Cellulose-Based Scaffolds with Prolonged Dexamethasone Release for Bone Tissue Engineering
Abstract
1. Introduction
2. Results and Discussion
2.1. Cellulose and Cellulose/HAp Scaffolds and Their Structure
2.2. Loading of Dexamethasone Sodium Phosphate into the Scaffolds and Its Release
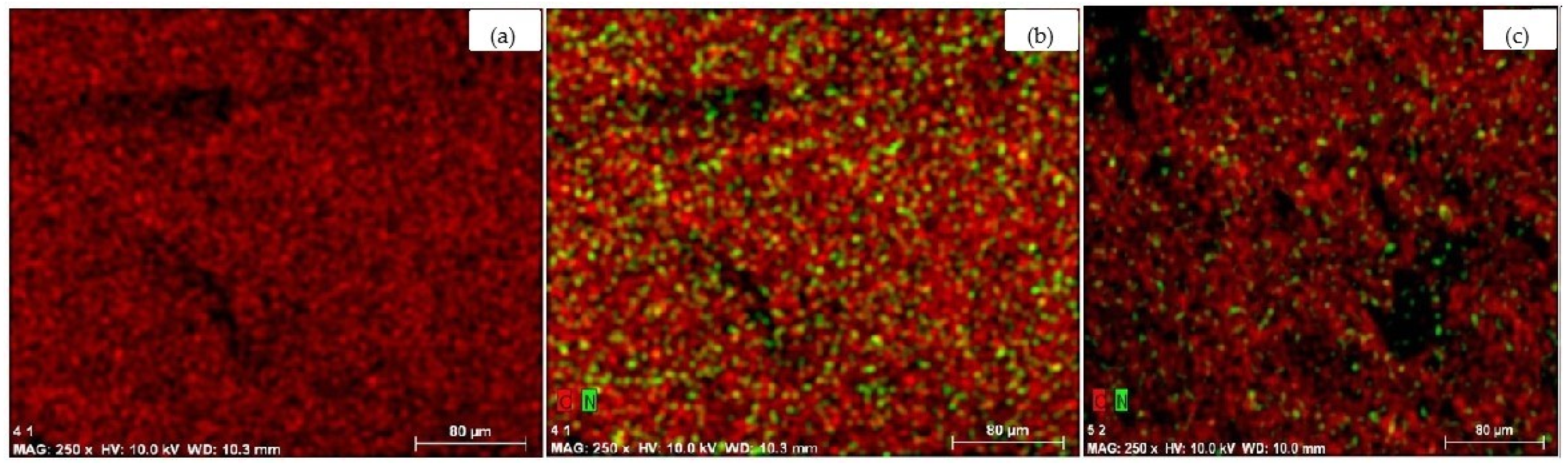
2.3. Amination of the Scaffolds and Their Properties
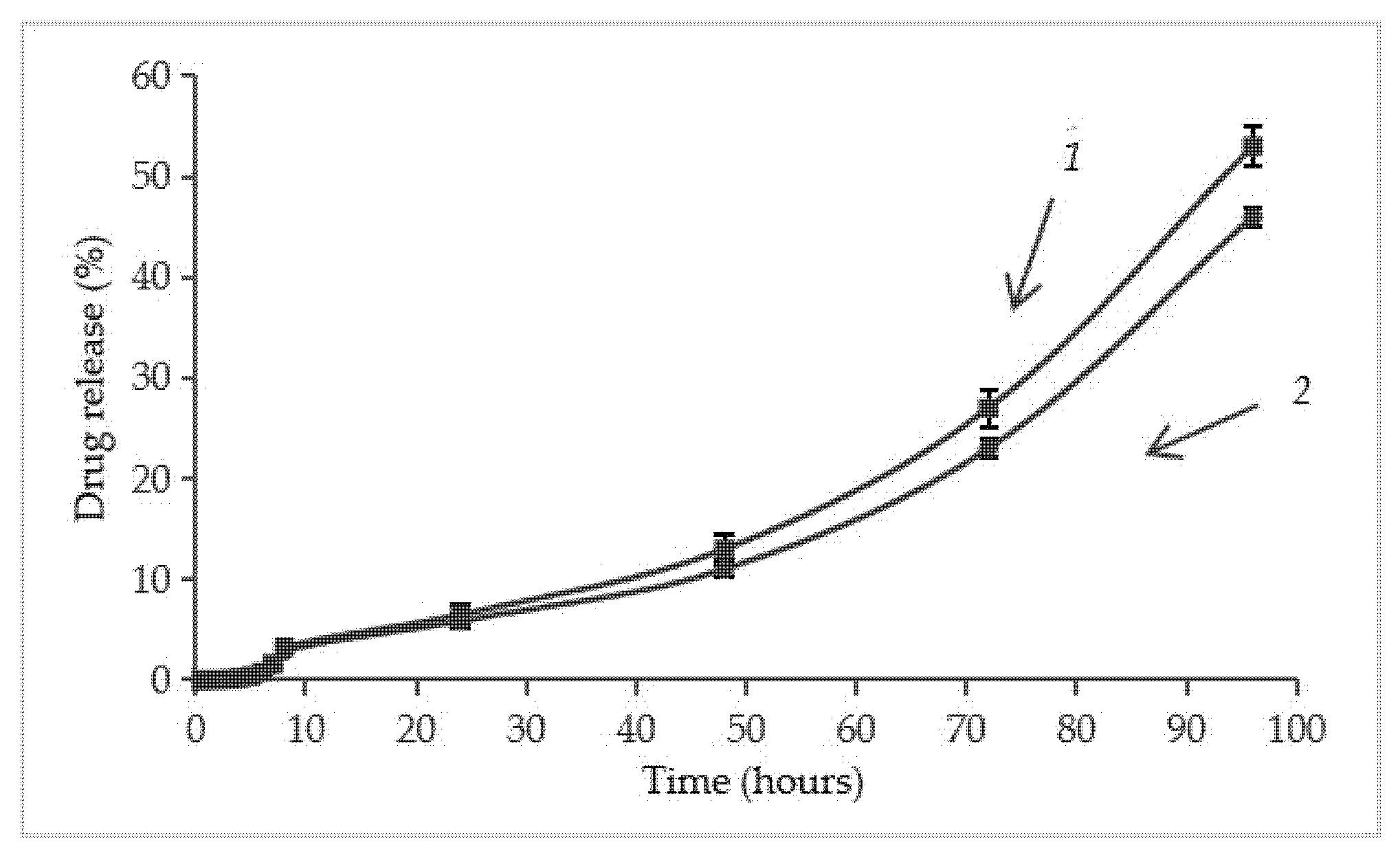
2.4. Immobilization of Dexamethasone Sodium Phosphate into the Aminated Scaffolds and Its Release
3. Materials and Methods
3.1. Materials
3.2. Preparation of Cellulose and Cellulose/HAp Gels
3.3. Preparation of Cellulose and Cellulose/HAp Scaffolds
3.4. Micro-Computed Tomography
3.5. Scanning Electron Microscopy and Electron Dispersive Spectroscopy
3.6. Preparation of the Scaffolds with Amino Groups
3.7. Loading the Scaffolds with Dexamethasone Sodium Phosphate
3.8. Evaluation of Dexamethasone Sodium Phosphate Release
3.9. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HAp | Hydroxyapatite |
| PLLA | Poly(lactic acid) |
| PLGA | Poly(lactic-co-glycolic acid) |
| ClDEAE·HCl | 2-chloro-N,N-diethylethylamine hydrochloride |
References
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Gage, M.; Liporace, F.; Egol, K.; McLaurin, T. Management of Bone Defects in Orthopedic Trauma. Bull. Hosp. Jt. Dis. 2018, 76, 4–8. [Google Scholar] [PubMed]
- Tit, D.M.; Bungau, S.G.; Iovan, C.; Nistor-Cseppento, D.C.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone Tissue Engineering: A Review in Bone Biomimetics and Drug Delivery Strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; Magariños, B.; Ferreira-Gonçalves, T.; Starbird-Pérez, R.; Álvarez-Lorenzo, C.; Reis, C.P.; Ardao, I.; García-González, C.A. Vancomycin-loaded methylcellulose aerogel scaffolds for advanced bone tissue engineering. Carbohydr. Polym. 2024, 324, 121536. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, C.; Li, R.; Yu, D.; Ding, Q. Nanocellulose based hydrogel or aerogel scaffolds for tissue engineering. Cellulose 2021, 28, 7497–7520. [Google Scholar] [CrossRef]
- Palaveniene, A.; Songailiene, K.; Baniukaitiene, O.; Tamburaci, S.; Kimna, C.; Tihminlioğlu, F.; Liesiene, J. The effect of biomimetic coating and cuttlebone microparticle reinforcement on the osteoconductive properties of cellulose-based scaffolds. Int. J. Biol. Macromol. 2020, 152, 1194–1204. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nazemi, Z.; Salehi, A.O.M.; Seyfoori, A.; John, J.V.; Nourbakhsh, M.S.; Akbari, M. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioact. Mater. 2022, 26, 137–163. [Google Scholar] [CrossRef]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef]
- Chen, L.; Tang, C.Y.; Chen, D.Z.; Wong, C.T.; Tsui, C.P. Fabrication and characterization of poly-D-L-lactide/nano-hydroxyapatite composite scaffolds with poly(ethylene glycol) coating and dexamethasone releasing. Compos. Sci. Technol. 2011, 71, 1842–1849. [Google Scholar] [CrossRef]
- Canto, I.; Mckean, R.; Charnley, M.; Blackwood, K.A.; Fiorica, C.; Ryans, A.J.; MacNeil, S. Development of an ibuprofen-releasing biodegradable PLA/PGA electrospun scaffold for tissue regeneration. Biotechnol. Bioeng. 2010, 105, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Bose, S. Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: In vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. Appl. Mater. Interfaces 2014, 6, 9955–9965. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, N.M.; Cheng, H.; Hill, P.S.; Guerreiro, J.D.T.; Dang, T.T.; Ma, M.; Watson, S.; Hwang, N.S.; Langer, R.; Anderson, D.G. Localized delivery of dexamethasone from electrospun fibers reduces the foreign body response. Biomacromolecules 2012, 13, 3031–3038. [Google Scholar] [CrossRef]
- Lee, J.B.; Jeong, S.M.; Kim, K.J.; Cho, D.H.; Kwon, I.K.; Yoon, I.C.; Choi, K.; Suh, J.K.F.; Park, J.H.; Park, Y.D.; et al. Osteogenic differentiation of human adipose-derived stem cells (hADSCs) on a dexamethasone eluting nanofiber scaffolds. J. Tissue Eng. Regen. Med. 2009, 6, 371–379. [Google Scholar]
- Tang, G.W.; Yang, Y.F.; Sun, A.P.; Song, T.T.; Zhao, Y.H.; Yuan, X.B.; Yuan, X.Y.; Fan, Y.B.; Wang, M. Controlled release of dexamethasone from porous PLGA scaffolds under cyclic loading. Sci. China Chem. 2010, 53, 594–598. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.W.; Suh, H. Sustained release of ascorbate-2-phosphate and dexamethasone from porous PLGA scaffolds for bone tissue engineering using mesenchymal stem cells. Biomater 2003, 24, 4671–4679. [Google Scholar] [CrossRef]
- Samorezov, J.E.; Alsberg, E. Spatial regulation of controlled bioactive factor delivery for bone tissue engineering. Adv. Drug Deliv. Rev. 2014, 23, 2–18. [Google Scholar] [CrossRef]
- Katzung, B.G. Basic and Clinical Pharmacology, 9th ed.; Lange Medical Books: New York, NY, USA, 2003; pp. 640–717. [Google Scholar]
- Yoon, J.J.; Kim, J.H.; Park, T.G. Dexamethasone-releasing biodegradable polymer scaffolds fabricated by a gas-foaming/salt-leaching method. Biomater 2003, 24, 2323–2329. [Google Scholar] [CrossRef]
- Majrashi, M.; Kotowska, A.; Scurr, D.; Hicks, J.M.; Ghaemmaghami, A.; Yang, J. Sustained release of dexamethasone from 3D-printed scaffolds modulates macrophage activation and enhances osteogenic differentiation. ASC Appl. Mater. Interfaces 2023, 15, 56623–56638. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wang, Z.; Qi, Y.; Li, L.; Zhang, P.; Chen, X.; Huang, Y. Composite PLA/PEG/nHA/dexamethasone scaffold prepared by 3D printing for bone regeneration. Macromol. Biosci. 2018, 18, 1800068. [Google Scholar] [CrossRef]
- Tsiapla, A.-R.; Karagkiozaki, V.; Bakola, V.; Pappa, F.; Gkertsiou, P.; Pavlidou, E.; Logothetidis, S. Biomimetic and biodegradable cellulose acetate scaffolds loaded with dexamethasone for bone implants. Beilstein J. Nanotechnol. 2018, 9, 1986–1994. [Google Scholar] [CrossRef]
- Sarkar, C.; Chowdhuri, A.R.; Garai, S.; Chakraborty, J.; Sahu, S.K. Three-dimensional cellulose-hydroxyapatite nanocomposite enriched with dexamethasone loaded metal-organic framework: A local drug delivery system for bone tissue engineering. Cellulose 2019, 26, 7253–7269. [Google Scholar] [CrossRef]
- Liesiene, J.; Kiselioviene, S.; Maruška, A.S.; Baniukaitiene, O. Preparation and characterization of a highly porous, rigid cellulose-based hydrogel for biomedical and biotechnological applications. New J. Chem. 2025, in press. [CrossRef]
- Daugela, P.; Pranskunas, M.; Juodzbalys, G.; Liesiene, J.; Baniukaitiene, O.; Afonso, A.; Sousa Gomes, P. Novel cellulose/hydroxyapatite scaffolds for bone tissue regeneration: In vitro and in vivo study. J. Tissue Eng. Regen. Med. 2018, 12, 1195–1208. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Wake, M.C.; Patrick, C.W.; Mikos, A.G. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 1994, 3, 339–343. [Google Scholar] [CrossRef]
- Kim, K.; Yeatts, A.; Dean, D.; Fisher, J.P. Stereolithographic bone scaffold design parameters: Osteogenic differentiation and signal expression. Tissue Eng. Part B Rev. 2010, 16, 523–539. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Lin, L.; Li, S.; Hou, W.; He, Y.; Sheng, L.; Yi, C.; Zhang, X.; Li, H.; et al. Large-pore-size Ti6Al4V scaffolds with different pore structures for vascularized bone regeneration. Mater. Sci. Eng. C 2021, 131, 112499. [Google Scholar] [CrossRef]
- Cleynenbreugel, T.V.; Schrooten, J.; Oosterwyck, H.V.; Sloten, J.V. Micro-CT-based screening of biomechanical and structural properties of bone tissue engineering scaffolds. Med. Biol. Eng. Comput. 2006, 44, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Anglov, T.; Petersen, I.M.; Kristiansen, J. Uncertainty of nitrogen determination by Kjeldahl method. Accredit. Qual. Assur. 1999, 4, 504–510. [Google Scholar] [CrossRef]


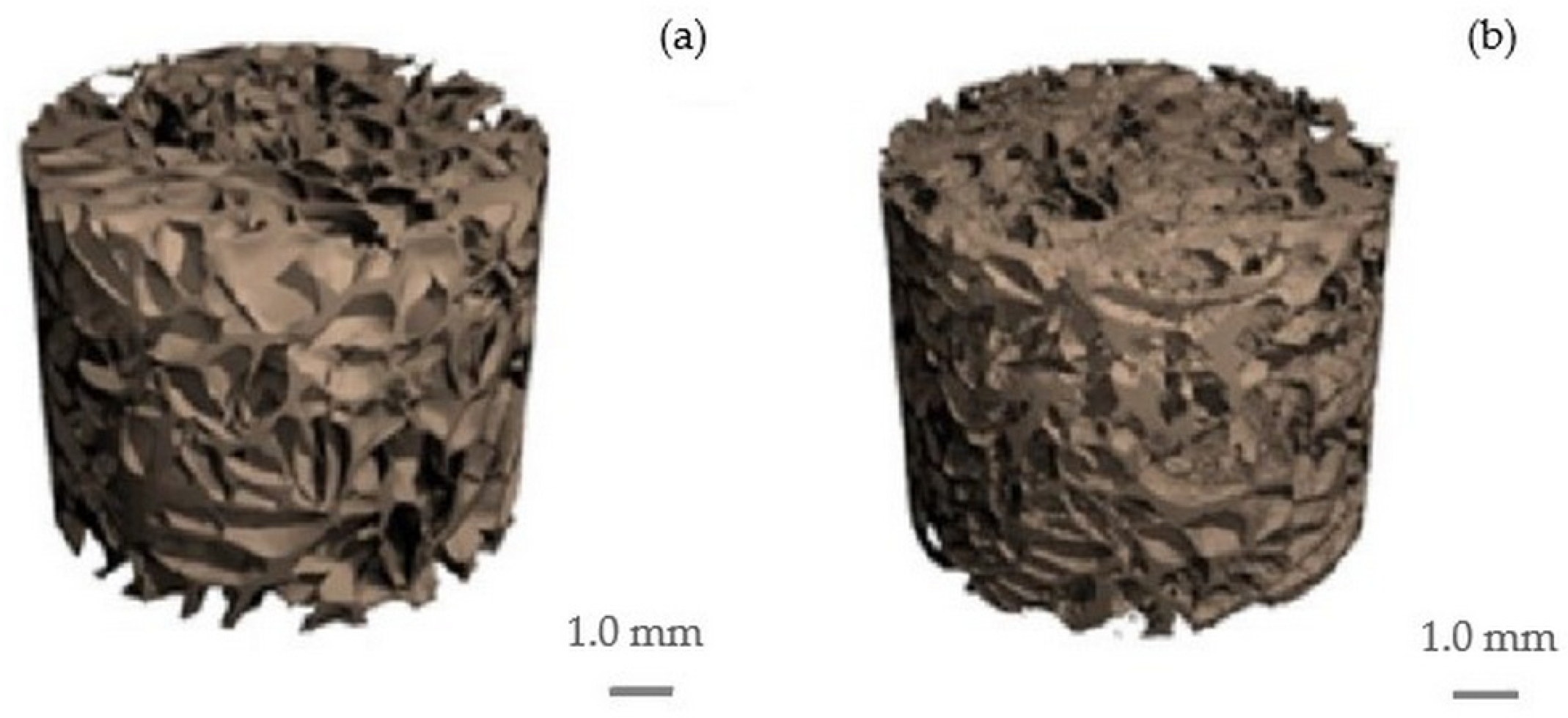
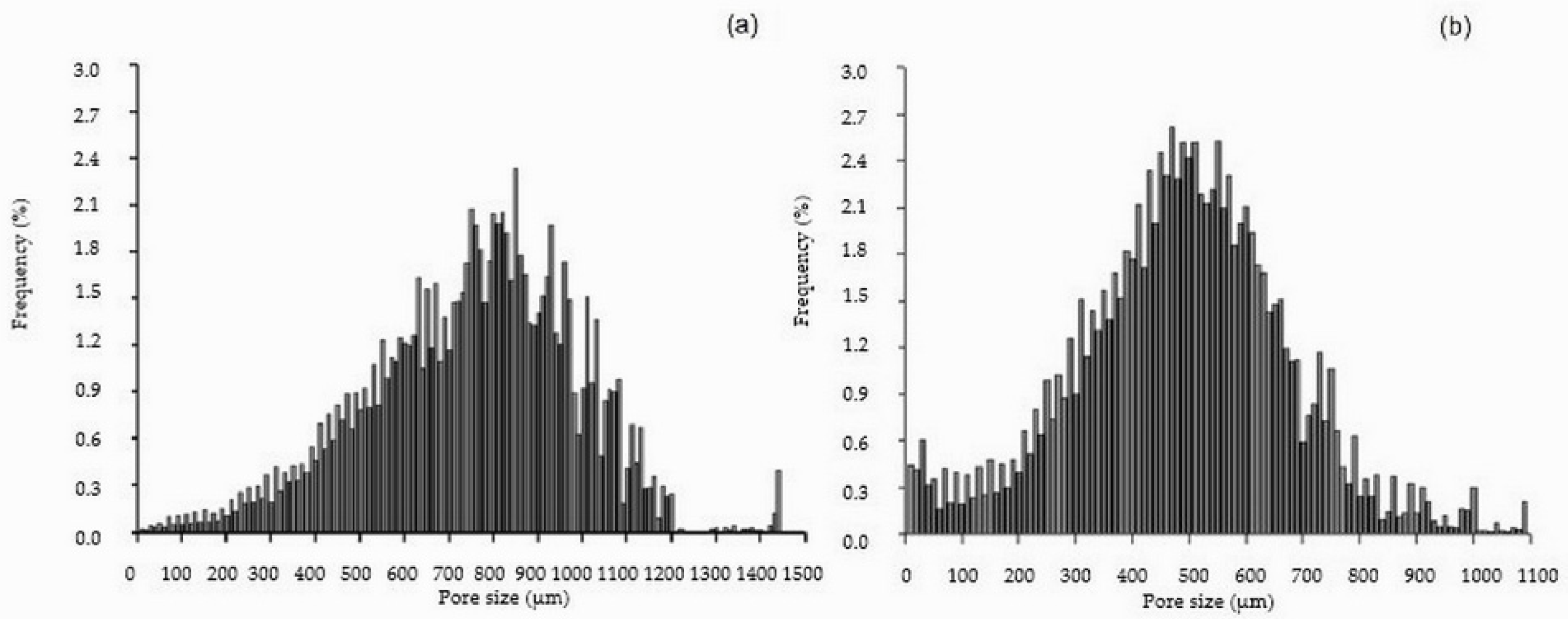
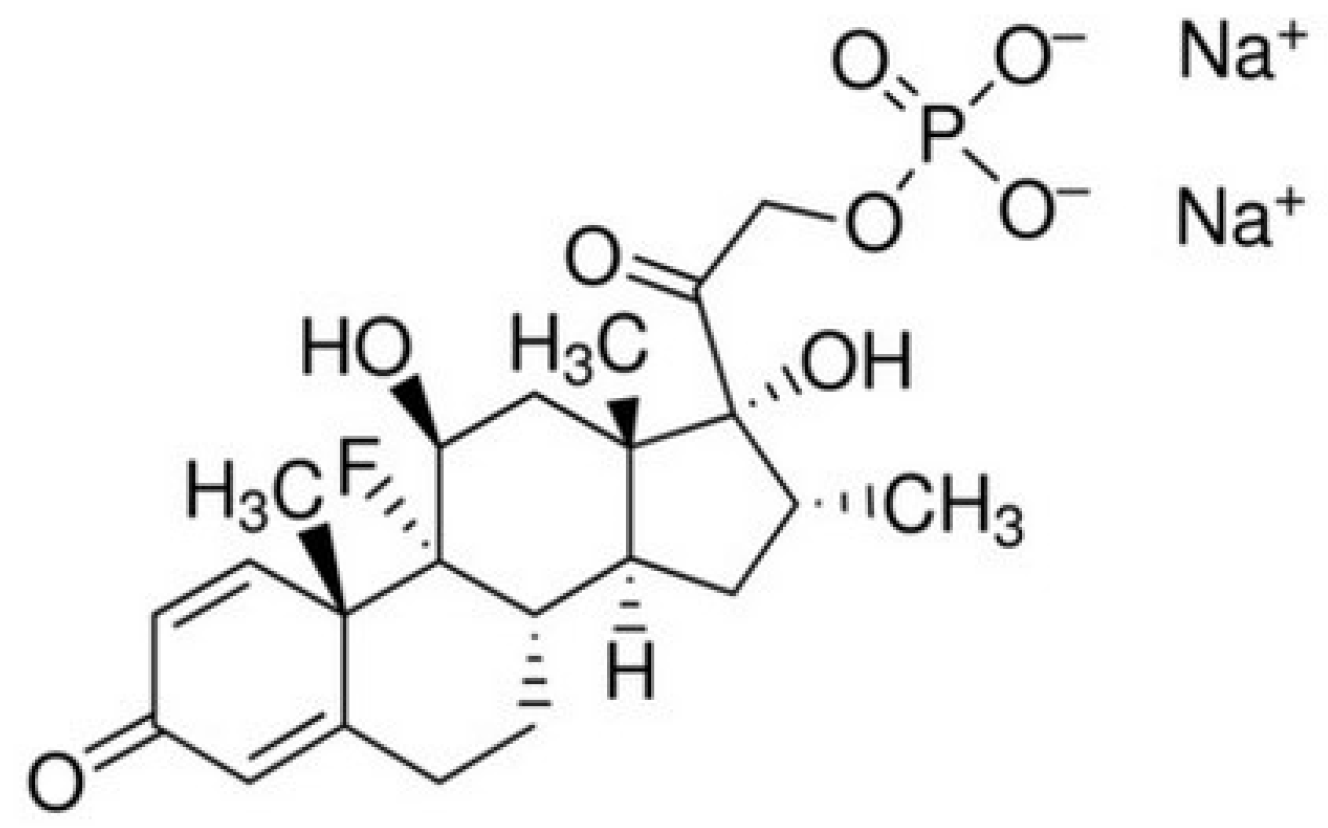

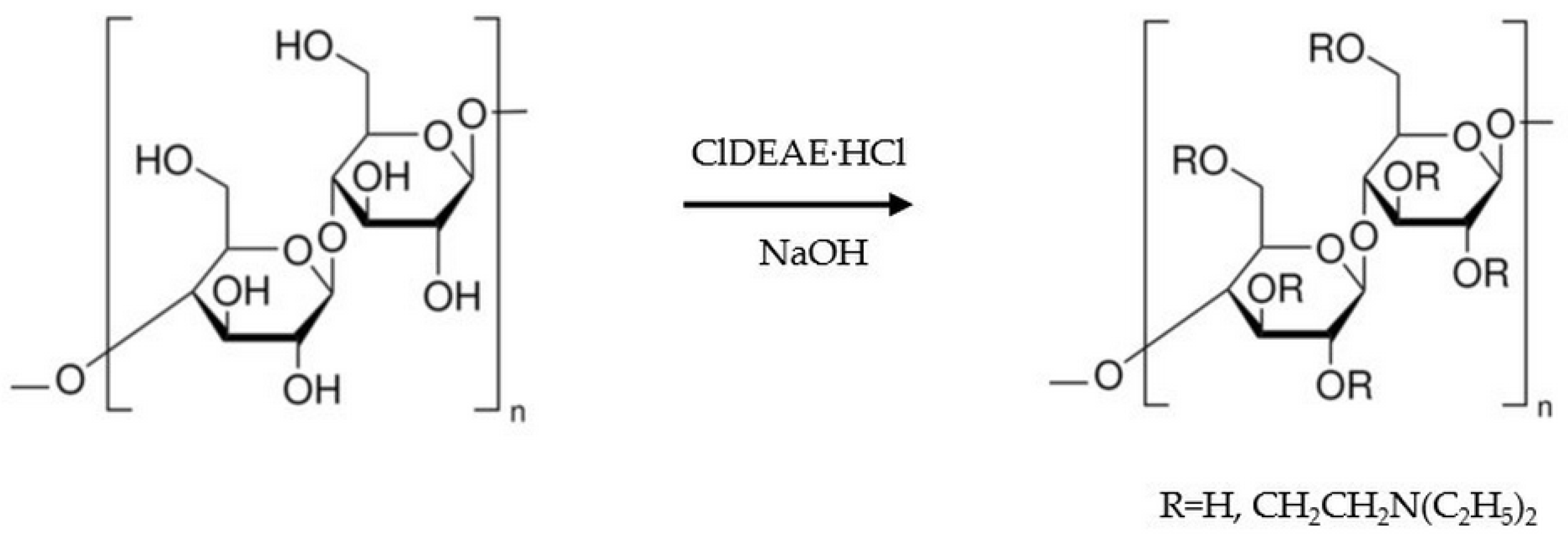

| Sample | Structural Parameters | ||||
|---|---|---|---|---|---|
| Xv, % | P, % | SS, mm−1 | L, µm | D, µm | |
| Cellulose scaffold | 25 ± 1 | 75 ± 1 | 15 ± 2 | 210 ± 26 | 750 ± 68 |
| Cellulose/HAp scaffold | 28 ± 1 | 72 ± 2 | 19 ± 1 | 120 ± 10 | 490 ± 94 |
| Pore Size, µm | Pores, % | |
|---|---|---|
| Cellulose Scaffold | Cellulose/HAp Scaffold | |
| ≥10 <300 | 3 | 16 |
| ≥300 <400 | 4 | 14 |
| ≥400 <500 | 7 | 22 |
| ≥500 <600 | 10 | 22 |
| ≥600 <700 | 14 | 15 |
| ≥700 <800 | 18 | 7 |
| ≥800 <900 | 18 | 2 |
| ≥900 <1000 | 14 | 1 |
| ≥1000 <1100 | 9 | 1 |
| ≥1100 <1500 | 3 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liesienė, J.; Baniukaitiene, O.; Minseviciene, I. Cellulose-Based Scaffolds with Prolonged Dexamethasone Release for Bone Tissue Engineering. Molecules 2025, 30, 2760. https://doi.org/10.3390/molecules30132760
Liesienė J, Baniukaitiene O, Minseviciene I. Cellulose-Based Scaffolds with Prolonged Dexamethasone Release for Bone Tissue Engineering. Molecules. 2025; 30(13):2760. https://doi.org/10.3390/molecules30132760
Chicago/Turabian StyleLiesienė, Jolanta, Odeta Baniukaitiene, and Ieva Minseviciene. 2025. "Cellulose-Based Scaffolds with Prolonged Dexamethasone Release for Bone Tissue Engineering" Molecules 30, no. 13: 2760. https://doi.org/10.3390/molecules30132760
APA StyleLiesienė, J., Baniukaitiene, O., & Minseviciene, I. (2025). Cellulose-Based Scaffolds with Prolonged Dexamethasone Release for Bone Tissue Engineering. Molecules, 30(13), 2760. https://doi.org/10.3390/molecules30132760






