Fractional Coprecipitation of Drugs and Natural Extracts with Zinc Hydroxide
Abstract
1. Introduction
2. Results and Discussion
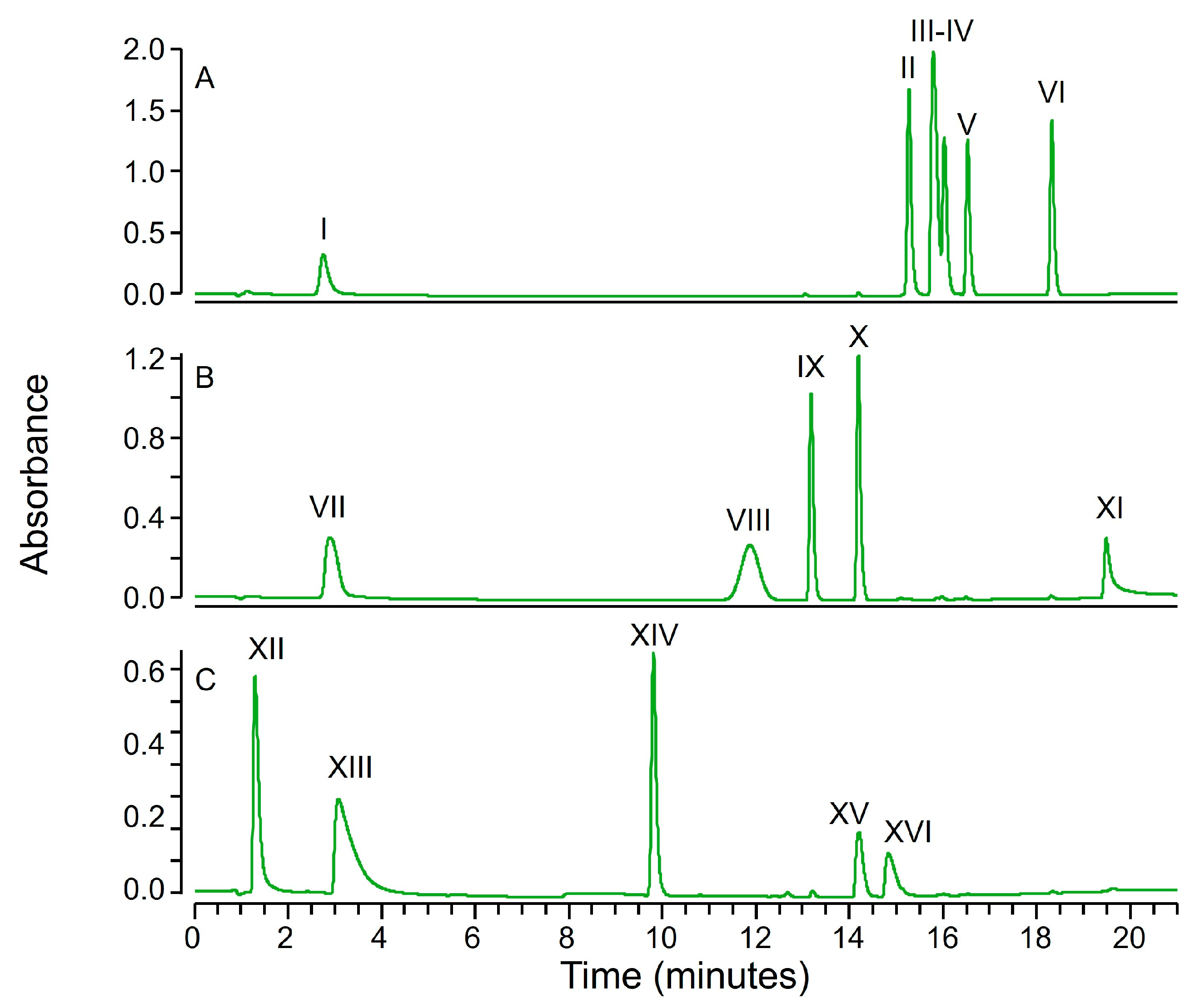
2.1. Fractional Coprecipitation of Drugs with Zinc Hydroxide
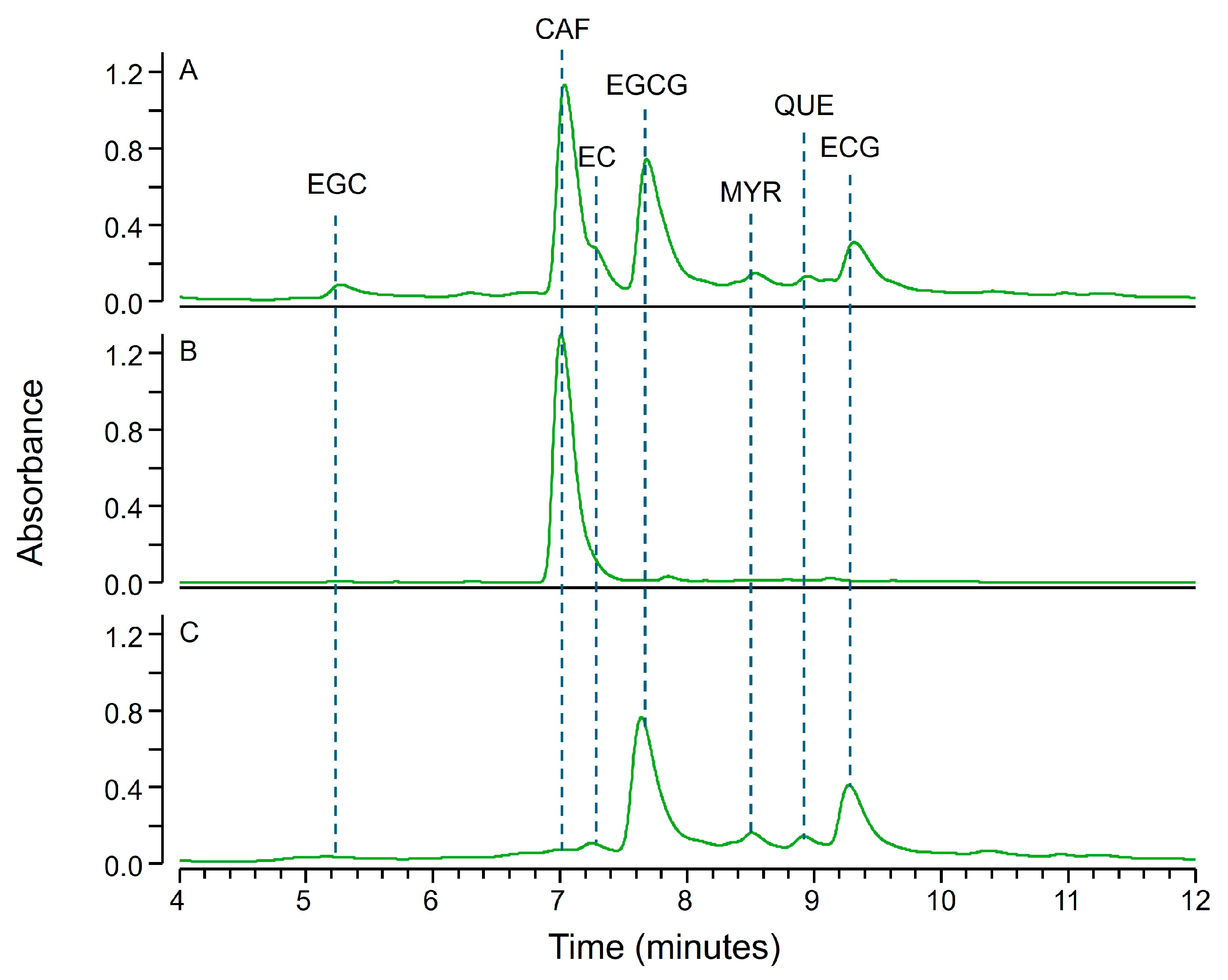
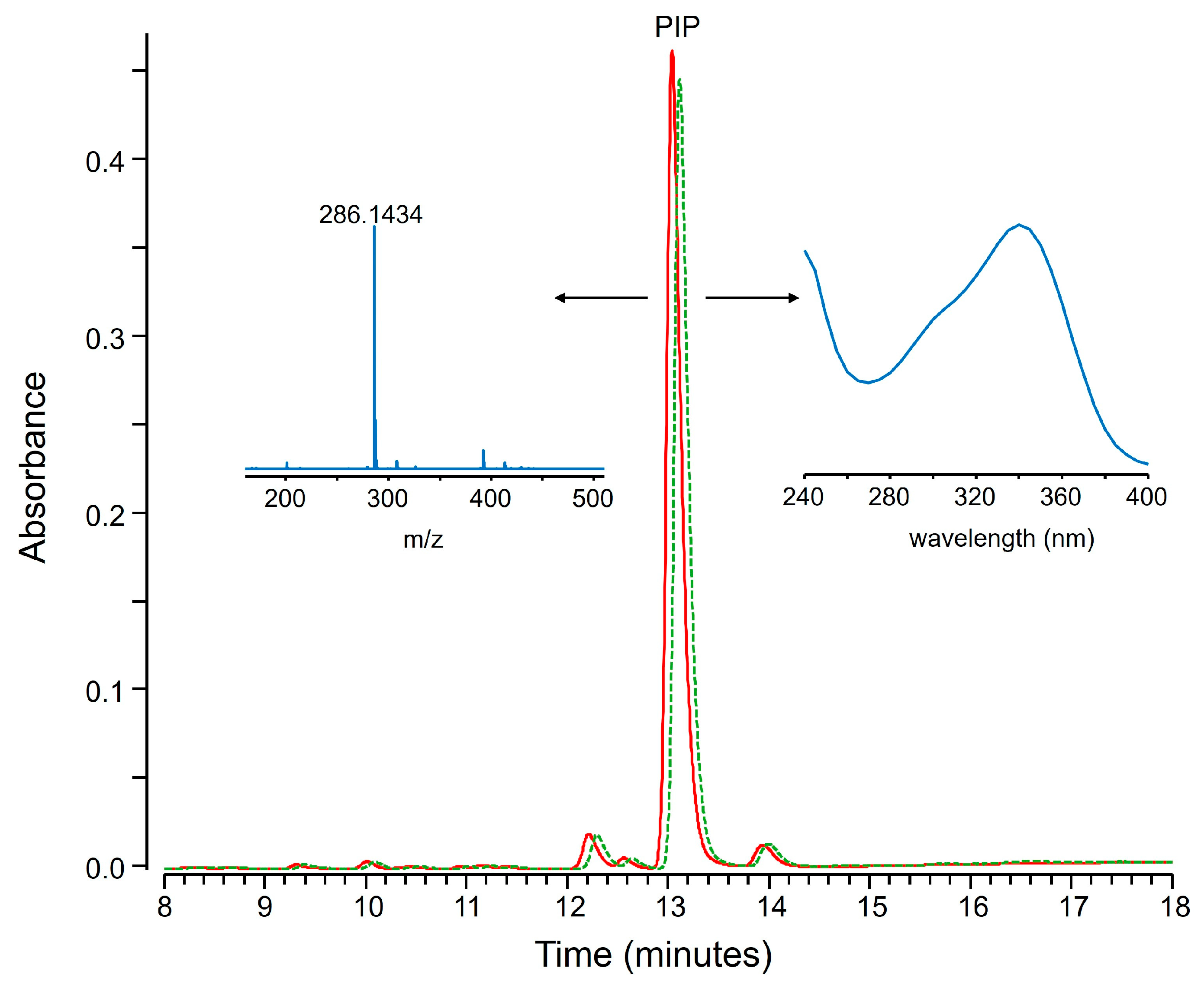
2.2. Fractional Coprecipitation of Natural Extracts with Zinc Hydroxide
3. Materials and Methods
3.1. Chemicals
3.2. Sample Preparation
3.2.1. Mixtures of Small Molecular Drugs
3.2.2. Extracts
3.2.3. Zinc Hydroxide Precipitation and Resuspension
3.3. Sample Analysis
3.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blais, J.F.; Djedidi, Z.; Cheikh, R.B.; Tyagi, R.D.; Mercier, G. Metals Precipitation from Effluents: Review. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2008, 12, 135–149. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Lai, H.; Ding, X.; Cui, M.; Zheng, J.; Chen, Z.; Pei, J.; Zhang, J. Mechanisms and influencing factors of biomineralization based heavy metal remediation: A review. Biogeotechnics 2023, 1, 100039. [Google Scholar] [CrossRef]
- Yu, F.; Huangfu, L.; Wang, C.; Li, C.; Yu, J.; Li, W.; Gao, S. Recovery of Fe and Al from red mud by a novel fractional precipitation process. Environ. Sci. Pollut. Res. 2020, 27, 14642–14653. [Google Scholar] [CrossRef]
- Yang, K.; Li, J.; Zhu, C.; Zhu, X.; Huang, W.; Wu, Z.; Fang, Z. Separation and recovery of valuable elements from acid leachate of spent carbon cathode by fractional precipitation method. J. Environ. Chem. Eng. 2023, 11, 110288. [Google Scholar] [CrossRef]
- Tasaki-Handa, Y.; Abe, Y.; Ooi, K.; Narita, H.; Tanaka, M.; Wakisaka, A. Environmentally friendly separation of dysprosium and neodymium by fractional precipitation of coordination polymers. RSC Adv. 2014, 4, 20496–20498. [Google Scholar] [CrossRef]
- Howard, P.; Parikh, R.S. Solution properties of cellulose triacetate. I. Fractional precipitation. J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 407–418. [Google Scholar] [CrossRef]
- Cragg, L.H.; Switzer, D.F. The Fractional Precipitation of Gr-S: The Effect of Concentration of the Solution on the Efficiency of Fractionation. Can. J. Chem. 1953, 31, 868–880. [Google Scholar] [CrossRef]
- Pernas, A.J.; Smidsrod, O.; Larsen, B.; Haug, A. Chemical heterogeneity of carrageenans as shown by fractional precipitation with potassium chloride. Acta Chem. Scand. 1967, 21, 98–110. [Google Scholar] [CrossRef][Green Version]
- Yen, D.R.; Raghavan, S.; Merrill, E.W. Fractional Precipitation of Star Poly(ethylene oxide). Macromolecules 1996, 29, 8977–8978. [Google Scholar] [CrossRef]
- Doonan, S. Bulk purification by fractional precipitation. Methods Mol. Biol. 2004, 244, 117–124. [Google Scholar] [CrossRef]
- Englard, S.; Seifter, S. Precipitation techniques. Methods Enzymol. 1990, 182, 285–300. [Google Scholar] [CrossRef]
- Burgess, R.R. Protein precipitation techniques. Methods Enzymol. 2009, 463, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Duong-Ly, K.C.; Gabelli, S.B. Salting out of proteins using ammonium sulfate precipitation. Methods Enzymol. 2014, 541, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Cohn, E.J.; Strong, L.E.; Hughes, W.L.; Mulford, D.J.; Ashworth, J.N.; Melin, M.; Taylor, H.L. Preparation and Properties of Serum and Plasma Proteins. IV. A System for the Separation into Fractions of the Protein and Lipoprotein Components of Biological Tissues and Fluids. J. Am. Chem. Soc. 1946, 68, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Macritchie, F. Effects of temperature on dissolution and precipitation of proteins and polyamino acids. J. Colloid Interface Sci. 1973, 45, 235–241. [Google Scholar] [CrossRef]
- Ohara, I.; Ariyoshi, S. Comparison of Protein Precipitants for the Determination of Free Amino Acids in Plasma. Agric. Biol. Chem. 1979, 43, 1473–1478. [Google Scholar] [CrossRef]
- Polson, C.; Sarkar, P.; Incledon, B.; Raguvaran, V.; Grant, R. Optimization of protein precipitation based upon effectiveness of protein removal and ionization effect in liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 785, 263–275. [Google Scholar] [CrossRef]
- Blanchard, J. Evaluation of the relative efficacy of various techniques for deproteinizing plasma samples prior to high-performance liquid chromatographic analysis. J. Chromatogr. 1981, 226, 455–460. [Google Scholar] [CrossRef]
- Souverain, S.; Rudaz, S.; Veuthey, J.L. Protein precipitation for the analysis of a drug cocktail in plasma by LC-ESI-MS. J. Pharm. Biomed. Anal. 2004, 35, 913–920. [Google Scholar] [CrossRef]
- Salina, E.; Regazzoni, L. Protein Precipitation by Metal Hydroxides as a Convenient and Alternative Sample Preparation Procedure for Bioanalysis. Molecules 2024, 30, 2. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gautam, N.; Alnouti, Y. Analyte recovery in LC-MS/MS bioanalysis: An old issue revisited. Anal. Chim. Acta 2022, 1198, 339512. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.F.; Barker, S.A.; Humphreys, J.D. Insoluble complexes of amino-acids, peptides, and enzymes with metal hydroxides. J. Chem. Soc. Perkin Trans. 1 1976, 962–967. [Google Scholar] [CrossRef]
- Ilin, A.; Raiko, T. Practical Approaches to Principal Component Analysis in the Presence of Missing Values. J. Mach. Learn. Res. 2010, 11, 1957–2000. [Google Scholar]
- Wei, W.; Cherukupalli, S.; Jing, L.; Liu, X.; Zhan, P. Fsp(3): A new parameter for drug-likeness. Drug Discov. Today 2020, 25, 1839–1845. [Google Scholar] [CrossRef]
- Wangkarn, S.; Grudpan, K.; Khanongnuch, C.; Pattananandecha, T.; Apichai, S.; Saenjum, C. Development of HPLC Method for Catechins and Related Compounds Determination and Standardization in Miang (Traditional Lanna Fermented Tea Leaf in Northern Thailand). Molecules 2021, 26, 6052. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res. Int. 2001, 34, 223–227. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef]
- Regazzoni, L.; Saligari, F.; Marinello, C.; Rossoni, G.; Aldini, G.; Carini, M.; Orioli, M. Coffee silver skin as a source of polyphenols: High resolution mass spectrometric profiling of components and antioxidant activity. J. Funct. Foods 2016, 20, 472–485. [Google Scholar] [CrossRef]
- Navarra, G.; Moschetti, M.; Guarrasi, V.; Mangione, M.R.; Militello, V.; Leone, M. Simultaneous Determination of Caffeine and Chlorogenic Acids in Green Coffee by UV/Vis Spectroscopy. J. Chem. 2017, 2017, 6435086. [Google Scholar] [CrossRef]
- Villanueva-Bermejo, D.; Zahran, F.; Troconis, D.; Villalva, M.; Reglero, G.; Fornari, T. Selective precipitation of phenolic compounds from Achillea millefolium L. extracts by supercritical anti-solvent technique. J. Supercrit. Fluids 2017, 120, 52–58. [Google Scholar] [CrossRef]
- Min-Kyoung, L. Improvement of the Fractional Precipitation Process for the Purification of (+)-Dihydromyricetin. Microbiol. Biotechnol. Lett. 2014, 42, 25–31. [Google Scholar] [CrossRef]
- Ranatunge, I.; Adikary, S.; Dasanayake, P.; Fernando, C.D.; Soysa, P. Development of a Rapid and Simple Method to Remove Polyphenols from Plant Extracts. Int. J. Anal. Chem. 2017, 2017, 7230145. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.; Mila, I.; Scalbert, A. Precipitation of Metal Ions by Plant Polyphenols: Optimal Conditions and Origin of Precipitation. J. Agric. Food Chem. 1996, 44, 599–606. [Google Scholar] [CrossRef]
- Dyer, J.A.; Scrivner, N.C.; Dentel, S.K. A practical guide for determining the solubility of metal hydroxides and oxides in water. Environ. Prog. 1998, 17, 1–8. [Google Scholar] [CrossRef]
- Keller, B.O.; Sui, J.; Young, A.B.; Whittal, R.M. Interferences and contaminants encountered in modern mass spectrometry. Anal. Chim. Acta 2008, 627, 71–81. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 9, 9. [Google Scholar]
- Richardson, P.; Hoare, M.; Dunnill, P. A new biochemical engineering approach to the fractional precipitation of proteins. Biotechnol. Bioeng. 1990, 36, 354–366. [Google Scholar] [CrossRef]


| PC1 | PC2 | |
|---|---|---|
| RES | 0.30 | 0.27 |
| SAG | 0.48 | 0.16 |
| SBG | 0.43 | −0.18 |
| LogD | 0.16 | −0.48 |
| CHA | 0.50 | 0.08 |
| LogS | −0.03 | 0.49 |
| HLB | −0.10 | 0.40 |
| Fsp3 | 0.32 | −0.04 |
| TPSA | −0.32 | −0.01 |
| POL | −0.03 | −0.49 |
| Compound | SUP | PRE | ||
|---|---|---|---|---|
| Mean (%) | SD | Mean (%) | SD | |
| I | 4.5 | 0.1 | 99.3 | 0.1 |
| III | 2.2 | 0.1 | 127.8 | 0.7 |
| VI | 21.2 | 0.1 | 58.4 | 3.4 |
| XV | 15.7 | 0.2 | 85.6 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzese, A.; Regazzoni, L. Fractional Coprecipitation of Drugs and Natural Extracts with Zinc Hydroxide. Molecules 2025, 30, 2699. https://doi.org/10.3390/molecules30132699
Franzese A, Regazzoni L. Fractional Coprecipitation of Drugs and Natural Extracts with Zinc Hydroxide. Molecules. 2025; 30(13):2699. https://doi.org/10.3390/molecules30132699
Chicago/Turabian StyleFranzese, Andrea, and Luca Regazzoni. 2025. "Fractional Coprecipitation of Drugs and Natural Extracts with Zinc Hydroxide" Molecules 30, no. 13: 2699. https://doi.org/10.3390/molecules30132699
APA StyleFranzese, A., & Regazzoni, L. (2025). Fractional Coprecipitation of Drugs and Natural Extracts with Zinc Hydroxide. Molecules, 30(13), 2699. https://doi.org/10.3390/molecules30132699






