Systematic Investigations of the Huperzine A—Producing Endophytic Fungi of Huperzia serrata in China and Fermentation Optimization Using OSMAC Strategy
Abstract
1. Introduction
2. Results
2.1. Fingerprint Analysis Reveals Composition and Relative Proportions
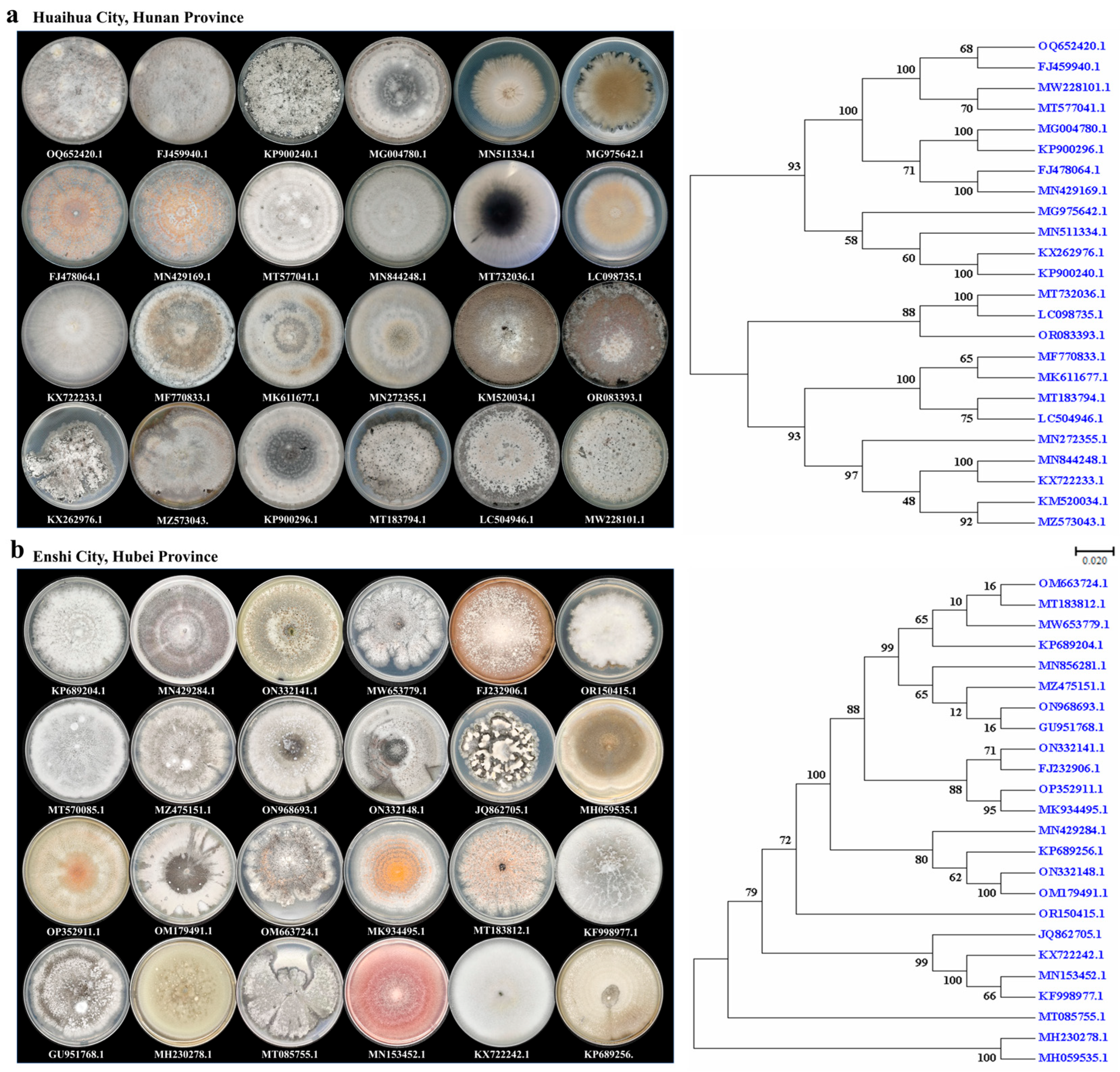
2.2. Morphology of Endophytic Fungi in H. serrata
2.3. Identification Based on ITS Sequences and Phylogenetic Analysis
2.4. Isolation of Endophytic Fungi from H. serrata
2.5. Screening of HupA-Producing Endophytic Fungi
2.5.1. Detection of Alkaloid Precipitator
2.5.2. HPLC and LC-MS Analysis
2.6. Inhibition Activity of Purified HupA on AChE
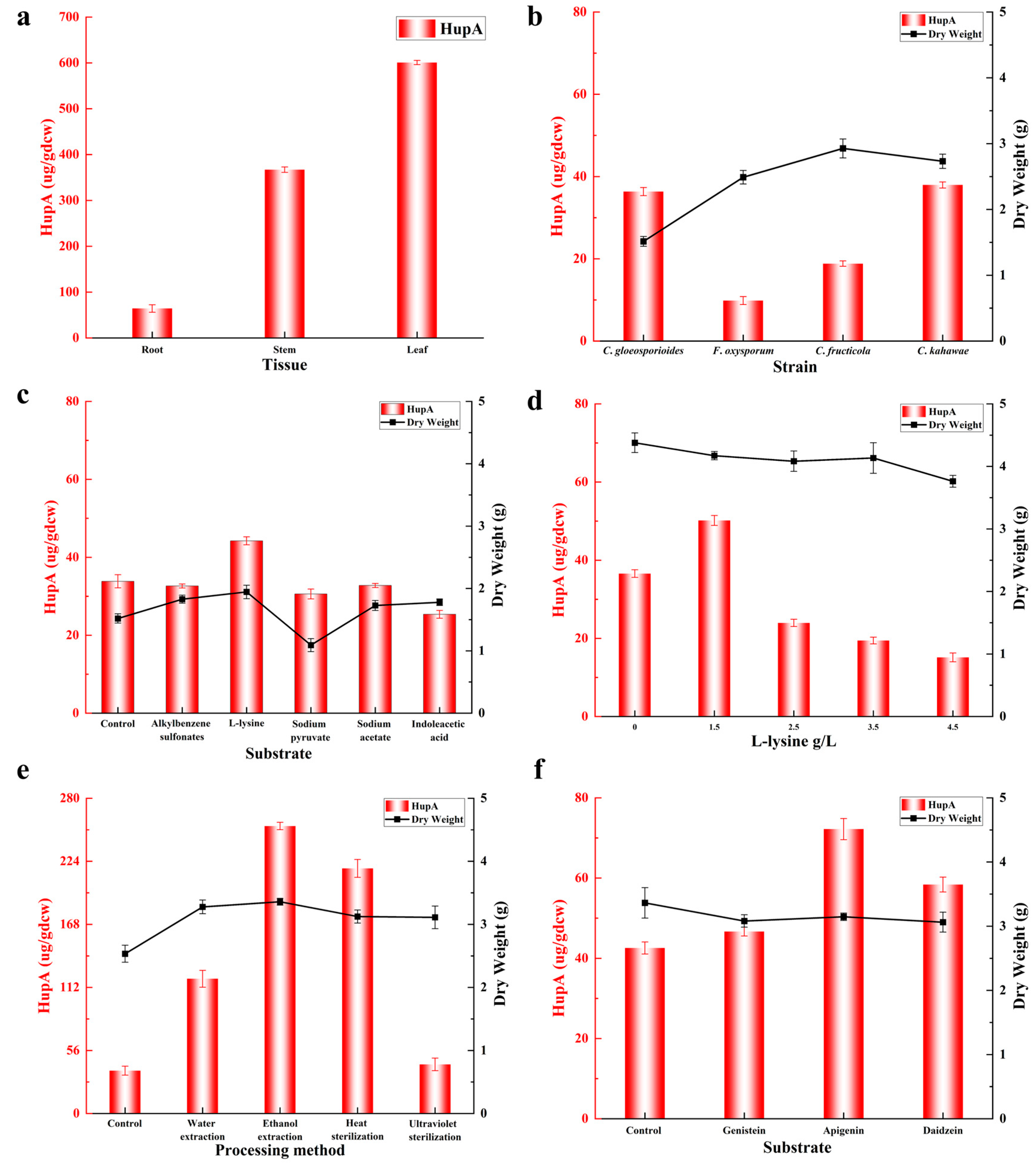
2.7. Strategies for Improving HupA Production in Endophytic Fungi
2.7.1. Selective Medium
2.7.2. Effects of Biological Precursor and Inducers on HupA Production
2.7.3. Optimization of L-Lysine Concentration for Improving HupA Production
2.7.4. Effects of H. serrata Extracts on HupA Production
2.7.5. Effects of Flavonoids on HupA Production
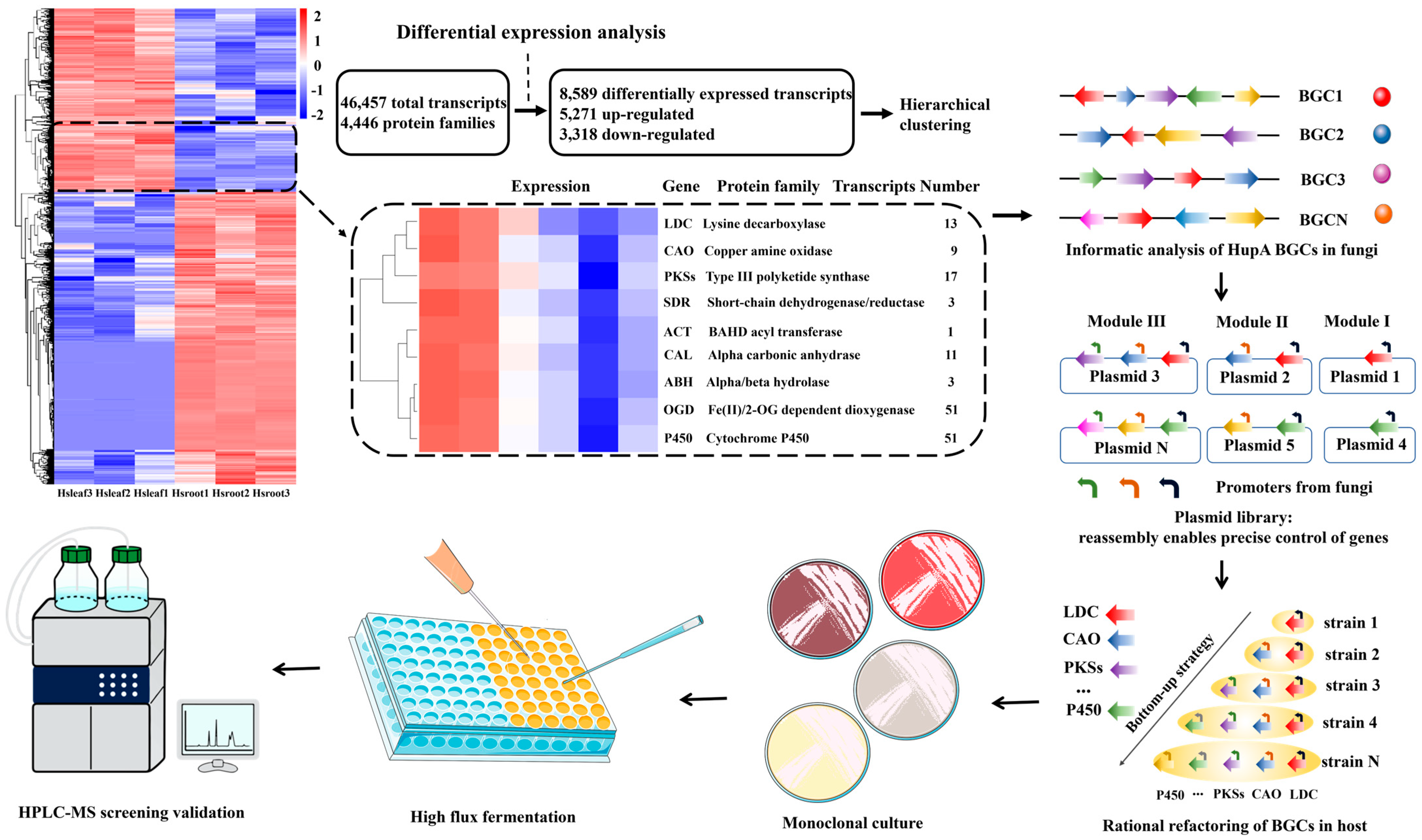
2.8. Transcriptome Sequencing of H. serrata
2.9. H. serrata Extracts Upregulated the HupA Biosynthesis Genes in C. kahawae
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Fingerprint Analysis
4.3. Isolation of Endophytic Fungi
4.4. Identification of Endophytic Fungi
4.5. Shake Flask Fermentation of Endophytic Fungi
4.6. H. serrata and Its Extracts
4.7. HupA Extraction from Endophytic Fungi
4.8. Analytical Method
4.8.1. Alkaloid Precipitator
4.8.2. HPLC and LC-MS Analysis of HupA
4.9. In Vitro Assay of AChE Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ossenkoppele, R.; Kant, R.V.D.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhou, H.; Zhang, M.; Su, J.; Wang, X.; Li, S.; Yang, Z.; Kang, Z.; Zhou, R. C3N nanodots inhibits Aβ peptides aggregation pathogenic path in Alzheimer’s disease. Nat. Commun. 2023, 14, 5718. [Google Scholar] [CrossRef] [PubMed]
- Warfield, A.E.; Gupta, P.; Ruhmann, M.M.; Jeffs, Q.L.; Guidone, G.C.; Rhymes, H.W.; Thompson, M.I.; Todd, W.D. A brainstem to circadian system circuit links Tau pathology to sundowning-related disturbances in an Alzheimer’s disease mouse model. Nat. Commun. 2023, 14, 5027. [Google Scholar] [CrossRef]
- Johnson, E.C.B.; Bian, S.; Haque, R.U.; Carter, E.K.; Watson, C.M.; Gordon, B.A.; Ping, L.; Duong, D.M.; Epstein, M.P.; McDade, E.; et al. Cerebrospinal fluid proteomics define the natural history of autosomal dominant Alzheimer’s disease. Nat. Med. 2023, 29, 1979–1988. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Tian, P.; Tan, T. Delineating biosynthesis of huperzine A, A plant-derived medicine for the treatment of Alzheimer’s disease. Biotechnol. Adv. 2022, 60, 108026. [Google Scholar] [CrossRef]
- Nett, R.S.; Dho, Y.; Low, Y.Y.; Sattely, E.S. A metabolic regulon reveals early and late acting enzymes in neuroactive Lycopodium alkaloid biosynthesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2102949118. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, X.; Yang, J.; Wang, Y.; Yao, K.; Huo, Q.; Fu, Y.; Wei, Y.; Guo, B. The temporal and spatial endophytic fungal community of Huperzia serrata: Diversity and relevance to huperzine A production by the host. BMC Microbiol. 2022, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Nett, R.S.; Dho, Y.; Tsai, C.; Passow, D.; Grundman, J.M.; Low, Y.Y.; Sattely, E.S. Plant carbonic anhydrase-like enzymes in neuroactive alkaloid biosynthesis. Nature 2023, 624, 182–191. [Google Scholar] [CrossRef]
- Han, W.X.; Han, Z.W.; Jia, M.; Zhang, H.; Li, W.Z.; Yang, L.B.; Liang, F.; Han, L.; Zhao, N.; Li, X.F. Five novel and highly efficient endophytic fungi isolated from Huperzia serrata expressing huperzine A for the treatment of Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 9159–9177. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Z.; Wang, Y.; Yang, H.; Zeng, Q.; Zhu, D. Efficient strategy for maintaining and enhancing the huperzine A production of Shiraia sp. Slf14 through inducer elicitation. J. Ind. Microbiol. Biotechnol. 2014, 41, 1175–1179. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Dou, M.; Liu, D.; Lin, W.; Fan, A. Discovery of a hybrid molecule with phytotoxic activity by genome mining, heterologous expression, and OSMAC strategy. J. Agric. Food Chem. 2024, 72, 18520–18527. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Li, C.; Shu, Y.; Wang, J.; Wang, C.; Zhu, L.; Yang, Y.; Liu, J.; He, B.; Cai, L.; et al. Steroids and dihydroisocoumarin glycosides from Xylaria sp. by the one strain many compounds strategy and their bioactivities. Chin. J. Nat. Med. 2023, 21, 154–160. [Google Scholar] [CrossRef]
- Liu, T.; Ren, Z.; Chunyu, W.X.; Li, G.D.; Chen, X.; Zhang, Z.T.L.; Sun, H.B.; Wang, M.; Xie, T.P.; Wang, M.; et al. Exploration of diverse secondary metabolites from Streptomyces sp. YINM00001, using genome mining and one strain many compounds approach. Front. Microbiol. 2022, 13, 831174. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zou, L.; Lei, X.; Su, J.; Yang, R.; Xie, W.; Li, W.; Chen, G. OSMAC strategy integrated with molecular networking discovery peniciacetals A−I, nine new meroterpenoids from the mangrove-derived fungus Penicillium sp. HLLG-122. Bioorg. Chem. 2023, 130, 106271. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) principle to marine microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef]
- Wu, Y.M.; Yang, X.Q.; Li, S.Y.; Chen, J.X.; Wang, T.; Sun, J.; Yang, Y.B.; Ding, Z.T. Chlorinated cyclopentene derivatives and antifungal activities from Periconia sp. induced by the one strain many compounds strategy and host plant culture. J. Agric. Food Chem. 2022, 70, 8653–8661. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, D.L.; Liu, G.H.; Fu, X.; Gu, Y.C.; Ding, L.S.; Zhou, Y. Antifungal halogenated cyclopentenones from the endophytic fungus Saccharicola bicolor of Bergenia purpurascens by the one strain many compounds strategy. J. Agric. Food Chem. 2019, 68, 185–192. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Q.M.; Yan, X.; Hu, C.; Wang, W.; Wang, R.K.; Luo, X.; Zhang, X.W. Bioactivity-guided isolation of cytotoxic phenanthrenes from Spiranthes sinensis. J. Agric. Food Chem. 2019, 67, 7274–7280. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Wang, Y.Q.; Su, B.J.; Wang, H.S.; Liao, H.B.; Liang, D. New enantiomeric lignans and new meroterpenoids with nitric oxide release inhibitory activity from Piper puberulum. Bioorg. Chem. 2022, 119, 105522. [Google Scholar] [CrossRef]
- Le, T.T.M.; Hoang, A.T.H.; Le, T.T.B.; Vo, T.T.B.; Quyen, D.V.; Chu, H.H. Isolation of endophytic fungi and screening of huperzine A–producing fungus from Huperzia serrata in vietnam. Sci. Rep. 2019, 9, 16152. [Google Scholar] [CrossRef]
- Ma, X.; Gang, D.R. The Lycopodium alkaloids. Nat. Prod. Rep. 2004, 21, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Yang, M.; Su, J. Research on endophytic fungi for producing huperzine A on a large-scale. Crit. Rev. Microbiol. 2020, 46, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Jain, R.; Bhardwaj, P.; Thakur, A.; Kumari, M.; Bhushan, S.; Kumar, S. Plant probiotics-endophytes pivotal to plant health. Microbiol. Res. 2022, 263, 127148. [Google Scholar] [CrossRef] [PubMed]
- Joyner, P.M. Protein adducts and protein oxidation as molecular mechanisms of flavonoid bioactivity. Molecules 2021, 26, 5102. [Google Scholar] [CrossRef]
- Lotfi, M.S.; Rassouli, F.B. Natural flavonoid apigenin, an effective agent against nervous system cancers. Mol. Neurobiol. 2024, 61, 5572–5583. [Google Scholar] [CrossRef]
- Redkar, A.; Sabale, M.; Zuccaro, A.; Pietro, A.D. Determinants of endophytic and pathogenic lifestyle in root colonizing fungi. Curr. Opin. Plant Biol. 2022, 67, 102226. [Google Scholar] [CrossRef]
- Tiwari, P.; Kang, S.; Bae, H. Plant-endophyte associations: Rich yet under-explored sources of novel bioactive molecules and applications. Microbiol. Res. 2023, 266, 127241. [Google Scholar] [CrossRef]
- Ji, X.; Xia, Y.; Zhang, H.; Cui, J.L. The microscopic mechanism between endophytic fungi and host plants: From recognition to building stable mutually beneficial relationships. Microbiol. Res. 2022, 261, 127056. [Google Scholar] [CrossRef]
- Shrestha, N.; Zhang, X.C. Recircumscription of Huperzia serrata complex in China using morphological and climatic data. J. Syst. Evol. 2014, 53, 88–103. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Zaki, A.G.; Shatoury, E.H.E.; Ahmed, A.S.; Hagar, O.E.A.A. Production and enhancement of the acetylcholinesterase inhibitor, huperzine A, from an endophytic Alternaria brassicae AGF041. Appl. Microbiol. Biotechnol. 2019, 103, 5867–5878. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Elsevier Public Health Emergency Collection: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, P.; Tao, T.; Chen, Q.H. Drug brain distribution following intranasal administration of huperzine A in situ gel in rats. Acta Pharmacol. Sin. 2007, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Noushahi, H.A.; Zhang, Y.; Liu, J.; Cosoveanu, A.; Liu, Y.; Yan, L.; Zhang, J.; Shu, S. Endophytic fungal community of Huperzia serrata: Diversity and relevance to the production of huperzine A by the plant host. Molecules 2021, 26, 892. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Migas, P.; Stefanowicz, J.; Luczkiewicz, M.; Baranowska, M.K. Densitometric TLC analysis for the control of tropane and steroidal alkaloids in Lycium barbarum. Food Chem. 2017, 221, 535–540. [Google Scholar] [CrossRef]
- Ren, N.; Liu, J.; Yang, D.; Peng, Y.; Hong, J.; Liu, X.; Zhao, N.; Zhou, J.; Luo, Y. Indentification of vincamine indole alkaloids producing endophytic fungi isolated from Nerium indicum, Apocynaceae. Microbiol. Res. 2016, 192, 114–121. [Google Scholar] [CrossRef]
- Kassam, R.; Jaiswal, N.; Hada, A.; Phani, V.; Yadav, J.; Budhwar, R.; Godwin, J.; Chatterjee, M.; Bhat, C.G.; Mishra, J.; et al. Evaluation of Paecilomyces tenuis producing huperzine A for the management of root-knot nematode Meloidogyne incognita (Nematoda: Meloidogynidae). J. Pest Sci. 2022, 96, 723–743. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, Z.; Zhu, Q.; Tian, P. Systematic Investigations of the Huperzine A—Producing Endophytic Fungi of Huperzia serrata in China and Fermentation Optimization Using OSMAC Strategy. Molecules 2025, 30, 2704. https://doi.org/10.3390/molecules30132704
Li W, Wang Z, Zhu Q, Tian P. Systematic Investigations of the Huperzine A—Producing Endophytic Fungi of Huperzia serrata in China and Fermentation Optimization Using OSMAC Strategy. Molecules. 2025; 30(13):2704. https://doi.org/10.3390/molecules30132704
Chicago/Turabian StyleLi, Wei, Zhicheng Wang, Qiuyu Zhu, and Pingfang Tian. 2025. "Systematic Investigations of the Huperzine A—Producing Endophytic Fungi of Huperzia serrata in China and Fermentation Optimization Using OSMAC Strategy" Molecules 30, no. 13: 2704. https://doi.org/10.3390/molecules30132704
APA StyleLi, W., Wang, Z., Zhu, Q., & Tian, P. (2025). Systematic Investigations of the Huperzine A—Producing Endophytic Fungi of Huperzia serrata in China and Fermentation Optimization Using OSMAC Strategy. Molecules, 30(13), 2704. https://doi.org/10.3390/molecules30132704






