Removal of Azoxystrobin and Deltamethrin from Water Using Activated Biochar from Moringa oleifera L. Wood: Synthesis, Characterization, and Adsorption Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorbent Characterization
2.1.1. SEM Analysis
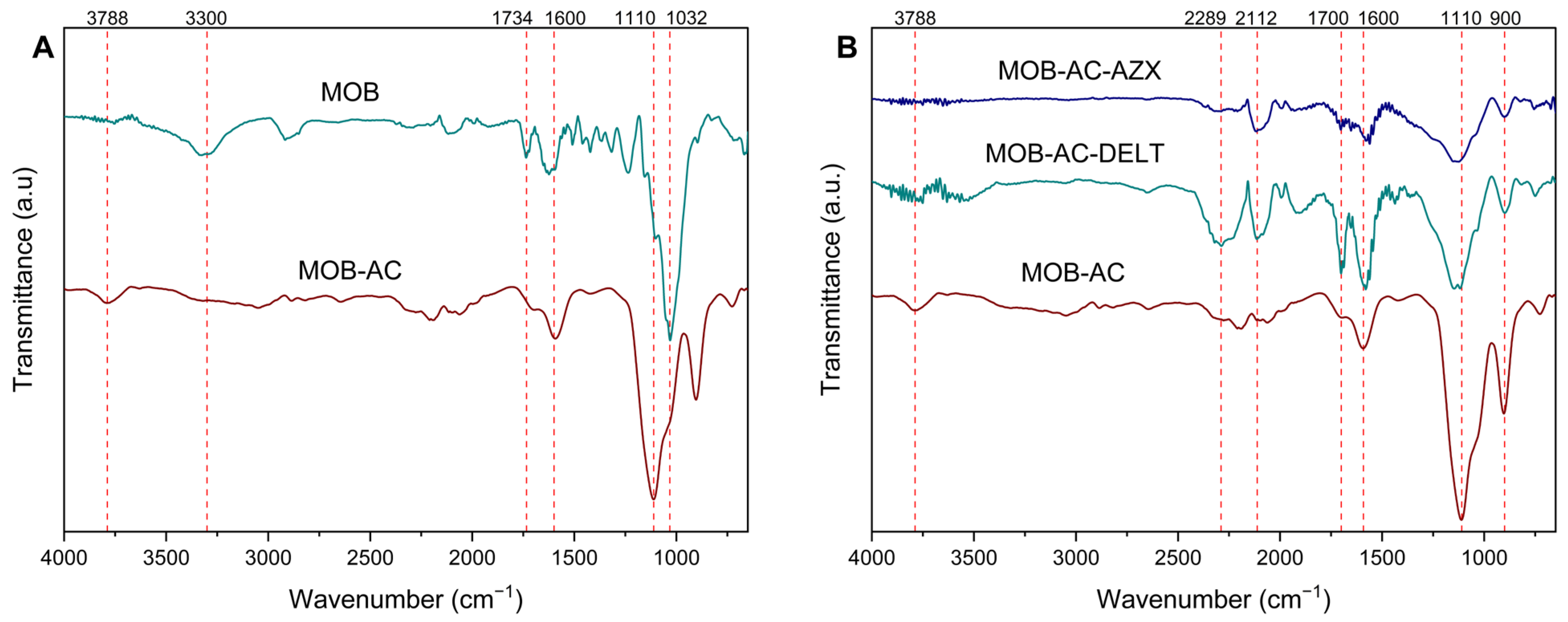
2.1.2. FTIR Analysis
2.1.3. Textural Properties Analysis
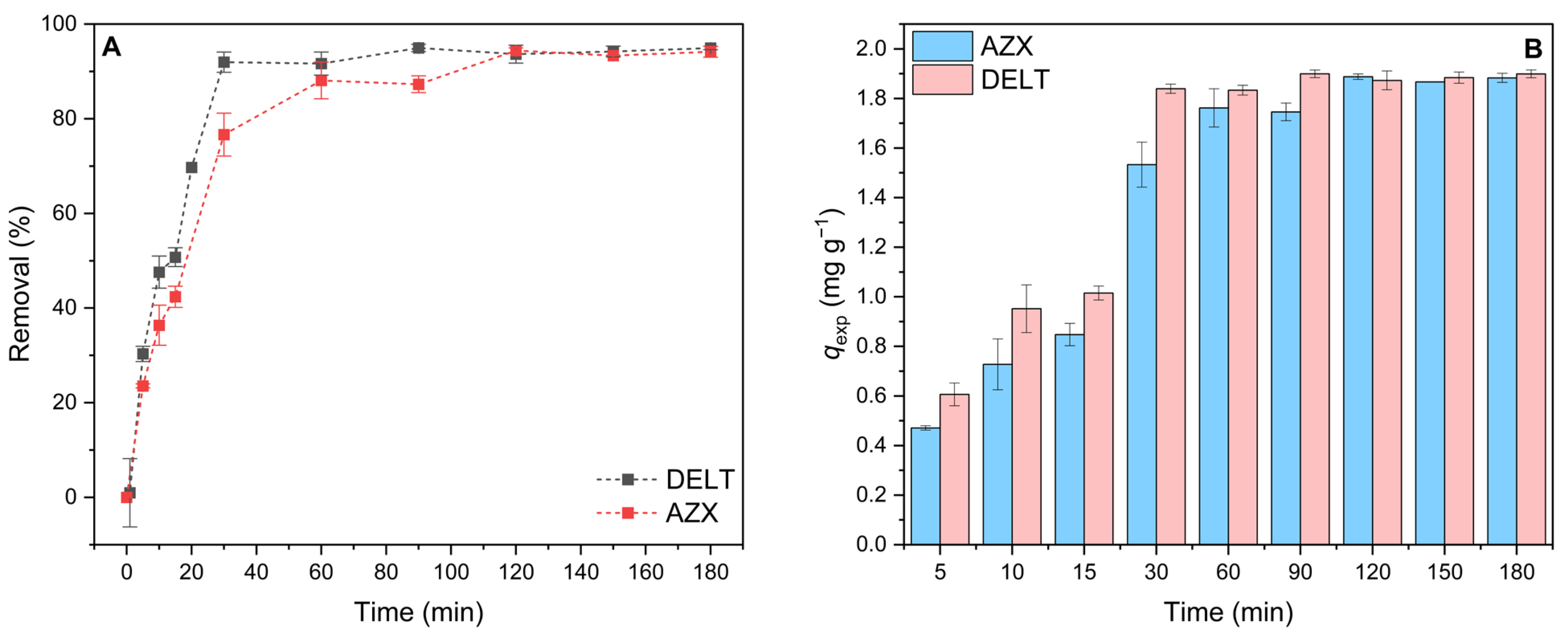
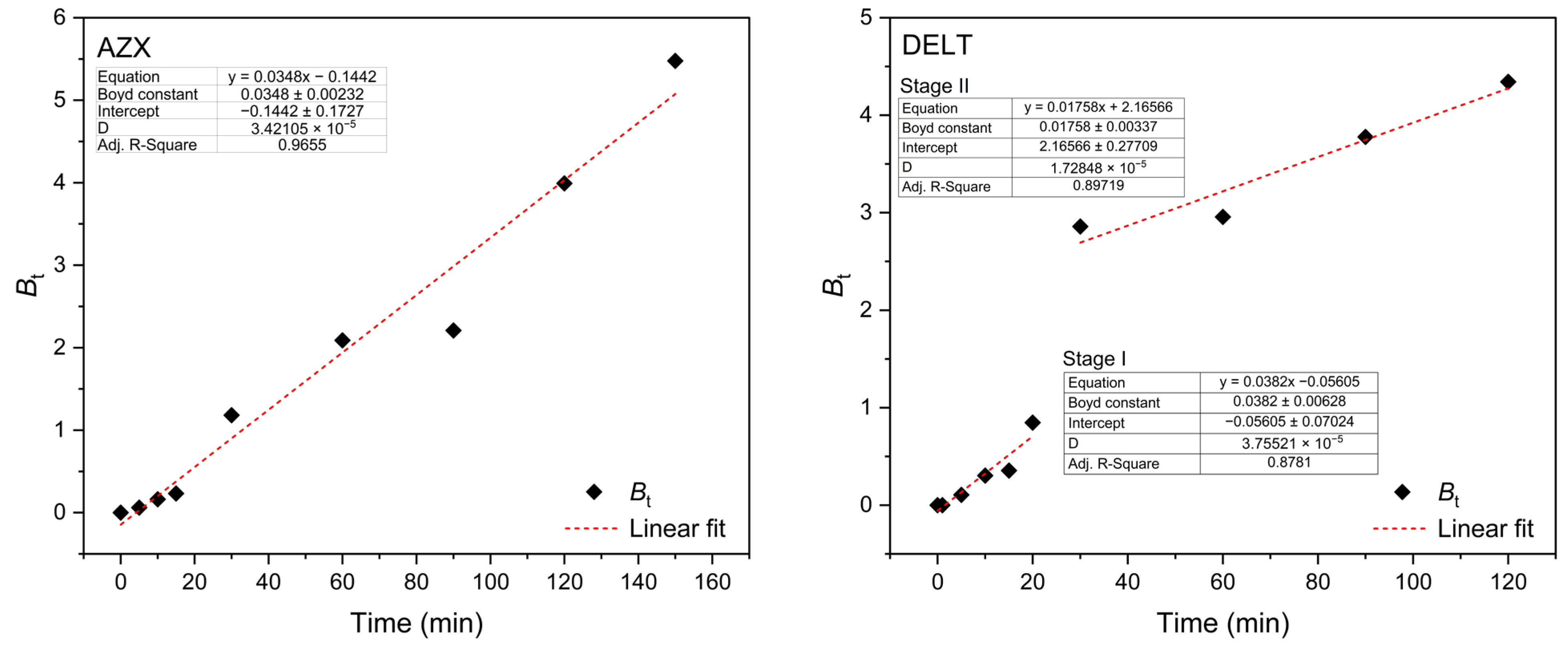
2.2. Kinetic Studies
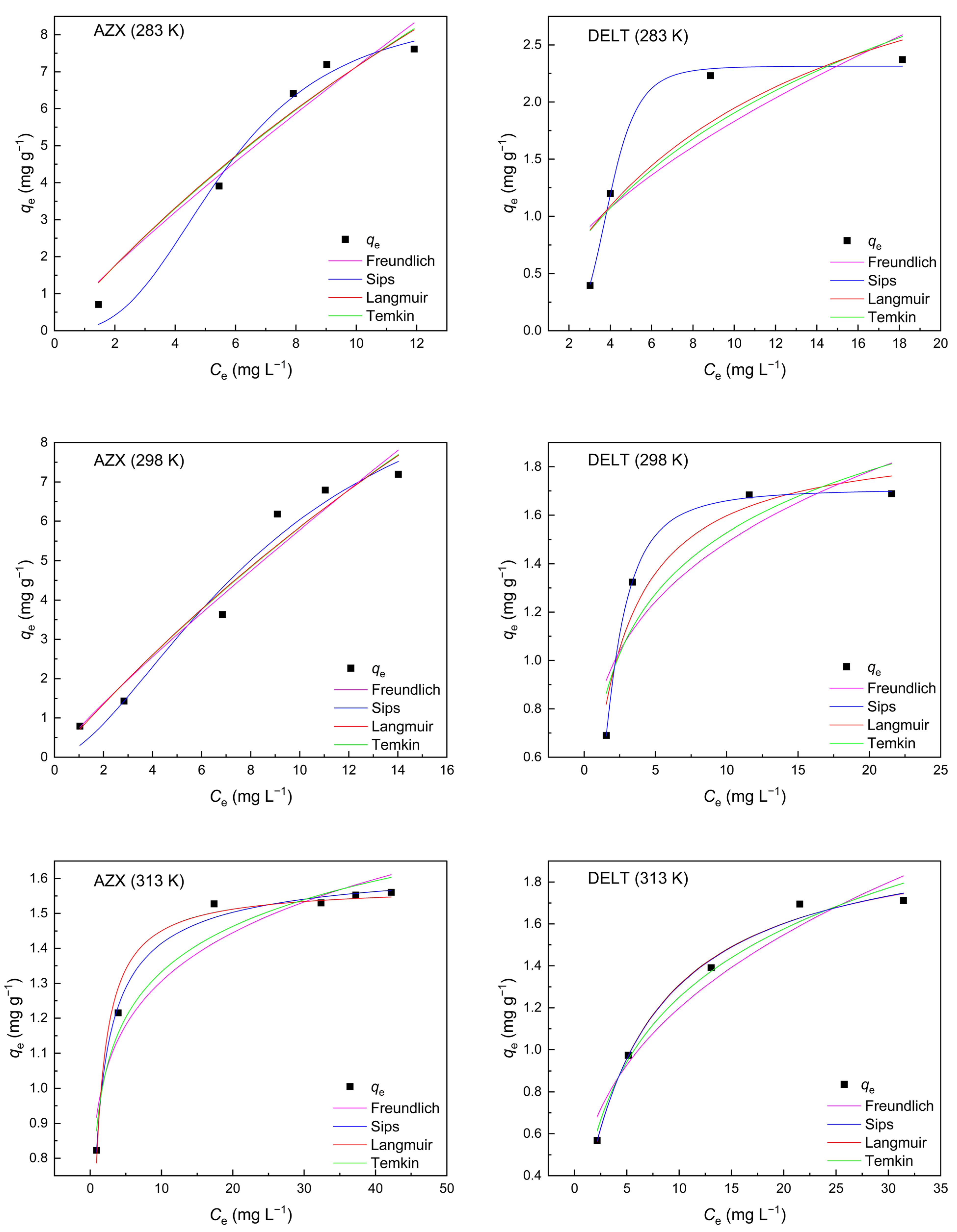
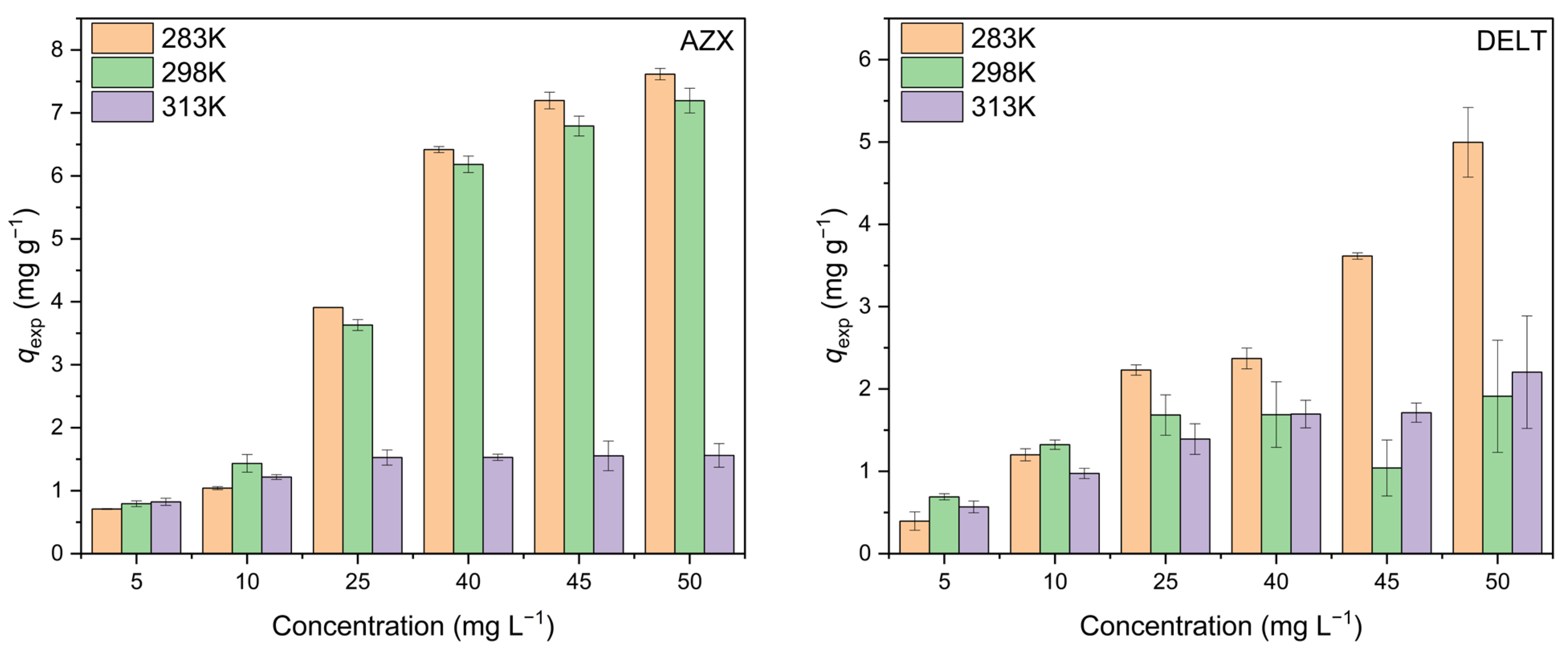
2.3. Adsorption Isotherms
2.4. Thermodynamic Studies
2.5. General Observations
3. Materials and Methods
3.1. Preparation of Activated Biochar from Moringa oleifera L. Wood
3.2. Adsorbent Characterization
3.3. Preparation of Pesticides Aqueous Solutions
3.4. Batch Adsorption Studies
3.4.1. Non-Linear Kinetic and Isotherm Models
3.4.2. Thermodynamic Parameters
3.5. Pesticide Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biswas, P.; Vellanki, B.P.; Kazmi, A.A. Investigating Biotic and Abiotic Degradation of Emerging Contaminants in Surface Water-Sediment Batch Systems. J. Clean. Prod. 2024, 467, 143019. [Google Scholar] [CrossRef]
- Salvestrini, S.; Fenti, A.; Qian, L.; Kopinke, F.-D. Oxidation of Organic Pollutants over MnO2 in Cold Water Assisted by Peroxydisulfate. Chem. Eng. J. 2024, 479, 147170. [Google Scholar] [CrossRef]
- Santos, Y.T.d.C.; Pereira Da Costa, G.; Marcell Coelho Menezes, J.; Victor Serra Nunes, J.; Hosseini-Bandegharaei, A.; Douglas Melo Coutinho, H.; Sena Júnior, D.; José De Paula Filho, F.; Nonato Pereira Teixeira, R. Adsorption of Zn(II) IONS by Ziziphus Joazeiro Barks in Aqueous Solutions. Results Chem. 2024, 7, 101339. [Google Scholar] [CrossRef]
- Hamadeen, H.M.; Elkhatib, E.A.; Badawy, M.E.I.; Abdelgaleil, S.A.M. Green Low Cost Nanomaterial Produced from Moringa Oleifera Seed Waste for Enhanced Removal of Chlorpyrifos from Wastewater: Mechanism and Sorption Studies. J. Environ. Chem. Eng. 2021, 9, 105376. [Google Scholar] [CrossRef]
- Çelekli, A.; Al-Nuaimi, A.I.; Bozkurt, H. Adsorption Kinetic and Isotherms of Reactive Red 120 on Moringa Oleifera Seed as an Eco-Friendly Process. J. Mol. Struct. 2019, 1195, 168–178. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Bezerra, C.d.O.; Quesada, H.B.; Alves Baptista, A.T.; Nishi, L.; Vieira, M.F.; Bergamasco, R. Modified Moringa Oleifera Lam. Seed Husks as Low-Cost Biosorbent for Atrazine Removal. Environ. Technol. 2019, 42, 1092–1103. [Google Scholar] [CrossRef]
- Gomes, H.D.O.; Freire, P.D.T.C.; Do Nascimento, R.F.; Pereira Teixeira, R.N. Removal of Contaminants from Water Using Moringa Oleifera Lam. as Biosorbent: An Overview of the Last Decade. J. Water Process Eng. 2022, 46, 102576. [Google Scholar] [CrossRef]
- Masekela, D.; Hintsho-Mbita, N.C.; Mabuba, N. Diethylamine Functionalised Moringa oleifera Leaves for the Removal of Chromium(VI) and Bacteria from Wastewater. Int. J. Environ. Anal. Chem. 2020, 102, 3002–3022. [Google Scholar] [CrossRef]
- Escobar, O.d.S.; Ferraz de Azevedo, C.; Swarowsky, A.; Adebayo, M.A.; Schadeck Netto, M.; Machado Machado, F. Utilization of Different Parts of Moringa Oleifera Lam. Seeds as Biosorbents to Remove Acid Blue 9 Synthetic Dye. J. Environ. Chem. Eng. 2021, 9, 105553. [Google Scholar] [CrossRef]
- Kalavathy, M.H.; Miranda, L.R. Moringa Oleifera—A Solid Phase Extractant for the Removal of Copper, Nickel and Zinc from Aqueous Solutions. Chem. Eng. J. 2010, 158, 188–199. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.V.R.; Rao, M.M.; Wang, M.C. Biosorption of Pb2+ from Aqueous Solutions by Moringa Oleifera Bark: Equilibrium and Kinetic Studies. J. Hazard. Mater. 2010, 174, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.H.K.; Ramana, D.K.V.; Seshaiah, K.; Reddy, A.V.R. Biosorption of Ni(II) from Aqueous Phase by Moringa Oleifera Bark, a Low Cost Biosorbent. Desalination 2011, 268, 150–157. [Google Scholar] [CrossRef]
- Holanda, M.A.S.; Coelho Menezes, J.M.; Coutinho, H.D.M.; Teixeira, R.N.P. Effectiveness of Biochar as an Adsorbent for Pesticides: Systematic Review and Meta-Analysis. J. Environ. Manag. 2023, 345, 118719. [Google Scholar] [CrossRef]
- Fan, M.; Shao, Y.; Wang, Y.; Sun, J.; He, H.; Guo, Y.; Zhang, S.; Wang, S.; Li, B.; Hu, X. Evolution of Pore Structure and Functionalities of Activated Carbon and Phosphorous Species in Activation of Cellulose with H3PO4. Renew. Energy 2025, 240, 122151. [Google Scholar] [CrossRef]
- Neme, I.; Gonfa, G.; Masi, C. Activated Carbon from Biomass Precursors Using Phosphoric Acid: A Review. Heliyon 2022, 8, e11940. [Google Scholar] [CrossRef]
- Yang, H.; Chen, P.; Chen, W.; Li, K.; Xia, M.; Xiao, H.; Chen, X.; Chen, Y.; Wang, X.; Chen, H. Insight into the Formation Mechanism of N, P Co-Doped Mesoporous Biochar from H3PO4 Activation and NH3 Modification of Biomass. Fuel Process. Technol. 2022, 230, 107215. [Google Scholar] [CrossRef]
- Mainali, K.; Garcia-Perez, M. Effect of H3PO4 and NaOH Additives on the Co-Carbonization of Cellulose and N-Containing Compounds to Produce N-Doped Chars. J. Anal. Appl. Pyrolysis 2023, 169, 105837. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in Aquatic Environments and Their Removal by Adsorption Methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, M.; Cao, J.; She, Y.; Cao, Z.; Hao, Z.; Jin, F.; Wang, J.; Abd El-Aty, A.M. A Method for the Rapid Determination of Pesticides Coupling Thin-Layer Chromatography and Enzyme Inhibition Principles. Food Chem. 2023, 416, 135822. [Google Scholar] [CrossRef]
- Silva, R.d.O.; De Menezes, M.G.G.; De Castro, R.C.; Nobre, C.D.A.; Milhome, M.A.L.; Do Nascimento, R.F. Efficiency of ESI and APCI Ionization Sources in LC-MS/MS Systems for Analysis of 22 Pesticide Residues in Food Matrix. Food Chem. 2019, 297, 124934. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Y.; Zhang, J.; Min, S. A Fast Determination of Insecticide Deltamethrin by Spectral Data Fusion of UV–Vis and NIR Based on Extreme Learning Machine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119119. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Hou, K.; Zhou, T.; Wang, X.; Zhang, J.; Cheng, C.; Du, Z.; Li, B.; Wang, J.; Wang, J.; et al. Characterization of the Responses of Soil Micro-Organisms to Azoxystrobin and the Residue Dynamics of Azoxystrobin in Wheat–Corn Rotation Fields over Two Years. Chemosphere 2023, 318, 137918. [Google Scholar] [CrossRef] [PubMed]
- Trostanetsky, A.; Quinn, E.; Rapaport, A.; Harush, A.; Gottlieb, D. Efficacy of Deltamethrin Emulsifiable Concentrate against Stored-Product Insects. J. Stored Prod. Res. 2023, 101, 102072. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.H.; Hassan, S.M.; Arief, M.M.H.; Mohammad, S.G. Validation of Quantitative Method for Azoxystrobin Residues in Green Beans and Peas. Food Chem. 2015, 182, 246–250. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Khan, M.S.; Othmani, A.; Khanday, W.A.; Gökkuş, Ö.; Osagie, C.; Ahmaruzzaman, M.; Mishra, S.R.; et al. Sustainable Remediation Technologies for Removal of Pesticides as Organic Micro-Pollutants from Water Environments: A Review. Appl. Surf. Sci. Adv. 2024, 19, 100558. [Google Scholar] [CrossRef]
- Wu, H.; Gao, J.; Xie, M.; Wu, J.; Song, R.; Yuan, X.; Wu, Y.; Ou, D. Chronic Exposure to Deltamethrin Disrupts Intestinal Health and Intestinal Microbiota in Juvenile Crucian Carp. Ecotoxicol. Environ. Saf. 2022, 241, 113732. [Google Scholar] [CrossRef]
- Kovačević, M.; Stjepanović, N.; Zelić, L.; Lončarić, Ž. Multigenerational and Transgenerational Effects of Azoxystrobin on Folsomia Candida. Environ. Pollut. 2023, 336, 122398. [Google Scholar] [CrossRef]
- Veneciano, R.I.; Parra, V.S.; Quiroz, W.; Fuentes, E.; Aguilar, L.F.; Bravo, M.A. Deltamethrin Determination in Natural Water Samples via Photochemically-Induced Fluorescence Coupled to Third-Order Multivariate Calibration. Microchem. J. 2020, 159, 105561. [Google Scholar] [CrossRef]
- Do Rego, E.L.; De Souza, J.R.; Nakamura, T.C.; Portela, J.F.; Diniz, P.H.G.D.; Da Silva, J.D.S. Pesticides in Surface Water of the Ondas River Watershed, Western Bahia, Brazil: Spatial-Seasonal Distribution and Risk Assessment. Chemosphere 2024, 354, 141659. [Google Scholar] [CrossRef]
- Yimer, T.F.; Ayele, D.T.; Brihanu, Y.K.; Akele, M.L.; Kassaw, M.M.; Alemu, A.K.; Ayitegeb, D.Y.; Birhan, T.A.; Amare, Z.M.; Semegn, A.A.; et al. Ecological Risk Assessment of Organochlorine Pesticide Residues in Sediment Samples from Lake Tana and Hayqe in Northwest Ethiopia. Emerg. Contam. 2024, 10, 100354. [Google Scholar] [CrossRef]
- ANVISA. Programa de Análise de Resíduos de Agrotóxicos em Alimentos (PARA). Relatório dos Resultados das Análises de Amostras Monitoras No Ciclo 2023; ANVISA: Brasília, Brazil, 2024; p. 152. [Google Scholar]
- Reddy, D.H.K.; Lee, S.M.; Seshaiah, K. Removal of Cd(II) and Cu(II) from Aqueous Solution by Agro Biomass: Equilibrium, Kinetic and Thermodynamic Studies. Environ. Eng. Res. 2012, 17, 125–132. [Google Scholar] [CrossRef]
- Souza, H.K.S.; Fagundes Klen, M.R.; Wernke, G.; Mantovani, D.; Nishi, L.; Shimabuku-Biadola, Q.L.; Vieira, M.F.; Bergamasco, R.; Vieira, A.M.S. Improvement of Adsorption Conditions of Different Parts of Moringa Oleifera on the Perception of Diuron Removal from Contaminated Waters. Desalination Water Treat. 2019, 171, 331–343. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.V.R.; Lee, S.M. Optimization of Cd(II), Cu(II) and Ni(II) Biosorption by Chemically Modified Moringa Oleifera Leaves Powder. Carbohydr. Polym. 2012, 88, 1077–1086. [Google Scholar] [CrossRef]
- Tavares, F.d.O.; Moraes Pinto, L.A.; Marinozi Vicentini, J.C.; Vieira, M.F.; Bergamasco, R.; Vieira, A.M.S. Analysis of the Influence of Natural Adsorbent Functionalization Moringa Oleifera for Pb(II) Removal from Contaminated Water. Environ. Prog. Sustain. Energy 2019, 39, e13318. [Google Scholar] [CrossRef]
- Swelam, A.; Saied, S.; Hafez, A. Removal Comparative Study for Cd(II) Ions from Polluted Solutions by Adsorption and Coagulation Techniques Using Moringa Oleifera Seeds. Egypt. J. Chem. 2019, 62, 1899–1917. [Google Scholar] [CrossRef]
- Coldebella, P.F.; Fagundes-Klen, M.R.; Nishi, L.; Valverde, K.C.; Cavalcanti, E.B.; Andreo dos Santos, O.A.; Bergamasco, R. Potential Effect of Chemical and Thermal Treatment on the Kinetics, Equilibrium, and Thermodynamic Studies for Atrazine Biosorption by the Moringa Oleifera Pods. Can. J. Chem. Eng. 2017, 95, 961–973. [Google Scholar] [CrossRef]
- Adebayo, G.B.; Jamiu, W.; Okoro, H.K.; Okeola, F.O.; Adesina, A.K.; Feyisetan, O.A. Kinetics, Thermodynamics and Isothermal Modelling of Liquid Phase Adsorption of Methylene Blue onto Moringa Pod Husk Activated Carbon. S. Afr. J. Chem. 2019, 72, 263–273. [Google Scholar] [CrossRef]
- Al-Kindi, G.; Al Haidari, H. The Removal of Ibuprofen Drugs Residues from Municipal Wastewater by Moringa Oleifera Seeds. J. Ecol. Eng. 2021, 22, 83–94. [Google Scholar] [CrossRef]
- Gañán, P.; Cruz, J.; Garbizu, S.; Arbelaiz, A.; Mondragon, I. Stem and Bunch Banana Fibers from Cultivation Wastes: Effect of Treatments on Physico-chemical Behavior. J. Appl. Polym. Sci. 2004, 94, 1489–1495. [Google Scholar] [CrossRef]
- Kaushik, A.; Singh, M. Isolation and Characterization of Cellulose Nanofibrils from Wheat Straw Using Steam Explosion Coupled with High Shear Homogenization. Carbohydr. Res. 2011, 346, 76–85. [Google Scholar] [CrossRef]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent Progress on Biosorption of Heavy Metals from Liquids Using Low Cost Biosorbents: Characterization, Biosorption Parameters and Mechanism Studies. Clean Soil Air Water 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Elangovan, R.; Philip, L.; Chandraraj, K. Biosorption of Chromium Species by Aquatic Weeds: Kinetics and Mechanism Studies. J. Hazard. Mater. 2008, 152, 100–112. [Google Scholar] [CrossRef]
- Keereerak, A.; Chinpa, W. A Potential Biosorbent from Moringa Oleifera Pod Husk for Crystal Violet Adsorption: Kinetics, Isotherms, Thermodynamic and Desorption Studies. ScienceAsia 2020, 46, 186–194. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Salvestrini, S.; Grilli, E.; Coppola, E. Investigating the Sorption/Desorption of the Cationic Herbicide Paraquat in Clay Minerals Using Batch and Electro–Ultrafiltration Techniques. Environments 2024, 11, 53. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Kinetics of Solute Adsorption at Solid/Aqueous Interfaces: Searching for the Theoretical Background of the Modified Pseudo-First-Order Kinetic Equation. Langmuir 2008, 24, 5393–5399. [Google Scholar] [CrossRef]
- Plazinski, W. Applicability of the Film-Diffusion Model for Description of the Adsorption Kinetics at the Solid/Solution Interfaces. Appl. Surf. Sci. 2010, 256, 5157–5163. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Kinetic Study of Dye Sorption by Biosorbent Waste Product Pith. Resour. Conserv. Recycl. 1999, 25, 171–193. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Aharoni, C.; Tompkins, F.C. Kinetics of Adsorption and Desorption and the Elovich Equation. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1970; Volume 21, pp. 1–49, ISBN 978-0-12-007821-9. [Google Scholar]
- Xiao, Y.; Azaiez, J.; Hill, J.M. Erroneous Application of Pseudo-Second-Order Adsorption Kinetics Model: Ignored Assumptions and Spurious Correlations. Ind. Eng. Chem. Res. 2018, 57, 2705–2709. [Google Scholar] [CrossRef]
- Mudhoo, A.; Pittman, C.U. Adsorption Data Modeling and Analysis under Scrutiny: A Clarion Call to Redress Recently Found Troubling Flaws. Chem. Eng. Res. Des. 2023, 192, 371–388. [Google Scholar] [CrossRef]
- Yao, C.; Chen, T. A Film-Diffusion-Based Adsorption Kinetic Equation and Its Application. Chem. Eng. Res. Des. 2017, 119, 87–92. [Google Scholar] [CrossRef]
- Hu, Q.; Lan, R.; He, L.; Liu, H.; Pei, X. A Critical Review of Adsorption Isotherm Models for Aqueous Contaminants: Curve Characteristics, Site Energy Distribution and Common Controversies. J. Environ. Manag. 2023, 329, 117104. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Matubayasi, N. Cooperative Sorption on Porous Materials. Langmuir 2021, 37, 10279–10290. [Google Scholar] [CrossRef]
- Brião, G.d.V.; Hashim, M.A.; Chu, K.H. The Sips Isotherm Equation: Often Used and Sometimes Misused. Sep. Sci. Technol. 2023, 58, 884–892. [Google Scholar] [CrossRef]
- Salvestrini, S.; Bollinger, J.-C. Revisiting the Extended van’t Hoff Equation: Comments on “Highly-Efficient Nitrogen Self-Doped Biochar for Versatile Dyes’ Removal Prepared from Soybean Cake via a Simple Dual-Templating Approach and Associated Thermodynamics”. J. Clean. Prod. 2022, 373, 133632. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Gürsoy, N.; Şimşek, S.; Özer, A.; Karakuş, N. Removal of Food Dyes from Aqueous Solution by Chitosan-Vermiculite Beads. Int. J. Biol. Macromol. 2020, 148, 635–646. [Google Scholar] [CrossRef]
- Raji, Y.; Nadi, A.; Mechnou, I.; Saadouni, M.; Cherkaoui, O.; Zyade, S. High Adsorption Capacities of Crystal Violet Dye by Low-Cost Activated Carbon Prepared from Moroccan Moringa Oleifera Wastes: Characterization, Adsorption and Mechanism Study. Diam. Relat. Mater. 2023, 135, 109834. [Google Scholar] [CrossRef]
- Ranote, S.; Chauhan, G.S.; Joshi, V. Etherified Moringa Oleifera Gum as Rapid and Effective Dye Adsorbents. Chem. Eng. J. 2020, 387, 124055. [Google Scholar] [CrossRef]
- Viotti, P.V.; Moreira, W.M.; dos Santos, O.A.A.; Bergamasco, R.; Vieira, A.M.S.; Vieira, M.F. Diclofenac Removal from Water by Adsorption on Moringa Oleifera Pods and Activated Carbon: Mechanism, Kinetic and Equilibrium Study. J. Clean. Prod. 2019, 219, 809–817. [Google Scholar] [CrossRef]
- Shahwan, T. Critical Insights into the Limitations and Interpretations of the Determination of ∆Go, ∆Ho, and ∆So of Sorption of Aqueous Pollutants on Different Sorbents. Colloid Interface Sci. Commun. 2021, 41, 100369. [Google Scholar] [CrossRef]
- Oba, O.A.; Pasaoglulari Aydinlik, N. Preparation of Mesoporous Activated Carbon from Novel African Walnut Shells (AWS) for Deltamethrin Removal: Kinetics and Equilibrium Studies. Appl. Water Sci. 2022, 12, 149. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Shawaqfeh, A.T.; Lafi, W.K. Adsorption of Pesticides from Aqueous Solutions Using Oil Shale Ash. Desalination 2007, 208, 294–305. [Google Scholar] [CrossRef]
- Ettish, M.N.; El-Sayyad, G.S.; Wong, D.; Elsayed, M.A.; Abuzalat, O. Efficient Removal of Deltamethrin (Pyrethroid Ester Insecticide) from Water Using Novel Chemically Activated Carbon Derived from the Inner Stem Bark of C. Verum Tree. Appl. Surf. Sci. Adv. 2022, 9, 100245. [Google Scholar] [CrossRef]
- Li, Y.; Zhen, D.; Liu, F.; Zhang, X.; Gao, Z.; Wang, J. Adsorption of Azoxystrobin and Pyraclostrobin onto Degradable and Non-Degradable Microplastics: Performance and Mechanism. Sci. Total Environ. 2024, 912, 169453. [Google Scholar] [CrossRef]
- Malhat, F.; Abdallah, O.I.; Hussien, M.; Youssef, A.M.; Alminderej, F.M.; Saleh, S.M. Enhanced Adsorption of Azoxystrobin from Water by As-Prepared Silica Nanoparticles. Coatings 2023, 13, 1286. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics 1. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.; Heller, W. The Adsorption of Cis- and Trans-Azobenzene. J. Am. Chem. Soc. 1939, 61, 2228–2230. [Google Scholar] [CrossRef]
- Chu, K.H. Revisiting the Temkin Isotherm: Dimensional Inconsistency and Approximate Forms. Ind. Eng. Chem. Res. 2021, 60, 13140–13147. [Google Scholar] [CrossRef]
- Temkin, M.I. The Kinetics of Some Industrial Heterogeneous Catalytic Reactions. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1979; Volume 28, pp. 173–291, ISBN 978-0-12-007828-8. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A Critical Review of the Estimation of the Thermodynamic Parameters on Adsorption Equilibria. Wrong Use of Equilibrium Constant in the Van’t Hoof Equation for Calculation of Thermodynamic Parameters of Adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Gomes, H.d.O.; Bento, E.C.L.D.S.; Santos, C.R.F.D.; Cardoso, R.D.S.; Souza, C.D.O.D.; Oliveira, L.C.C.D.; Costa, J.G.M.D.; Nascimento, R.F.D.; Teixeira, R.N.P. Comparative Study Between VWD and FLD Detector in HPLC System for Azoxystrobin Quantification in Water. Chromatographia 2023, 86, 605–615. [Google Scholar] [CrossRef]

| MOB | MOB-AC | |
|---|---|---|
| Total surface area (m2 g−1) | 1.875 | 188.5 |
| External surface area (m2 g−1) | 1.045 | 15.31 |
| Total pore volume (cm3 g−1) | 0.0036 | 0.0353 |
| Average pore diameter (nm) | 3.36 | 3.53 |
| Kinetic Model | Parameters | Pesticide | |
|---|---|---|---|
| Azoxystrobin | Deltamethrin | ||
| PFO | qe (mg g−1) | 1.860 ± 0.036 | 1.901 ± 0.043 |
| k1 | 0.049 ± 0.004 | 0.067 ± 0.006 | |
| Adj. R2 | 0.982 | 0.983 | |
| 0.00561 | 0.00911 | ||
| RSS | 0.03928 | 0.09113 | |
| PSO | qe (mg g−1) | 2.133 ± 0.081 | 2.107 ± 0.087 |
| k2 | 0.027 ± 0.005 | 0.042 ± 0.009 | |
| Adj. R2 | 0.966 | 0.967 | |
| 0.01081 | 0.01782 | ||
| RSS | 0.07568 | 0.17819 | |
| Elovich | α | 0.232 ± 0.088 | 0.421 ± 0.201 |
| β | 2.228 ± 0.317 | 2.490 ± 0.387 | |
| Adj. R2 | 0.917 | 0.922 | |
| 0.02658 | 0.04239 | ||
| RSS | 0.18608 | 0.42393 | |
| Isotherm Model | Parameters | Azoxystrobin | Deltamethrin | ||||
|---|---|---|---|---|---|---|---|
| 283 K | 298 K | 313 K | 283 K | 298 K | 313 K | ||
| Langmuir | qmax (mg g−1) | 30.27 | 34.13 | 1.580 | 4.078 | 1.865 | 2.064 |
| KL (L mg−1) | 0.031 | 0.021 | 1.120 | 0.091 | 0.523 | 0.173 | |
| Adj. R2 | 0.943 | 0.948 | 0.979 | 0.743 | 0.912 | 0.990 | |
| 0.47406 | 0.40463 | 0.00184 | 0.22239 | 0.01701 | 0.0024 | ||
| RSS | 1.42217 | 1.6185 | 0.00738 | 0.44479 | 0.05103 | 0.0072 | |
| Freundlich | KF (mg g−1 (L mg−1)−1/n) | 0.953 | 0.744 | 0.934 | 0.481 | 0.906 | 0.512 |
| n | 1.144 | 1.123 | 6.860 | 1.722 | 5.132 | 2.710 | |
| Adj. R2 | 0.928 | 0.942 | 0.912 | 0.648 | 0.660 | 0.940 | |
| 0.59268 | 0.45466 | 0.00776 | 0.30372 | 0.06528 | 0.01449 | ||
| RSS | 1.77803 | 1.81863 | 0.03102 | 0.60745 | 0.19585 | 0.04347 | |
| Temkin II | qT (mg g−1) | 13.89 | 15.93 | 0.188 | 1.417 | 0.294 | 0.500 |
| KT (L mg−1) | 0.067 | 0.044 | 119.6 | 0.284 | 13.95 | 1.117 | |
| Adj. R2 | 0.941 | 0.948 | 0.949 | 0.715 | 0.747 | 0.977 | |
| 0.4865 | 0.40976 | 0.00448 | 0.24634 | 0.04856 | 0.00554 | ||
| RSS | 1.45949 | 1.63906 | 0.01792 | 0.49269 | 0.14568 | 0.01663 | |
| Sips | KS (L mg−1) | 0.007 | 0.026 | 1.061 | 3.854 × 10−4 | 0.271 | 0.165 |
| βS | 2.876 | 1.648 | 0.732 | 5.714 | 2.088 | 0.654 | |
| qmax (mg g−1) | 8.756 | 11.20 | 1.661 | 2.314 | 1.711 | 2.931 | |
| Adj. R2 | 0.972 | 0.947 | 0.991 | 0.992 | 0.999 | 0.999 | |
| 0.2311 | 0.41649 | 7.5354 × 10−4 | 0.00705 | 1.3121 × 10−4 | 7.9215 × 10−6 | ||
| RSS | 0.4622 | 1.24946 | 0.00226 | 0.00705 | 2.6243 × 10−4 | 0.0079 | |
| Temp (K) | K° | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) |
|---|---|---|---|---|
| Azoxystrobin | ||||
| 283 | 2735 | −17.67 | 123 ± 36 | 497 ± 122 |
| 298 | 10,811 | −25.12 | ||
| 313 | 428,123 | −32.58 | ||
| Deltamethrin | ||||
| 283 | 195 | −14.89 | 153 ± 95 | 593 ± 318 |
| 298 | 136,462 | −23.78 | ||
| 313 | 88,361 | −32.68 | ||
| Pesticide | Adsorbent | qmax, mg g−1 (Temperature) | Reference |
|---|---|---|---|
| Deltamethrin | Moringa oleifera wood activated carbon | 4.078 (283 K) | Present study |
| KOH-modified African walnut shell | 57.64 (not specified) | [64] | |
| Oil shale ash | 10.96 (298 K) | [65] | |
| C. Verum tree activated carbon | 89.30 (313 K) | [66] | |
| Azoxystrobin | Moringa oleifera wood activated carbon | 34.13 (298 K) | Present study |
| UV-aged polyethylene | 235.4 (298 K) | [67] | |
| As-prepared silica nanoparticles | 0.85 (283 K) | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, H.; Bento, E.; Tavares, M.D.; Santos, Y.; da Costa, J.G.; do Nascimento, R.; Salvestrini, S.; Teixeira, R. Removal of Azoxystrobin and Deltamethrin from Water Using Activated Biochar from Moringa oleifera L. Wood: Synthesis, Characterization, and Adsorption Study. Molecules 2025, 30, 2757. https://doi.org/10.3390/molecules30132757
Gomes H, Bento E, Tavares MD, Santos Y, da Costa JG, do Nascimento R, Salvestrini S, Teixeira R. Removal of Azoxystrobin and Deltamethrin from Water Using Activated Biochar from Moringa oleifera L. Wood: Synthesis, Characterization, and Adsorption Study. Molecules. 2025; 30(13):2757. https://doi.org/10.3390/molecules30132757
Chicago/Turabian StyleGomes, Hiago, Ellen Bento, Maria Dayrine Tavares, Yannice Santos, José Galberto da Costa, Ronaldo do Nascimento, Stefano Salvestrini, and Raimundo Teixeira. 2025. "Removal of Azoxystrobin and Deltamethrin from Water Using Activated Biochar from Moringa oleifera L. Wood: Synthesis, Characterization, and Adsorption Study" Molecules 30, no. 13: 2757. https://doi.org/10.3390/molecules30132757
APA StyleGomes, H., Bento, E., Tavares, M. D., Santos, Y., da Costa, J. G., do Nascimento, R., Salvestrini, S., & Teixeira, R. (2025). Removal of Azoxystrobin and Deltamethrin from Water Using Activated Biochar from Moringa oleifera L. Wood: Synthesis, Characterization, and Adsorption Study. Molecules, 30(13), 2757. https://doi.org/10.3390/molecules30132757









