Structure–Function Relationship of Novel Tetrakis (Mercapto-Terphenyl)Benzene Cobalt (II) Phthalocyanines: Synthesis and Computational Evaluation
Abstract
1. Introduction
2. Experimental
2.1. Characterization Techniques
2.2. Synthesis Studies
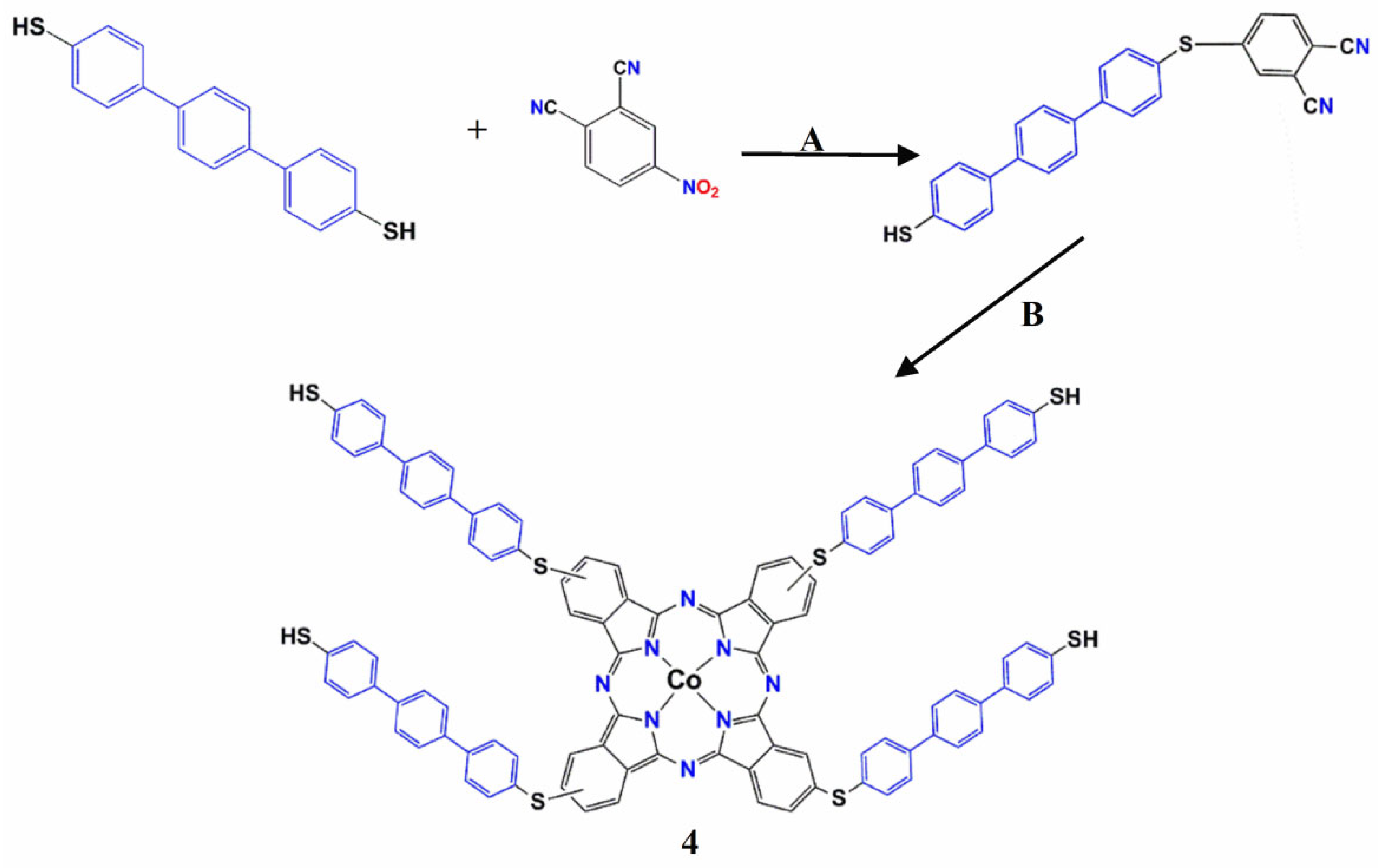
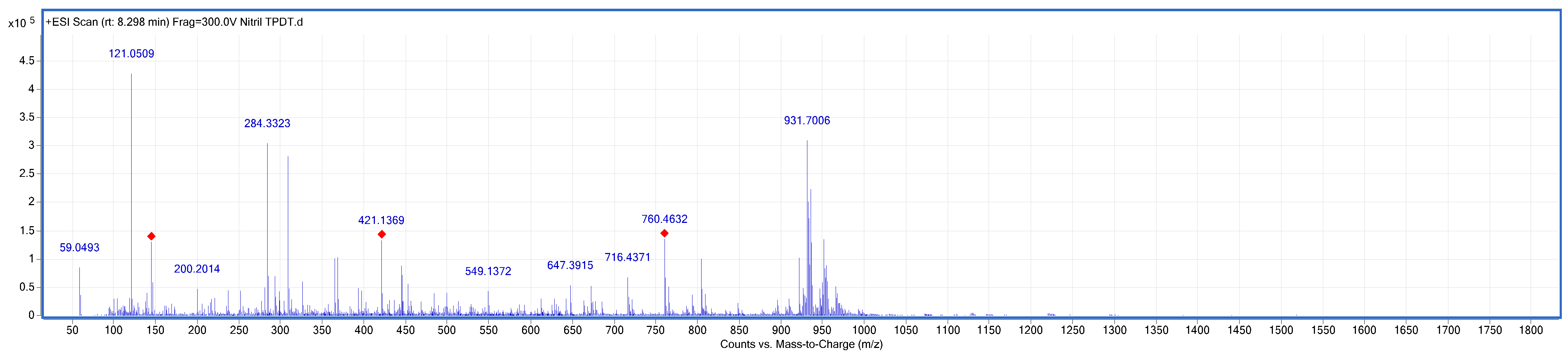
2.2.1. Synthesis of Phthalonitrile (3) 4-[(4′′-Mercapto-[1,1′:4′,1′′-Terphenly]-4-Yl)Thio) Phthalonitrile
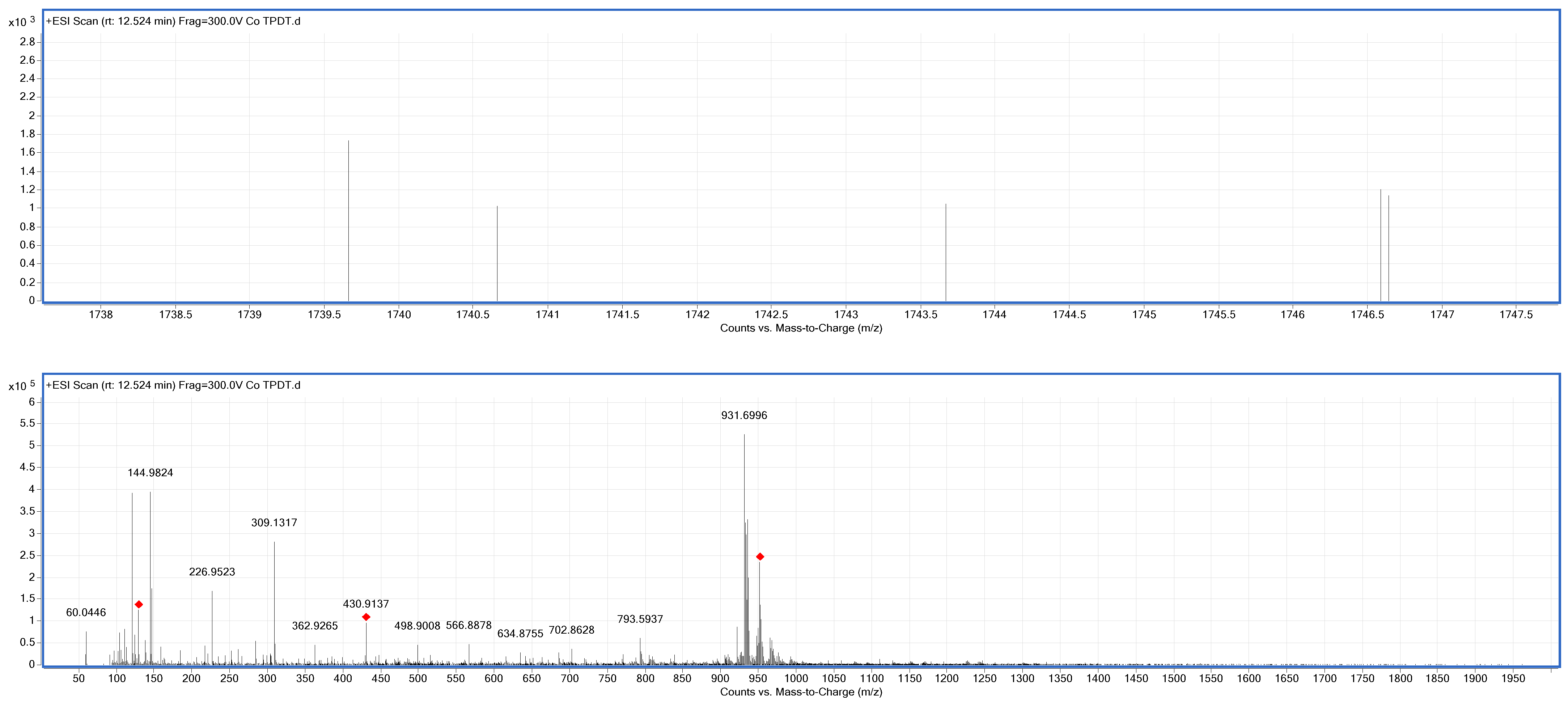
2.2.2. Synthesis of Compound (4) 2, 9(10), 16(17), 23(24)-Tetrakis-(2-(E-(2-(E-2-Mercaptobenzyliedene) Mercaptoterphenly-2-Thio Cobalt(II)
2.3. Computational Details
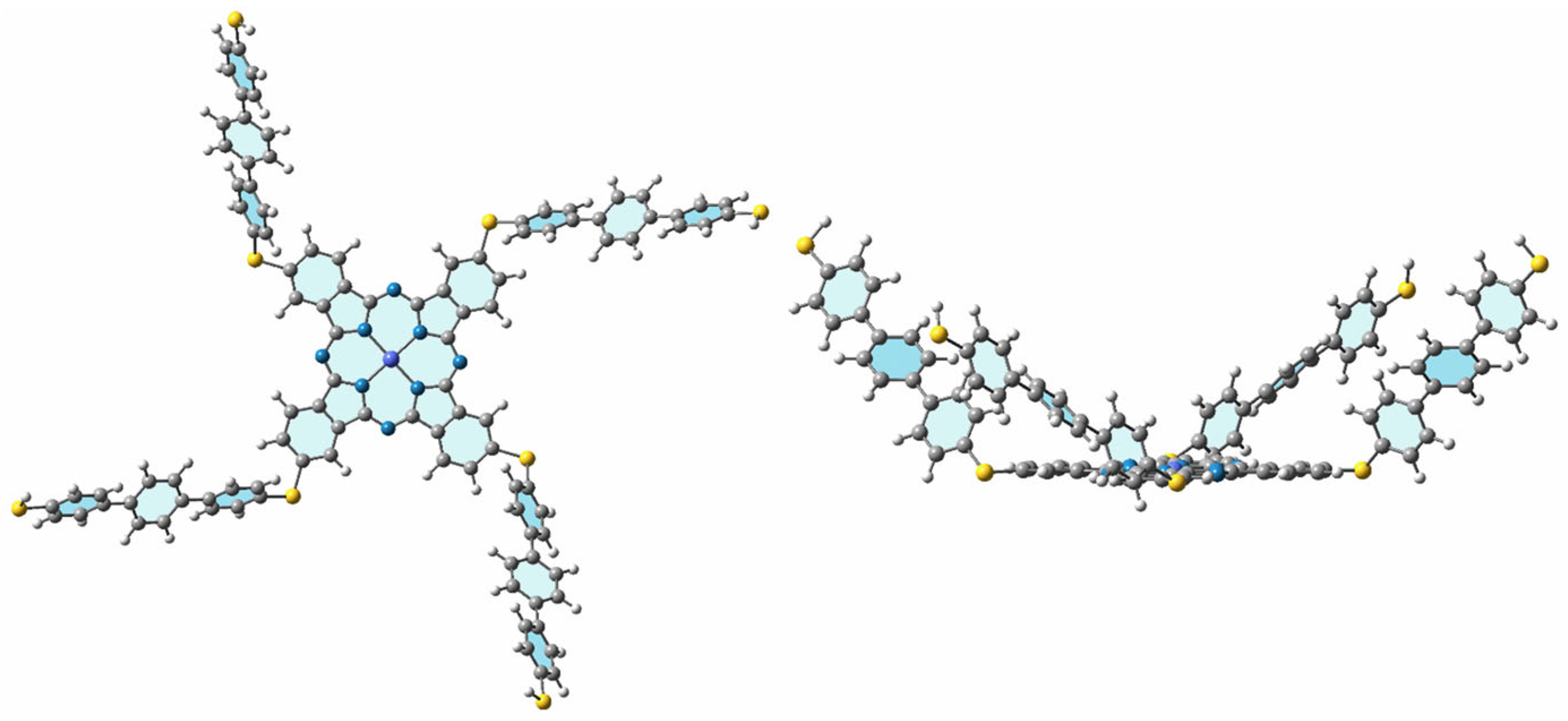
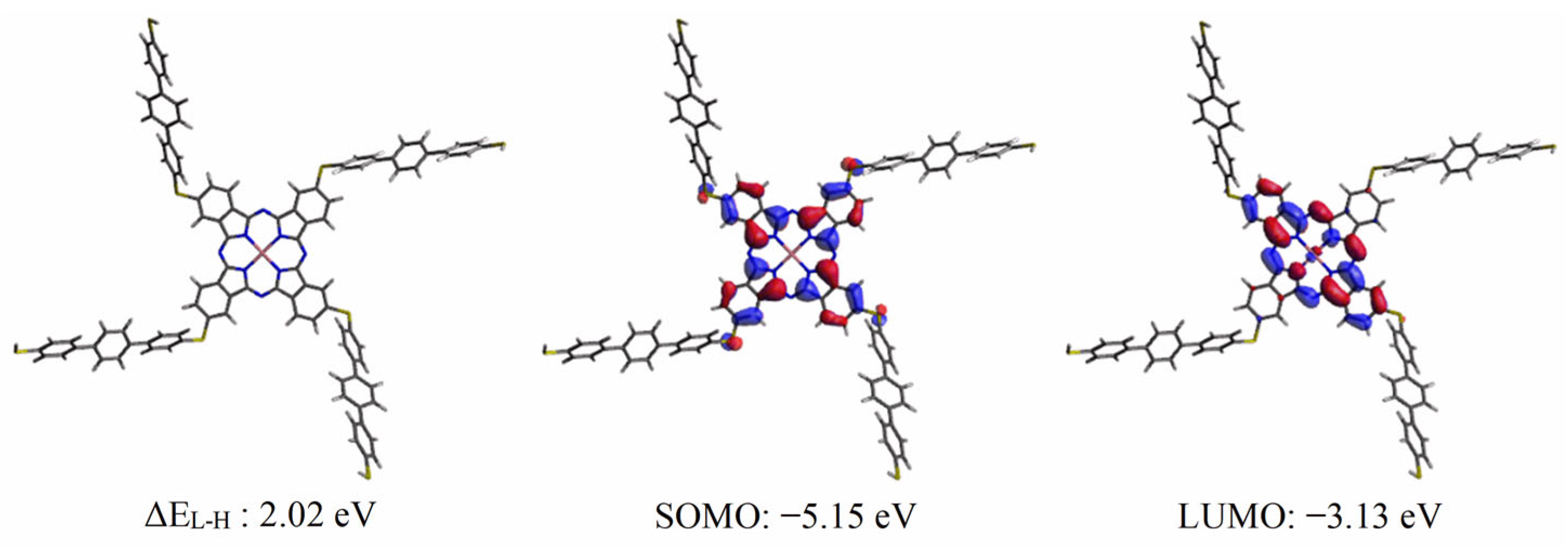
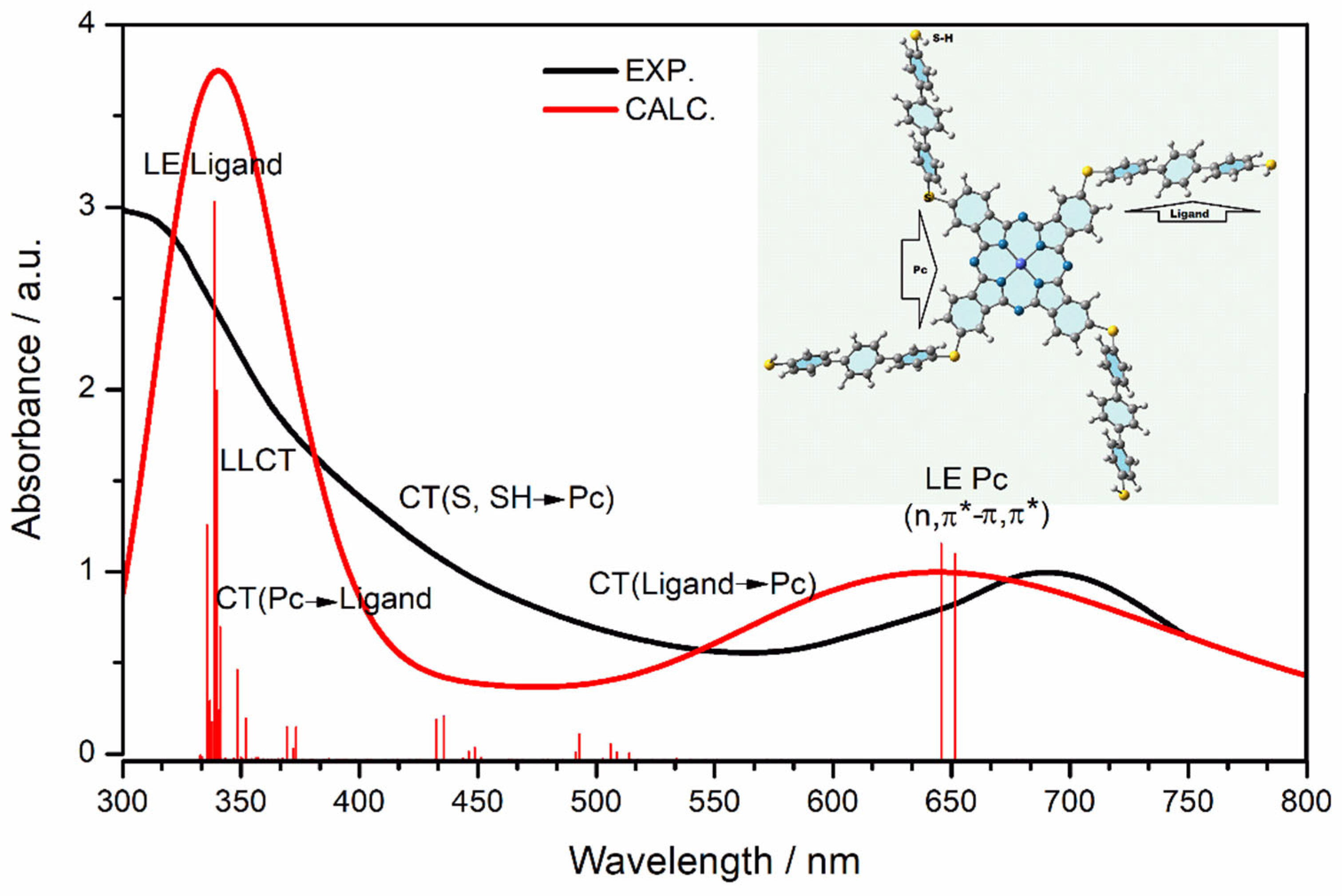
3. Results and Discussion
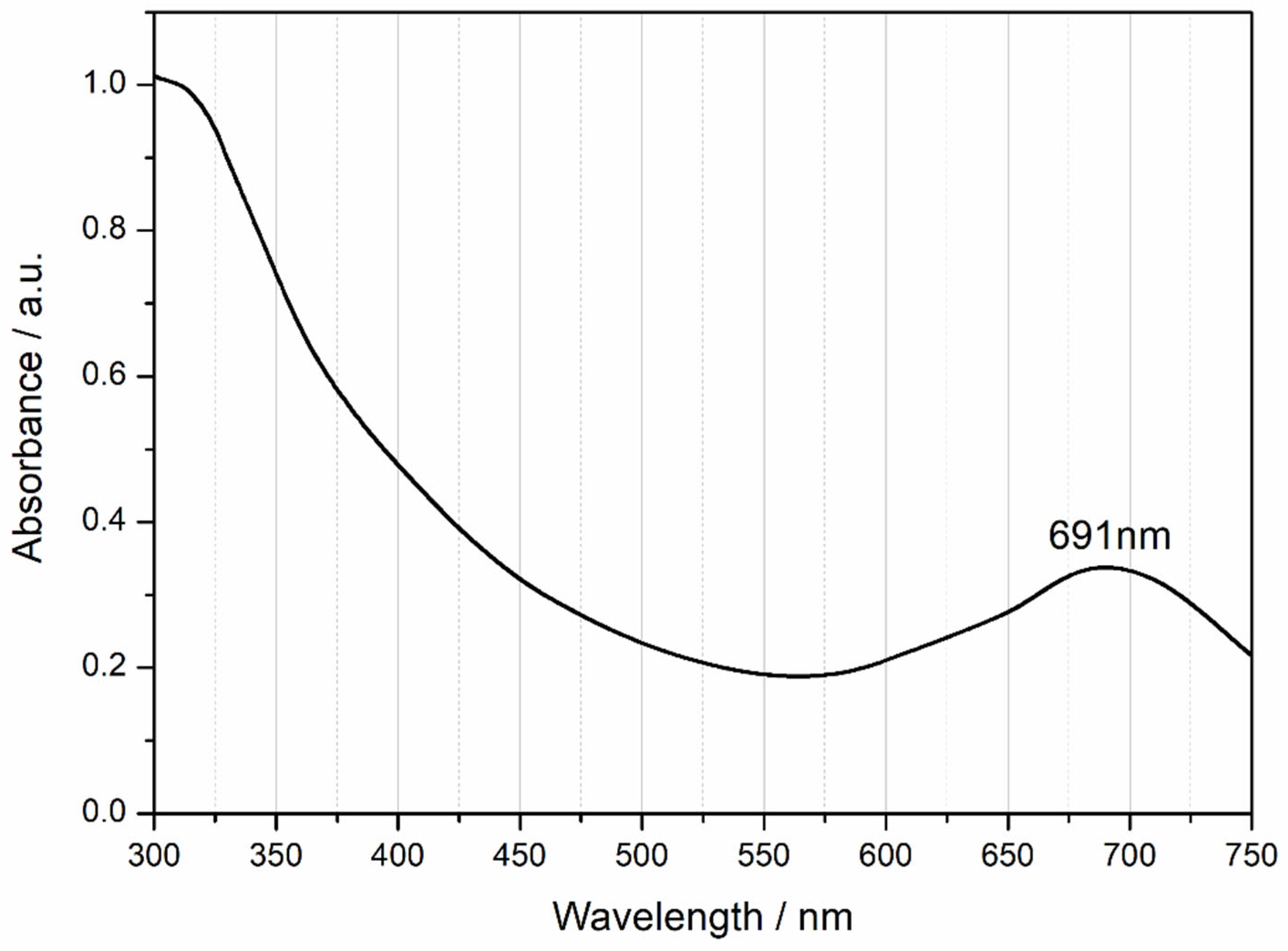
3.1. Chemical Synthesis Studies
3.2. Computational Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautrot, J.E.; Hodge, P.; Cupertino, D.; Helliwell, M. 2,6-Diaryl-9,10-Anthraquinones as Models for Electron-Accepting Polymers. New J. Chem. 2007, 31, 1585–1593. [Google Scholar] [CrossRef]
- Tolbin, A.Y.; Ivanov, A.V.; Tomilova, L.G.; Zefirov, N.S. Preparation of 1,2-Bis(3,4-Dicyanophenoxymethyl)Benzene and the Binuclear Zinc Phthalocyanine Derived from It. Mendeleev Commun. 2002, 12, 96–97. [Google Scholar] [CrossRef]
- Kaya, Y.; Erçağ, A.; Koca, A. New Square-Planar Nickel(II)-Triphenylphosphine Complexes Containing ONS Donor Ligands: Synthesis, Characterization, Electrochemical and Antioxidant Properties. J. Mol. Struct. 2020, 1206, 127653. [Google Scholar] [CrossRef]
- Gómez-Lara, J.; González-Rolón, B.; Cogordan, J.A.; Ortíz, A.; Espinosa-Pérez, G.; Ríos, S. Synthesis of Saturated and Unsaturated Curtis-Type Ni Derivatives as 2,6-Dihydroxyanthraquinonates. Crystal and Molecular Structure. Thin Film Measurements of Conductivity and Ab Initio Calculations of Electronic States. Chem. Mater. 2000, 12, 3570–3577. [Google Scholar] [CrossRef]
- Son, E.J.; Kim, J.H.; Kim, K.; Park, C.B. Quinone and Its Derivatives for Energy Harvesting and Storage Materials. J. Mater. Chem. A 2016, 4, 11179–11202. [Google Scholar] [CrossRef]
- Langdon-Jones, E.E.; Pope, S.J.A. The Coordination Chemistry of Substituted Anthraquinones: Developments and Applications. Coord. Chem. Rev. 2014, 269, 32–53. [Google Scholar] [CrossRef]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M.; Zaczyńska, E. Azaphenothiazines—Promising Phenothiazine Derivatives. An Insight into Nomenclature, Synthesis, Structure Elucidation and Biological Properties. Eur. J. Med. Chem. 2017, 138, 774–806. [Google Scholar] [CrossRef]
- Correia, H.M.G.; Ramos, M.M.D. Computer Simulation of Electron Transfer in Molecular Electronic Devices. Mater. Sci. Eng. C 2005, 25, 682–686. [Google Scholar] [CrossRef]
- Wu, H.-L.; Shin, M.; Liu, Y.-M.; See, K.A.; Gewirth, A.A. Thiol-Based Electrolyte Additives for High-Performance Lithium-Sulfur Batteries. Nano Energy 2017, 32, 50–58. [Google Scholar] [CrossRef]
- Farrugia, V.M.; Birau, M.M.; Iftime, G.; Abraham, B.E. Alizarin-Based Polymer Colorants. US 9181389B2, November 2015. [Google Scholar]
- Shimizu, T.; Kaneko, I.; Watanabe, M. Method of Preventing Polymer-Scale Formation. US Patent 4,933,399, September 1991. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. G16_C01 2016, Gaussian 16, Revision C.01; Gaussian, Inc.: Wallin, UK, 2016.
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, version 6.0.16; Semichem Inc.: Shawnee Mission, KS, USA, 2016.
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Gross, E.K.U.; Kohn, W. Time-Dependent Density-Functional Theory. In Density Functional Theory of Many-Fermion Systems; Löwdin, P.-O., Ed.; Academic Press: Cambridge, MA, USA, 1990; Volume 21, pp. 255–291. ISBN 0065-3276. [Google Scholar]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A Complete Basis Set Model Chemistry. I. The Total Energies of Closed-shell Atoms and Hydrides of the First-row Elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF Version of the PCM Solvation Method: An Overview of a New Method Addressed to Study Molecular Solutes at the QM Ab Initio Level. J. Mol. Struct. THEOCHEM 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Bhat, N.G.; Narayana, B. Complexes of Ag(I), TI(I), Zn(II), Hg(II), Cd(II), Pd(II), Co(II) and Ni(II) with 4-[(4-Dimethylamino-benzylidene)-amino]-5-ethyl-2,4-dihydro-[1,2,4]Triazole-3-thione (HDEDT) and 4-[(Benzylidene)Amino]-6-(T-butyl)-4H-[1,2,4]-triazine-3-thione-5-one (HBBTT). Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Tadros, A.B. Marine Coatings Based on Metal Complexes of 4-Amino-3-Hydrazino-5-Thio-1,2,4-Triazole. J. Chem. Technol. Biotechnol. 1989, 45, 213–221. [Google Scholar] [CrossRef]
- Sener, S.; Acar, N.; Dumludag, F. Electrical, Spectroscopic and DFT Studies of Synthesized Bridged Bis (2-Oxy Benzylidene) Hydrazine-Carbonothioly-Hydrazono Cobalt(II)Phthalocyanine. Polyhedron 2024, 247, 116738. [Google Scholar] [CrossRef]
- Şener, S.; Tahir Bayraç, A.; Bilgenur Şener, B.; Tozlu, C.; Acar, N.; Salih, B.; Yüksel, M.; Bekaroğlu, Ö. Synthesis, Characterization, and DFT Study of Novel Metallo Phtalocyanines with Four Carboranyl Clusters as Photosensitisers for the Photodynamic Therapy of Breast Cancer Cells. Eur. J. Pharm. Sci. 2019, 129, 124–131. [Google Scholar] [CrossRef]
- Cavdan, S.E.; Acar-Selcuki, N.; Kaya, D.; Ekicibil, A.; Sener, S. Synthesized Tetrakis (2-Oxy Benzylidene)—Hyroanthracen-2-Yloxy Zinc and Cobalt (II) Phthalocyanines: Structural, DFT and SEM-EDS Study. Inorganica Chim. Acta 2025, 581, 122614. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Fukui, K. Role of Frontier Orbitals in Chemical Reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sener, S.; Acar-Selcuki, N. Structure–Function Relationship of Novel Tetrakis (Mercapto-Terphenyl)Benzene Cobalt (II) Phthalocyanines: Synthesis and Computational Evaluation. Molecules 2025, 30, 2693. https://doi.org/10.3390/molecules30132693
Sener S, Acar-Selcuki N. Structure–Function Relationship of Novel Tetrakis (Mercapto-Terphenyl)Benzene Cobalt (II) Phthalocyanines: Synthesis and Computational Evaluation. Molecules. 2025; 30(13):2693. https://doi.org/10.3390/molecules30132693
Chicago/Turabian StyleSener, Sevil, and Nursel Acar-Selcuki. 2025. "Structure–Function Relationship of Novel Tetrakis (Mercapto-Terphenyl)Benzene Cobalt (II) Phthalocyanines: Synthesis and Computational Evaluation" Molecules 30, no. 13: 2693. https://doi.org/10.3390/molecules30132693
APA StyleSener, S., & Acar-Selcuki, N. (2025). Structure–Function Relationship of Novel Tetrakis (Mercapto-Terphenyl)Benzene Cobalt (II) Phthalocyanines: Synthesis and Computational Evaluation. Molecules, 30(13), 2693. https://doi.org/10.3390/molecules30132693




