrokaempferol, also known as aromadendrin, is a flavonoid found in various plants and medicinal herbs [82,96]. This compound exhibits a broad range of pharmacological activities, including potent antioxidant, anti-inflammatory, and antidiabetic effects [73,97]. Aromadendrin has been identified in extracts from the bark and roots of C. vitifolium [17,98].
Pinobanksin is recognized for its diverse biological properties, such as antioxidant, antimicrobial, and anti-inflammatory activities, and is commonly found in Lavandula stoechas [21,71].
(−)-8-Prenylnaringenin (8-prenylnaringenin) is a powerful phytoestrogen primarily found in hops (Humulus lupulus) [99]. Celebrated for its wide range of biological activities and significant health benefits, this compound is among the most potent phytoestrogens and is used to alleviate menopausal and postmenopausal symptoms associated with declining hormonal levels in women [100,101].
Morachalcone, a type of chalcone, is present in several medicinal plants, including Glycyrrhiza glabra [102], Campomanesia adamantium [103], Cochlospermum tinctorium [104], and Sorbus commixta Hedl [105]. Known for its remarkable biological properties, morachalcone has demonstrated significant antioxidant, antibacterial, antifungal, antiviral, and anticancer activities in various studies [16,46,106].
The phenolic compounds identified in the extracts under study demonstrate significant antioxidant properties, protecting cells from oxidative damage [107]. Numerous studies have confirmed the antioxidant potential of these phenolic phytochemicals and phenolic acids, and our findings further underscore their importance in C. angolense [108,109,110].
Embelin (2,5-dihydroxy-3-undecyl-1,4-benzoquinone), a natural compound derived from Embelia ribes, exhibits a wide range of pharmacological activities, which are partly attributed to its ability to scavenge free radicals [111,112]. Its therapeutic spectrum includes antioxidant, anti-inflammatory, anticonvulsant, antifertility, anti-implantation, hepatoprotective, analgesic, wound-healing, and antibacterial effects [113,114]. Moreover, embelin has been reported as an inhibitor of cancer cell growth, making it a promising anticancer candidate despite its limited clinical applicability due to low water solubility [115].
Antiarol (3,4,5-trimethoxyphenol) is another natural compound, isolated from Cochlospermum vitifolium, Antiaris toxicaria, and Mentha aquatica, known for its antioxidant properties and potential applications in various biological and pharmaceutical contexts [17,30,116].
Zingerone (vanillylacetone) is recognized for its antioxidant and antidiarrheal properties [117]. It effectively scavenges free radicals and reactive oxygen species, and inhibits enzymes involved in their production [118]. Similarly, (+)-[6]-gingerol, a major bioactive constituent of ginger, is celebrated for its diverse biological activities—including antioxidant, anti-inflammatory, antimicrobial, anticancer, neuroprotective, cardiovascular and respiratory protective, anti-obesity, antidiabetic, anti-nausea, and antiemetic effects [109,119]. This phytochemical has also been identified in the lichen Parmotrema hypoleucinum from Algeria [120], highlighting its value as a dietary molecule with significant health benefits, particularly in tumor treatment and the inhibition of liver and gallbladder inflammation [121].
Oleuropein, which imparts the characteristic bitter taste to olives and is the primary contributor to the anti-inflammatory activity of olive leaf extract, is one of the most extensively studied compounds. Along with its derivative hydroxytyrosol, oleuropein is known for its potent antioxidant properties and a wide array of health benefits, including anti-inflammatory, anti-atherogenic, anticancer, antimicrobial, antiviral, and protective effects against cardiovascular and metabolic diseases [122,123,124]. A recent study on Lavandula pedunculata reported the presence of oleuropein and demonstrated its antioxidant, anti-inflammatory, and antimicrobial activities [21].
Coumarandione (dicoumarol) is a phenol-derived coumarin, a secondary metabolite present in various plants such as guaco and fennel [107]. This natural compound functions as an anticoagulant and was historically significant as the first oral drug used to prevent blood clot formation. In addition to its anticoagulant properties, dicoumarol and other coumarins exhibit antioxidant activity and can act as enzyme inhibitors [3,48].
Herniarin, another phenol-derived coumarin naturally occurring in certain plant species [8], also displays antioxidant properties. It has been shown to reduce radiation-induced cytotoxicity and genotoxicity in human lymphocytes [125]. Identified in the aqueous extract of Glycyrrhiza glabra, herniarin has been used therapeutically to treat hepatitis by lowering liver transaminase levels [102]. Moreover, German chamomile flowers, particularly the late liguliflorae, have been reported to contain both herniarin and umbelliferone (7-hydroxycoumarin), which may contribute to chamomile’s allergenic potential [46,126]. Herniarin is also being explored as a potential chemoprotective agent against cisplatin-induced genotoxicity [127].
The presence of these bioactive phytochemicals in the studied extracts may help explain the traditional use of this plant against hepatitis in the Cuanza Norte Province [128]. In general, phytochemicals such as flavonoids and phenolics are known for their antioxidant activity, which helps protect cells from oxidative damage caused by free radicals. Increasing attention is being given to these compounds for their potential to counteract the harmful effects of free radicals and mitigate chronic diseases associated with aging [62,69,129].
In addition to flavonoids and phenolics, other classes of bioactive phytochemicals identified in this study—including alkaloids, terpenes, tannins, and quinones—exhibit significant antioxidant properties [66]. Alkaloids, which play a crucial role in plant defense against insects and herbivores, are also highly valued in medicine for their therapeutic benefits. Well-known examples include morphine and nicotine [130]. For instance, elaeococanin C, an alkaloid reported in the leaves of Elaeocarpus kalziensis Schltr, demonstrates notable biological activity and is also present in Averrhoa bilimbi [30,131].
Similarly, evocarpine, a quinolone alkaloid primarily isolated from the fruit and herb of Evodia rutaecarpa, exhibits slight toxicity and has shown a pronounced antagonistic effect against various mycobacterial strains [132,133]. Hygrine, a ketonic alkaloid naturally occurring in coca leaves (Erythroxylum coca) and other plants, is of considerable interest due to its mydriatic action [134]. Additionally, edpetiline, a natural alkaloid found in Petilium eduardi, is noted for its anti-inflammatory and antioxidant properties and is predominantly used in research settings [135].
Terpenes are renowned for their aromatic properties and are widely used in the perfume, cosmetics, food, and pharmaceutical industries. They also fulfill important ecological roles, such as attracting pollinators or repelling herbivores [136]. Ganoderic acid, a triterpenoid derived from lanosterol and found in Ganoderma lucidum and other Ganoderma species, displays a range of pharmacological activities including anticancer, antiviral, antioxidant, and platelet aggregation inhibition properties [137]. These compounds have been extensively studied in traditional medicine for their potential health benefits, with some studies reporting anti-HIV-1 activity, hepatoprotective, antihypertensive, and cholesterol-lowering effects [138,139,140].
Umbellatolide B, a lactone and monoterpenoid classified as an 11-noriridoid, is another notable phytochemical present in various plants. Isolated from Morinda umbellata, it has been investigated for its potential antimicrobial and anti-inflammatory activities, and is traditionally used to prevent and treat inflammation, cancer, neurological disorders, and age-related conditions [141,142].
Farnesylacetone is a terpenic ketone renowned for its anti-inflammatory and antifungal properties, which underlie its frequent use in traditional medicine and cosmetic products for therapeutic benefits Pterosin B, a sesquiterpenoid found in ferns such as Pteridium aquilinum, has attracted research interest due to its intriguing pharmacological properties and its effects on chondrogenic cells [143,144].
Lucialdehyde B is a triterpene extracted from the fruiting bodies of certain plants; it exhibits notable cytotoxic activity, with studies focusing on its potential against cancers such as breast cancer and sarcoma. In C. angolense, this compound, identified in the bark, has demonstrated antiviral and cytotoxic effects against various cancer cell lines [145].
Farnesolic acid is another natural terpenoid present in diverse sources, including the leaves of Myrcia alagoensis (Myrtaceae) and even marine organisms like crustaceans. It serves as an important precursor in the biosynthesis of hormones and other bioactive compounds [146]. Beyond its role in insect juvenile hormone biosynthesis [147], farnesolic acid exhibits antimicrobial, anti-inflammatory, and potential antitumor properties by modulating various biological pathways [135,148].
Urechitol B, a natural terpenoid found in the root extracts of Pentalinon andrieuxii—a plant used in traditional Mayan medicine—is influenced by the flowering stage, which affects its production [149,150].
Loliolide, a ubiquitous monoterpenoid lactone first described in 1974, has been isolated from both plants and animals. It is also present in several marine brown algae such as Sargassum horneri, Sargassum ringgoldianum subsp. coreanum, and Undaria pinnatifida, where it exhibits antioxidant, antifungal, antibacterial, and anticancer activities [151]. In the medicinal plant Lactuca serriola, loliolide has been associated with a range of biological effects, including hepatoprotection, antioxidant, antivenom, allelopathic, sedative, anticonvulsant, antiepileptic, anti-inflammatory, and anticancer activities [30].
Ellagic acid rhamnoside is the sole tannin identified in the ethanolic extract of the bark, noted for its antimicrobial activity [152]. Research on ellagic acid and its derivatives from various plant species has revealed a spectrum of pharmacological properties, including antioxidant, antimicrobial, anticancer, antidepressant, cardiovascular protective, antidiabetic, neuroprotective, and anti-inflammatory effects [153].
Our investigation also uncovered additional bioactive phytochemicals with diverse pharmacological activities that fall outside the previously mentioned classes. Ostopanic acid, also known as (E,E)-7,12-dioxooctadeca-8,10-dienoic acid, is a cytotoxic fatty acid initially isolated from the stems and fruits of Ostodes paniculata and later from the hallucinogenic mushroom Gymnopilus spectabilis [154]. This compound has demonstrated potential in inhibiting the growth of certain cancer cells, such as P-388 lymphocytic leukemia cells [129,155].
Sorbic acid (2,4-hexadienoic acid) is a natural organic compound found in the fruits and seeds of Sorbus aucuparia [38]. Primarily employed as a preservative in the food and beverage industry, it effectively inhibits bacteria, yeasts, and fungi by reducing their capacity for amino acid absorption. Notably, the literature indicates that sorbic acid poses minimal health risks and is not associated with cancer; it was first isolated in 1859 from the unripe fruits of the rowan tree (Sorbus aucuparia), which explains its name [155].
Valerenic acid, identified in the studied leaves, is a major component of the volatile oils and roots of the medicinal plant Valeriana officinalis. This compound is renowned for its diverse pharmacological activities, particularly its sedative and anxiolytic properties, which make it a key ingredient in natural treatments for insomnia and anxiety [8,156].
Cinnamaldehyde, a natural compound found in cinnamon, exhibits a range of biological activities, including antimicrobial, anti-inflammatory, angiogenic, and wound-healing effects [110]. Widely used as a spice and in traditional herbal medicine, cinnamon (Cinnamomum species) owes much of its biological efficacy to cinnamaldehyde, the principal active component of its essential oil. This compound has been associated with antimicrobial, antioxidant, and antidiabetic activities [28,157]. Furthermore, several studies indicate that when cinnamaldehyde is combined with other phytochemicals such as eugenol, thymol, and carvacrol, it exhibits enhanced, synergistic biological effects [158,159]. In our study, this compound was detected in the leaves and bark of C. angolense and has also been identified in the root oils of C. angolense as well as in the fruit oils of Alchornea cordifolia [116,160].
Benzaldehyde is another naturally occurring product with significant biological activity. Commonly used as a flavoring and fragrance agent in foods, perfumes, and the dye industry, benzaldehyde also exhibits notable pharmacological properties and has even been described as a bee repellent [12,161].
Studies on C. angolense extracts have shown that Borututu root contains high levels of antioxidant compounds, directly contributing to its pharmacological potential. These findings can be correlated with previous studies on bioactive phytochemicals identified in medicinal plants of the same species and other related species, emphasizing their significant antioxidant activities in the acetone and ethanol extracts of the leaves, bark, and roots [162,163,164,165].
Ultimately, our findings underscore that numerous phytochemicals present in the analyzed extracts exhibit antioxidant activity comparable to quercetin and curcumin. These bioactive compounds not only neutralize free radicals—thereby safeguarding cells and tissues from oxidative damage—but also contribute to the prevention of various chronic diseases [166,167,168]. This robust antioxidant capacity likely underpins the plant’s longstanding traditional use among rural communities in Angola, particularly in the Cuanza Norte region, where its therapeutic benefits are highly valued. Furthermore, our results pave the way for future research into the development of novel, plant-based therapeutic agents that capitalize on these bioactive properties.
3. Materials and Methods
3.1. Sample Collection and Authentication
Roots, barks, and leaves of Cochlospermum angolense Welw ex Oliv were collected in March 2017 in the Kwanza Norte Province, near Ndalatando, Angola, with the consent of local traditional authorities and in accordance with the United Nations Convention on Biodiversity (Figure 2). All samples were gathered during the flowering season (coordinates S9°16.493′, E15°01.203′; altitude: 791 m) (Figure 3). The species was authenticated by botanist Christin Heinze from the University of Dresden, Germany (Vaucher DR 044693). The samples were dried in a location shielded from direct sunlight and stored in sealed bags. A reference sample from each was subsequently deposited at the Laboratory of Fiber Materials and Environmental Technologies (FibEnTech), University of Beira Interior, Covilhã, Portugal.

Figure 2.
Angolan Cochlospermum angolense Welw ex Oliv.
Figure 3.
The topography of Angola, indicating provincial boundaries and region of study.
3.2. Chemicals and Reagents
All chemicals and reagents used in this study were of analytical grade. These included n-hexane, acetone, ethanol, chloroform, ethyl acetate, curcumin, quercetin, dimethyl sulfoxide (DMSO), 2,2-diphenyl-1-picrylhydrazyl (DPPH), sodium nitroprusside dihydrate, phosphoric acid, sulfanilamide, N-(1-naphthyl)ethylenediamine dihydrochloride, sodium phosphate dibasic, potassium chloride, nitro blue tetrazolium dihydrochloride, β-nicotinamide adenine dinucleotide (NADH), phenazine methosulfate, 2-thiobarbituric acid, ferrous sulfate heptahydrate, trichloroacetic acid (TCA), and sodium lauryl sulfate (SDS). All reagents were purchased from Sigma-Aldrich, Steinheim, Germany.
3.3. Samples Preparation and Extraction
Organic hexane extracts were first obtained via Soxhlet extraction. Specifically, 308.97 g of leaves, 544.31 g of bark, and 720.82 g of roots from Cochlospermum angolense were extracted over a 24-h period using approximately 2500 mL of n-hexane. Following extraction, the samples were filtered, and the solvent was removed under vacuum using a rotary evaporator set at 35 °C.
After hexane extraction, the residual plant materials were transferred into 20-L flasks, and acetone was added at a ratio of 25 g of plant material to 100 mL of acetone. The extraction was allowed to proceed for one week. The resulting acetone extracts were then filtered and evaporated under vacuum in a rotary evaporator at 40 °C until dry.
Subsequently, the same plant materials were subjected to ethanol extraction under similar conditions. Ethanol was added at a ratio of 25 g of plant material to 100 mL of ethanol, and the extraction was maintained for one week. After filtration, the ethanol extracts were evaporated under vacuum in a rotary evaporator at 40 °C until dryness.
All organic extracts were then weighed, and the different masses along with their respective yields (calculated by comparing the mass of the extracts to the initial mass of the plant material) are presented in Table 3.

Table 3.
The different masses (g) and yields (%) of the acetone and ethanolic extracts from the leaves, bark, and roots.
3.4. Evaluation of Antioxidant Activity of the Extracts
3.4.1. 2,2-Diphenyl-1-picrylhydrazil Radical (DPPH•)-Scavenging Activity
The determination of the DPPH radical inhibition percentage was carried out following the methodology proposed by [169,170]. In each well of a 96-well microplate, 100 µL of DPPH and 100 µL of extracts, previously dissolved in DMSO, was added at concentrations ranging from 0.48 to 125 µg/mL. Quercetin was used as positive control. The microplate was incubated in the dark at room temperature for 30 min, and the absorbance was measured at a wavelength of 492 nm using an microplate reader (Promega Glo-max®-Multi+ reader, model E9032, Promega, Madison, WI, USA). All experiments were performed in triplicate. The percentage of DPPH radical scavenging was calculated using Equation (1):
where Acontrol is the absorbance of the reaction media without the test sample and Atest is the absorbance in presence of the essential oil or Quercetin. The IC50 values—the concentration of compound required to reduce the DPPH absorbance by 50%—were determined by linear regression analysis of the dose-response data.
3.4.2. Nitric Oxide Radical Assay
The nitric oxide (NO) scavenging activity was determined using a Griess Illosvoy reaction-based assay [171]. Briefly, 50 μL of the extract, 50 μL of a 10 mM sodium nitroprusside solution, and 50 μL of Griess reagent prepared in phosphate-buffered saline (PBS, pH 7.4) were mixed in a 96-well microplate. The extracts were previously dissolved in DMSO and tested at concentrations ranging from 0.48 to 125 μg/mL. The plate was incubated at 25 °C for 90 min, after which the absorbance was measured at 560 nm.
All measurements were performed in triplicate. The NO radical scavenging activity (%) was calculated using Formula (1).
3.4.3. Superoxide Radical Assay
The superoxide anion (O2•−) scavenging activity of extracts was determined using an NADH-PMS system, following the method described by [49], with slight modifications. The assay was conducted at various concentrations of extract or quercetin (0.48 to 125 µg/mL), and absorbance readings were taken at 560 nm using Promega Glo-max®-Multi+ reader (model E9032). The IC50 values obtained were then compared to those for quercetin. All samples were tested in triplicate. The percentage of O2•− activity was calculated using the same formula applied for the DPPH radical inhibition assay.
3.4.4. Lipid Peroxidation Inhibition Assay
A modified thiobarbituric acid reactive substances (TBARS) assay [172] was employed to measure lipid peroxidation, using egg yolk homogenates as the lipid-rich medium. In this adapted method, 100 μL of egg homogenate (diluted 1:25 v/v in PBS, pH 7.4) and 10 μL of extract or positive control (curcumin) at various concentrations (0.48 to 125 µg/mL) were added to a test tube along with 25 mmol/L of freshly prepared homogenate. The mixture was incubated at 37 °C for 15 min. The reaction was terminated by adding 50 μL of 15% (w/v) trichloroacetic acid (TCA), followed by centrifugation at 3500 rpm for 15 min. A 200 μL aliquot of the supernatant was mixed with 100 μL of 0.8% thiobarbituric acid (TBA) in 1.1% sodium dodecyl sulfate (SDS) and heated in a water bath at 95 °C for 30 min. After cooling, the absorbance of each sample was measured at 532 nm to quantify the MDA levels. The percentage inhibition of lipid peroxidation was calculated using Equation (2).
where Acontrol is the absorbance of an egg yolk emulsion in a blank buffer without the test sample and Atest is the absorbance of the egg yolk emulsion containing either the extracts or the standard substance (curcumin).
3.5. Profiling of Chemical Compounds by High-Performance Liquid Chromatography with Electrospray Ionization and Tandem Mass Spectrometry Detection (HPLC-ESI-MS-MS)
The analysis of phenolic compounds, including flavonoids (e.g., flavanones, glycosides, and methoxyflavones), coumarins, and phenols, as well as non-phenolic compounds such as alkaloids, terpenoids (acyclic monoterpenoids and sesquiterpenes), polyketides, benzoquinones, aromatic esters, and ascorbic acid derivatives, was carried out according to the method of Lahlou et al. [30], on an or-bitrap Thermo q-Exactive (Thermo Fisher Scientific, Waltham, MA, USA) mass spectrometer coupled to a Vanquish HPLC. A Kinetex XB-C18 (Phenomenex, Aschaffenburg, Germany) with a particle size of 2.6 microns, 100 mm in length, and a diameter of 2.1 mm was used as a column. The mobile phases were 0.1% formic aqueous solution (A) and acetonitrile (B). The gradient program (time (min), % B) was as follows: (0.00, 50); (20.00, 100); (25.00, 100); (35.00, 50). The flow rate was 0.200 mL min−1, and the injection volume was 10 µL. The ionization electrospray in positive mode was used. The following analysis parameters were electrospray voltage, −3.8 kV, sheath gas flow rate, 30; auxiliary gas unit flow rate, 10; drying gas temperature, 310 °C; capillary temperature, 320 °C; S-lens and RF level, 55. The acquisition was performed in a mass range from 100 to 1000 a.m.u. An auto MS2 program was used with a fragmentation voltage of 30 V.
3.6. Statistical Analysis
All determinations were made in triplicate, and values were expressed as mean ± standard deviation (SD). Statistical differences were determined by analysis of variance (ANOVA) and the least significant difference (LSD) test, with a p < 0.05 for comparison of means in each of the variables analyzed in the different trials. All statistical analyses were performed with GraphPad 9.0 software.
4. Conclusions
Cochlospermum angolense Welw ex Oliv contains a broad array of bioactive phytochemicals—including flavonoids, phenols, sesquiterpenoids, quinones, and alkaloids—that underpin the significant antioxidant activity observed in this study. These compounds play a crucial role in neutralizing free radicals and alleviating oxidative stress, thereby supporting the plant’s traditional use in Angolan medicine. In practice, various formulations such as infusions, pills, and syrups made from the leaves, bark, and roots are employed to harness these antioxidant, antimicrobial, and anti-inflammatory properties, particularly in the treatment of liver diseases.
Our research further expands the phytochemical profile of C. angolense by identifying several novel compounds, including (+)-abscisic acid, angustine B, pinobanksin, dihydrogenistein, (−)-8-prenylnaringenin, isoquercetin, samandarine, dihydromyricetin, and eupatoriocromene. The discovery of these compounds not only enhances our understanding of the plant’s chemical complexity but also suggests potential new avenues for therapeutic application. The high concentrations of flavonoids, phenols, and terpenes contribute to its robust antioxidant capacity, making C. angolense a promising candidate for development into conventional pharmaceutical agents.
Moreover, with this work, the use of traditional medicinal knowledge is complemented with scientific research results, providing a strong base for further research. The potent bioactivities observed in our extracts provide a compelling rationale for future studies. The objectives were accomplished; nevertheless, in future work, some topics may be addressed. Studies using in vivo models, and comprehensive toxicity testing, along with the optimal dosage for effectiveness, may be established for the extrapolation of the results to therapeutic applications. The exact mechanisms of action of the active compounds also remain unknown, as do the possible synergistic or antagonistic interactions among the phytochemicals present in the extracts. Future research should focus on exploring in vivo studies, toxicity, and the synergistic interactions among these phytochemicals, as well as their potential roles in the prevention or mitigation of chronic diseases associated with oxidative stress. Overall, our study lays a solid foundation for the continued exploration of C. angolense as a valuable resource for novel therapeutic agents in conventional medicine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30132768/s1.
Author Contributions
Conceptualization, N.S.; Chemical analysis methodology, J.M.R., N.S. and C.R.; Validation, J.M.R. and C.R.; Evaluation of the antioxidant activity, N.S., A.M.B. and E.G.D.L.; Investigation, N.S.; Writing—original draft preparation, N.S., A.M.R. and R.A.L.; References and statistics, N.S. and R.A.L.; Writing—review and editing, N.S., R.A.L., J.C., J.M.R., A.M.R. and L.S.; Supervision, J.M.R., J.C., D.D. and L.S.; Project administration, J.M.R., J.C. and L.S.; Funding acquisition, J.M.R. and D.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are also very grateful for the support granted by the Research Unit of Fiber Materials and Environmental Technologies (FibEnTech-UBI), through the Project reference UIDB/00195/2020, funded by the Fundação para a Ciência e a Tecnologia, IP/MCTES through national funds (PIDDAC) and DOI: 10.54499/UIDB/00195/2020 (https://doi.org/10.54499/UIDB/00195/2020). The support of the Chemistry Dep., Faculty of Science, and Fiber Materials and Environmental Technologies Research Unit (FibEnTech-UBI), University of Beira Interior, Portugal. The NMR spectrometers are part of the National NMR Facility supported by FCT-Portugal (ROTEIRO/0031/2013-PINFRA/22161/2016, cofinanced by FEDER through COMPETE 2020, POCI and PORL and FCT through PIDDAC). Ministerio de Ciencia e Innovación (PID2020-118303GB-I00/MCIN/AEI/10.13039/501100011033 and AMR for FPU fellowship), Junta de Castilla y León (SA076P20) and Universidad de Salamanca (Programa I 2025), Sistema Nacional de Investigación, SENACYT, Panamá (SNI-EGDL).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors are grateful for the Research Unit of Fiber Materials and Environmental Technologies (FibEnTech-UBI) through the Project reference UIDB/00195/2020, funded by the Fundação para a Ciência e a Tecnologia, IP/MCTES through national funds (PIDDAC) and DOI: 10.54499/UIDB/00195/2020 (https://doi.org/10.54499/UIDB/00195/2020) and Universidad de Salamanca (Programa propio I).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huntley, B.J.; Russo, V.; Lages, F.; Ferrand, N. Biodiversity of Angola: Science & Conservation: A Modern Synthesis; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Huntley, B.J. Ecology of Angola; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar]
- Almeida, M.Z.d. Plantas Medicinais Mara Zélia de Almeida, 3rd ed.; EDUFBA (Editora da Universidade Federal da Bahia): Salvador, Brasil, 2011. [Google Scholar]
- Neto, F.G.G.; Almeida, G.S.S.A.; Jesus, N.G.; Fonseca, M.R. Estudo Etnobotânico de plantas medicinais utilizadas pela Comunidade do Sisal no município de Catu, Bahia, Brasil. Rev. Bras. de Plantas Med. 2014, 16, 856–865. [Google Scholar] [CrossRef]
- Amaral, F.M.M.; Ribeiro, M.N.S.; Barbosa-Filho, J.M.; Reis, A.S.; Nascimento, F.R.F.; Macedo, R.O. Plants and chemical constituents with giardicidal activity. Rev. Bras. Farmacogn. 2006, 16, 696–720. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and Antimicrobial Activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. Essential Oils and Their Major Monoterpene Alcohols Alone and in Combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef] [PubMed]
- Haidara, M.; Bourdy, G.; De Tommasi, N.; Braca, A.; Traore, K.; Giani, S.; Sanogo, R. Medicinal plants used in Mali for the treatment of malaria and liver diseases. Nat. Prod. Commun. 2016, 11, 339–352. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Frías-Moreno, M.N.; Parra-Quezada, R.A.; González-Aguilar, G.; Ruíz-Canizales, J.; Molina-Corral, F.J.; Sepulveda, D.R.; Salas-Salazar, N.; Olivas, G.I. Quality, bioactive compounds, antioxidant capacity, and enzymes of raspberries at different maturity stages, effects of organic vs. Conventional fertilization. Foods 2021, 10, 953. [Google Scholar] [CrossRef]
- Canga, I.L.V.; Vita, P.; De Oliveira, A.I.d.F.T.; De Pinho, C.M.L.; González, M.Á.C. Ethnopharmacological study of medicinal plants from the province of Cuanza Norte (Angola). Rev. Context. Saúde 2022, 22, e13336. [Google Scholar] [CrossRef]
- Abourashed, E.A.; Fu, H.W. Hydroxybenzoic acids are significant contributors to the antioxidant effect of borututu bark, Cochlospermum angolensis Welw. ex Oliv. Antioxidants 2017, 6, 9. [Google Scholar] [CrossRef]
- Gebashe, F.; Aremu, A.O.; Van Staden, J.; Gruz, J.; Finnie, J.F. Phytochemical profiles and antioxidant activity of grasses used in South African traditional medicine. Plants 2020, 9, 371. [Google Scholar] [CrossRef]
- De Menezes Filho, A.C.P.; de Sousa, W.C.; Christofoli, M.; de Castro, C.F.S. Perfil químico e atividades antioxidante e antifúngica do óleo essencial da flor de Cochlospermum regium (Mart. ex Schrank.)–Pilg. (Bixaceae). Colloq. Agrar. 2020, 16, 89–101. [Google Scholar] [CrossRef]
- Arunachalam, K.; Damazo, A.S.; Pavan, E.; Oliveira, D.M.; Figueiredo, F.d.F.; Machado, M.T.M.; Balogun, S.O.; Soares, I.M.; Barbosa, R.d.S.; Alvim, T.d.C.; et al. Cochlospermum regium (Mart. ex Schrank) Pilg.: Evaluation of chemical profile, gastroprotective activity and mechanism of action of hydroethanolic extract of its xylopodium in acute and chronic experimental models. J. Ethnopharmacol. 2019, 233, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Ezeja, M.I.; Anaga, A.O. Anti-ulcerogenic activity of the methanol root bark extract of Cochlospermum planchonii (Hook f). Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Gulumian, M.; Yahaya, E.S.; Steenkamp, V. African Herbal Remedies with Antioxidant Activity: A Potential Resource Base for Wound Treatment. Evid. Based Complement. Altern. Med. 2018, 2018, 4089541. [Google Scholar] [CrossRef]
- Aguilar-Guadarrama, A.B.; Rios, M.Y. Flavonoids, Sterols and Lignans from Cochlospermum vitifolium and Their Relationship with Its Liver Activity. Molecules 2018, 23, 1952. [Google Scholar] [CrossRef]
- De Miranda Pedroso, T.F.; Bonamigo, T.R.; da Silva, J.; Vasconcelos, P.; Félix, J.M.; Cardoso, C.A.L.; Souza, R.I.C.; dos Santos, A.C.; Volobuff, C.R.F.; Formagio, A.S.N.; et al. Chemical constituents of Cochlospermum regium (Schrank) Pilg. root and its antioxidant, antidiabetic, antiglycation, and anticholinesterase effects in Wistar rats. Biomed. Pharmacother. 2019, 111, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Thanzami, K.; Malsawmtluangi, C.; Lalhlenmawia, H.; Seelan, T.V.; Palanisamy, S.; Kandasamy, R.; Pachuau, L. Characterization and in vitro antioxidant activity of Albizia stipulata Boiv. gum exudates. Int. J. Biol. Macromol. 2015, 80, 231–239. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Alves, M.J.; Pereira, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profile and antimicrobial activity of different dietary supplements based on Cochlospermum angolensis Welw. Ind. Crops Prod. 2015, 74, 412–416. [Google Scholar] [CrossRef]
- Carvalho, F.; Lahlou, R.A.; Silva, L.R. Phenolic Compounds from Cherries and Berries for Chronic Disease Management and Cardiovascular Risk Reduction. Nutrients 2024, 16, 1597. [Google Scholar] [CrossRef]
- Inácio, M.C.; Paz, T.A.; Bertoni, B.W.; Vieira, M.A.R.; Marques, M.O.M.; Pereira, A.M.S. Histochemical investigation of Cochlospermum regium (Schrank) Pilg. leaves and chemical composition of its essential oil. Nat. Prod. Res. 2014, 28, 727–731. [Google Scholar] [CrossRef]
- Pereira, C.; Barreira, J.C.M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Barros, L.; Ferreira, I.C.F.R. New insights into the effects of formulation type and compositional mixtures on the antioxidant and cytotoxic activities of dietary supplements based-on hepatoprotective plants. Food Funct. 2014, 5, 2052–2060. [Google Scholar] [CrossRef][Green Version]
- Zhen, J.; Guo, Y.; Villani, T.; Carr, S.; Brendler, T.; Mumbengegwi, D.R.; Kong, A.N.T.; Simon, J.E.; Wu, Q. Phytochemical analysis and anti-inflammatory activity of the extracts of the african medicinal plant Ximenia caffra. J. Anal. Methods Chem. 2015, 2015, 948262. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar] [CrossRef]
- Ferrera, T.S.; Heldwein, A.B.; Dos Santos, C.O.; Somavilla, J.C.; Sautter, C.K. Substâncias fenólicas, flavonoides e capacidade antioxidante em erveiras sob diferentes coberturas do solo e sombreamentos. Rev. Bras. Plantas Med. 2016, 18, 588–596. [Google Scholar] [CrossRef]
- Koffi, J.A.; Silué, K.D.; Tano, D.K.; Dable, T.M.; Yavo, W. Evaluation of antiplasmodial activity of extracts from endemic medicinal plants used to treat malaria in Côte d’Ivoire. BioImpacts 2020, 10, 151–157. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Polat, D.Ç.; Şafak, E.K.; Yıldız, G.; Küpeli, A.; Selenkol, E.; Sobarzo-Sánchez, E. Phytochemical Composition and Biological Activities of Arctium minus (Hill) Bernh.: A Potential Candidate as Antioxidant, Enzyme Inhibitor, and Cytotoxic Agent. Antioxidants 2022, 11, 1852. [Google Scholar] [CrossRef]
- Hamrouni, H.; Othman, K.B.; Benmoussa, H.; Idoudi, S.; Najjaa, H.; Neffati, M.; Elfalleh, W. Phenolic Profiling, Antioxidant, and Antibacterial Activities of Selected Medicinal Plants from Tunisia. Chem. Biodivers. 2023, 20, 2–10. [Google Scholar] [CrossRef]
- Lahlou, R.A.; Gonçalves, A.C.; Bounechada, M.; Nunes, A.R.; Soeiro, P.; Alves, G.; Moreno, D.A.; Garcia-Viguera, C.; Raposo, C.; Silvestre, S.; et al. Antioxidant, Phytochemical, and Pharmacological Properties of Algerian Mentha aquatica Extracts. Antioxidants 2024, 13, 1512. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Li, Q.; Dai, H.; Zhou, Q.; Meng, X.; Chen, Z.; Zhang, X.; Zhang, Z.; Liu, Y.; et al. Metabolomic Profiling Using UPLC–MS/MS Revealed Differential Changes in Metabolites of Three Tea Plant Varieties and Their Potential Antioxidant Activities. J. Food Biochem. 2024, 2024, 1–14. [Google Scholar] [CrossRef]
- Lahlou, R.A.; Carvalho, F.; Pereira, M.J.; Lopes, J.; Silva, L.R. Overview of Ethnobotanical–Pharmacological Studies Carried Out on Medicinal Plants from the Serra da Estrela Natural Park: Focus on Their Antidiabetic Potential. Pharmaceutics 2024, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Guo, Z.; Bi, X.; Zhao, Y. Polyphenolic profile as well as anti-oxidant and anti-diabetes effects of extracts from freeze-dried black raspberries. J. Funct. Foods. 2017, 31, 179–187. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- de Oliveira Galvão, F.; da Silva Dantas, F.G.; de Lima Santos, C.R.; Marchioro, S.B.; Cardoso, C.A.L.; Wender, H.; Sangalli, A.; de Almeida-Apolonio, A.A.; de Oliveira, K.M.P. Cochlospermum regium (Schrank) pilger leaf extract inhibit methicillin-resistant Staphylococcus aureus biofilm formation. J. Ethnopharmacol. 2020, 261, 113167. [Google Scholar] [CrossRef]
- Pereira, C.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties, anti-hepatocellular carcinoma activity and hepatotoxicity of artichoke, milk thistle and borututu. Ind. Crops Prod. 2013, 49, 61–65. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Bhat, R. The sorbus spp.—Underutilised plants for foods and nutraceuticals: Review on polyphenolic phytochemicals and antioxidant potential. Antioxidants 2020, 9, 813. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Anumula, M.; Mondi, S.R.; Gottumukkula, K.M. A Review on Cochlospermum gossypium. Int. J. Pharm. Biol. Sci. 2019, 9, 374–377. [Google Scholar]
- Calderaro, A.; Patanè, G.T.; Tellone, E.; Barreca, D.; Ficarra, S.; Misiti, F.; Laganà, G. The Neuroprotective Potentiality of Flavonoids on Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 14835. [Google Scholar] [CrossRef]
- Mahendra, C.; Murali, M.; Manasa, G.; Ponnamma, P.; Abhilash, M.R.; Lakshmeesha, T.R.; Satish, A.; Amruthesh, K.N.; Sudarshana, M.S. Antibacterial and antimitotic potential of bio-fabricated zinc oxide nanoparticles of Cochlospermum religiosum (L.). Microb. Pathog. 2017, 110, 620–629. [Google Scholar] [CrossRef]
- Cornélio Favarin, D.; Robison De Oliveira, J.; Jose Freire De Oliveira, C.; De Paula Rogerio, A. Potential effects of medicinal plants and secondary metabolites on acute lung injury. Biomed Res. Int. 2013, 2013, 17–23. [Google Scholar] [CrossRef]
- De Sá, M.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Han, L.; Sun, B.S. Extraction yields and anti-oxidant activity of proanthocyanidins from different parts of grape pomace: Effect of mechanical treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Apolonio, A.A.; Cupozak-Pinheiro, W.J.; Berres, V.M.; Dantas, F.G.S.; Svidzinski, T.I.E.; Oliveira, K.M.P.; Chang, M.R. Control of Cryptococcus Gattii Biofilms by an Ethanolic Extract of Cochlospermum Regium (Schrank) Pilger Leaves. Sci. World, J. 2018, 2018, 5764187. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, V.V.; dos Santos Ramos, A.A.; da Silva, B.B.; Bauab, M.M. Antimicrobial activity of natural products against Helicobacter pylori: A review. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 54. [Google Scholar] [CrossRef]
- Leme, D.E.M.; Rodrigues, A.B.; De Almeida-Apolonio, A.A.; Dantas, F.G.D.S.; Negri, M.F.N.; Svidzinski, T.I.E.; Mota, J.D.S.; Cardoso, C.A.L.; Oliveira, K.M.P. De In Vitro Control of Uropathogenic Microorganisms with the Ethanolic Extract from the Leaves of Cochlospermum regium (Schrank) Pilger. Evid. Based Complement. Altern. Med. 2017, 2017, 4687154. [Google Scholar] [CrossRef]
- Sousa, C.M.D.M.; Silva, H.R.E.; Vieira, G.M.; Ayres, M.C.C.; Da Costa, C.L.S.; Araújo, D.S.; Cavalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.D.M.; Brandão, M.S.; et al. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim. Nova 2007, 30, 351–355. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Ellagic Acid and Derivatives from Cochlospermum angolensis Welw. Extracts: HPLC–DAD–ESI/MS n Profiling, Quantification and In Vitro Anti-depressant, Anti-cholinesterase and Anti-oxidant Activities. Phytochem. Anal. 2013, 24, 534–540. [Google Scholar] [CrossRef]
- Köksal, E.; Tohma, H.; Kılıç, Ö.; Alan, Y.; Aras, A.; Gülçin, I.; Bursal, E. Assessment of antimicrobial and antioxidant activities of nepeta trachonitica: Analysis of its phenolic compounds using HPLC-MS/MS. Sci. Pharm. 2017, 85, 24. [Google Scholar] [CrossRef]
- Reis Mansur, M.C.P.P.; Leitão, S.G.; Cerqueira-Coutinho, C.; Vermelho, A.B.; Silva, R.S.; Presgrave, O.A.F.; Leitão, Á.A.C.; Leitão, G.G.; Ricci-Júnior, E.; Santos, E.P. In vitro and in vivo evaluation of efficacy and safety of photoprotective formulations containing antioxidant extracts. Braz. J. Pharmacogn. 2016, 26, 251–258. [Google Scholar] [CrossRef]
- Carvalho, R.S.; Carollo, C.A.; de Magalhães, J.C.; Palumbo, J.M.C.; Boaretto, A.G.; Nunes e Sá, I.C.; Ferraz, A.C.; Lima, W.G.; de Siqueira, J.M.; Ferreira, J.M.S. Antibacterial and antifungal activities of phenolic compound-enriched ethyl acetate fraction from Cochlospermum regium (mart. Et. Schr.) Pilger roots: Mechanisms of action and synergism with tannin and gallic acid. S. Afr. J. Bot. 2018, 114, 181–187. [Google Scholar] [CrossRef]
- Tchicaillat-Landou, M.; Petit, J.; Gaiani, C.; Miabangana, E.S.; Kimbonguila, A.; Nzikou, J.M.; Scher, J.; Matos, L. Ethnobotanical study of medicinal plants used by traditional healers for the treatment of oxidative stress-related diseases in the Congo Basin. J. Herb. Med. 2018, 13, 76–90. [Google Scholar] [CrossRef]
- Presber, W.; Hegenscheid, B.; Friedmann-Alvermann, B.; Dorge, S.; Voigt, G.; Hiller, K.; Hils, J.; May, A.; Bothig, B. Antiviral activity of extracts from Cochlospermum angolense Welw. Pharmazie 1987, 42, 707–708. [Google Scholar]
- Presber, W.; Herrmann, D.K.; Hegenscheid, B. The effect of an extract from Cochlospermum angolense (“Burututu”) on Plasmodium berghei in the mouse malaria suppression test. Angew. Parasitol. 1991, 32, 7–9. [Google Scholar] [PubMed]
- Presber, W.; Hegenscheid, B.; Hernandez-Alvarez, H.; Herrmann, D.; Brendel, C. Inhibition of the growth of Plasmodium falciparum and Plasmodium berghei in vitro by an extract of Cochlospermum angolense (Welw.). Acta Trop. 1992, 50, 331–338. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.P.; Da Silva, T.L.T.; De Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Granato, D.; Koot, A.; Schnitzler, E.; van Ruth, S.M. Authentication of geographical origin and crop system of grape juices by phenolic compounds and antioxidant activity using chemometrics. J. Food Sci. 2015, 80, C584–C593. [Google Scholar] [CrossRef] [PubMed]
- Tchacondo, T.; Karou, S.D.; Batawila, K.; Agban, A.; Ouro-Bang’na, K.; Anani, K.T.; Gbeassor, M.; de Souza, C. Herbal remedies and their adverse effects in Tem tribe traditional medicine in Togo. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 45–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Çekiç, Ç.; Özgen, M. Comparison of antioxidant capacity and phytochemical properties of wild and cultivated red raspberries (Rubus idaeus L.). J. Food Compos. Anal. 2010, 23, 540–544. [Google Scholar] [CrossRef]
- Chidewe, C.K.; Chirukamare, P.; Nyanga, L.K.; Zvidzai, C.J.; Chitindingu, K. Phytochemical Constituents and the Effect of Processing on Antioxidant Properties of Seeds of an Underutilized Wild Legume B auhinia P etersiana. J. Food Biochem. 2016, 40, 326–334. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Jatau, A.I.; Khalid, G.M.; Alshargi, O.Y. Traditional uses, phytochemistry, and pharmacological activities of Cochlospermum tinctorium A. Rich (Cochlospermaceae): A review. Futur. J. Pharm. Sci. 2021, 7, 20. [Google Scholar] [CrossRef]
- Abubakar, I.B.; Malami, I.; Muhammad, A.; Salihu Shinkafi, T.; Shehu, D.; Maduabuchi Aja, P. A review of the medicinal uses and biological activity of Piliostigma thonningii (Schum). Milne-Redh. RPS Pharm. Pharmacol. Rep. 2024, 3, rqae004. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C.F.R. Analytical Tools Used to Distinguish Chemical Profiles of Plants Widely Consumed as Infusions and Dietary Supplements: Artichoke, Milk Thistle, and Borututu. Food Anal. Methods. 2014, 7, 1604–1611. [Google Scholar] [CrossRef]
- Anaga, A.O.; Oparah, N. Investigation of the methanol root extract of Cochlospermum planchonii for pharmacological activities in vitro and in vivo. Pharm. Biol. 2009, 47, 1027–1034. [Google Scholar] [CrossRef]
- Ahmed, T.S.; Magaji, M.G.; Yaro, A.H.; Musa, A.M.; Adamu, A.K. Aqueous methanol extracts of Cochlospermum tinctorium (A. Rich) possess analgesic and anti-inflammatory activities. J. Young Pharm. 2011, 3, 237–242. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Da Mota, R.V.; Cordenunsi, B.R.; Lajolo, F.M. Physico-chemical characterization and bioactive compounds of blackberry fruits (Rubus sp.) grown in Brazil. Cienc. Tecnol. Aliment. 2008, 28, 702–708. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Sabarathinam, S. Unraveling the therapeutic potential of quercetin and quercetin-3-O-glucuronide in Alzheimer’s disease through network pharmacology, molecular docking, and dynamic simulations. Sci. Rep. 2024, 14, 14852. [Google Scholar] [CrossRef] [PubMed]
- Ez Zoubi, Y.; Bousta, D.; Farah, A. A Phytopharmacological review of a Mediterranean plant: Lavandula stoechas L. Clin. Phytoscience 2020, 6, 9. [Google Scholar] [CrossRef]
- Dankwa, B.; Broni, E.; Enninful, K.S.; Kwofie, S.K.; Wilson, M.D. Consensus docking and MM-PBSA computations identify putative furin protease inhibitors for developing potential therapeutics against COVID-19. Struct. Chem. 2022, 33, 2221–2241. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Lee, J.J.; Kim, I.S.; Kim, Y.; Myung, C.S. Stimulation of glucose uptake and improvement of insulin resistance by aromadendrin. Pharmacology 2011, 88, 266–274. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Tolba, M.F.; Karonen, M. Antioxidant and hepatoprotective activities of flavonoids from Bauhinia hookeri. Rec. Nat. Prod. 2016, 10, 812–817. [Google Scholar]
- Chahal, A.; Saini, A.K.; Chhillar, A.K.; Saini, R.V. Natural antioxidants as defense system against cancer. Asian J. Pharm. Clin. Res. 2018, 11, 38–44. [Google Scholar] [CrossRef]
- Mezghani, I.; Bargougui, K.; Salah, H.B.; Frikha, D.; Zouari, N.; Chaieb, M. LC-ESI-MS characterization, antioxidant and antibacterial properties of various solvent extracts from Tricholaena teneriffae L. S. Afr. J. Bot. 2021, 143, 116–122. [Google Scholar] [CrossRef]
- Manga, H.M.; Brkic, D.; Marie, D.E.P.; Quetin-Leclercq, J. In vivo anti-inflammatory activity of Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg. (Euphorbiaceae). J. Ethnopharmacol. 2004, 92, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, O.S.; Kim, B.Y.; Jeong, S.J. Apigetrin from scutellaria baicalensis georgi inhibits neuroinflammation in BV-2 microglia and exerts neuroprotective effect in HT22 hippocampal cells. J. Med. Food. 2016, 19, 1032–1040. [Google Scholar] [CrossRef]
- Farag, M.A.; Sakna, S.T.; El-Fiky, N.M.; Shabana, M.M.; Wessjohann, L.A. Phytochemical, antioxidant and antidiabetic evaluation of eight Bauhinia L. species from Egypt using UHPLC-PDA-qTOF-MS and chemometrics. Phytochemistry 2015, 119, 41–50. [Google Scholar] [CrossRef]
- Cock, I.E.; Van Vuuren, S.F. A review of the traditional use of southern African medicinal plants for the treatment of fungal skin infections. J. Ethnopharmacol. 2020, 251, 112539. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.; Lee, S.J.; Ham, I.; Whang, W.K. Antioxidant activities of new flavonoids from Cudrania tricuspidata root bark. Arch. Pharm. Res. 2009, 32, 195–200. [Google Scholar] [CrossRef]
- Yang, Q.; Huan, R.; Meng, D.; Qi, J.; Xia, L. Progress in the study of anti-tumor effects and mechanisms of vitexin. Pharmacol. Rep. 2024, 77, 124–134. [Google Scholar] [CrossRef]
- Ranjan, R.; Kishore, K.; Ranjan, R.; Sheikh, T.J.; Jha, A.K.; Ojha, B.K.; Kumar, S.; Kumar, R. Nutraceutical Potential of Vitexin: A Flavone Glycoside. J. Phytopharm. 2023, 12, 44–50. [Google Scholar] [CrossRef]
- Yu, P.R.; Tseng, C.Y.; Hsu, C.C.; Chen, J.H.; Lin, H.H. In vitro and in vivo protective potential of quercetin-3-glucuronide against lipopolysaccharide-induced pulmonary injury through dual activation of nuclear factor-erythroid 2 related factor 2 and autophagy. Arch. Toxicol. 2024, 98, 1415–1436. [Google Scholar] [CrossRef]
- Mottaghi, S.; Abbaszadeh, H. The anticarcinogenic and anticancer effects of the dietary flavonoid, morin: Current status, challenges, and future perspectives. Phyther. Res. 2021, 35, 6843–6861. [Google Scholar] [CrossRef]
- Hong, D.G.; Lee, S.; Kim, J.; Yang, S.; Lee, M.; Ahn, J.; Lee, H.; Chang, S.-C.; Ha, N.-C.; Lee, J. Anti-Inflammatory and Neuroprotective Effects of Morin in an MPTP-Induced Parkinson’s Disease Model. Int. J. Mol. Sci. 2022, 23, 10578. [Google Scholar] [CrossRef] [PubMed]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Lauria, G.; Aiello, F.; Carullo, G.; Curcio, R.; Fiorillo, M.; Campiani, G.; Dolce, V.; Cappello, A.R. Exploiting Glycyrrhiza glabra L. (Licorice) Flavanones: Licoflavanone’s Impact on Breast Cancer Cell Bioenergetics. Int. J. Mol. Sci. 2024, 25, 7907. [Google Scholar] [CrossRef] [PubMed]
- Orallo, F.; Camiña, M.; Álvarez, E.; Basaran, H.; Lugnier, C. Implication of cyclic nucleotide phosphodiesterase inhibition in the vasorelaxant activity of the citrus-fruits flavonoid (±)-naringenin. Planta Med. 2005, 71, 99–107. [Google Scholar] [CrossRef]
- Sánchez-Salgado, J.C.; Estrada-Soto, S.; García-Jiménez, S.; Montes, S.; Gómez-Zamudio, J.; Villalobos-Molina, R. Analysis of flavonoids bioactivity for cholestatic liver disease: Systematic literature search and experimental approaches. Biomolecules 2019, 9, 102. [Google Scholar] [CrossRef]
- Martínez-Rodriguez, L.; Murguía-Hernández, K.; García-Juárez, I.; Uribe-Esquivel, M.; Gómez-Reyes, E. La historia oscura de la rosa amarilla: Un reporte de caso de toxicidad hepática asociado al consumo de Cochlospermum vitifolium como remedio herbolario. Rev. Gastroenterol. México. 2015, 80, 220–222. [Google Scholar] [CrossRef][Green Version]
- Sanchez-Salgado’a, J.C.; Ortiz-Andradea, R.R.; Aguirre-Crespoa, F.; Vergara-Galiciaa, J.; Leon-Rivera’b, I.S.; Montesc, R.; Villalobos-Molinad, S.E.-S. Cochlospermum 6 word. J. Ethnopharmacol. 2007, 109, 400–405. [Google Scholar]
- Assini, J.M.; Mulvihill, E.E.; Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Chhoker, S.S.; Sawyez, C.G.; Drangova, M.; Adams, A.C.; Kharitonenkov, A.; et al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology 2015, 156, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Deng, Z.; Rong, X.; Li, R.; You, Z.; Guo, X.; Cai, C.; Zhao, Y.; Gao, P.; Cao, G.; et al. Naringenin Prevents Oxidative Stress and Inflammation in LPS-Induced Liver Injury through the Regulation of LncRNA-mRNA in Male Mice. Molecules 2022, 28, 198. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kang, M.W.; Roh, J.H.; Choi, S.U.; Zee, O.P. Cytotoxic phenolic compounds from Chionanthus retusus. Arch. Pharm. Res. 2009, 32, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, N.H.; Kim, J.Y.; Park, J.H.; Shin, S.Y.; Kwon, Y.S.; Lee, H.J.; Kim, S.S.; Chun, W. Aromadendrin inhibits lipopolysaccharide-induced nuclear translocation of NF-κB and phosphorylation of JNK in RAW 264.7 macrophage cells. Biomol. Ther. 2013, 21, 216–221. [Google Scholar] [CrossRef]
- De Almeida, S.C.X.; De Lemos, T.L.G.; Silveira, E.R.; Pessoa, O.D.L. Volatile and non-volatile chemical constituents of Cochlospermum vitifolium (willdenow) sprengel. Quim. Nova 2005, 28, 57–60. [Google Scholar] [CrossRef]
- Zołnierczyk, A.K.; Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Woźniak, E.; Anioł, M. Isoxanthohumol—Biologically active hop flavonoid. Fitoterapia 2015, 103, 71–82. [Google Scholar] [CrossRef]
- Pohjanvirta, R.; Nasri, A. The Potent Phytoestrogen 8-Prenylnaringenin: A Friend or a Foe? Int. J. Mol. Sci. 2022, 23, 3168. [Google Scholar] [CrossRef]
- Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic perspectives of 8-prenylnaringenin, a potent phytoestrogen from hops. Molecules 2018, 23, 660. [Google Scholar] [CrossRef]
- Shirani, M.; Raeisi, R.; Heidari-Soureshjani, S.; Asadi-Samani, M.; Luther, T. A review for discovering hepatoprotective herbal drugs with least side effects on kidney. J. Nephropharmacology. 2017, 6, 38–48. [Google Scholar] [CrossRef]
- Campos, J.F.; de Toledo Espindola, P.P.; Torquato, H.F.V.; Vital, W.D.; Justo, G.Z.; Silva, D.B.; Carollo, C.A.; Souza, K.d.P.; Paredes-Gamero, E.J.; dos Santos, E.L. Leaf and root extracts from Campomanesia adamantium (Myrtaceae) promote apoptotic death of leukemic cells via activation of intracellular calcium and caspase-3. Front. Pharmacol. 2017, 8, 466. [Google Scholar] [CrossRef]
- Ballin, N.Z.; Traore, M.; Tinto, H.; Sittie, A.; Mølgaard, P.; Olsen, C.E.; Kharazmi, A.; Christensen, S.B. Antiplasmodial compounds from Cochlospermum tinctorium. J. Nat. Prod. 2002, 65, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, L.R.; Bae, M.S.; Kim, B.M.; Oh, G.S.; Chai, K.Y. A chalcone glycoside from the fruits of Sorbus commixta Hedl. Molecules 2009, 14, 5323–5327. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tielas, C.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Atividades Biológicas e Novas Aplicações das Chalconas. Planta Daninha. 2016, 34, 607–616. [Google Scholar] [CrossRef]
- Soares, S.E. Phenolic acids as antioxidants. Rev. Nutr. 2002, 15, 71–81. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Morais, A.L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Insights on the formulation of herbal beverages with medicinal claims according with their antioxidant properties. Molecules 2013, 18, 2851–2863. [Google Scholar] [CrossRef]
- De Oliveira, C.B.; Comunello, L.N.; Lunardelli, A.; Amaral, R.H.; Pires, M.G.S.; Da Silva, G.L.; Manfredini, V.; Vargas, C.R.; Gnoatto, S.C.B.; De Oliveira, J.R.; et al. Phenolic enriched extract of Baccharis trimera presents anti-inflammatory and antioxidant activities. Molecules 2012, 17, 1113–1123. [Google Scholar] [CrossRef]
- Hayashi, M.A.; Bizerra, F.C.; Ismael, P.; Silva, D. Antimicrobial compounds from natural sources. Front. Microbiol. 2013, 4, 3389. [Google Scholar] [CrossRef]
- Othman, S.N.N.; Lum, P.T.; Sekar, M.; Mazlan, N.A.; Yusri, P.Z.S.; Ghazali, N.F.; Idi, H.M.; Azman, S.; Ismail, M.; Noor, A.A.M. Molecules of Interest—Embelin—A Review. Res. J. Pharm. Technol. 2020, 13, 3485. [Google Scholar] [CrossRef]
- Joshi, R.; Kamat, J.P.; Mukherjee, T. Free radical scavenging reactions and antioxidant activity of embelin: Biochemical and pulse radiolytic studies. Chem. Biol. Interact. 2007, 167, 125–134. [Google Scholar] [CrossRef]
- Chitra, M.; Shyamala Devi, C.S.; Sukumar, E. Antibacterial activity of embelin. Fitoterapia 2003, 74, 401–403. [Google Scholar] [CrossRef]
- Mahendran, S.; Thippeswamy, B.S.; Veerapur, V.P.; Badami, S. Anticonvulsant activity of embelin isolated from Embelia ribes. Phytomedicine 2011, 18, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Danquah, M. Embelin and Its Derivatives: Design, Synthesis, and Potential Delivery Systems for Cancer Therapy. Pharmaceuticals 2022, 15, 1131. [Google Scholar] [CrossRef]
- DeFilipps, R.A.; Krupnick, G.A. The medicinal plants of Myanmar. PhytoKeys 2018, 102, 1–341. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H. Bin Bioactive compounds and bioactivities of ginger (zingiber officinale roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Hsiang, C.Y.; Lo, H.Y.; Huang, H.C.; Li, C.C.; Wu, S.L.; Ho, T.Y. Ginger extract and zingerone ameliorated trinitrobenzene sulphonic acid-induced colitis in mice via modulation of nuclear factor-κB activity and interleukin-1β signalling pathway. Food Chem. 2013, 136, 170–177. [Google Scholar] [CrossRef]
- Promdam, N.; Panichayupakaranant, P. [6]-Gingerol: A narrative review of its beneficial effect on human health. Food Chem. Adv. 2022, 1, 100043. [Google Scholar] [CrossRef]
- Kerboua, M.; Monia, A.A.; Samba, N.; Silva, L.; Raposo, C.; Díez, D.; Rodilla, J.M. Phytochemical Composition of Lichen Parmotrema hypoleucinum (J. Steiner) Hale from Algeria. Molecules 2022, 27, 5229. [Google Scholar] [CrossRef]
- Zahoor, A.; Yang, C.; Yang, Y.; Guo, Y.; Zhang, T.; Jiang, K.; Guo, S.; Deng, G. 6-Gingerol exerts anti-inflammatory effects and protective properties on LTA-induced mastitis. Phytomedicine 2020, 76, 153248. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Imeneo, M.; Luzza, F. Olive tree biophenols in inflammatory bowel disease: When bitter is better. Int. J. Mol. Sci. 2019, 20, 1390. [Google Scholar] [CrossRef]
- Coppa, C.F.S.C.; Rosim, R.E.; De Oliveira, C.A.F.; Da Costa Rodrigues, C.E.; Gonçalves, C.B. Extração de oleuropeína a partir de folhas de oliveira utilizando solvente hidroalcoólico. Braz. J. Food Technol. 2017, 20, e2016169. [Google Scholar] [CrossRef]
- Otero, D.M.; Oliveira, F.M.; Lorini, A.; da Fonseca Antunes, B.; Oliveira, R.M.; Zambiazi, R.C. Oleuropein: Methods for extraction, purifying and applying. Rev. Ceres 2020, 67, 315–329. [Google Scholar] [CrossRef]
- Al Fares, E.; Sanikidze, T.; Kalmakhelidze, S.; Topuria, D.; Mansi, L.; Kitson, S.; Molazadeh, M. The Alleviating Effect of Herniarin Against Ionizing Radiation-Induced Genotoxicity and Cytotoxicity in Human Peripheral Blood Lymphocytes. Curr. Radiopharm. 2022, 15, 141–147. [Google Scholar] [CrossRef]
- Paulsen, E.; Otkjær, A.; Andersen, K.E. The coumarin herniarin as a sensitizer in German chamomile [Chamomilla recutita (L.) Rauschert, Compositae]. Contact Dermat. 2010, 62, 338–342. [Google Scholar] [CrossRef]
- Salehcheh, M.; Safari, O.; Khodayar, M.J.; Mojiri-Forushani, H.; Cheki, M. The protective effect of herniarin on genotoxicity and apoptosis induced by cisplatin in bone marrow cells of rats. Drug Chem. Toxicol. 2022, 45, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Michel, T.; Nde, C.B.M.; Njuh, A.N.; Cisilotto, J.; Ndinteh, D.T.; Clyne, C.; Fernandez, X.; Creczynski-Pasa, T.B.; Njamen, D. Estrogen-like and tissue-selective effects of 7-methoxycoumarin from Ficus umbellata (Moraceae): An in vitro and in vivo study. BMC Complement. Altern. Med. 2017, 17, 383. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Shah, M.H.; Li, T.; Fu, X.; Guo, X.; Liu, R.H. Ethnomedicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. J. Ethnopharmacol. 2015, 162, 333–345. [Google Scholar] [CrossRef] [PubMed]
- De Assis, C.M.; Moreno, P.R.H.; Young, M.C.M.; De Arruda Campos, I.P.; Suffredini, I.B. Isolation and evaluation of the biological activity related to the major alkaloids in Tabernaemontana angulata Mart. ex Müll. Arg., Apocynaceae. Rev. Bras. Farmacogn. 2009, 19, 626–631. [Google Scholar] [CrossRef]
- Tufariello, J.J.; Ali, S.A. Elaeocarpus Alkaloids. The Synthesis of dl-Elaeokanine-A and dl-Elaeokanine-C. Tetrahedron Lett. 1979, 20, 4445–4448. [Google Scholar] [CrossRef]
- Adams, M.; Mahringer, A.; Kunert, O.; Fricker, G.; Efferth, T.; Bauer, R. Cytotoxicity and P-glycoprotein modulating effects of quinolones and indoloquinazolines from the Chinese herb Evodia rutaecarpa. Planta Med. 2007, 73, 1554–1557. [Google Scholar] [CrossRef]
- Hochfellner, C.; Evangelopoulos, D.; Zloh, M.; Wube, A.; Guzman, J.D.; Mchugh, T.D.; Kunert, O.; Bhakta, S.; Bucar, F. Antagonistic effects of indoloquinazoline alkaloids on antimycobacterial activity of evocarpine. J. Appl. Microbiol. 2015, 118, 864–872. [Google Scholar] [CrossRef]
- Rubio, N.; Moreda-Pineiro, A.; Bermejo Barrera, P.; Bermejo, A. Perfil de alcaloides de la hoja de coca en el fluido oral de un mascador de hoja de coca y un bebedor de té de coca. Acta Toxicológica Argent. 2019, 27, 72–80. [Google Scholar]
- Zhang, L.; Li, X.; Li, T.; Xiong, R.; Li, Y.; Yan, D.; Chen, P. Farnesoic acid methyltransferase 6 (BmFAMeT6) interrelates with moltinism of dominant trimolter in silkworm, Bombyx mori. Biologia 2021, 76, 2231–2240. [Google Scholar] [CrossRef]
- Felipe, L.O.; Bicas, J.L. Terpenos, aromas e a química dos compostos naturais. Química Nov. Na Esc. 2017, 39, 120–130. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Y.; Shang, H.; Luo, Y.; Tian, Y. Ganoderic Acid A and Its Amide Derivatives as Potential Anti-Cancer Agents by Regulating the p53-MDM2 Pathway: Synthesis and Biological Evaluation. Molecules 2023, 28, 2374. [Google Scholar] [CrossRef] [PubMed]
- Solon, S.; Carollo, C.A.; Brandão, L.F.G.; De MacEdo, C.D.S.; Klein, A.; Dias-Junior, C.A.; De Siqueira, J.M. Phenolic derivatives and other chemical compounds from. Quim. Nova 2012, 35, 1169–1172. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Yen, G.-C. Ganoderic Acid and Lucidenic Acid (Triterpenoid). Enzymes. 2014, 36, 33–56. [Google Scholar]
- Ban, N.K.; Giang, V.H.; Linh, T.M.; Lien, L.Q.; Ngoc, N.T.; Thao, D.T.; Nam, N.H.; Cuong, N.X.; Van Kiem, P.; Van Minh, C. Two new 11-noriridoids from the aerial parts of Morinda umbellata. Phytochem. Lett. 2013, 6, 267–269. [Google Scholar] [CrossRef]
- Kouda, R.; Yamamoto, K.; Katsuyama, A.; Ichikawa, S.; Yakushiji, F. Concise Synthesis of 11-Noriridoids via Pauson–Khand Reaction. Chem. Pharm. Bull. 2024, 72, c24-00190. [Google Scholar] [CrossRef]
- Dexter, H.R.; Allen, E.; Williams, D.M. A concise stereoselective synthesis of pterosin B. Tetrahedron Lett. 2018, 59, 4323–4325. [Google Scholar] [CrossRef]
- Yahara, Y.; Takemori, H.; Okada, M.; Kosai, A.; Yamashita, A.; Kobayashi, T.; Fujita, K.; Itoh, Y.; Nakamura, M.; Fuchino, H.; et al. Pterosin B prevents chondrocyte hypertrophy and osteoarthritis in mice by inhibiting Sik3. Nat. Commun. 2016, 7, 10959. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Z.; Chen, J.; Liu, B.; Wu, C.; Li, Y.; Xu, J.; Li, P. Lucialdehyde B suppresses proliferation and induces mitochondria-dependent apoptosis in nasopharyngeal carcinoma CNE2 cells. Pharm. Biol. 2023, 61, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Do Silva, A.N.; Uetanabaro, A.P.T.; Lucchese, A.M. Chemical composition and antibacterial activity of essential oils from Myrcia alagoensis (Myrtaceae). Nat. Prod. Commun. 2013, 8, 269–271. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, P.; Li, J.; Wang, Y.; Li, J.; Chen, P. A farnesoic acid O-methyltransferase (FAMeT) from Exopalaemon carinicauda is responsive to Vibrio anguillarum and WSSV challenge. Cell Stress Chaperones 2014, 19, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Holford, K.C.; Edwards, K.A.; Bendena, W.G.; Tobe, S.S.; Wang, Z.; Borst, D.W. Purification and characterization of a mandibular organ protein from the American lobster, Homarus americanus: A putative farnesoic acid O-methyltransferase. Insect Biochem. Mol. Biol. 2004, 34, 785–798. [Google Scholar] [CrossRef]
- Pires-Moreira, M.; Chan-Poot, S.; Aviles-Berzunza, E.; Garcia-Sosa, K.; Godoy-Hernandez, G.; Pena-Rodriguez, L.M. Influence of the Flowering Stage in the Production of Urechitol a in Agrobacterium Rhizogenes-Transformed Plants of Pentalinon Andrieuxii. Bot. Sci. 2024, 102, 438–446. [Google Scholar] [CrossRef]
- Yam-Puc, A.; Chee-González, L.; Escalante-Erosa, F.; Arunachalampillai, A.; Wendt, O.F.; Sterner, O.; Godoy-Hernández, G.; Peña-Rodríguez, L.M. Steroids from the root extract of Pentalinon andrieuxii. Phytochem. Lett. 2012, 5, 45–48. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A.; et al. Loliolide, a new therapeutic option for neurological diseases? In vitro neuroprotective and anti-inflammatory activities of a monoterpenoid lactone isolated from codium tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef]
- Fontaine, B.M.; Nelson, K.; Lyles, J.T.; Jariwala, P.B.; García-Rodriguez, J.M.; Quave, C.L.; Weinert, E.E. Identification of ellagic acid rhamnoside as a bioactive component of a complex botanical extract with anti-biofilm activity. Front. Microbiol. 2017, 8, 496. [Google Scholar] [CrossRef]
- Rwegoshora, F.; Mabiki, F.; Machumi, F.; Chacha, M.; Styrishave, B.; Cornett, C. A New Ellagic Acid Rhamnoside from Synadenium glaucescens and Its Cytotoxicity Evaluation. Chem. Nat. Compd. 2023, 59, 670–674. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, S.U.; Lee, K.R. Gymnopilin K: A new cytotoxic gymnopilin from Gymnopilus spectabilis. J. Antibiot. 2012, 65, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Laufer, H.; Borst, D.; Baker, F.C.; Carrasco, C.; Sinkus, M.; Reuter, C.C.; Tsai, L.W.; Schooley, D.A. Identification of a juvenile hormone-like compound in a crustacean. Science 1987, 235, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Figueiredo, C.S.S.e.S.; Viera de Oliveira, P.; De Silva Saminez, W.F.; Muniz Diniz, R.; Silva Rodrigues, J.F.; Maia da Silva, M.S.; Nascimento da Silva, L.C.; Grigolin Grisotto, M.A. Óleo essencial da Canela (Cinamaldeído) e suas aplicações biológicas. Rev. Investig. Biomed. 2018, 9, 192. [Google Scholar] [CrossRef][Green Version]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Giovanelli, S.; Cioni, P.L.; Flamini, G.; Pistelli, L. Evaluation of volatile constituents of Cochlospermum angolense. Nat. Prod. Commun. 2012, 7, 629–632. [Google Scholar] [CrossRef]
- Tlhapi, D.; Malebo, N.; Manduna, I.T.; Lautenschläger, T.; Mawunu, M. A Review of Medicinal Plants Used in the Management of Microbial Infections in Angola. Plants 2024, 13, 2991. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Kumar, G.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Mahomoodally, M.F.; Seebaluck-Sandoram, R.; Etienne, O.K.; Zengin, G. An insight into Cochlospermum planchonii extracts obtained by traditional and green extraction methods: Relation between chemical compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 147, 112226. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction—A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Kiendrebeogo, M.; Compaoré, M.; Meda, R.N.T.; Bacher, M.; Koenig, K.; Pacher, T.; Fuehrer, H.P.; Noedl, H.; Willcox, M.; et al. Quality assessment and antiplasmodial activity of West African Cochlospermum species. Phytochemistry 2015, 119, 51–61. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Abudunia, A.M.; Marmouzi, I.; Faouzi, M.E.A.; Ramli, Y.; Taoufik, J.; El Madani, N.; Essassi, E.M.; Salama, A.; Khedid, K.; Ansar, M.; et al. Activité anti-candidose, antibactérienne, cytotoxique et antioxydante des fleurs de Calendula arvensis. J. Mycol. Med. 2017, 27, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Heinze, C.; Dundão, M.D.F.; Neinhuis, C.; Lautenschläger, T. Economic Potential of Selected Native Plants from Cuanza Norte, Northern Angola. Econ. Bot. 2019, 73, 96–111. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant capacity and phenolic contents of some Mediterranean medicinal plants and their potential role in the inhibition of cyclooxygenase-1 and acetylcholinesterase activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Akagi, H.; Matsuo, N.; Suzuki, T. FREE RADICALS AND THE MAIN DIETARY ANTIOXIDANTS. Eisei Kagaku 1989, 35, 123–130. [Google Scholar] [CrossRef]
- Sabir, S.M.; Abbas, S.R.; Shahida, S.; Khan, M.F. In-Vitro antioxidant, anti-lipid peroxidative activities and In-Silico study of Terminalia chebula bioactive compounds. Clin. Phytoscience 2020, 6, 93. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Pombal, S.; Hernández, Y.; Diez, D.; Mondolis, E.; Mero, A.; Morán-Pinzón, J.; Guerrero, E.I.; Rodilla, J.M. Antioxidant Activity of Carvone and Derivatives against Superoxide Ion. Nat. Prod. Commun. 2017, 12, 653–655. [Google Scholar] [CrossRef]
- Tvete Inngjerdingen, K.; Ballo, N.; Zhang, B.-Z.; Malterud, K.E.; Michaelsen, T.E.; Diallo, D.; Smestad Paulsen, B. A comparison of bioactive aqueous extracts and polysaccharide fractions from roots of wild and cultivated Cochlospermum tinctorium A. Rich. Phytochemistry 2013, 93, 136–143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).