Photophysical Properties and Protein Binding Studies of Piperazine-Substituted Anthracene-BODIPY Dyads for Antimicrobial Photodynamic Therapy
Abstract
1. Introduction
2. Results and Discussion
2.1. Photophysical Properties
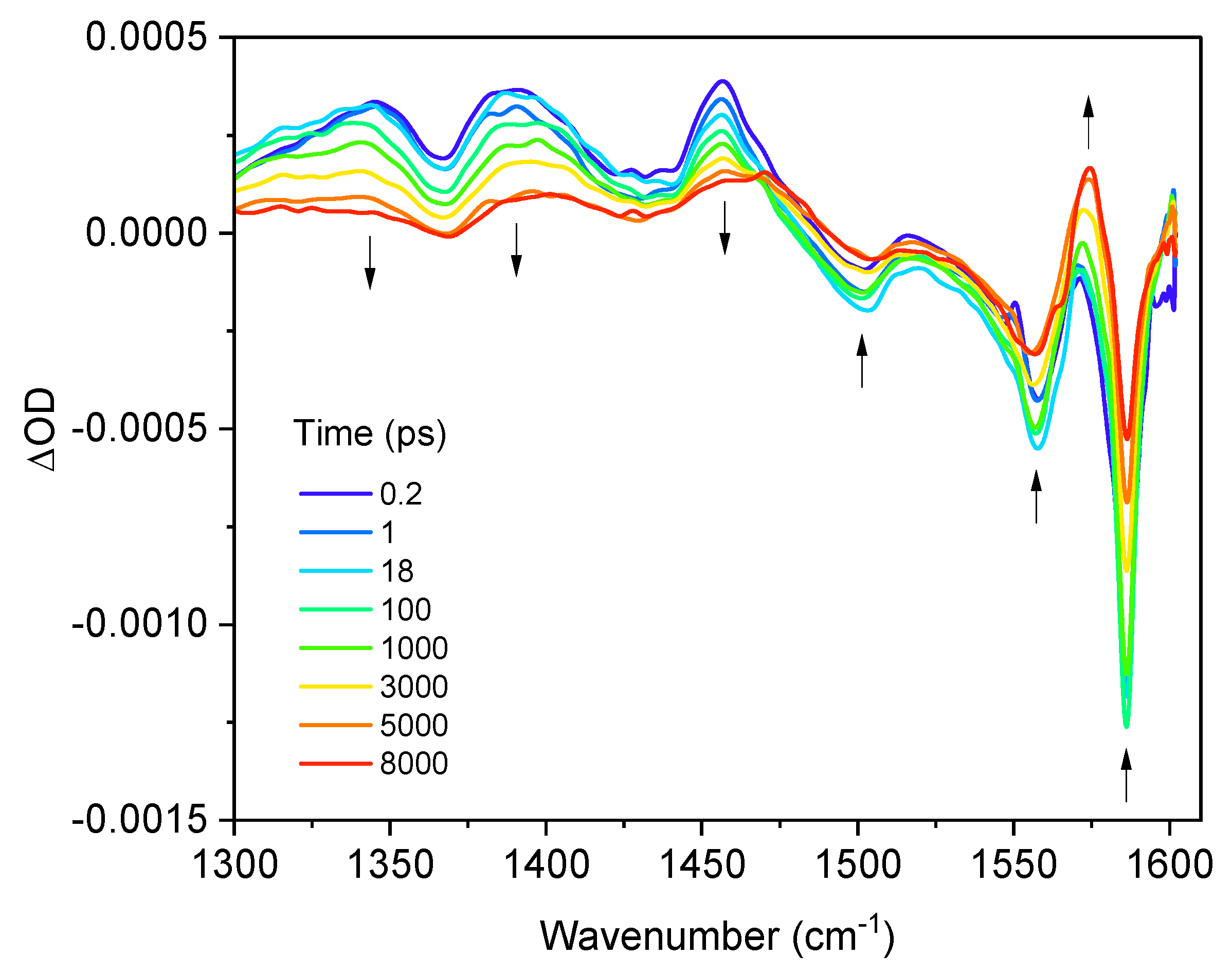
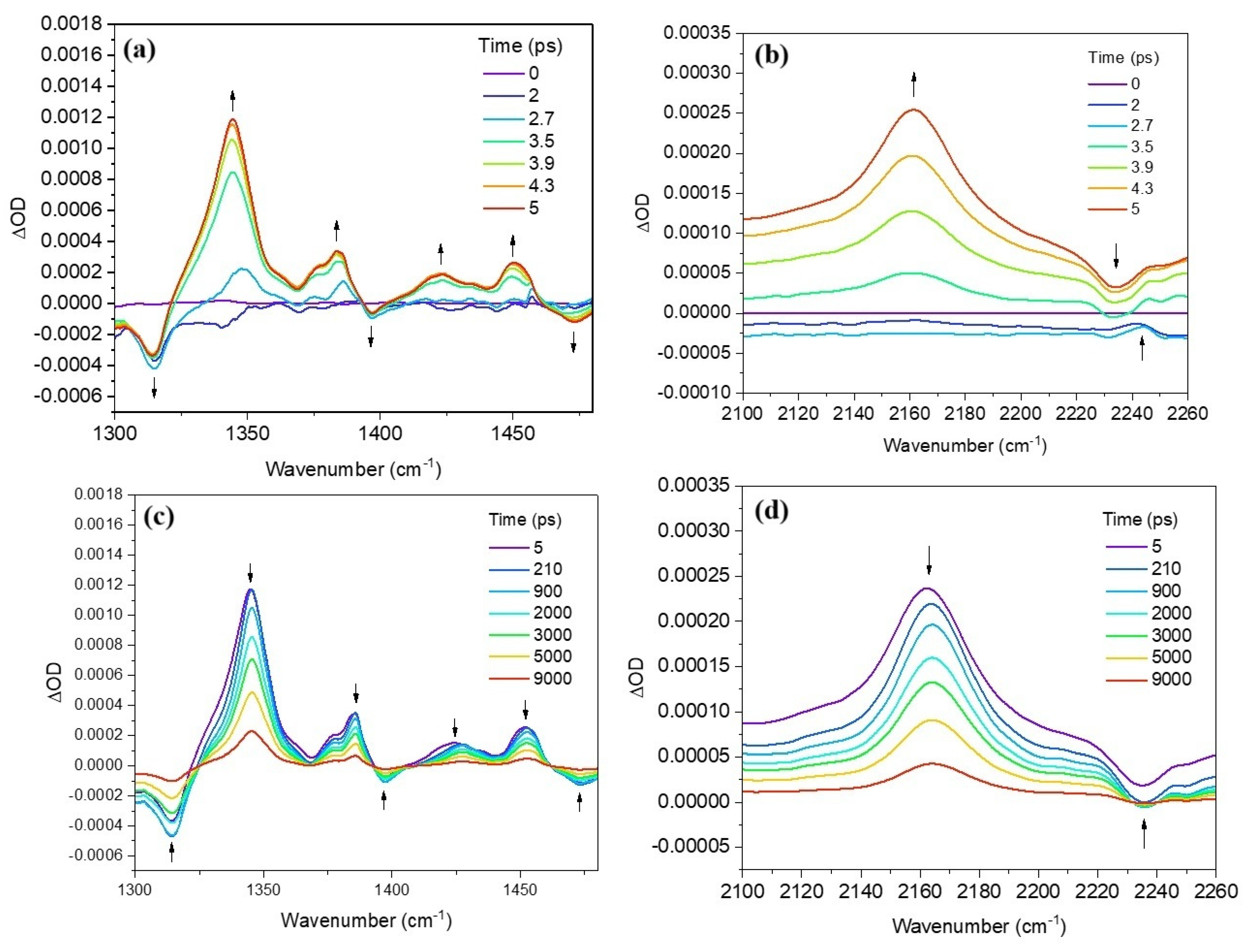
2.2. Time-Resolved Infrared Spectroscopy (TRIR)
2.3. Nanosecond Transient Absorption Spectroscopy (TAS)
2.4. Quantification of Singlet Oxygen
2.5. Biological Studies
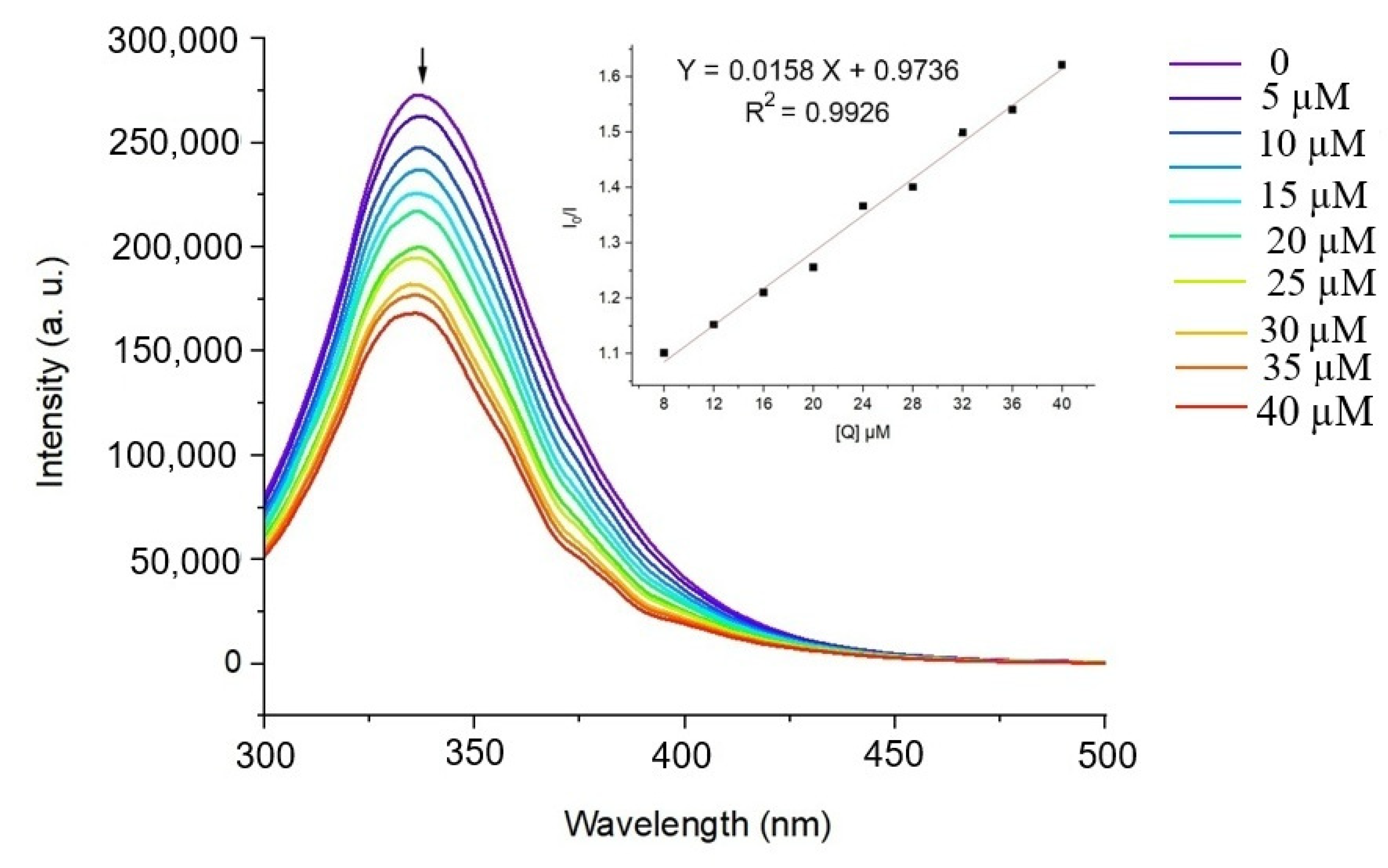
2.5.1. BSA-Binding Studies
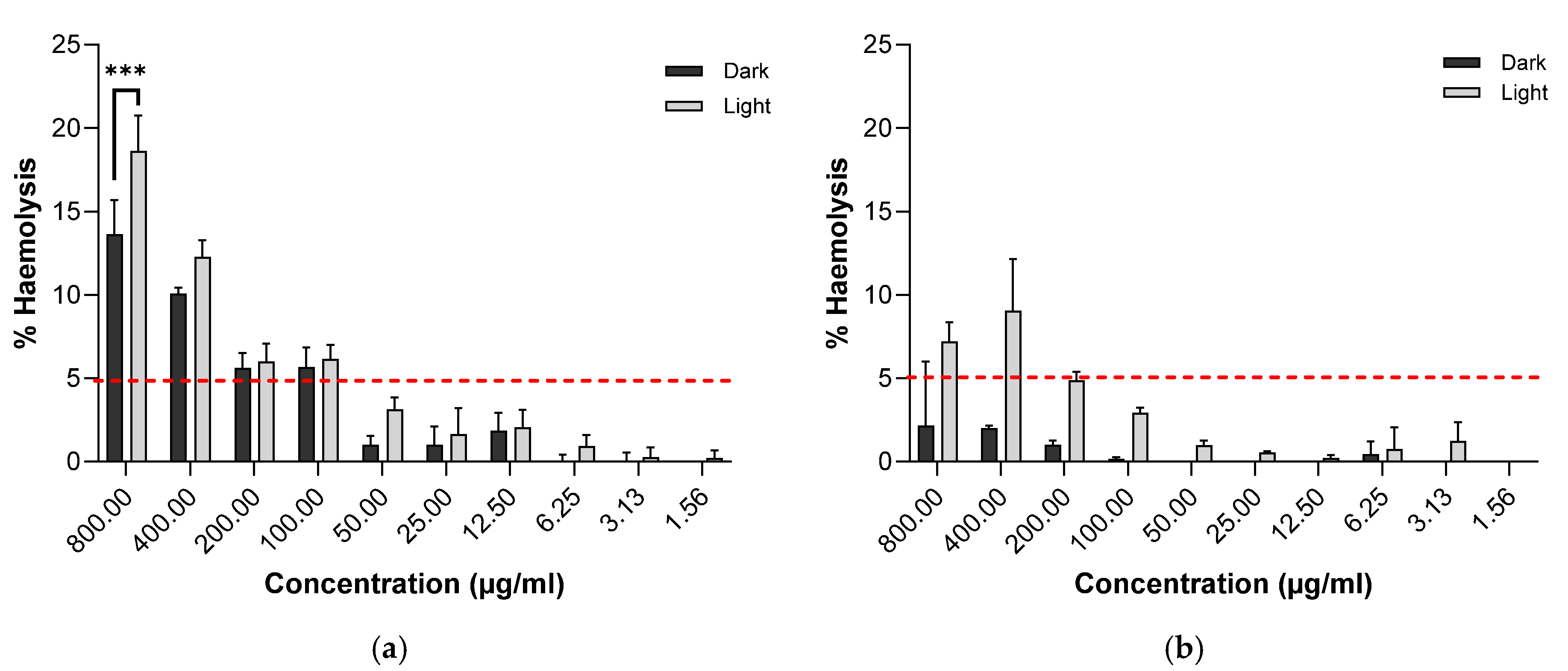
2.5.2. Biocompatibility Investigation
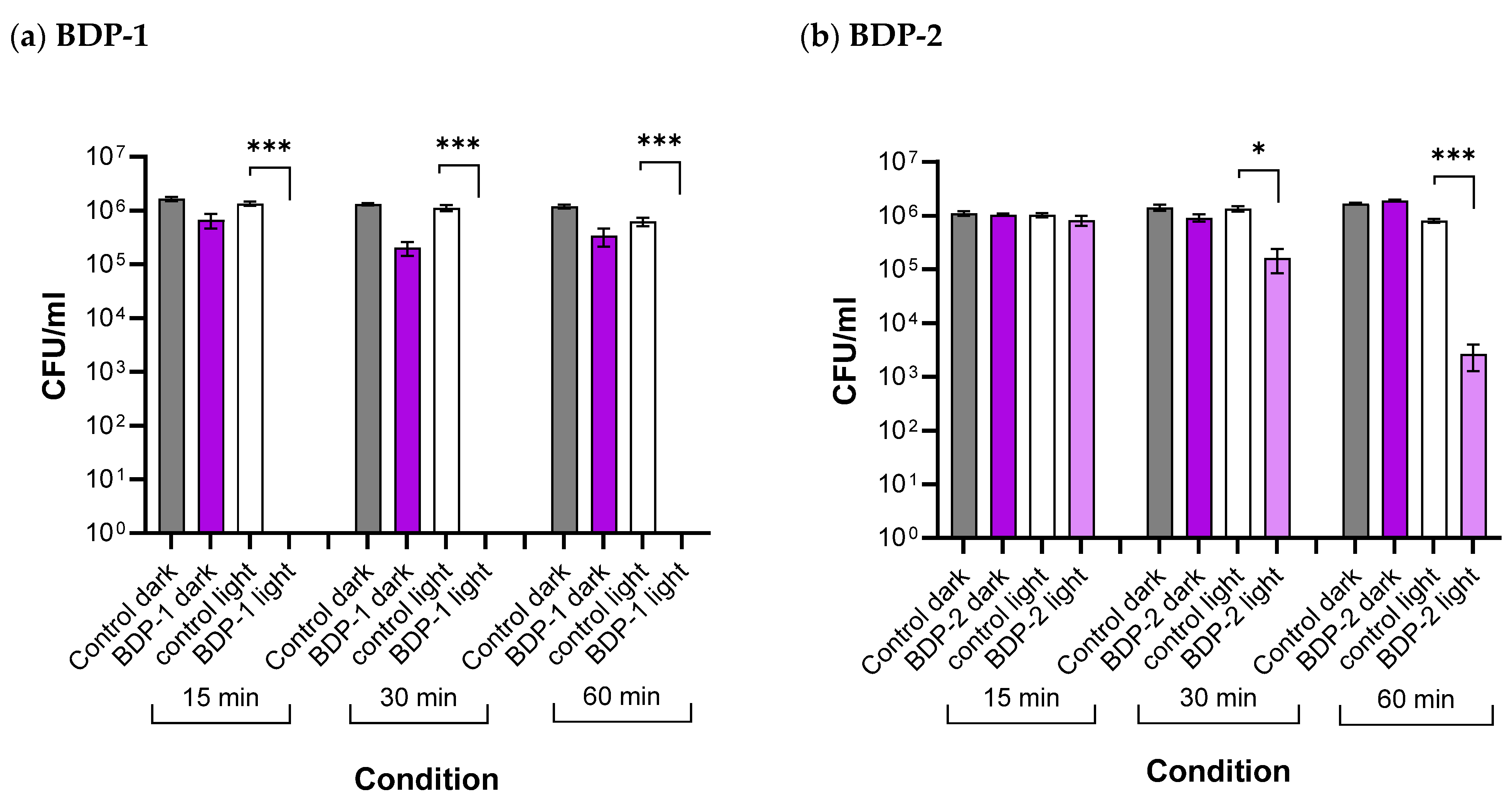
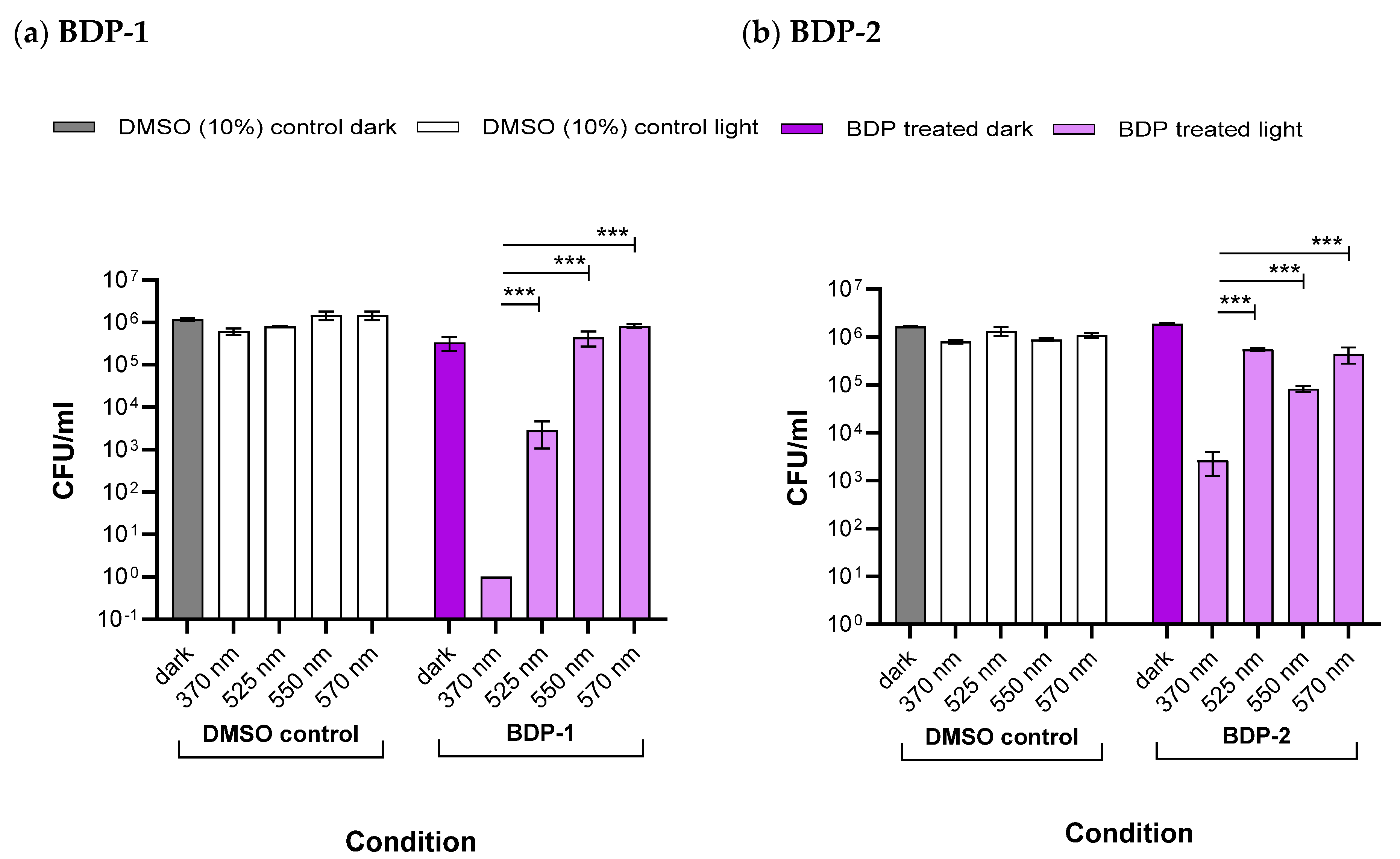
2.5.3. Antibacterial Studies
3. Materials and Methods
3.1. Protein Binding Studies
3.2. Singlet Oxygen Studies
3.3. Antimicrobial Studies
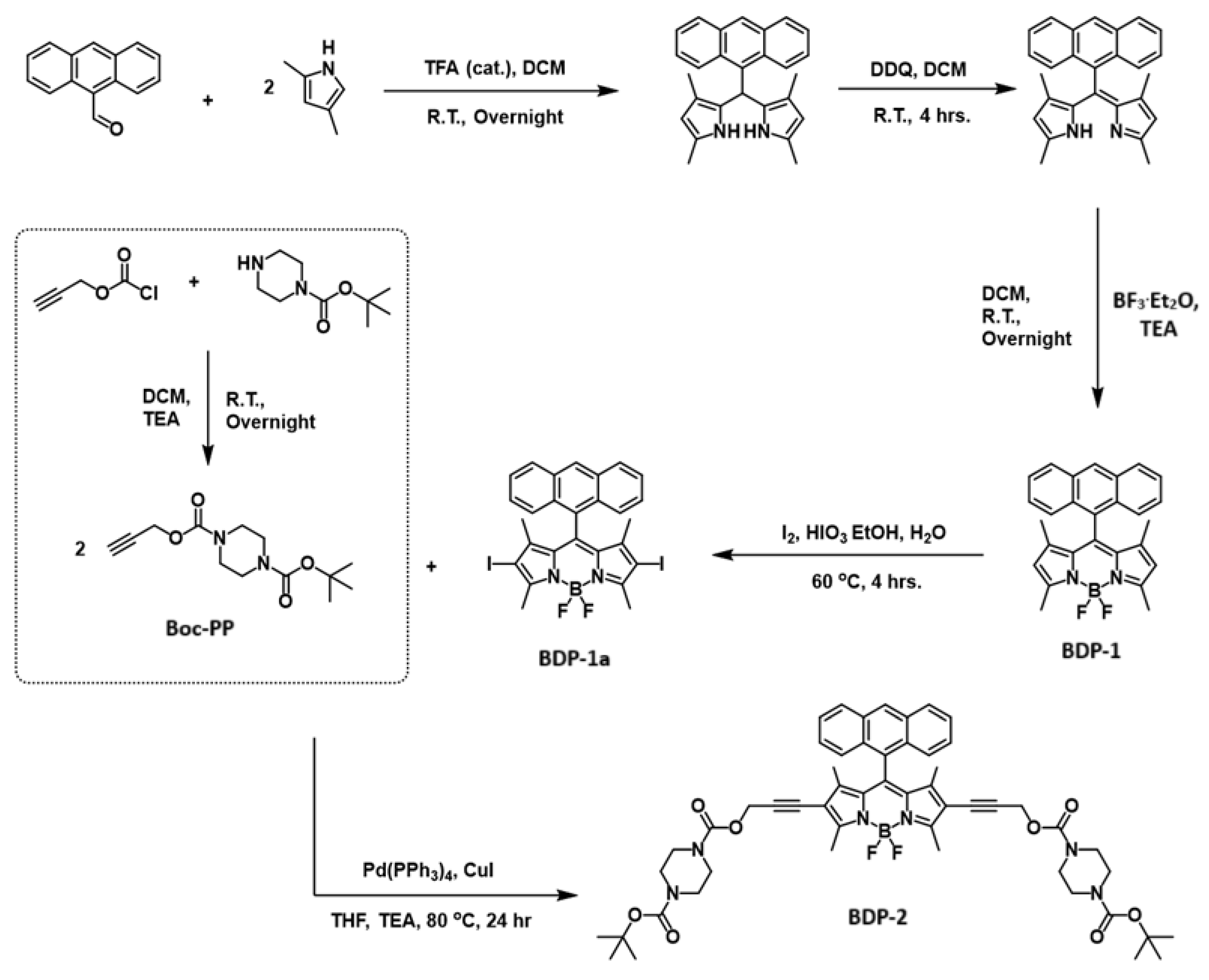
3.4. Synthesis
3.4.1. Synthesis of 1-(tert-Butyl) 4-(Prop-2-yn-1-yl) Piperazine-1,4-Dicarboxylate (Boc-PP)
3.4.2. Synthesis of BDP-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO|No Time to Wait: Securing the Future from Drug-Resistant Infections. Available online: https://www.who.int/publications/i/item/no-time-to-wait-securing-the-future-from-drug-resistant-infections (accessed on 21 October 2019).
- Home|AMR Review. Available online: https://amr-review.org/ (accessed on 19 March 2020).
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, L.; Hughes, D.F.; Pryce, M.T. Covalent Organic Frameworks: Advancing Antimicrobial Photodynamic Therapy for next-Generation Treatments. Coord. Chem. Rev. 2025, 528, 216424. [Google Scholar] [CrossRef]
- Fallon, M.; Lalrempuia, R.; Tabrizi, L.; Brandon, M.P.; McGarry, R.; Cullen, A.; Fernández-Alvarez, F.J.; Pryce, M.T.; Fitzgerald-Hughes, D. Novel Cyclometalated Iridium (III) Complexes as Antibacterial Agents for Photodynamic Inactivation. J. Photochem. Photobiol. Chem. 2025, 462, 116218. [Google Scholar] [CrossRef]
- Tabrizi, L.; McGarry, R.; Turzanska, K.; Varvarezos, L.; Fallon, M.; Brannigan, R.; Costello, J.T.; Fitzgerald-Hughes, D.; Pryce, M.T. Porphyrin-Polymer as a Photosensitizer Prodrug for Antimicrobial Photodynamic Therapy and Biomolecule Binding Ability. Biomacromolecules 2024, 25, 7736–7749. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, A.; Biddulph, J.; Tabrizi, L.; Fitzgerald-Hughes, D.; Pryce, M.T. Chapter Eleven—Photoactive Organometallic Compounds as Antimicrobial Agents. In Advances in Inorganic Chemistry; Ford, P.C., van Eldik, R., Eds.; Biomedical Applications of Inorganic Photochemistry; Academic Press: Cambridge, MA, USA, 2022; Volume 80, pp. 381–409. [Google Scholar]
- Hamblin, M.R.; Hasan, T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial Photodynamic Inactivation: A Bright New Technique to Kill Resistant Microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.C.; Ogilby, P.R. Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef]
- Piskorz, J.; Porolnik, W.; Kucinska, M.; Dlugaszewska, J.; Murias, M.; Mielcarek, J. BODIPY-Based Photosensitizers as Potential Anticancer and Antibacterial Agents: Role of the Positive Charge and the Heavy Atom Effect. ChemMedChem 2021, 16, 399–411. [Google Scholar] [CrossRef]
- Gorman, A.; Killoran, J.; O’Shea, C.; Kenna, T.; Gallagher, W.M.; O’Shea, D.F. In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy. J. Am. Chem. Soc. 2004, 126, 10619–10631. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.M.K.; Kim, G.; Kwon, N.; Kim, C.-Y.; Park, S.; Yoon, J. Molecular Design of Highly Efficient Heavy-Atom-Free Triplet BODIPY Derivatives for Photodynamic Therapy and Bioimaging. Angew. Chem. Int. Ed. 2020, 59, 8957–8962. [Google Scholar] [CrossRef]
- Dong, Y.; Dick, B.; Zhao, J. Twisted Bodipy Derivative as a Heavy-Atom-Free Triplet Photosensitizer Showing Strong Absorption of Yellow Light, Intersystem Crossing, and a High-Energy Long-Lived Triplet State. Org. Lett. 2020, 22, 5535–5539. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, M.; Zhang, X.-F.; Shi, M.; Jia, M.; Hu, X.; Liu, L.; Wang, T. Minimizing the Electron Donor Size of Donor–Acceptor-Type Photosensitizer: Twisted Intramolecular Charge-Transfer-Induced Triplet State and Singlet Oxygen Formation. J. Phys. Chem. C 2020, 124, 23556–23558. [Google Scholar] [CrossRef]
- Callaghan, S.; Filatov, M.A.; Savoie, H.; Boyle, R.W.; Senge, M.O. In Vitro Cytotoxicity of a Library of BODIPY-Anthracene and -Pyrene Dyads for Application in Photodynamic Therapy. Photochem. Photobiol. Sci. 2019, 18, 495–504. [Google Scholar] [CrossRef]
- Filatov, M.A. Heavy-Atom-Free BODIPY Photosensitizers with Intersystem Crossing Mediated by Intramolecular Photoinduced Electron Transfer. Org. Biomol. Chem. 2019, 18, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, J. Bodipy–Anthracene Dyads as Triplet Photosensitizers: Effect of Chromophore Orientation on Triplet-State Formation Efficiency and Application in Triplet–Triplet Annihilation Upconversion. Org. Lett. 2017, 19, 4492–4495. [Google Scholar] [CrossRef]
- Halimehjani, A.Z.; Dehghan, F.; Tafakori, V.; Amini, E.; Hooshmand, S.E.; Nosood, Y.L. Synthesis of Novel Antibacterial and Antifungal Dithiocarbamate-Containing Piperazine Derivatives via Re-Engineering Multicomponent Approach. Heliyon 2022, 8, e09564. [Google Scholar] [CrossRef]
- Chaudhary, P.; Kumar, R.; Verma, A.K.; Singh, D.; Yadav, V.; Chhillar, A.K.; Sharma, G.L.; Chandra, R. Synthesis and Antimicrobial Activity of N-Alkyl and N-Aryl Piperazine Derivatives. Bioorg. Med. Chem. 2006, 14, 1819–1826. [Google Scholar] [CrossRef]
- Salem, M.E.; Fares, I.M.Z.; Ghozlan, S.A.S.; Abdel-Aziz, M.M.; Abdelhamid, I.A.; Elwahy, A.H.M. Facile Synthesis and Antimicrobial Activity of Bis(Fused 4H-Pyrans) Incorporating Piperazine as Novel Hybrid Molecules: Michael’s Addition Approach. J. Heterocycl. Chem. 2022, 59, 1907–1926. [Google Scholar] [CrossRef]
- Faizan, M.; Kumar, R.; Mazumder, A.; Salahuddin; Kukreti, N.; Kumar, A.; Chaitanya, M.V.N.L. The Medicinal Chemistry of Piperazines: A Review. Chem. Biol. Drug Des. 2024, 103, e14537. [Google Scholar] [CrossRef]
- Romanelli, M.N.; Manetti, D.; Braconi, L.; Dei, S.; Gabellini, A.; Teodori, E. The Piperazine Scaffold for Novel Drug Discovery Efforts: The Evidence to Date. Expert Opin. Drug Discov. 2022, 17, 969–984. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Carter, D.C. Atomic Structure and Chemistry of Human Serum Albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef]
- Khaibrakhmanova, D.; Nikiforova, A.; Sedov, I. Binding Constants of Substituted Benzoic Acids with Bovine Serum Albumin. Pharmaceuticals 2020, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Fossum, C.J.; Johnson, B.O.V.; Golde, S.T.; Kielman, A.J.; Finke, B.; Smith, M.A.; Lowater, H.R.; Laatsch, B.F.; Bhattacharyya, S.; Hati, S. Insights into the Mechanism of Tryptophan Fluorescence Quenching Due to Synthetic Crowding Agents: A Combined Experimental and Computational Study. ACS Omega 2023, 8, 44820–44830. [Google Scholar] [CrossRef]
- Patra, S.; Santhosh, K.; Pabbathi, A.; Samanta, A. Diffusion of Organic Dyes in Bovine Serum Albumin Solution Studied by Fluorescence Correlation Spectroscopy. RSC Adv. 2012, 2, 6079–6086. [Google Scholar] [CrossRef]
- Yallur, B.C.; Katrahalli, U.; Krishna, P.M.; Hadagali, M.D. BSA Binding and Antibacterial Studies of Newly Synthesized 5,6-Dihydroimidazo[2,1-b]Thiazole-2-Carbaldehyde. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 117192. [Google Scholar] [CrossRef] [PubMed]
- Çetindere, S. Photophysics of BODIPY Dyes: Recent Advances. In Photophysics, Photochemical and Substitution Reactions—Recent Advances; IntechOpen: London, UK, 2020; ISBN 978-1-83968-224-7. [Google Scholar]
- Agazzi, M.L.; Ballatore, M.B.; Reynoso, E.; Quiroga, E.D.; Durantini, E.N. Synthesis, Spectroscopic Properties and Photodynamic Activity of Two Cationic BODIPY Derivatives with Application in the Photoinactivation of Microorganisms. Eur. J. Med. Chem. 2017, 126, 110–121. [Google Scholar] [CrossRef]
- Chen, K.; Dong, Y.; Zhao, X.; Imran, M.; Tang, G.; Zhao, J.; Liu, Q. Bodipy Derivatives as Triplet Photosensitizers and the Related Intersystem Crossing Mechanisms. Front. Chem. 2019, 7, 821. [Google Scholar] [CrossRef]
- Dong, Y.; Sukhanov, A.A.; Zhao, J.; Elmali, A.; Li, X.; Dick, B.; Karatay, A.; Voronkova, V.K. Spin–Orbit Charge-Transfer Intersystem Crossing (SOCT-ISC) in Bodipy-Phenoxazine Dyads: Effect of Chromophore Orientation and Conformation Restriction on the Photophysical Properties. J. Phys. Chem. C 2019, 123, 22793–22811. [Google Scholar] [CrossRef]
- Stranius, K.; Iashin, V.; Nikkonen, T.; Muuronen, M.; Helaja, J.; Tkachenko, N. Effect of Mutual Position of Electron Donor and Acceptor on Photoinduced Electron Transfer in Supramolecular Chlorophyll–Fullerene Dyads. J. Phys. Chem. A 2014, 118, 1420–1429. [Google Scholar] [CrossRef]
- Petrushenko, I.K.; Petrushenko, K.B. Effect of Meso-Substituents on the Electronic Transitions of BODIPY Dyes: DFT and RI-CC2 Study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sukhanov, A.A.; Toffoletti, A.; Sadiq, F.; Zhao, J.; Barbon, A.; Voronkova, V.K.; Dick, B. Insights into the Efficient Intersystem Crossing of Bodipy-Anthracene Compact Dyads with Steady-State and Time-Resolved Optical/Magnetic Spectroscopies and Observation of the Delayed Fluorescence. J. Phys. Chem. C 2019, 123, 265–274. [Google Scholar] [CrossRef]
- Wei, Z.; Sharma, S.; Philip, A.M.; Sengupta, S.; Grozema, F.C. Excited State Dynamics of BODIPY-Based Acceptor–Donor–Acceptor Systems: A Combined Experimental and Computational Study. Phys. Chem. Chem. Phys. 2021, 23, 8900–8907. [Google Scholar] [CrossRef] [PubMed]
- Shakerizadeh-Shirazi, F.; Hemmateenejad, B.; Mehranpour, A.M. Determination of the Empirical Solvent Polarity Parameter ET(30) by Multivariate Image Analysis. Anal. Methods 2013, 5, 891–896. [Google Scholar] [CrossRef]
- Dai, Y.; Dellai, A.; Bassan, E.; Bellatreccia, C.; Gualandi, A.; Anselmi, M.; Cozzi, P.G.; Ceroni, P.; Negri, F. Solvent and Alkyl Substitution Effects on Charge-Transfer Mediated Triplet State Generation in BODIPY Dyads: A Combined Computational and Experimental Study. Photochem. Photobiol. Sci. 2024, 23, 451–462. [Google Scholar] [CrossRef]
- Suhina, T.; Amirjalayer, S.; Woutersen, S.; Bonn, D.; Brouwer, A.M. Ultrafast Dynamics and Solvent-Dependent Deactivation Kinetics of BODIPY Molecular Rotors. Phys. Chem. Chem. Phys. 2017, 19, 19998–20007. [Google Scholar] [CrossRef]
- Filatov, M.A.; Karuthedath, S.; Polestshuk, P.M.; Savoie, H.; Flanagan, K.J.; Sy, C.; Sitte, E.; Telitchko, M.; Laquai, F.; Boyle, R.W.; et al. Generation of Triplet Excited States via Photoinduced Electron Transfer in Meso-Anthra-BODIPY: Fluorogenic Response toward Singlet Oxygen in Solution and in Vitro. J. Am. Chem. Soc. 2017, 139, 6282–6285. [Google Scholar] [CrossRef]
- Balanikas, E.; Reymond-Joubin, M.; Vauthey, E. Excited-State Symmetry Breaking in Solvent Mixtures. J. Phys. Chem. Lett. 2024, 15, 2447–2452. [Google Scholar] [CrossRef] [PubMed]
- Cullen, A.A.; Rajagopal, A.; Heintz, K.; Heise, A.; Murphy, R.; Sazanovich, I.V.; Greetham, G.M.; Towrie, M.; Long, C.; Fitzgerald-Hughes, D.; et al. Exploiting a Neutral BODIPY Copolymer as an Effective Agent for Photodynamic Antimicrobial Inactivation. J. Phys. Chem. B 2021, 125, 1550–1557. [Google Scholar] [CrossRef]
- Cullen, A.A.; Heintz, K.; O’Reilly, L.; Long, C.; Heise, A.; Murphy, R.; Karlsson, J.; Gibson, E.; Greetham, G.M.; Towrie, M.; et al. A Time-Resolved Spectroscopic Investigation of a Novel BODIPY Copolymer and Its Potential Use as a Photosensitiser for Hydrogen Evolution. Front. Chem. 2020, 8, 584060. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Wu, S.; Wang, Z.; Wu, W.; Ma, J.; Guo, S.; Huang, L. Intramolecular RET Enhanced Visible Light-Absorbing Bodipy Organic Triplet Photosensitizers and Application in Photooxidation and Triplet–Triplet Annihilation Upconversion. J. Am. Chem. Soc. 2013, 135, 10566–10578. [Google Scholar] [CrossRef] [PubMed]
- Raskolupova, V.I.; Popova, T.V.; Zakharova, O.D.; Nikotina, A.E.; Abramova, T.V.; Silnikov, V.N. Human Serum Albumin Labelling with a New BODIPY Dye Having a Large Stokes Shift. Molecules 2021, 26, 2679. [Google Scholar] [CrossRef]
- Das, S.; Dey, S.; Patra, S.; Bera, A.; Ghosh, T.; Prasad, B.; Sayala, K.D.; Maji, K.; Bedi, A.; Debnath, S. BODIPY-Based Molecules for Biomedical Applications. Biomolecules 2023, 13, 1723. [Google Scholar] [CrossRef] [PubMed]
- El Gammal, R.N.; Elmansi, H.; El-Emam, A.A.; Belal, F.; Elzahhar, P.A.; Belal, A.S.F.; Hammouda, M.E.A. Insights on the In-Vitro Binding Interaction between Donepezil and Bovine Serum Albumin. BMC Chem. 2023, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Kummur, K.N.; Panda, S.M.; Patil, M.B.; Tripathy, U.; Sidarai, A.H. Revealing the Interaction Mechanism between Bovine Serum Albumin (BSA) and a Fluorescent Coumarin Derivative: A Multispectroscopic and in Silico Approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 318, 124466. [Google Scholar] [CrossRef]
- Theoretical Research on Excited States: Ultraviolet and Fluorescence Spectra of Aromatic Amino Acids|Interdisciplinary Sciences: Computational Life Sciences. Available online: https://link.springer.com/article/10.1007/s12539-020-00395-3 (accessed on 20 January 2025).
- Verma, R.; Pyreddy, S.; Redmond, C.E.; Qazi, F.; Khalid, A.; O’Brien-Simpson, N.M.; Shukla, R.; Tomljenovic-Hanic, S. Detection and Identification of Amino Acids and Proteins Using Their Intrinsic Fluorescence in the Visible Light Spectrum. Anal. Chim. Acta 2023, 1282, 341925. [Google Scholar] [CrossRef]
- Minamide, M.; Tsurushima, M.; Koga, R.; Hasegawa, K.; Kurosawa, Y.; Tsuchida, T.; Goto, S. Distinguishing the Transitions of Fluorescence Spectra of Tryptophan-134 and 213 in BSA Induced by Bindings of UV Filters, Oxybenzone-3, and Avobenzone. Bull. Chem. Soc. Jpn. 2024, 97, uoae058. [Google Scholar] [CrossRef]
- Ahmad, B.; Parveen, S.; Khan, R.H. Effect of Albumin Conformation on the Binding of Ciprofloxacin to Human Serum Albumin: A Novel Approach Directly Assigning Binding Site. Biomacromolecules 2006, 7, 1350–1356. [Google Scholar] [CrossRef]
- Zornić, S.; Simović Marković, B.; Franich, A.A.; Janjić, G.V.; Jadranin, M.B.; Avdalović, J.; Rajković, S.; Živković, M.D.; Arsenijević, N.N.; Radosavljević, G.D.; et al. Characterization, Modes of Interactions with DNA/BSA Biomolecules and Anti-Tumor Activity of Newly Synthesized Dinuclear Platinum(II) Complexes with Pyridazine Bridging Ligand. J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem. 2024, 29, 51–73. [Google Scholar] [CrossRef]
- Karanlık, C.C.; Karanlık, G.; Erdoğmuş, A. Water-Soluble Meso-Thienyl BODIPY Therapeutics: Synthesis, Characterization, Exploring Photophysicochemical and DNA/BSA Binding Properties. J. Photochem. Photobiol. Chem. 2023, 438, 114581. [Google Scholar] [CrossRef]
- Chakraborty, G.; Ray, A.K.; Singh, P.K.; Pal, H. Non-Covalent Interaction of BODIPY-Benzimidazole Conjugate with Bovine Serum Albumin–A Photophysical and Molecular Docking Study. J. Photochem. Photobiol. Chem. 2019, 377, 220–227. [Google Scholar] [CrossRef]
- Pang, W.; Lv, J.; Du, S.; Wang, J.; Wang, J.; Zeng, Y. Preparation of Curcumin-Piperazine Coamorphous Phase and Fluorescence Spectroscopic and Density Functional Theory Simulation Studies on the Interaction with Bovine Serum Albumin. Mol. Pharm. 2017, 14, 3013–3024. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wen, Y.; Xiao, Y.; Yu, G.; Liu, Y.; Qian, X. Bulky 4-Tritylphenylethynyl Substituted Boradiazaindacene: Pure Red Emission, Relatively Large Stokes Shift and Inhibition of Self-Quenching. Chem. Commun. 2008, 39, 4777–4779. [Google Scholar] [CrossRef]





| Solvent | abs (nm) | em (nm) | Δ (cm−1) | ɸFl | τ1 (ns) | τ2 (µs) | ɸΔ | |
|---|---|---|---|---|---|---|---|---|
| BDP-1 | DCM | 507 | 516 | 344 | 0.14 a | 4.20 (510 nm) | 82 a | 0.82 a |
| THF | 505 | 518 | 497 | 0.15 | 5.69 | 48/166 b | 0.31 a | |
| MeCN | 503 | 512 | 349 | 0.01 a | 3.62/5.38 | 18/105 b | 0.86 a | |
| BDP-2 | DCM | 552 | 572 | 633 | 0.19 | 4.06 | 44/230 c | 0.85 |
| THF | 552 | 568 | 510 | 0.30 | 4.09 | 49/213 b | 0.25 | |
| MeCN | 549 | 571 | 702 | 0.01 | 2.59/5.35 | 31/138 b | 0.81 |
| Solvent | abs (nm) | τ1 (µs) | τ2 (µs) | ɸΔ | |
|---|---|---|---|---|---|
| BDP-1 | DCM | 507 | - b | 82 a | 0.82 a |
| THF | 505 | 48 c | 166 c | 0.31 a | |
| MeCN | 503 | 18 c | 105 c | 0.86 a | |
| BDP-2 | DCM | 552 | 44 d | 230 d | 0.85 |
| THF | 552 | 49 c | 213 c | 0.25 | |
| MeCN | 549 | 31 c | 138 c | 0.81 |
| Wavelength (nm) | Light Intensity (mW/cm2) | Light Dose (mJ/cm2) | Log Reduction in CFU·mL−1 a | |
|---|---|---|---|---|
| BDP-1 | BDP-2 | |||
| 370 | 0.70 | 2523 | 5.18 | 2.68 |
| 525 | 0.05 | 196 | 2.11 | 0.37 |
| 550 | 6.12 | 22,018 | 0.61 | 0.75 |
| 570 | 0.02 | 64 | 0.23 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Sullivan, S.; Tabrizi, L.; Turzańska, K.; Clark, I.P.; Fitzgerald-Hughes, D.; Pryce, M.T. Photophysical Properties and Protein Binding Studies of Piperazine-Substituted Anthracene-BODIPY Dyads for Antimicrobial Photodynamic Therapy. Molecules 2025, 30, 2727. https://doi.org/10.3390/molecules30132727
O’Sullivan S, Tabrizi L, Turzańska K, Clark IP, Fitzgerald-Hughes D, Pryce MT. Photophysical Properties and Protein Binding Studies of Piperazine-Substituted Anthracene-BODIPY Dyads for Antimicrobial Photodynamic Therapy. Molecules. 2025; 30(13):2727. https://doi.org/10.3390/molecules30132727
Chicago/Turabian StyleO’Sullivan, Stephen, Leila Tabrizi, Kaja Turzańska, Ian P. Clark, Deirdre Fitzgerald-Hughes, and Mary T. Pryce. 2025. "Photophysical Properties and Protein Binding Studies of Piperazine-Substituted Anthracene-BODIPY Dyads for Antimicrobial Photodynamic Therapy" Molecules 30, no. 13: 2727. https://doi.org/10.3390/molecules30132727
APA StyleO’Sullivan, S., Tabrizi, L., Turzańska, K., Clark, I. P., Fitzgerald-Hughes, D., & Pryce, M. T. (2025). Photophysical Properties and Protein Binding Studies of Piperazine-Substituted Anthracene-BODIPY Dyads for Antimicrobial Photodynamic Therapy. Molecules, 30(13), 2727. https://doi.org/10.3390/molecules30132727







