Impact of Chemicals and Processing Treatments on Thermo-Mechanical Recycling of Polyester Textiles
Abstract
1. Introduction
2. Methodology
- Identify potential risks with recycling contaminated PET textile waste during each stage of the manufacturing process;
- List and categorise the most common additives and contaminants present during the whole life cycle of a textile product;
- Give examples of the effects that various classes of contaminants may have on recycling;
- Present less chemically intensive alternative textile production methods with a critical analysis of their advantages and limitations where possible.
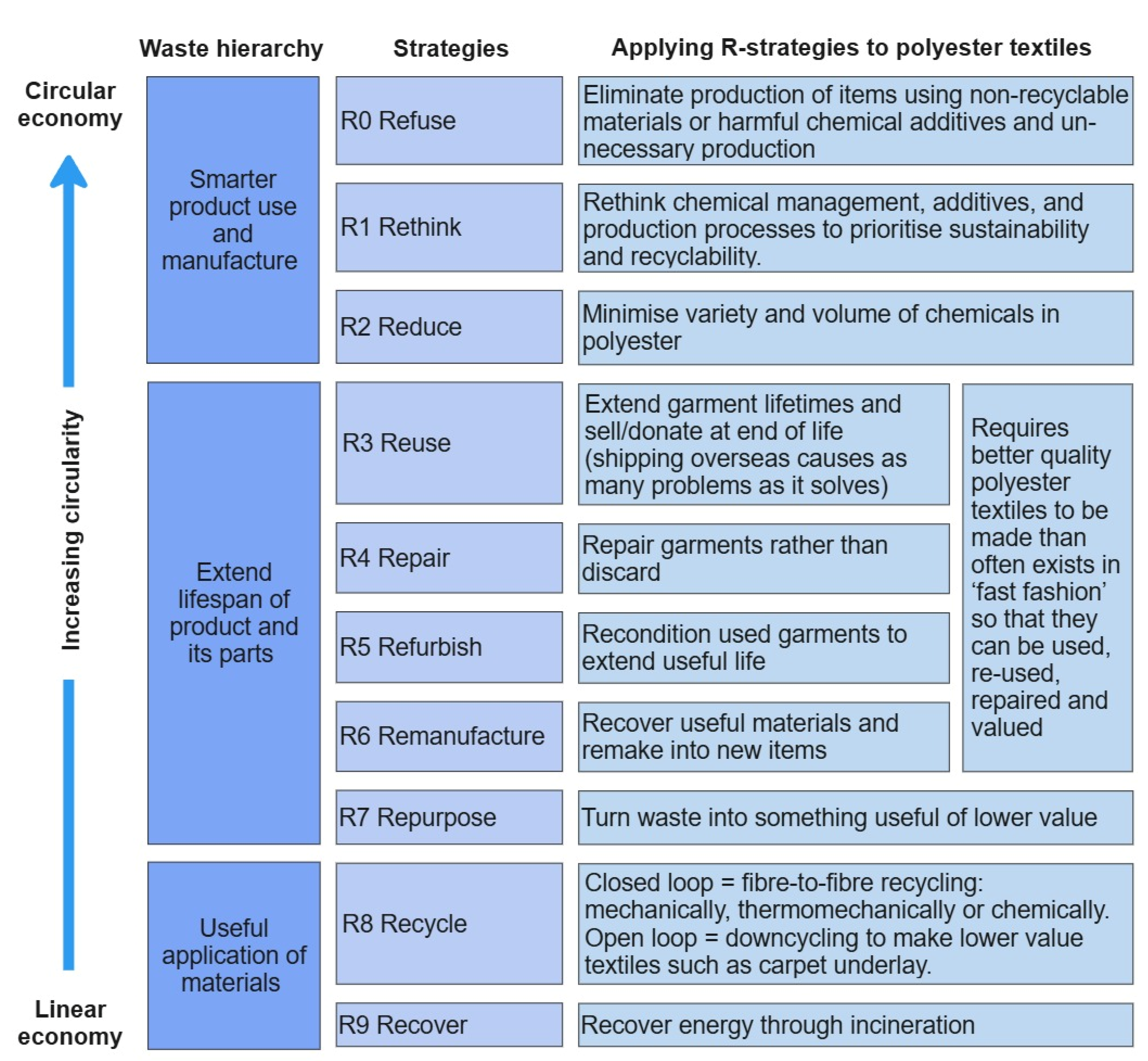
3. Additives in Textiles
3.1. Polyester Structures
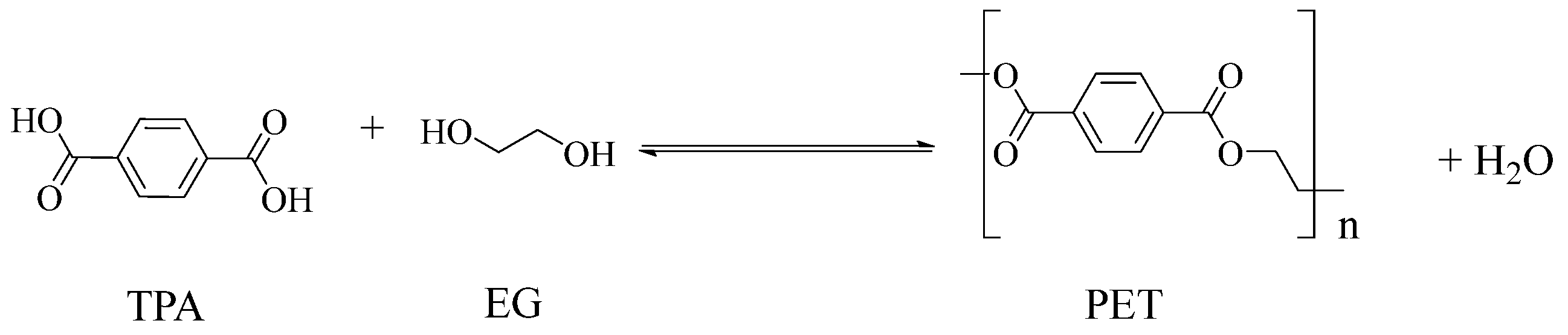
| Polyester * | Diacid(s) | Diol(s) (b) | Selected Properties |
|---|---|---|---|
Poly(ethylene terephthalate) (PET)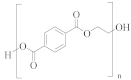 | TPA or DMT (a) | EG | --- |
Poly(butylene terephthalate) (PBT)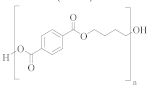 | TPA or DMT | BD | Greater alkaline hydrolysis resistance, crystallises faster [57] |
Poly(trimethlyene terephthalate) (PTT)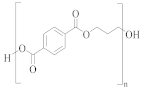 | TPA or DMT | PD | Greater alkaline hydrolysis resistance, crystallises faster [57] |
Poly(cyclohexanedimethylene terephthalate) (PCT) | TPA or DMT | CHDM | Processing temperatures > 300 °C, near decomposition temperature [58] |
Poly(ethylene naphthalate) (PEN)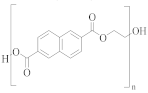 | naphthalene-2,6-dicarboxylic acid | EG | Greater alkaline hydrolysis resistance |
Poly(ethylene 2,5-furandicarboxylate) (PEF)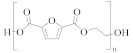 | TPA or DMT | EG | Lower melting temperature, derived from biomass [59] |
| Polyester | Diacid(s) (a) | Diol(s) | Selected Properties |
|---|---|---|---|
Cationic dyeable PET (CDP)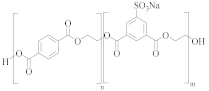 | SIPE and TPA | EG | Lower resistance to thermo-oxidative degradation [61] |
poly(ethylene terephthalate-co-diethylene terephthalate)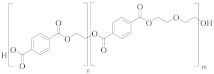 | TPA or DMT | EG and DEG (1–4 mol%) | Lower resistance to thermo-oxidative degradation [53,54] |
poly(ethylene terephthalate-co-isophthalate) (CPET) | isophthalic acid (IPA) (<5 mol%) and TPA or DMT | EG | |
poly(ethylene-co-1,3-butylene terephthalate)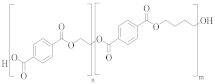 | TPA or DMT | EG and butane-1,4-diol (<5 mol%) | Greater alkaline hydrolysis resistance, crystallises faster [57] |
poly(ethylene-co-1,3-propylene terephthalate)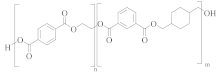 | TPA or DMT | EG and propane-1,3-diol (<5 mol%) | Greater alkaline hydrolysis resistance, crystallises faster [57] |
poly(ethylene-co-1,4-cyclohexanedimethanol terephthalate) (PETG)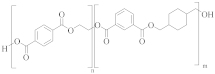 | TPA or DMT | EG and CHDM (≤50 mol%) | Greater hydrolysis resistance [62] |
Poly(cyclohexanedimethlyene terephthalate-co-isophthalate) (PCTA)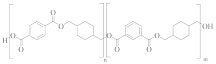 | TPA and IPA (>35 mol%) | CHDM | Greater hydrolysis resistance [58] |
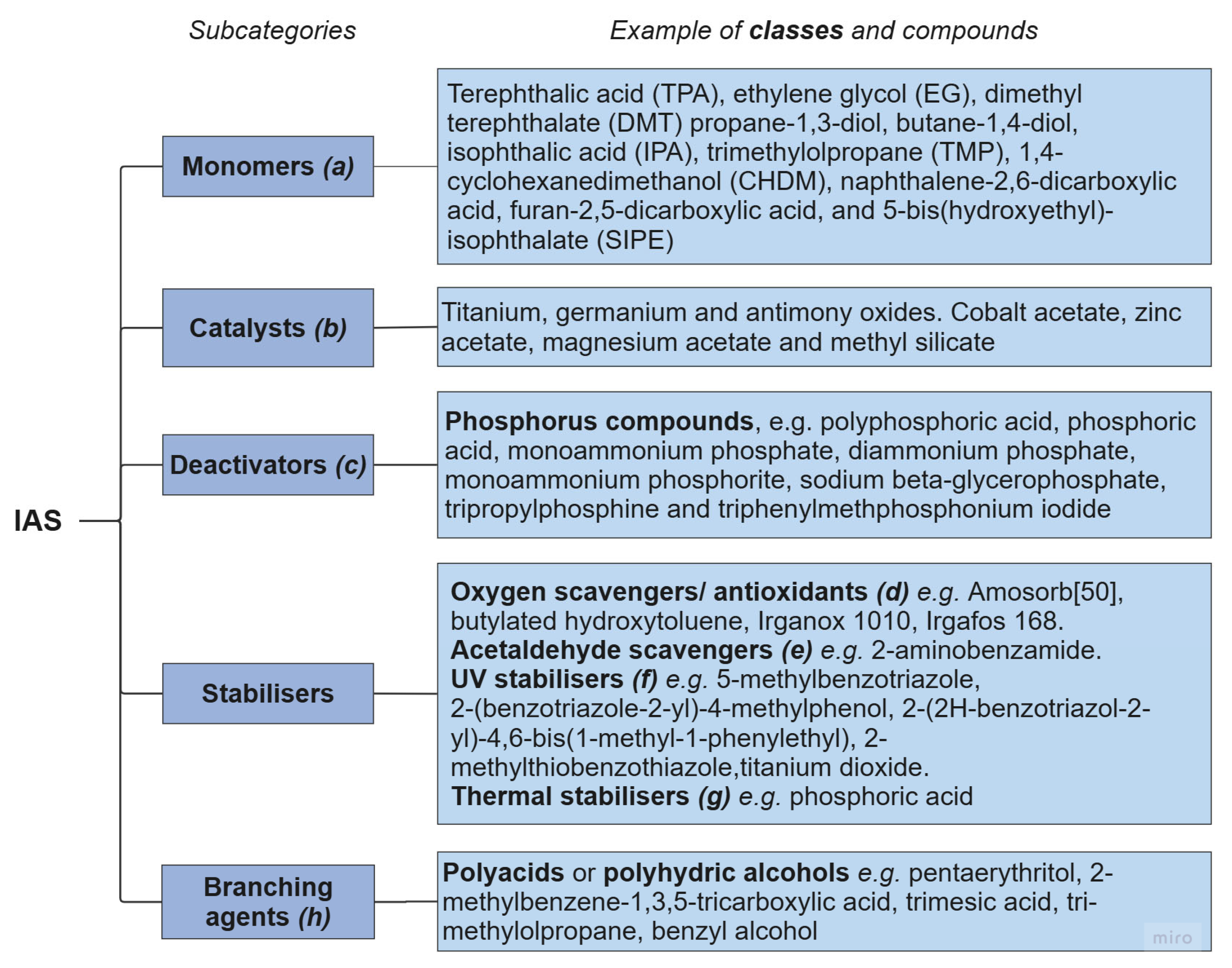
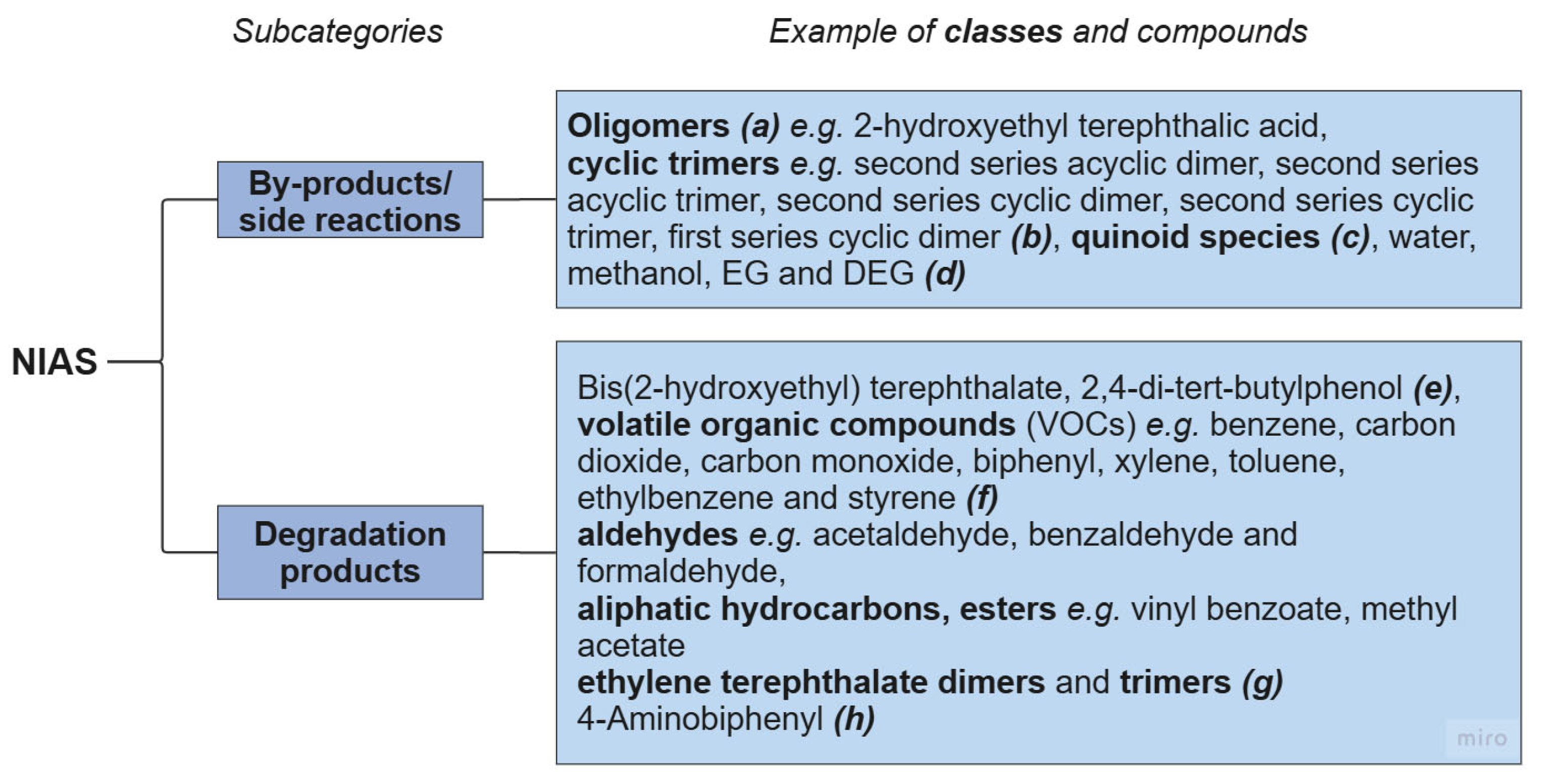
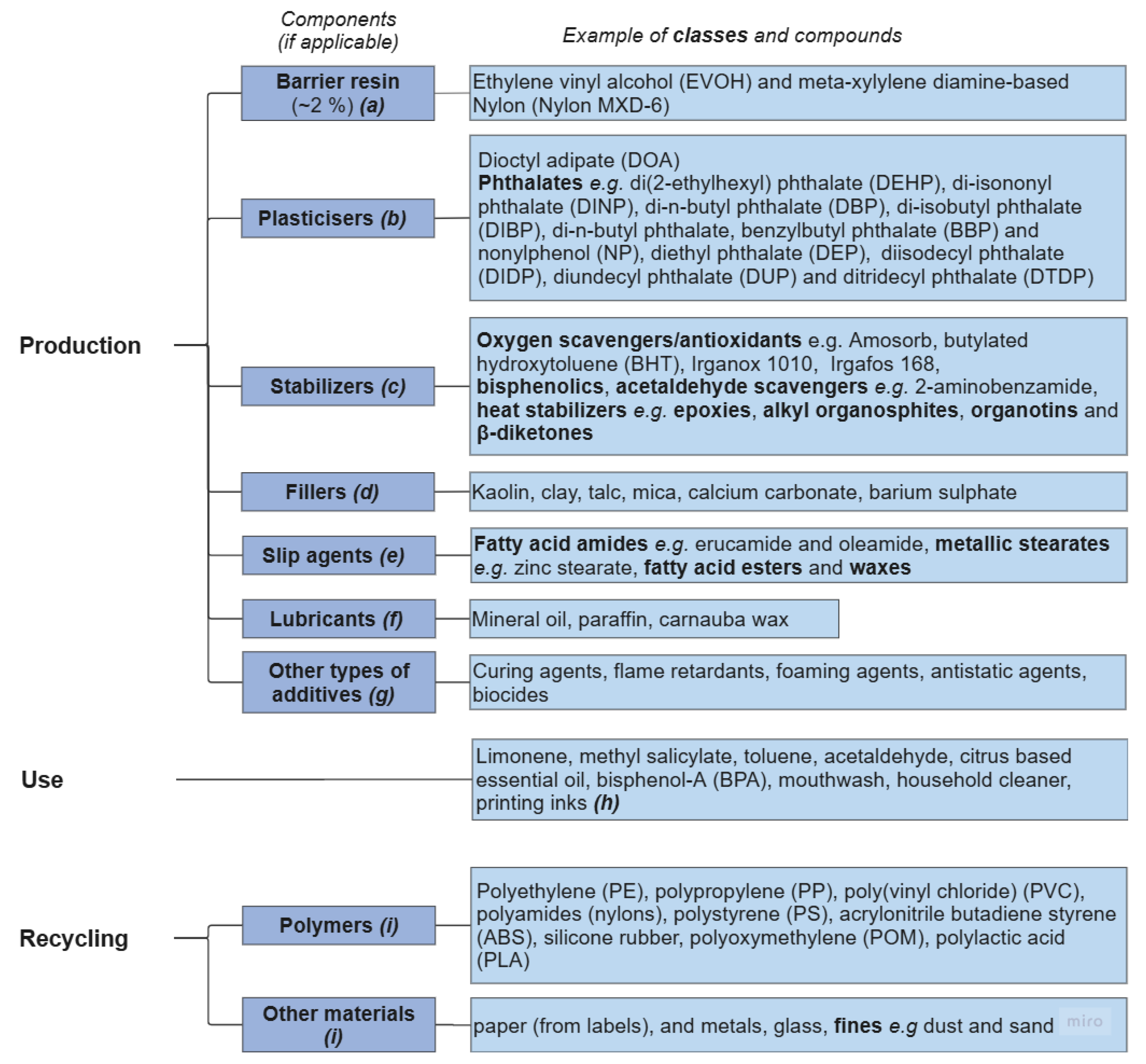
3.2. Contaminants from Synthesis and Polymer Processing
3.3. Recycled PET (rPET) in Textiles
3.4. Processing Polyester Yarns for Fabric Production
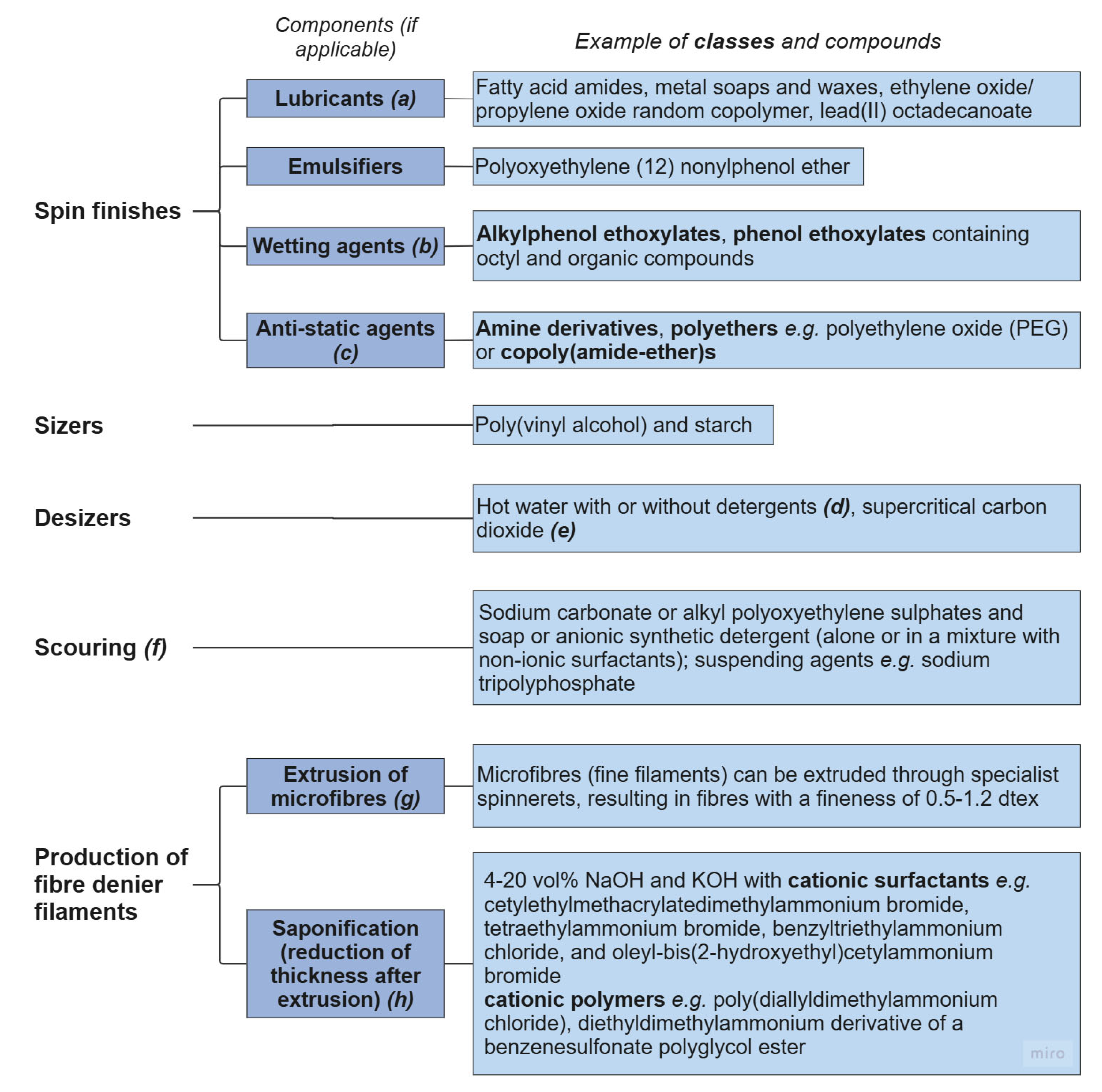
3.4.1. Spin Finishes, Sizing, and Pretreatment
3.4.2. Chemical Reduction of Linear Density (Optional Process)
3.4.3. Heat Setting for Textured Multi-Filaments
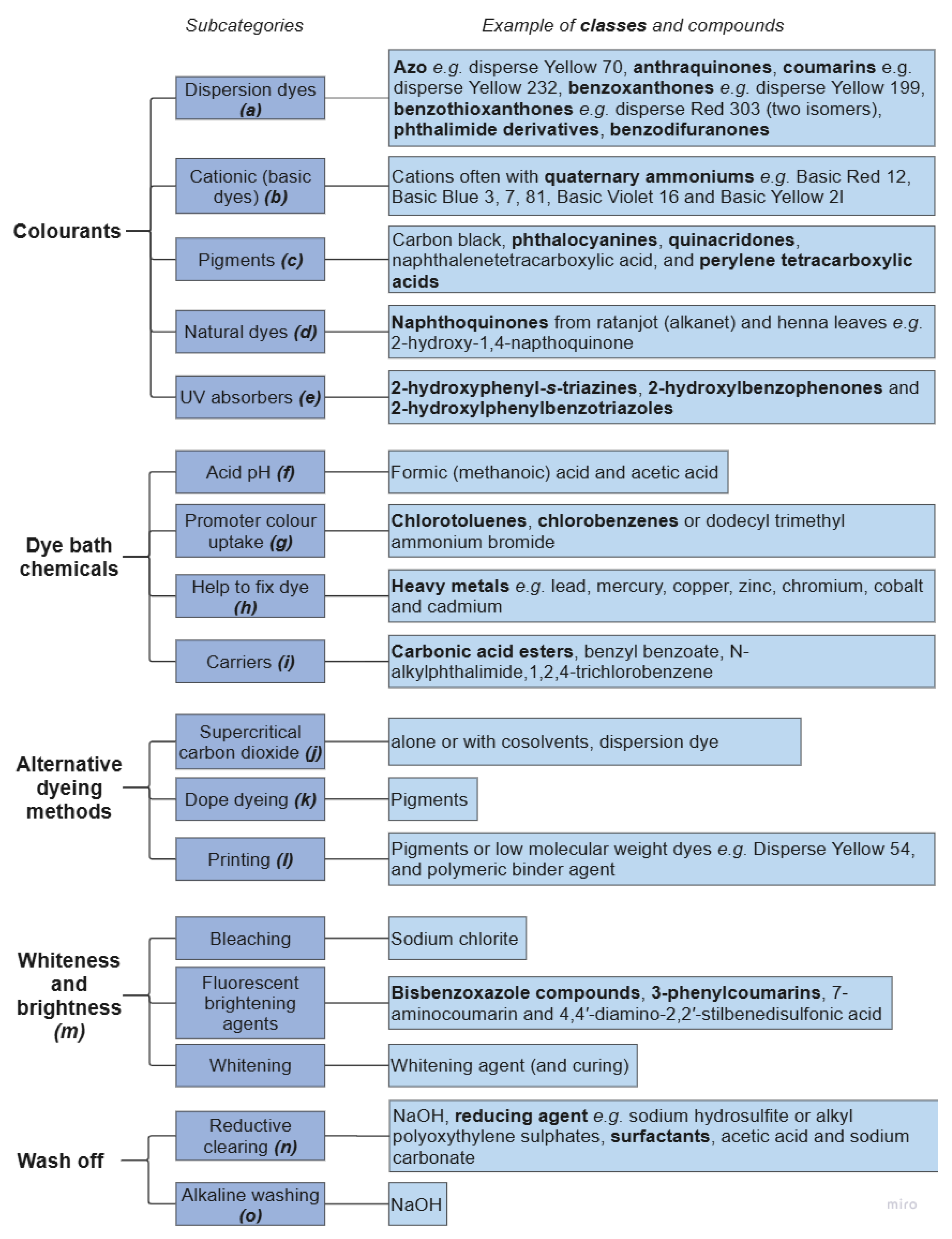
3.5. Colouration of Polyester Yarns and Fabric
3.6. Fabric Finishing Agents
3.6.1. Flame Retardants
3.6.2. Wetting/Wicking
3.6.3. Stain- and Water-Repellent Additives
3.6.4. Thermal and Mechanical Finishes for Polyester Fabric
3.7. Use Phase
3.8. Contamination During Recycling
4. Recycling Challenges
5. Outlook
- Textile production relies on specific additives for processing and in finishing treatments to increase durability. Completely removing chemicals in textiles is not feasible. Instead, research needs to be conducted to understand which chemicals catalyse degradation and find alternatives.
- Many of the less chemically intensive alternatives are more environmentally sustainable but currently have infrastructural issues and/or cost barriers to implementation.
- Some additives can increase resistance to certain types of degradation. Identifying these chemicals and encouraging their use in textiles could potentially increase the quality of recycled products.
- Polyester textiles should be designed to be recycled; chemicals and contaminants should be minimised and standardised wherever possible. Any chemicals used in PET production should be communicated to the brands, upstream manufacturers, and consumers in a permanent and readable manner.
- A ‘plastic textiles’ treaty, like the plastics treaty currently in negotiation, could benefit recyclers and the environment.
- PET specific recommendations:
- Limit DEG content in the original polymer as it significantly reduces the molecular weight of PET during reprocessing.
- Minimise PVC contamination. The hydrochloric acid formed during degradation can catalyse the hydrolysis of PET, reducing molecular weight.
- Avoid any concentrated alkaline treatment. The resulting increase in the number of carboxylic end groups is known to catalyse the hydrolysis of PET.
- Wicking and wetting agents should be avoided in favour of specially extruded (rather than alkaline-treated) channelled microfibre polyester, which exhibits similar properties.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BD | butane-1,4-diol |
| BHET | bis(2-hydroxyethyl) terephthalate |
| BPA | bisphenol-A |
| CHDM | 1,4-cyclohexanedimethanol |
| DEG | diethylene glycol |
| DEHP | di(2-ethylhexyl) phthalate |
| DINP | di-isononyl phthalate |
| DMT | dimethyl terephthalate |
| ECHA | European Chemicals Agency |
| EG | ethylene glycol |
| EPR | extended producer responsibility |
| FTOHs | fluorotelomer alcohols |
| IAS | intentionally added substances |
| IPA | isophthalic acid |
| Na2CO3 | sodium carbonate |
| NaOH | sodium hydroxide |
| NIAS | non-intentionally added substances |
| NIR | near-infrared |
| NP | nonylphenol |
| OP | octylphenol |
| PBT | poly(butylene terephthalate) |
| PD | propane-1,3-diol |
| PE | polyethylene |
| PEN | poly(ethylene naphthalate) |
| PET | poly(ethylene terephthalate) |
| PFAS | poly- and per-fluoroalkyl substances |
| PP | polypropylene |
| PS | polystyrene |
| PTT | poly(trimethlyene terephthalate) |
| PVC | poly(vinyl chloride) |
| RFID | radio frequency identification |
| rPET | recycled polyethylene terephthalate |
| ScCO2 | supercritical CO2 |
| SCFP | side-chain-fluorinated polymers |
| SIPE | 5-bis(hydroxyethyl)-isophthalate |
| TiO2 | titanium dioxide |
| TPA | terephthalic acid |
References
- Niinimäki, K.; Peters, G.; Dahlbo, H.; Perry, P.; Rissanen, T.; Gwilt, A. The environmental price of fast fashion. Nat. Rev. Earth Environ. 2020, 1, 189–200. [Google Scholar] [CrossRef]
- Le, K. Textile Recycling Technologies, Colouring and Finishing Methods; Solid Waste Services; The University of British Columbia: Vancouver, BC, Canada, 2018; pp. 23–50. [Google Scholar]
- Hayes, L.L. Synthetic textile innovations: Polyester fiber-to-fiber recycling for the advancement of sustainability. AATCC Rev. 2011, 11, 37–41. [Google Scholar]
- Candido, R.G. 17—Recycling of textiles and its economic aspects. In Fundamentals of Natural Fibres and Textiles; Mondal, M.I.H., Ed.; Woodhead Publishing: London, UK, 2021; pp. 599–624. [Google Scholar]
- Juanga-Labayen, J.P.; Labayen, I.V.; Yuan, Q. A Review on Textile Recycling Practices and Challenges. Textiles 2022, 2, 174–188. [Google Scholar] [CrossRef]
- Siliņa, L.; Dāboliņa, I.; Lapkovska, E. Sustainable textile industry—Wishful thinking or the new norm: A review. J. Eng. Fibers Fabr. 2024, 19, 1–27. [Google Scholar] [CrossRef]
- Wicker, A. Fast Fashion Is Creating an Environmental Crisis. Newsweek 1 September 2016. Available online: https://www.newsweek.com/2016/09/09/old-clothes-fashion-waste-crisis-494824.html (accessed on 1 May 2025).
- Guo, Z.; Eriksson, M.; de la Motte, H.; Adolfsson, E. Circular recycling of polyester textile waste using a sustainable catalyst. J. Clean. Prod. 2021, 283, 124579. [Google Scholar] [CrossRef]
- Potting, J.; Hekkert, M.P.; Worrell, E.; Hanemaaijer, A. Circular Economy: Measuring Innovation in the Product Chain; PBL Netherlands Assessment Agency: The Hague, The Netherlands, 2017. [Google Scholar]
- Enking, J.; Becker, A.; Schu, G.; Gausmann, M.; Cucurachi, S.; Tukker, A.; Gries, T. Recycling processes of polyester-containing textile waste—A review. Resour. Conserv. Recycl. 2025, 219, 108256. [Google Scholar] [CrossRef]
- Sandin, G.; Peters, G.M. Environmental impact of textile reuse and recycling—A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Celep, G.; Tetik, G.D.; Yilmaz, F. Limitations of Textile Recycling: The Reason behind the Development of Alternative Sustainable Fibers. In Next-Generation Textiles; Ibrahim, H., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Abbas-Abadi, M.S.; Tomme, B.; Goshayeshi, B.; Mynko, O.; Wang, Y.; Roy, S.; Kumar, R.; Baruah, B.; De Clerck, K.; De Meester, S.; et al. Advancing Textile Waste Recycling: Challenges and Opportunities Across Polymer and Non-Polymer Fiber Types. Polymers 2025, 17, 628. [Google Scholar] [CrossRef]
- Blum, P. Circular Fashion: Making the Fashion Industry Sustainable; Laurence King Publishing: London, UK, 2021. [Google Scholar]
- Gupta, R.; Kushwaha, A.; Dave, D.; Mahanta, N.R. Chapter 10—Waste management in fashion and textile industry: Recent advances and trends, life-cycle assessment, and circular economy. In Emerging Trends to Approaching Zero Waste; Hussain, C.M., Singh, S., Goswami, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–242. [Google Scholar]
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef]
- Barnard, E.; Rubio Arias, J.J.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Tavares, A.A.; Silva, D.F.A.; Lima, P.S.; Andrade, D.L.A.C.S.; Silva, S.M.L.; Canedo, E.L. Chain extension of virgin and recycled polyethylene terephthalate. Polym. Test. 2016, 50, 26–32. [Google Scholar] [CrossRef]
- Elander, M.; Ljungkvist, H. Critical Aspects in Design for Fiber-to-Fiber Recycling of Textiles; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016. [Google Scholar]
- Keßler, L.; Matlin, S.A.; Kümmerer, K. The contribution of material circularity to sustainability—Recycling and reuse of textiles. Curr. Opin. Green Sustain. Chem. 2021, 32, 100535. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Shilpa, G.N.; Jayprakash, V.L.; Prashant, R.G.; Krishna, R.; Anil, K.K. Degradation and Recyclability of Poly (Ethylene Terephthalate). In Polyester; Hosam El-Din, M.S., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 75–97. [Google Scholar]
- Ronkay, F.; Molnár, B.; Szabó, E.; Marosi, G.; Bocz, K. Water boosts reactive toughening of PET. Polym. Degrad. Stab. 2022, 203, 110052. [Google Scholar] [CrossRef]
- Zhang, J.M.; Hua, Q.; Reynolds, C.T.; Zhao, Y.; Dai, Z.; Bilotti, E.; Tang, J.; Peijs, T. Preparation of High Modulus Poly(Ethylene Terephthalate): Influence of Molecular Weight, Extrusion, and Drawing Parameters. Int. J. Polym. Sci. 2017, 2017, 2781425. [Google Scholar] [CrossRef]
- Textile Exchange. Materials Market Report 2024. Available online: https://textileexchange.org/knowledge-center/reports/materials-market-report-2024.pdf (accessed on 25 April 2025).
- Braun, G.; Som, C.; Schmutz, M.; Hischier, R. Environmental Consequences of Closing the Textile Loop—Life Cycle Assessment of a Circular Polyester Jacket. Appl. Sci. 2021, 11, 2964. [Google Scholar] [CrossRef]
- Palacios-Mateo, C.; van der Meer, Y.; Seide, G. Analysis of the polyester clothing value chain to identify key intervention points for sustainability. Environ. Sci. Eur. 2021, 33, 2. [Google Scholar] [CrossRef]
- Schönberger, H.; Schäfer, T. Best Available Techniques in Textile Industry; 20094329; Publications Office of the European Union: Berlin, Germany, 2003. [Google Scholar]
- Carlsson, J.; Iadaresta, F.; Eklund, J.; Avagyan, R.; Östman, C.; Nilsson, U. Suspect and non-target screening of chemicals in clothing textiles by reversed-phase liquid chromatography/hybrid quadrupole-Orbitrap mass spectrometry. Anal. Bioanal. Chem. 2022, 414, 1403–1413. [Google Scholar] [CrossRef]
- Xia, C.; Diamond, M.L.; Peaslee, G.F.; Peng, H.; Blum, A.; Wang, Z.; Shalin, A.; Whitehead, H.D.; Green, M.; Schwartz-Narbonne, H.; et al. Per- and Polyfluoroalkyl Substances in North American School Uniforms. Environ. Sci. Technol. 2022, 56, 13845–13857. [Google Scholar] [CrossRef]
- van der Veen, I.; Hanning, A.-C.; Stare, A.; Leonards, P.E.G.; de Boer, J.; Weiss, J.M. The effect of weathering on per- and polyfluoroalkyl substances (PFASs) from durable water repellent (DWR) clothing. Chemosphere 2020, 249, 126100. [Google Scholar] [CrossRef]
- Abdallah, M.A.-E.; Drage, D.S.; Sharkey, M.; Berresheim, H.; Harrad, S. A rapid method for the determination of brominated flame retardant concentrations in plastics and textiles entering the waste stream. J. Sep. Sci. 2017, 40, 3873–3881. [Google Scholar] [CrossRef]
- Xue, J.; Liu, W.; Kannan, K. Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing. Environ. Sci. Technol. 2017, 51, 5279–5286. [Google Scholar] [CrossRef] [PubMed]
- Luongo, G.; Iadaresta, F.; Moccia, E.; Östman, C.; Crescenzi, C. Determination of aniline and quinoline compounds in textiles. J. Chromatogr. A 2016, 1471, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Avagyan, R.; Luongo, G.; Thorsén, G.; Östman, C. Benzothiazole, benzotriazole, and their derivates in clothing textiles—A potential source of environmental pollutants and human exposure. Environ. Sci. Pollut. Res. 2015, 22, 5842–5849. [Google Scholar] [CrossRef] [PubMed]
- Rovira, J.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Human exposure to trace elements through the skin by direct contact with clothing: Risk assessment. Environ. Res. 2015, 140, 308–316. [Google Scholar] [CrossRef]
- Brüschweiler, B.J.; Küng, S.; Bürgi, D.; Muralt, L.; Nyfeler, E. Identification of non-regulated aromatic amines of toxicological concern which can be cleaved from azo dyes used in clothing textiles. Regul. Toxicol. Pharmacol. 2014, 69, 263–272. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.P.; Kooi, M.W.; te Biesebeek, J.D. Consumer Exposure to Chemicals in Indoor Environment: A Specific Focus on Chemicals from Textile Products; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2010. [Google Scholar]
- Tuzen, M.; Onal, A.; Soylak, M. Determination of trace heavy metals in some textile products produced in Turkey. Bull. Chem. Soc. Ethiop. 2008, 22, 379–384. [Google Scholar] [CrossRef]
- El Darai, T.; Ter-Halle, A.; Blanzat, M.; Despras, G.; Sartor, V.; Bordeau, G.; Lattes, A.; Franceschi, S.; Cassel, S.; Chouini-Lalanne, N.; et al. Chemical recycling of polyester textile wastes: Shifting towards sustainability. Green Chem. 2024, 26, 6857–6885. [Google Scholar] [CrossRef]
- Ndagano, U.N.; Cahill, L.; Smullen, C.; Gaughran, J.; Kelleher, S.M. The Current State-of-the-Art of the Processes Involved in the Chemical Recycling of Textile Waste. Molecules 2025, 30, 299. [Google Scholar] [CrossRef]
- Stubbe, B.; Van Vrekhem, S.; Huysman, S.; Tilkin, R.G.; De Schrijver, I.; Vanneste, M. White Paper on Textile Fibre Recycling Technologies. Sustainability 2024, 16, 618. [Google Scholar] [CrossRef]
- Bundesinstitut für Risikobewertung BfR. Introduction to the Problems Surrounding Garment Textiles. Available online: http://www.bfr.bund.de/cm/349/introduction-to-the-problems-surrounding-garment-textiles.pdf (accessed on 25 April 2025).
- Castillo Castillo, A.; Brophy, K.; Von Holstein, I. Addressing Plastic Additives; Faculty of Engineeringm, Imperial College London: London, UK, 2023. [Google Scholar]
- Undas, A.K.; Groenen, M.; Peters, R.J.B.; van Leeuwen, S.P.J. Safety of recycled plastics and textiles: Review on the detection, identification and safety assessment of contaminants. Chemosphere 2023, 312, 137175. [Google Scholar] [CrossRef]
- Militký, J. 13—Tensile failure of polyester fibers. In Handbook of Properties of Textile and Technical Fibres, 2nd ed.; Bunsell, A.R., Ed.; Woodhead Publishing: London, UK, 2018; pp. 421–514. [Google Scholar]
- Veit, D. Polyester. In Fibers: History, Production, Properties, Market; Springer International Publishing: Cham, Switzerland, 2022; pp. 625–648. [Google Scholar]
- Odian, G. Step Polymerization. In Principles of Polymerization; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 39–197. [Google Scholar]
- Menczel, J.D. 10—Poly(ethylene naphthalate) [poly(ethylene-2,6-naphthalene dicarboxylate)]. In Thermal Analysis of Textiles and Fibers; Jaffe, M., Menczel, J.D., Eds.; Woodhead Publishing: London, UK, 2020; pp. 191–196. [Google Scholar]
- Doshi, Y. Polyethylene Naphthalate Market Analysis & Forecast: 2025–2032. Available online: https://www.coherentmarketinsights.com/market-insight/polyethylene-naphthalate-market-2907 (accessed on 18 June 2025).
- Kohan Textile Journal. Cationic Dyeable Polyester; A Great Fiber of Enormous Potential. Available online: https://kohantextilejournal.com/cationic-dyeable-polyester-a-great-fiber-of-enormous-potential (accessed on 18 June 2025).
- Decon. Cationic Dyeable Yarn. Available online: https://www.polyestermfg.com/cationic-dyeable-yarn/ (accessed on 18 June 2025).
- Nayak, S.; Labde, J.; Geedh, S.; Jaisingh, S.K.; Rao, K.; Venkatachalam, S.; Kelkar, A.K. Study on degradation reactions in polyethylene terephthalate containing 5-sulpho isophthalyl moieties. J. Appl. Polym. Sci. 2010, 118, 2791–2800. [Google Scholar] [CrossRef]
- Fiorillo, C.; Trossaert, L.; Bezeraj, E.; Debrie, S.; Ohnmacht, H.; Van Steenberge, P.H.M.; D’Hooge, D.R.; Edeleva, M. Molecular and material property variations during the ideal degradation and mechanical recycling of PET. RSC Sustain. 2024, 2, 3596–3637. [Google Scholar] [CrossRef]
- Lecomte, H.A.; Liggat, J.J. Degradation mechanism of diethylene glycol units in a terephthalate polymer. Polym. Degrad. Stab. 2006, 91, 681–689. [Google Scholar] [CrossRef]
- Rieckmann, T.; Besse, K.; Frei, F.; Völker, S. Quantification of Colour Formation in PET Depending on SSP Residence Time, Temperature, and Oxygen Concentration. Macromol. Symp. 2013, 333, 162–171. [Google Scholar] [CrossRef]
- Feldman, D.; Barbalata, A. Polyesters. In Synthetic Polymers: Technology, Properties, Applications; Chapman & Hall: London, UK, 1996. [Google Scholar]
- İçoğlu, H.I. Comparative Analysis of PET, PTT and PBT Yarns Hydrolyzed by Alkali. Text. Appar. 2022, 32, 57–64. [Google Scholar] [CrossRef]
- Lim, H.C.A. Chapter 20—Thermoplastic Polyesters. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 527–543. [Google Scholar]
- Cui, Y.; Deng, C.; Fan, L.; Qiu, Y.; Zhao, L. Progress in the biosynthesis of bio-based PET and PEF polyester monomers. Green Chem. 2023, 25, 5836–5857. [Google Scholar] [CrossRef]
- Mather, R.R.; Wardman, R.H.; Rana, S. Synthetic Fibres. In The Chemistry of Textile Fibres, 3rd ed.; Royal Society of Chemistry: London, UK, 2023. [Google Scholar]
- Hu, Y.; Wang, Y.; Zhang, X.; Qian, J.; Xing, X.; Wang, X. Regenerated cationic dyeable polyester deriving from poly(ethylene terephthalate) waste. Polym. Degrad. Stab. 2020, 179, 109261. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, W.; Zhang, J. Alkali resistance of poly(ethylene terephthalate) (PET) and poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate) (PETG) copolyesters: The role of composition. Polym. Degrad. Stab. 2015, 120, 232–243. [Google Scholar] [CrossRef]
- Kaiho, S.; Hmayed, A.A.R.; Delle Chiaie, K.R.; Worch, J.C.; Dove, A.P. Designing Thermally Stable Organocatalysts for Poly(ethylene terephthalate) Synthesis: Toward a One-Pot, Closed-Loop Chemical Recycling System for PET. Macromolecules 2022, 55, 10628–10639. [Google Scholar] [CrossRef]
- Dhaka, V.; Singh, S.; Anil, A.G.; Sunil Kumar Naik, T.S.; Garg, S.; Samuel, J.; Kumar, M.; Ramamurthy, P.C.; Singh, J. Occurrence, toxicity and remediation of polyethylene terephthalate plastics. A review. Environ. Chem. Lett. 2022, 20, 1777–1800. [Google Scholar] [CrossRef]
- Kim, D.-J.; Lee, K.-T. Determination of monomers and oligomers in polyethylene terephthalate trays and bottles for food use by using high performance liquid chromatography-electrospray ionization-mass spectrometry. Polym. Test. 2012, 31, 490–499. [Google Scholar] [CrossRef]
- Nichols, C.S.; Moore, T.C.; Edwards, W.L. Method of Catalyst Deactivation in Continuous Polyethylene Terephthalate Production. WO1997044376A1 Patent 27 November 1997. [Google Scholar]
- Barker, R.H. Additives in fibers and fabrics. Environ. Health Perspect. 1975, 11, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Thoden van Velzen, U.; Brouwer, M.; Molenveld, K. Technical Quality of rPET; Rapport 1661; Wageningen UR Food & Biobased Research: Wageningen, The Netherlands, 2016. [Google Scholar]
- Gerassimidou, S.; Lanska, P.; Hahladakis, J.N.; Lovat, E.; Vanzetto, S.; Geueke, B.; Groh, K.J.; Muncke, J.; Maffini, M.; Martin, O.V.; et al. Unpacking the complexity of the PET drink bottles value chain: A chemicals perspective. J. Hazard. Mater. 2022, 430, 128410. [Google Scholar] [CrossRef]
- Nerin, C.; Alfaro, P.; Aznar, M.; Domeño, C. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Anal. Chim. Acta 2013, 775, 14–24. [Google Scholar] [CrossRef]
- Mather, R.R.; Wardman, R.H.; Rana, S. Chapter 8: Enhancement of Fibre Performance by Surface Modification. In The Chemistry of Textile Fibres; Royal Society of Chemistry: London, UK, 2023; pp. 315–371. [Google Scholar]
- Jaffe, M.; Easts, A.J.; Feng, X. 8—Polyester fibers. In Thermal Analysis of Textiles and Fibers; Jaffe, M., Menczel, J.D., Eds.; Woodhead Publishing: London, UK, 2020; pp. 133–149. [Google Scholar]
- Mohammadi Avarzman, A.; Rafizadeh, M.; Afshar Taromi, F. Branched polyester based on the polyethylene tere/iso phthalate and trimellitic anhydride as branching agent. Polym. Bull. 2021, 79, 6099–6121. [Google Scholar] [CrossRef]
- Biver, M.; Turner, A.; Filella, M. Antimony release from polyester textiles by artificial sweat solutions: A call for a standardized procedure. Regul. Toxicol. Pharmacol. 2021, 119, 104824. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Wang, Y.; Gan, X.; Wang, N. Preparation and Properties of Opaque Polyethylene Terephthalate/TiO2 Filaments. Mater. Sci. 2021, 27, 325–329. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Z.; Kong, F.; Ma, X. The effect of metal catalyst on the discoloration of poly(ethylene terephthalate) in thermo-oxidative degradation. Polym. Degrad. Stab. 2010, 95, 53–58. [Google Scholar] [CrossRef]
- Nishioji, H.; Hashimoto, H.; Iida, Y.; Saisho, H. EXAFS Study of a Germanium Catalyst in PET Polymer. Jpn. J. Appl. Phys. 1993, 32, 517. [Google Scholar] [CrossRef]
- Wadey, B.L. Plasticizers. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 441–456. [Google Scholar]
- Jensen, A.A.; Knudsen, H.N. Total health assessment of chemicals in indoor climate from various consumer products. Surv. Chem. Subst. Consum. Prod. 2006, 75. [Google Scholar]
- Saini, A.; Thaysen, C.; Jantunen, L.; McQueen, R.H.; Diamond, M.L. From Clothing to Laundry Water: Investigating the Fate of Phthalates, Brominated Flame Retardants, and Organophosphate Esters. Environ. Sci. Technol. 2016, 50, 9289–9297. [Google Scholar] [CrossRef] [PubMed]
- Pivnenko, K.; Eriksen, M.K.; Martín-Fernández, J.A.; Eriksson, E.; Astrup, T.F. Recycling of plastic waste: Presence of phthalates in plastics from households and industry. Waste Manag. 2016, 54, 44–52. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, W.A. New advances in poly(ethylene terephthalate) polymerization and degradation. Polym. Int. 2002, 51, 923–930. [Google Scholar] [CrossRef]
- Coniglio, M.; Fioriglio, C.; Laganà, P. Non-Intentionally Added Substances in PET-Bottled Mineral Water; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Textile Exchange. Materials Market Report 2023. Available online: https://textileexchange.org/knowledge-center/documents/materials-market-report-2023/ (accessed on 31 March 2025).
- Hussain, M.; Ashraf, M.; Kaleem Ullah, H.M.; Akram, S. Recycling in Textiles; Springer Nature: Cham, Switzerland, 2023; pp. 177–212. [Google Scholar]
- Farah, S.; Kunduru, K.R.; Basu, A.; Domb, A.J. 8—Molecular Weight Determination of Polyethylene Terephthalate. In Poly(Ethylene Terephthalate) Based Blends, Composites and Nanocomposites; Visakh, P.M., Liang, M., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 143–165. [Google Scholar]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Laursen, S.; Hansen, J.; Pommer, K. Survey of Chemical Compounds in Textile Fabrics; Survey of Chemicals in Consumer Products, No. 23; Danish Environmental Protection Agency: Odense, Denmark, 2003. [Google Scholar]
- Keresztes, S.; Tatár, E.; Czégény, Z.; Záray, G.; Mihucz, V.G. Study on the leaching of phthalates from polyethylene terephthalate bottles into mineral water. Sci. Total Environ. 2013, 458–460, 451–458. [Google Scholar] [CrossRef]
- Dreolin, N.; Aznar, M.; Moret, S.; Nerin, C. Development and validation of a LC–MS/MS method for the analysis of bisphenol a in polyethylene terephthalate. Food Chem. 2019, 274, 246–253. [Google Scholar] [CrossRef]
- Qu, M.; Lu, D.; Deng, H.; Wu, Q.; Han, L.; Xie, Z.; Qin, Y.; Schubert, D.W. A comprehensive study on recycled and virgin PET melt-spun fibers modified by PMDA chain extender. Mater. Today Commun. 2021, 29, 103013. [Google Scholar] [CrossRef]
- Salazar-Beltrán, D.; Hinojosa-Reyes, L.; Palomino-Cabello, C.; Turnes-Palomino, G.; Hernández-Ramírez, A.; Guzmán-Mar, J.L. Determination of phthalate acid esters plasticizers in polyethylene terephthalate bottles and its correlation with some physicochemical properties. Polym. Test. 2018, 68, 87–94. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, G.; Lei, K.; LeBlanc, G.A.; An, L. Phthalate Esters and Their Potential Risk in PET Bottled Water Stored under Common Conditions. Int. J. Environ. Res. Public Health 2019, 17, 141. [Google Scholar] [CrossRef]
- PET Resin Association (PETRA). The Science Behind PET. Available online: https://petresin.org/the-science-behind-pet/ (accessed on 18 June 2025).
- Lim, J.; Ahn, Y.; Cho, H.; Kim, J. Optimal strategy to sort plastic waste considering economic feasibility to increase recycling efficiency. Process Saf. Environ. Prot. 2022, 165, 420–430. [Google Scholar] [CrossRef]
- Itim, B.; Philip, M. Effect of multiple extrusions and influence of PP contamination on the thermal characteristics of bottle grade recycled PET. Polym. Degrad. Stab. 2015, 117, 84–89. [Google Scholar] [CrossRef]
- Paci, M.; La Mantia, F.P. Influence of small amounts of polyvinylchloride on the recycling of polyethyleneterephthalate. Polym. Degrad. Stab. 1999, 63, 11–14. [Google Scholar] [CrossRef]
- Pavan, M.; Samant, L.; Mahajan, S.; Kaur, M. Role of Chemicals in Textile Processing and Its Alternatives. Springer Nature: Singapore, 2024; pp. 55–72. [Google Scholar]
- Rovira, J.; Domingo, J.L. Human health risks due to exposure to inorganic and organic chemicals from textiles: A review. Environ. Res. 2019, 168, 62–69. [Google Scholar] [CrossRef]
- Khan, W.U.; Ahmed, S.; Dhoble, Y.; Madhav, S. A critical review of hazardous waste generation from textile industries and associated ecological impacts. J. Indian Chem. Soc. 2023, 100, 100829. [Google Scholar] [CrossRef]
- Sano, Y.; Lee, C.W.; Kimura, Y.; Saegusa, T. A novel spin-finishing method for antistatic modification of polyester fiber. Surface reaction onto amine-containing copoly(amide-ethers) blended with polyester fiber. Die Angew. Makromol. Chem. 1997, 246, 109–123. [Google Scholar] [CrossRef]
- Antony, A.; Raj, A.; Ramachandran, J.P.; Ramakrishnan, R.M.; Wallen, S.L.; Raveendran, P. Sizing and Desizing of Cotton and Polyester Yarns Using Liquid and Supercritical Carbon Dioxide with Nonfluorous CO2-Philes as Size Compounds. ACS Sustain. Chem. Eng. 2018, 6, 12275–12280. [Google Scholar] [CrossRef]
- Roy Choudhury, A.K. 3—Pre-treatment and preparation of textile materials prior to dyeing. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing Limited: London, UK, 2011; Volume 1, pp. 64–149. [Google Scholar]
- Srinivasan, J. 6—Engineering finer and softer textile yarns. In Technical Textile Yarns; Alagirusamy, R., Das, A., Eds.; Woodhead Publishing: London, UK, 2010; pp. 185–214. [Google Scholar]
- Čorak, I.; Tarbuk, A.; Đorđević, D.; Višić, K.; Botteri, L. Sustainable Alkaline Hydrolysis of Polyester Fabric at Low Temperature. Materials 2022, 15, 1530. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Chen, T.-K. Effect of surface treatment on PET spinning and the yarn property. Colloids Surf. A Physicochem. Eng. Asp. 2007, 295, 75–80. [Google Scholar] [CrossRef]
- Hatch, K.L. Preparatory and final finishes. In Textile Science; West Publishing Company: Eagan, MN, USA, 1993; p. 386. [Google Scholar]
- Nguyen, T.N.; Rangel, A.; Grainger, D.W.; Migonney, V. Influence of spin finish on degradation, functionalization and long-term storage of polyethylene terephthalate fabrics dedicated to ligament prostheses. Sci. Rep. 2021, 11, 4258. [Google Scholar] [CrossRef]
- Rahman, M.; East, G. The Effect of Spin Finishes on Hydrolysis Rates of Medical Grade Polyester. J. Appl. Biomater. Biomech. 2009, 7, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Yu, D.; Zhao, K. Durable Moisture-wicking and Fast-dry Polyester Fabric Prepared by UV-induced Click Reaction. Fibers Polym. 2020, 21, 111–118. [Google Scholar] [CrossRef]
- Phaneuf, M.D.; Quist, W.C.; Bide, M.J.; LoGerfo, F.W. Modification of polyethylene terephthalate (dacron) via denier reduction: Effects on material tensile strength, weight, and protein binding capabilities. J. Appl. Biomater. 1995, 6, 289–299. [Google Scholar] [CrossRef]
- Gawish, S.M.; Mosleh, S.; Ramadan, A.M. Synthesis of a new cationic surfactant for the alkaline hydrolysis of solvent-pretreated polyester fabrics. J. Appl. Polym. Sci. 2002, 85, 1652–1660. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Karayannidis, G.P. Effect of carboxylic end groups on thermooxidative stability of PET and PBT. Polym. Degrad. Stab. 1999, 63, 213–218. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, M.J.; Lee, B.S.; Lee, K.S. Effects of thermomigration on the washfastness of disperse dyes having different molecular size. Fibers Polym. 2004, 5, 39–43. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Sasaki, K.; Hirogaki, K.; Tabata, I.; Nakane, K. Supercritical fluid dyeing of polyester fabrics using polymeric nanofibers loaded with disperse dye. J. Supercrit. Fluids 2024, 211, 106289. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Su, Y.; Wei, M.; Li, H.; Liu, J. Determination of restricted dyes in textile raw material solid wastes by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2023, 1711, 464447. [Google Scholar] [CrossRef]
- Chavan, R.B. 16—Environmentally friendly dyes. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing: London, UK, 2011; Volume 1, pp. 515–561. [Google Scholar]
- Clark, M. 1—Fundamental principles of dyeing. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing: London, UK, 2011; Volume 1, pp. 3–27. [Google Scholar]
- Arora, A.; Rastogi, D.; Gupta, D.; Gulrajani, M. Dyeing parameters of hydroxynaphthoquinones extracted from Arnebia nobilis Rech.f. Indian J. Fibre Text. Res. 2012, 37, 91–97. [Google Scholar]
- Purwar, S. Application of natural dye on synthetic fabrics: A review. Int. J. Home Sci. 2016, 2, 283–287. [Google Scholar]
- Elnagar, K.; Abou Elmaaty, T.; Raouf, S. Dyeing of Polyester and Polyamide Synthetic Fabrics with Natural Dyes Using Ecofriendly Technique. J. Text. 2014, 363079. [Google Scholar] [CrossRef]
- Gurudatt, K.; De, P.; Rakshit, A.K.; Bardhan, M.K. Dope-dyed Polyester Fibers from Recycled PET Wastes for Use in Molded Automotive Carpets. J. Ind. Text. 2005, 34, 167–179. [Google Scholar] [CrossRef]
- Pająk, A.; Rybiński, P.; Janowska, G.; Kucharska-Jastrząbek, A. The thermal properties and the flammability of pigmented elastomeric materials. J. Therm. Anal. Calorim. 2014, 117, 789–798. [Google Scholar] [CrossRef]
- Gulrajani, M.L. 10—Disperse dyes. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing: London, UK, 2011; Volume 1, pp. 365–394. [Google Scholar]
- Sk, M.S.; Akram, W.; Mia, R.; Fang, J.; Kabir, S.M.M. Fabrication of UV-Protective Polyester Fabric with Polysorbate 20 Incorporating Fluorescent Color. Polymers 2022, 14, 4366. [Google Scholar] [CrossRef]
- Herbst, W.; Hunger, K. 1.8 Areas of Application for Organic Pigments In Industrial Organic Pigments, 3rd ed.; WILEY-VCH: Hoboken, NJ, USA, 2004. [Google Scholar]
- Mekonnen, B.; Asaye, A.; Wolela, D.; Dessie, A. Alkaline Hydrolysis of Polyester Fabric and Dyeing with Natural Colorants Extracted from Henna Leaves. Adv. Res. Text. Eng. 2023, 8, 7. [Google Scholar] [CrossRef]
- Moura, J.C.V.P.; Oliveira-Campos, A.M.F.; Griffiths, J. The effect of additives on the photostability of dyed polymers. Dye. Pigment. 1997, 33, 173–196. [Google Scholar] [CrossRef]
- European Outdoor Group. Chemicals in Textile Production; European Outdoor Group: Zug, Switzerland, 2022. [Google Scholar]
- Freeman, H.S.; Mason, M.E.; Lye, J. Disperse dyes containing a built-in oxalanilide stabilizer. Dye. Pigment. 1999, 42, 53–63. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Lin, H.-m.; Lee, M.-J. Solubility of disperse yellow 54 in supercritical carbon dioxide with or without cosolvent. Fluid Phase Equilibria 2007, 260, 287–294. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Kumar, N. The reduction clearing of dyed polyester. Part 1: Colour strength. Dye. Pigment. 2008, 76, 799–809. [Google Scholar] [CrossRef]
- Li, H.; Pei, L.; Zhang, H.; Wang, Z.; Saleem, M.A.; Alebeid, O.K.; Wang, J. Extraction of Cyclic Oligomer and Their Influence on Polyester Dyeing in a Silicone Waterless Dyeing System. Polymers 2021, 13, 3687. [Google Scholar] [CrossRef] [PubMed]
- Eren, H.A.; Anis, P. Surface Trimer Removal of Polyester Fibers by Ozone Treatment. Text. Res. J. 2009, 79, 652–656. [Google Scholar] [CrossRef]
- Paranjape, M.; Athalye, A. Eco-friendly Polyester Reduction Clearing: Examining Cutting-edge Approaches. Adv. Res. Text. Eng. 2024, 9, 1103. [Google Scholar]
- Zhan, Y.; Zhao, X.; Wang, W. Synthesis of phthalimide disperse dyes and study on the interaction energy. Dye. Pigment. 2017, 146, 240–250. [Google Scholar] [CrossRef]
- Kajiwara, N.; Sueoka, M.; Ohiwa, T.; Takigami, H. Determination of flame-retardant hexabromocyclododecane diastereomers in textiles. Chemosphere 2009, 74, 1485–1489. [Google Scholar] [CrossRef]
- Bascucci, C.; Duretek, I.; Lehner, S.; Holzer, C.; Gaan, S.; Hufenus, R.; Gooneie, A. Investigating thermomechanical recycling of poly(ethylene terephthalate) containing phosphorus flame. Polym. Degrad. Stab. 2022, 195, 109783. [Google Scholar] [CrossRef]
- Chen, J.; Dul, S.; Lehner, S.; Jovic, M.; Gaan, S.; Heuberger, M.; Hufenus, R.; Gooneie, A. Mechanical recycling of PET containing mixtures of phosphorus flame retardants. J. Mater. Sci. Technol. 2024, 194, 167–179. [Google Scholar] [CrossRef]
- Schmiermund, T. Flame Retardants. In The Chemistry Knowledge for Firefighters; Schmiermund, T., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2023; pp. 533–538. [Google Scholar]
- Tagliaro, I.; Mariani, M.; Akbari, R.; Contardi, M.; Summa, M.; Saliu, F.; Nisticò, R.; Antonini, C. PFAS-free superhydrophobic chitosan coating for fabrics. Carbohydr. Polym. 2024, 333, 121981. [Google Scholar] [CrossRef]
- Aldegunde-Louzao, N.; Lolo-Aira, M.; Herrero-Latorre, C. Phthalate esters in clothing: A review. Environ. Toxicol. Pharmacol. 2024, 108, 104457. [Google Scholar] [CrossRef]
- Mao, N.; Du, M. 10—Sol–gel-based treatments of textiles for water repellence. In Waterproof and Water Repellent Textiles and Clothing; Williams, J., Ed.; Woodhead Publishing: London, UK, 2018; pp. 233–265. [Google Scholar]
- The Lycra Company. About COOLMAX® Technology. Available online: https://www.lycra.com/en/frequently-asked-questions-coolmax/about-coolmax-technology (accessed on 20 June 2025).
- Zhang, X. 2—Antistatic and conductive textiles. In Functional Textiles for Improved Performance, Protection and Health; Pan, N., Sun, G., Eds.; Woodhead Publishing: London, UK, 2011; pp. 27–44. [Google Scholar]
- Ertekin, G.; Marmaralı, A. The Effect of Heat-Setting Conditions on the Performance Characteristics of Warp Knitted Spacer Fabrics. J. Eng. Fibers Fabr. 2016, 11, 64–71. [Google Scholar] [CrossRef]
- Conway, R. 7—Technical textile finishing. In Handbook of Technical Textiles, 2nd ed.; Horrocks, A.R., Anand, S.C., Eds.; Woodhead Publishing: London, UK, 2016; pp. 189–210. [Google Scholar]
- Das, B.; Das, A.; Kothari, V.K.; Fanguiero, R.; de Araújo, M. Effect of fibre diameter and cross-sectional shape on moisture transmission through fabrics. Fibers Polym. 2008, 9, 225–231. [Google Scholar] [CrossRef]
- Jabbar, A.; Bryant, M.; Armitage, J.; Tausif, M. Oxygen plasma treatment to mitigate the shedding of fragmented fibres (microplastics) from polyester textiles. Clean. Eng. Technol. 2024, 23, 100851. [Google Scholar] [CrossRef]
- Gabardo, R.S.; De Carvalho Cotre, D.S.; Lis Arias, M.J.; Moisés, M.P.; Martins Ferreira, B.T.; Samulewski, R.B.; Hinestroza, J.P.; Bezerra, F.M. Surface Modification of Polyester Fabrics by Ozone and Its Effect on Coloration Using Disperse Dyes. Materials 2021, 14, 3492. [Google Scholar] [CrossRef]
- Abrishami, S.; Shirali, A.; Sharples, N.; Kartal, G.E.; Macintyre, L.; Doustdar, O. Textile Recycling and Recovery: An Eco-friendly Perspective on Textile and Garment Industries Challenges. Text. Res. J. 2024, 94, 2815–2834. [Google Scholar] [CrossRef]
- Xia, D.; Zhang, H.; Ju, Y.; Xie, H.-b.; Su, L.; Ma, F.; Jiang, J.; Chen, J.; Francisco, J.S. Spontaneous Degradation of the “Forever Chemicals” Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) on Water Droplet Surfaces. J. Am. Chem. Soc. 2024, 146, 11266–11271. [Google Scholar] [CrossRef]
- Lohmann, R.; Letcher, R.J. The universe of fluorinated polymers and polymeric substances and potential environmental impacts and concerns. Curr. Opin. Green Sustain. Chem. 2023, 41, 100795. [Google Scholar] [CrossRef]
- Rodgers, K.M.; Swartz, C.H.; Occhialini, J.; Bassignani, P.; McCurdy, M.; Schaider, L.A. How Well Do Product Labels Indicate the Presence of PFAS in Consumer Items Used by Children and Adolescents? Environ. Sci. Technol. 2022, 56, 6294–6304. [Google Scholar] [CrossRef]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef]
- Spyrakis, F.; Dragani, T.A. The EU’s Per- and Polyfluoroalkyl Substances (PFAS) Ban: A Case of Policy over Science. Toxics 2023, 11, 721. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Crone, B.C.; Speth, T.F.; Wahman, D.G.; Smith, S.J.; Abulikemu, G.; Kleiner, E.J.; Pressman, J.G. Occurrence of Per- and Polyfluoroalkyl Substances (PFAS) in Source Water and Their Treatment in Drinking Water. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2359–2396. [Google Scholar] [CrossRef] [PubMed]
- Kahoush, M.; Kadi, N. Towards sustainable textile sector: Fractionation and separation of cotton/polyester fibers from blended textile waste. Sustain. Mater. Technol. 2022, 34, e00513. [Google Scholar] [CrossRef]
- Horn, S.; Mölsä, K.M.; Sorvari, J.; Tuovila, H.; Heikkilä, P. Environmental sustainability assessment of a polyester T-shirt—Comparison of circularity strategies. Sci. Total Environ. 2023, 884, 163821. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.K.; Andrady, A.L.; Bornman, J.F.; Aucamp, P.J.; Bais, A.F.; Banaszak, A.T.; Barnes, P.W.; Bernhard, G.H.; Bruckman, L.S.; Busquets, R.; et al. Plastics in the environment in the context of UV radiation, climate change and the Montreal Protocol: UNEP Environmental Effects Assessment Panel, Update 2023. Photochem. Photobiol. Sci. 2024, 23, 629–650. [Google Scholar] [CrossRef]
- Guan, L.-Y.; Shi, M.-W.; Long, J.-J. One-step method for stain proofing finishing of polyester fabric in supercritical carbon dioxide. J. CO2 Util. 2023, 67, 102316. [Google Scholar] [CrossRef]
- Luongo, G.; Avagyan, R.; Hongyu, R.; Östman, C. The washout effect during laundry on benzothiazole, benzotriazole, quinoline, and their derivatives in clothing textiles. Environ. Sci. Pollut. Res. 2016, 23, 2537–2548. [Google Scholar] [CrossRef]
- Foundation, C.E. Clothing Labels: Accurate or Not? Available online: https://www.circle-economy.com/resources/clothing-labels-accurate-or-not (accessed on 20 June 2025).
- Nørup, N.; Pihl, K.; Damgaard, A.; Scheutz, C. Evaluation of a European textile sorting centre: Material flow analysis and life cycle inventory. Resour. Conserv. Recycl. 2019, 143, 310–319. [Google Scholar] [CrossRef]
- Riba, J.-R.; Cantero, R.; Canals, T.; Puig, R. Circular economy of post-consumer textile waste: Classification through infrared spectroscopy. J. Clean. Prod. 2020, 272, 123011. [Google Scholar] [CrossRef]
- Riba, J.-R.; Cantero, R.; Riba-Mosoll, P.; Puig, R. Post-Consumer Textile Waste Classification through Near-Infrared Spectroscopy, Using an Advanced Deep Learning Approach. Polymers 2022, 14, 2475. [Google Scholar] [CrossRef]
- van Duijn, H. #whatisinmyclothes: The Truth Behind the Label. Available online: https://www.fashionrevolution.org/whatsinmyclothes-the-truth-behind-the-label/ (accessed on 19 May 2025).
- European Commission. Regulation (EU) No 1007/2011 of the European Parliament and of the Council of 27 September 2011 on Textile Fibre Names and Related Labelling and Marking of the Fibre Composition of Textile Products; European Commission: Brussels, Belgium, 2011; pp. 1–64. [Google Scholar]
- FibriTe, Kye Features, London, United Kingdom, Matoha Instrumentation Ltd. 2024. Available online: https://matoha.com/web/content/14524 (accessed on 22 June 2025).
- Welle, F. Twenty years of PET bottle to bottle recycling—An overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Pinter, E.; Welle, F.; Mayrhofer, E.; Pechhacker, A.; Motloch, L.; Lahme, V.; Grant, A.; Tacker, M. Circularity Study on PET Bottle-To-Bottle Recycling. Sustainability 2021, 13, 7370. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Zhang, Q.; Zuo, C.; Shi, H. An Overview of Chemical Additives on (Micro)Plastic Fibers: Occurrence, Release, and Health Risks. Rev. Environ. Contam. Toxicol. 2022, 260, 22. [Google Scholar] [CrossRef]
- Sandvik, I.M.; Stubbs, W. Circular fashion supply chain through textile-to-textile recycling. J. Fash. Mark. Manag. Int. J. 2019, 23, 366–381. [Google Scholar] [CrossRef]
- Chen, X.; Memon, H.A.; Wang, Y.; Marriam, I.; Tebyetekerwa, M. Circular Economy and Sustainability of the Clothing and Textile Industry. Mater. Circ. Econ. 2021, 3, 12. [Google Scholar] [CrossRef]
- Tange, L.; Van Houwelingen, J.A.; Peeters, J.R.; Vanegas, P. Recycling of flame retardant plastics from WEEE, technical and environmental challenges. Adv. Prod. Eng. Manag. 2013, 8, 67–77. [Google Scholar] [CrossRef][Green Version]
- Ellen MacArthur Foundation. Redesigning the Future of Fashion. Available online: https://www.ellenmacarthurfoundation.org/topics/fashion/overview?gad_source=1&gad_campaignid=18406820618&gbraid=0AAAAACb4JAcUQyRyzNZTDmbFpDxcVnYkB&gclid=Cj0KCQjwjdTCBhCLARIsAEu8bpLQ2Lk28jBoYNlIKBOoXpDGmNvz-IDQLdHxFM_7q-RJkdilt9ArsIsaAp3WEALw_wcB (accessed on 20 June 2025).
- Textile Exchange. Making Textile-to-Textile Recycling a Reality with SuperCircle. Available online: https://textileexchange.org/textile-to-textile-recycling-supercircle/ (accessed on 20 June 2025).
| Contaminant | Effect on rPET Properties |
|---|---|
| Ink, PVC | Increased reduction in intrinsic viscosity/molar mass |
| Amosorb, PS, PVC, TiO2 | Yellowing |
| PS, PP | Increased haze |
| PS, PP, EVOH, PVC | Hinders polycondensation during SSP |
| PLA, EVOH, PVC | Faster crystallisation |
| PP, PLA | Particle contamination |
| PVC | Benzene formation |
| EVOH | Cross-linking |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Standring, Z.; Macintyre, L.; Jiang, G.; Bucknall, D.; Arrighi, V. Impact of Chemicals and Processing Treatments on Thermo-Mechanical Recycling of Polyester Textiles. Molecules 2025, 30, 2758. https://doi.org/10.3390/molecules30132758
Standring Z, Macintyre L, Jiang G, Bucknall D, Arrighi V. Impact of Chemicals and Processing Treatments on Thermo-Mechanical Recycling of Polyester Textiles. Molecules. 2025; 30(13):2758. https://doi.org/10.3390/molecules30132758
Chicago/Turabian StyleStandring, Zara, Lisa Macintyre, Gigi Jiang, David Bucknall, and Valeria Arrighi. 2025. "Impact of Chemicals and Processing Treatments on Thermo-Mechanical Recycling of Polyester Textiles" Molecules 30, no. 13: 2758. https://doi.org/10.3390/molecules30132758
APA StyleStandring, Z., Macintyre, L., Jiang, G., Bucknall, D., & Arrighi, V. (2025). Impact of Chemicals and Processing Treatments on Thermo-Mechanical Recycling of Polyester Textiles. Molecules, 30(13), 2758. https://doi.org/10.3390/molecules30132758








