The Fate of Contaminants of Emerging Concern in an Upflow Anaerobic Sludge Blanket Reactor Coupled with Constructed Wetlands for Decentralized Domestic Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Conventional WWTP
2.2. Pilot System
2.3. Wastewater Influent Characteristics
2.4. Operational Parameters of the Pilot System
2.5. Sampling Campaigns
2.6. Organic Micropollutants Analytical Methods
2.7. Data Analysis
2.8. Risk Assessment Analysis
3. Results and Discussion
3.1. Conventional Pollutants Removal Efficiency of the Pilot System
3.2. Occurrence of Target CECs in Influent Wastewater
| Compound | Ιnfluent Concentration (ng/L) in Municipal WWTPs | Ref. |
|---|---|---|
| IBU | Greece: 2800–25,400, 12,500 (range, mean) | [37] |
| Sweden: 6900 ± 900 (mean ± SD) | [34] | |
| USA: 16,433–96,519 (range) | [30] | |
| China: 268–2240, 628, 811 (range, median, average) | [29] | |
| Slovenia: 4.61–77.9, 43.9, 100% (range, average, frequency of detection) | [28] | |
| NPX | Greece: n.d.–2000, 1500 (range, mean) | [37] |
| Sweden: 4900 ± 480 (mean ± SD) | [34] | |
| USA: 15,544–45,386 (range) | [30] | |
| China: 1.63–20.4, 11.0, 11.4 (range, median, average) | [29] | |
| Slovenia: 81.3–1290, 361, 100% (range, average, frequency of detection) | [28] | |
| DCF | Greece: n.d.–3900, 2000 (range, mean) | [37] |
| Sweden: 230 ± 9 (mean ± SD) | [34] | |
| China: 128.6–1027.1 (range) | [35] | |
| Slovenia: 2.09–48.5, 15.5, 96.2% (range, average, frequency of detection) | [28] | |
| KFN | China: 100.6–7881.0 (range) | [35] |
| China: 13.0–1030, 236, 299 (range, median, average) | [29] | |
| Slovenia: 0.534–692, 55.3, 84.6% (range, average, frequency of detection) | [28] | |
| TCS | Greece: n.d.–1000, 800 (range, mean) | [37] |
| USA: 179–2523 (range) | [30] | |
| China: BDL 1–62.9, BDL, 5.30 (range, median, average) | [29] | |
| Slovenia: 5.27–9.68, 6.64, 50% (range, average, frequency of detection) | [28] | |
| BPA | Slovenia: 11.2–489, 95.7, 73.1% (range, average, frequency of detection) | [28] |
| China: 836.9 ± 87.2 (mean ± SD) | [39] | |
| 5TTR | Australia: 6758 ± 1438 (mean ± SD) | [40] |
| Greece: 3579 ± 179 (mean ± SD) | [32] | |
| CBTR | Australia: 1196 ± 301 (mean ± SD) | [40] |
| Greece: 3875 ± 833 (mean ± SD) | [32] | |
| XTR | Greece: n.d.–55.3, 27, 79% (range, average, frequency of detection) | [31] |
| Greece: 2767 ± 1106 (mean ± SD) | [32] | |
| OH-BTH | Greece: 256–908, 503, 100% (range, average, frequency of detection) | [31] |
| Greece: 7076 ± 2275 (mean ± SD) | [32] |
3.3. Influence of Operational Conditions on Total Removal of Pilot with VSSF UNSAT CW (Line A) and the Conventional WWTP
3.4. Contribution of Each Treatment Stage of the Pilot System to the Overall Performance and Removal Mechanisms
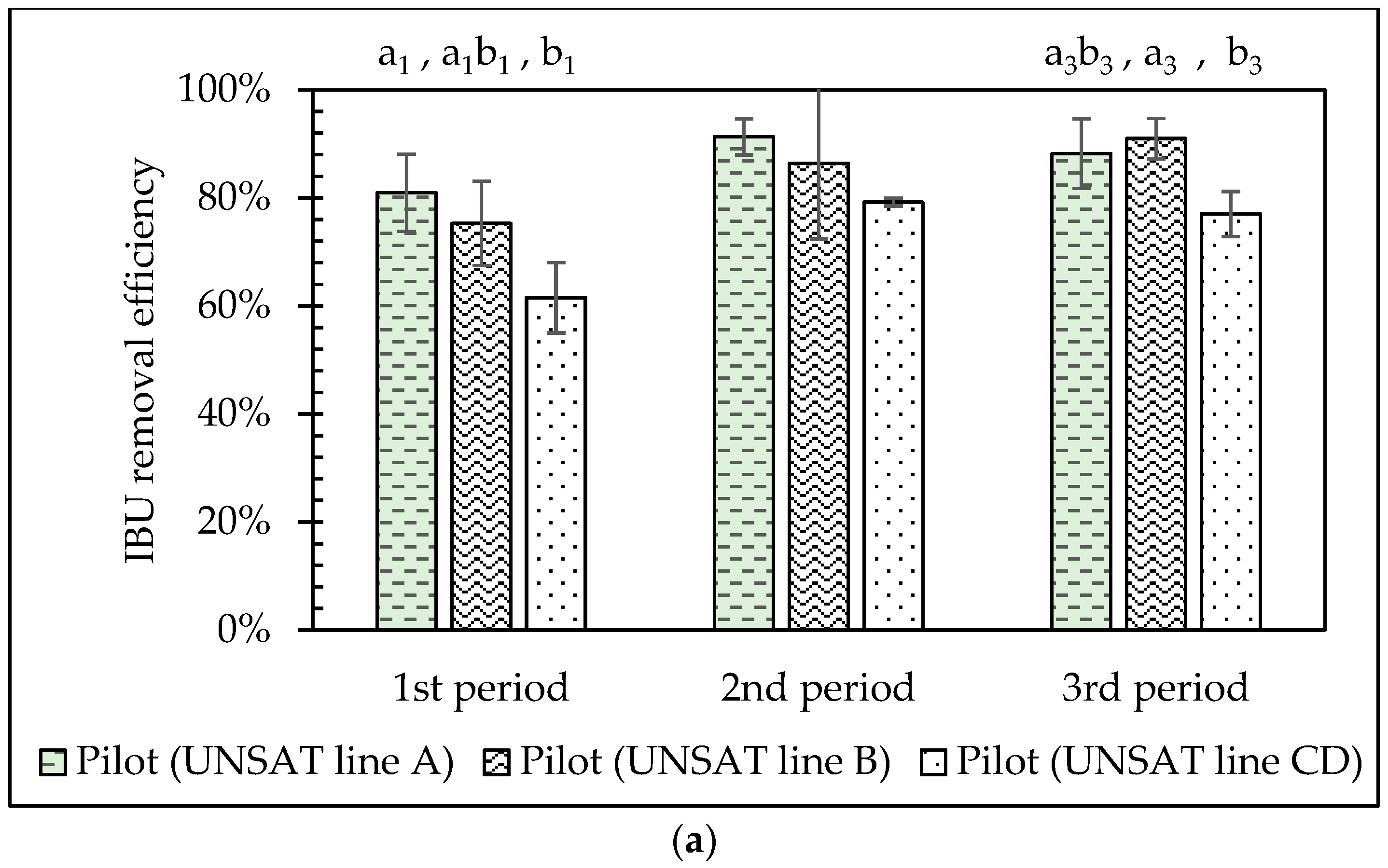
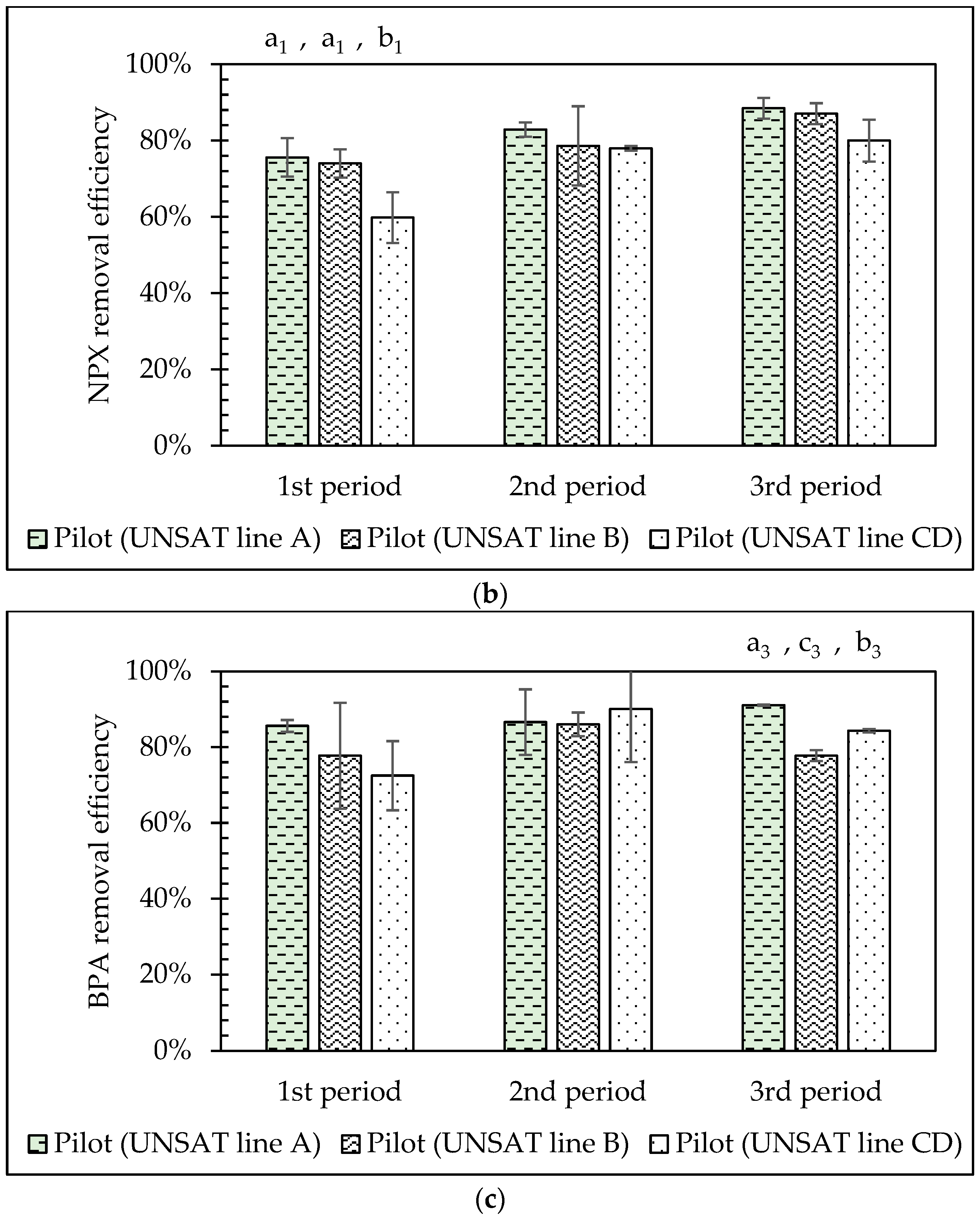
3.5. Comparison of the VSSF UNSAT CW Lines (A, B, C&D) Performance
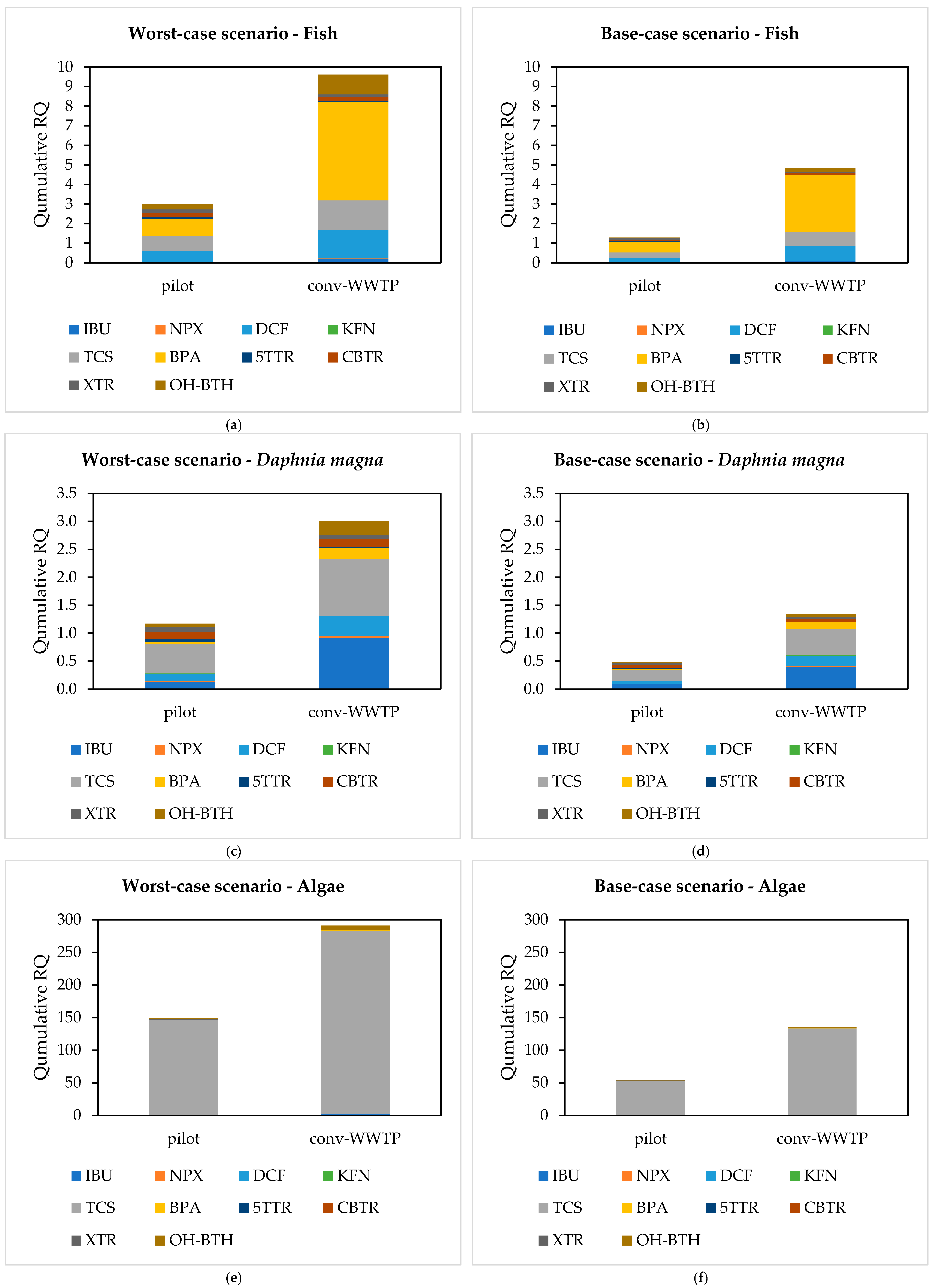
3.6. Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UASB | Upflow anaerobic sludge blanket reactor |
| VSSF | Vertical subsurface flow |
| CW | Constructed wetland |
| CECs | Contaminants of emerging concern |
| EDCs | Endocrine disrupting chemicals |
| SAT | Saturated (vertical subsurface flow constructed wetland) |
| UNSAT | Unsaturated (vertical subsurface flow constructed wetland) |
| Conv-WWTP | Conventional wastewater treatment plant |
| BTRs | Benzotriazoles |
| XTR | Xylytriazole |
| CBTR | 5-chlorobenzotriazole |
| 5TTR | 5-methyl-1H benzotriazole |
| BTH | Benzothiazole |
| OH-BTH | 2 hydroxybenzothiazole |
| TCS | Triclosan |
| BPA | Bisphenol A |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| IBU | Ibuprofen |
| NPX | Naproxen |
| DCF | Diclofenac |
| KFN | Ketoprofen |
| RQ | Risk Quotient |
References
- Kanaujiya, D.K.; Paul, T.; Sinharoy, A.; Pakshirajan, K. Biological Treatment Processes for the Removal of Organic Micropollutants from Wastewater: A Review. Curr. Pollut. Rep. 2019, 5, 112–128. [Google Scholar] [CrossRef]
- Seintos, T.; Koukoura, A.; Statiris, E.; Noutsopoulos, C.; Mamais, D.; Μasi, F.; Prado, O.; Rizzo, A.; Bartroli, A.; Stasinakis, A.S.; et al. Long-Term Operation of an Upflow Anaerobic Sludge Blanket Reactor Coupled with a Two-Stage Constructed Wetland for Domestic Wastewater Treatment. Chem. Eng. J. 2024, 500, 157216. [Google Scholar] [CrossRef]
- Askari, S.S.; Giri, B.S.; Basheer, F.; Izhar, T.; Ahmad, S.A.; Mumtaz, N. Enhancing Sequencing Batch Reactors for Efficient Wastewater Treatment across Diverse Applications: A Comprehensive Review. Environ. Res. 2024, 260, 119656. [Google Scholar] [CrossRef]
- Lau, P.L.; Trzcinski, A.P. A Review of Modified and Hybrid Anaerobic Baffled Reactors for Municipal Wastewater Treatment with a Focus on Emerging Contaminants. Environ. Sci. 2024, 10, 1335–1354. [Google Scholar] [CrossRef]
- Ji, J.; Kakade, A.; Yu, Z.; Khan, A.; Liu, P.; Li, X. Anaerobic Membrane Bioreactors for Treatment of Emerging Contaminants: A Review. J. Environ. Manag. 2020, 270, 110913. [Google Scholar] [CrossRef]
- Arias, A.; Alvarino, T.; Allegue, T.; Suárez, S.; Garrido, J.M.; Omil, F. An Innovative Wastewater Treatment Technology Based on UASB and IFAS for Cost-Efficient Macro and Micropollutant Removal. J. Hazard. Mater. 2018, 359, 113–120. [Google Scholar] [CrossRef]
- Johnson, J.L.; Dodder, N.G.; Mladenov, N.; Steinberg, L.; Richardot, W.H.; Hoh, E. Comparison of Trace Organic Chemical Removal Efficiencies between Aerobic and Anaerobic Membrane Bioreactors Treating Municipal Wastewater. ACS ES&T Water 2024, 4, 1381–1392. [Google Scholar] [CrossRef]
- Hartl, M.; García-Galán, M.J.; Matamoros, V.; Fernández-Gatell, M.; Rousseau, D.P.L.; Du Laing, G.; Garfí, M.; Puigagut, J. Constructed Wetlands Operated as Bioelectrochemical Systems for the Removal of Organic Micropollutants. Chemosphere 2021, 271, 129593. [Google Scholar] [CrossRef]
- Nan, X.; Lavrnić, S.; Toscano, A. Potential of Constructed Wetland Treatment Systems for Agricultural Wastewater Reuse under the EU Framework. J. Environ. Manag. 2020, 275, 111219. [Google Scholar] [CrossRef]
- Venditti, S.; Brunhoferova, H.; Hansen, J. Behaviour of 27 Selected Emerging Contaminants in Vertical Flow Constructed Wetlands as Post-Treatment for Municipal Wastewater. Sci. Total Environ. 2022, 819, 153234. [Google Scholar] [CrossRef]
- Reyes Contreras, C.; López, D.; Leiva, A.M.; Domínguez, C.; Bayona, J.M.; Vidal, G. Removal of Organic Micropollutants in Wastewater Treated by Activated Sludge and Constructed Wetlands: A Comparative Study. Water 2019, 11, 2515. [Google Scholar] [CrossRef]
- Gebru, S.B.; Werkneh, A.A. Applications of Constructed Wetlands in Removing Emerging Micropollutants from Wastewater: Occurrence, Public Health Concerns, and Removal Performances—A Review. S. Afr. J. Chem. Eng. 2024, 48, 395–416. [Google Scholar] [CrossRef]
- Overton, O.C.; Olson, L.H.; Das Majumder, S.; Shwiyyat, H.; Foltz, M.E.; Nairn, R.W. Wetland Removal Mechanisms for Emerging Contaminants. Land 2023, 12, 472. [Google Scholar] [CrossRef]
- Ávila, C.; Bayona, J.M.; Martín, I.; Salas, J.J.; García, J. Emerging Organic Contaminant Removal in a Full-Scale Hybrid Constructed Wetland System for Wastewater Treatment and Reuse. Ecol. Eng. 2015, 80, 108–116. [Google Scholar] [CrossRef]
- Reyes-Contreras, C.; Matamoros, V.; Ruiz, I.; Soto, M.; Bayona, J.M. Evaluation of PPCPs Removal in a Combined Anaerobic Digester-Constructed Wetland Pilot Plant Treating Urban Wastewater. Chemosphere 2011, 84, 1200–1207. [Google Scholar] [CrossRef]
- Sánchez, M.; Ruiz, I.; Soto, M. The Potential of Constructed Wetland Systems and Photodegradation Processes for the Removal of Emerging Contaminants—A Review. Environments 2022, 9, 116. [Google Scholar] [CrossRef]
- Sánchez, M.; Ramos, D.R.; Fernández, M.I.; Aguilar, S.; Ruiz, I.; Canle, M.; Soto, M. Removal of Emerging Pollutants by a 3-Step System: Hybrid Digester, Vertical Flow Constructed Wetland and Photodegradation Post-Treatments. Sci. Total Environ. 2022, 842, 156750. [Google Scholar] [CrossRef]
- de Melo, A.F.S.R.; de Oliveira, J.F.; Fia, F.R.L.; Fia, R.; de Matos, M.P.; Sanson, A.L. Microcontaminants Removal in Constructed Wetlands with Different Baffle Arrangements and Cultivated with Pennisetum Setaceum. Water Air Soil Pollut. 2022, 233, 322. [Google Scholar] [CrossRef]
- Nivala, J.; van Afferden, M.; Hasselbach, R.; Langergraber, G.; Molle, P.; Rustige, H.; Nowak, J. The New German Standard on Constructed Wetland Systems for Treatment of Domestic and Municipal Wastewater. Water Sci. Technol. 2018, 78, 2414–2426. [Google Scholar] [CrossRef]
- Samaras, V.G.; Thomaidis, N.S.; Stasinakis, A.S.; Lekkas, T.D. An Analytical Method for the Simultaneous Trace Determination of Acidic Pharmaceuticals and Phenolic Endocrine Disrupting Chemicals in Wastewater and Sewage Sludge by Gas Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2011, 399, 2549–2561. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Ajibola, A.; Kannan, K.; Thomaidis, N.S. Occurrence and Removal Efficiencies of Benzotriazoles and Benzothiazoles in a Wastewater Treatment Plant in Greece. Sci. Total Environ. 2013, 452–453, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Mazioti, A.A.; Stasinakis, A.S.; Gatidou, G.; Thomaidis, N.S.; Andersen, H.R. Sorption and Biodegradation of Selected Benzotriazoles and Hydroxybenzothiazole in Activated Sludge and Estimation of Their Fate during Wastewater Treatment. Chemosphere 2015, 131, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Thomaidi, V.S.; Stasinakis, A.S.; Borova, V.L.; Thomaidis, N.S. Is There a Risk for the Aquatic Environment Due to the Existence of Emerging Organic Contaminants in Treated Domestic Wastewater? Greece as a Case-Study. J. Hazard. Mater. 2015, 283, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Dotro, G.; Langergraber, G.; Molle, P.; Nivala, J.; Puigagut, J.; Stein, O.; von Sperling, M. Treatment Wetlands; IWA Publishing: London, UK, 2017; ISBN 9781780408774. [Google Scholar]
- Leitão, R.C. Robustness of UASB Reactors Treating Sewage Under Tropical Conditions; Wageningen University and Research: Gelderland, The Netherlands, 2004; ISBN 9798383016176. [Google Scholar]
- Mainardis, M.; Buttazzoni, M.; Goi, D. Up-Flow Anaerobic Sludge Blanket (Uasb) Technology for Energy Recovery: A Review on State-of-the-Art and Recent Technological Advances. Bioengineering 2020, 7, 43. [Google Scholar] [CrossRef]
- Baggiotto, C.; Decezaro, S.T.; Lutterbeck, C.A.; Friedrich, M.; Ramírez, R.J.M.G.; Wolff, D.B. Nitrogen Removal in Vertical Flow Constructed Wetlands: The Influence of Recirculation and Partial Saturation. Ecol. Eng. 2025, 212, 107519. [Google Scholar] [CrossRef]
- Česen, M.; Heath, D.; Krivec, M.; Košmrlj, J.; Kosjek, T.; Heath, E. Seasonal and Spatial Variations in the Occurrence, Mass Loadings and Removal of Compounds of Emerging Concern in the Slovene Aqueous Environment and Environmental Risk Assessment. Environ. Pollut. 2018, 242, 143–154. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Hu, A.; Rashid, A.; Ashfaq, M.; Wang, Y.; Wang, H.; Luo, H.; Yu, C.-P.; Sun, Q. Monitoring, Mass Balance and Fate of Pharmaceuticals and Personal Care Products in Seven Wastewater Treatment Plants in Xiamen City, China. J. Hazard. Mater. 2018, 354, 81–90. [Google Scholar] [CrossRef]
- D’Alessio, M.; Onanong, S.; Snow, D.D.; Ray, C. Occurrence and Removal of Pharmaceutical Compounds and Steroids at Four Wastewater Treatment Plants in Hawai’i and Their Environmental Fate. Sci. Total Environ. 2018, 631–632, 1360–1370. [Google Scholar] [CrossRef]
- Stasinakis, A.S.; Thomaidis, N.S.; Arvaniti, O.S.; Asimakopoulos, A.G.; Samaras, V.G.; Ajibola, A.; Mamais, D.; Lekkas, T.D. Contribution of Primary and Secondary Treatment on the Removal of Benzothiazoles, Benzotriazoles, Endocrine Disruptors, Pharmaceuticals and Perfluorinated Compounds in a Sewage Treatment Plant. Sci. Total Environ. 2013, 463–464, 1067–1075. [Google Scholar] [CrossRef]
- Koukoura, A.; Seintos, T.; Statiris, E.; Barka, E.; Gatidou, G.; Noutsopoulos, C.; Malamis, S.; Mamais, D.; Masi, F.; Rizzo, A.; et al. Comparing the Performance of Microbial Electrochemical Assisted and Aerated Treatment Wetlands in Pilot-Scale: Removal of Major Pollutants and Organic Micropollutants. Sci. Total Environ. 2024, 951, 175550. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P. Occurrence and Removal of Pharmaceuticals in Wastewater Treatment Plants. Process Saf. Environ. Prot. 2021, 150, 532–556. [Google Scholar] [CrossRef]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and Removal of Pharmaceuticals in a Municipal Sewage Treatment System in the South of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Cagnetta, G.; Duan, L.; Yang, J.; Deng, S.; Huang, J.; Wang, Y.; Yu, G. Typical Pharmaceuticals in Major WWTPs in Beijing, China: Occurrence, Load Pattern and Calculation Reliability. Water Res. 2018, 140, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of Pharmaceutical Residues, Personal Care Products, Lifestyle Chemicals, Illicit Drugs and Metabolites in Wastewater and Receiving Surface Waters of Krakow Agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Occurrence and Removal of PPCPs in Municipal and Hospital Wastewaters in Greece. J. Hazard. Mater. 2010, 179, 804–817. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal Occurrence, Removal, Mass Loading and Environmental Risk Assessment of 55 Pharmaceuticals and Personal Care Products in a Municipal Wastewater Treatment Plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- Nie, Y.; Qiang, Z.; Zhang, H.; Ben, W. Fate and Seasonal Variation of Endocrine-Disrupting Chemicals in a Sewage Treatment Plant with A/A/O Process. Sep. Purif. Technol. 2012, 84, 9–15. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Ying, G.-G.; Shareef, A.; Kookana, R.S. Occurrence and Removal of Benzotriazoles and Ultraviolet Filters in a Municipal Wastewater Treatment Plant. Environ. Pollut. 2012, 165, 225–232. [Google Scholar] [CrossRef]
- Chand, N.; Suthar, S.; Kumar, K.; Singh, V. Removal of Pharmaceuticals by Vertical Flow Constructed Wetland with Different Configurations: Effect of Inlet Load and Biochar Addition in the Substrate. Chemosphere 2022, 307, 135975. [Google Scholar] [CrossRef]
- Zhang, X.; Jing, R.; Feng, X.; Dai, Y.; Tao, R.; Vymazal, J.; Cai, N.; Yang, Y. Removal of Acidic Pharmaceuticals by Small-Scale Constructed Wetlands Using Different Design Configurations. Sci. Total Environ. 2018, 639, 640–647. [Google Scholar] [CrossRef]
- Nakada, N.; Tanishima, T.; Shinohara, H.; Kiri, K.; Takada, H. Pharmaceutical Chemicals and Endocrine Disrupters in Municipal Wastewater in Tokyo and Their Removal during Activated Sludge Treatment. Water Res. 2006, 40, 3297–3303. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Matamoros, V.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Assessment of Full-Scale Natural Systems for the Removal of PPCPs from Wastewater in Small Communities. Water Res. 2010, 44, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-S.; Ying, G.-G.; Shareef, A.; Kookana, R.S. Biodegradation of Three Selected Benzotriazoles under Aerobic and Anaerobic Conditions. Water Res. 2011, 45, 5005–5014. [Google Scholar] [CrossRef]
- Reemtsma, T.; Fiehn, O.; Kalnowski, G.; Jekel, M. Microbial Transformations and Biological Effects of Fungicide-Derived Benzothiazoles Determined in Industrial Wastewater. Environ. Sci. Technol. 1995, 29, 478–485. [Google Scholar] [CrossRef]
- Kraševec, I.; Prosen, H. Solid-Phase Extraction of Polar Benzotriazoles as Environmental Pollutants: A Review. Molecules 2018, 23, 2501. [Google Scholar] [CrossRef]
- Stasinakis, A.S. Review on the Fate of Emerging Contaminants during Sludge Anaerobic Digestion. Bioresour. Technol. 2012, 121, 432–440. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A Review on Emerging Contaminants in Wastewaters and the Environment: Current Knowledge, Understudied Areas and Recommendations for Future Monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; McDonald, J.A.; Khan, S.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Development of a Predictive Framework to Assess the Removal of Trace Organic Chemicals by Anaerobic Membrane Bioreactor. Bioresour. Technol. 2015, 189, 391–398. [Google Scholar] [CrossRef]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of Pharmaceuticals and Personal Care Products in Aquatic Plant-Based Systems: A Review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef]
- Queiroz, F.B.; Brandt, E.M.F.; Aquino, S.F.; Chernicharo, C.A.L.; Afonso, R.J.C.F. Occurrence of Pharmaceuticals and Endocrine Disruptors in Raw Sewage and Their Behavior in UASB Reactors Operated at Different Hydraulic Retention Times. Water Sci. Technol. 2012, 66, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, L.; García-Galán, M.J.; Aquino, S.F.; Afonso, R.J.d.C.F.; Ferrer, I.; Passos, F.; Mota, C.R. Can High Rate Algal Ponds Be Used as Post-Treatment of UASB Reactors to Remove Micropollutants? Chemosphere 2020, 248, 125969. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Wu, J.; Rich, S.L.; Richardson, R.E.; Helbling, D.E. Differential Biotransformation of Micropollutants in Conventional Activated Sludge and Up-Flow Anaerobic Sludge Blanket Processes. Environ. Sci. 2024, 10, 936–948. [Google Scholar] [CrossRef]
- Alvarino, T.; Suárez, S.; Garrido, M.; Lema, J.M.; Omil, F. A UASB Reactor Coupled to a Hybrid Aerobic MBR as Innovative Plant Configuration to Enhance the Removal of Organic Micropollutants. Chemosphere 2016, 144, 452–458. [Google Scholar] [CrossRef]
- Lyu, T.; Zhang, L.; Xu, X.; Arias, C.A.; Brix, H.; Carvalho, P.N. Removal of the Pesticide Tebuconazole in Constructed Wetlands: Design Comparison, Influencing Factors and Modelling. Environ. Pollut. 2018, 233, 71–80. [Google Scholar] [CrossRef]
- Felis, E.; Sochacki, A.; Magiera, S. Degradation of Benzotriazole and Benzothiazole in Treatment Wetlands and by Artificial Sunlight. Water Res. 2016, 104, 441–448. [Google Scholar] [CrossRef]
- Matamoros, V.; Jover, E.; Bayona, J.M. Occurrence and Fate of Benzothiazoles and Benzotriazoles in Constructed Wetlands. Water Sci. Technol. 2010, 61, 191–198. [Google Scholar] [CrossRef]
- Papaevangelou, V.A.; Gikas, G.D.; Tsihrintzis, V.A.; Antonopoulou, M.; Konstantinou, I.K. Removal of Endocrine Disrupting Chemicals in HSF and VF Pilot-Scale Constructed Wetlands. Chem. Eng. J. 2016, 294, 146–156. [Google Scholar] [CrossRef]
- Ávila, C.; Matamoros, V.; Reyes-Contreras, C.; Piña, B.; Casado, M.; Mita, L.; Rivetti, C.; Barata, C.; García, J.; Bayona, J.M. Attenuation of Emerging Organic Contaminants in a Hybrid Constructed Wetland System under Different Hydraulic Loading Rates and Their Associated Toxicological Effects in Wastewater. Sci. Total Environ. 2014, 470–471, 1272–1280. [Google Scholar] [CrossRef]
- Kahl, S.; Nivala, J.; van Afferden, M.; Müller, R.A.; Reemtsma, T. Effect of Design and Operational Conditions on the Performance of Subsurface Flow Treatment Wetlands: Emerging Organic Contaminants as Indicators. Water Res. 2017, 125, 490–500. [Google Scholar] [CrossRef]
- Matamoros, V.; Arias, C.; Brix, H.; Bayona, J.M. Removal of Pharmaceuticals and Personal Care Products (PPCPs) from Urban Wastewater in a Pilot Vertical Flow Constructed Wetland and a Sand Filter. Environ. Sci. Technol. 2007, 41, 8171–8177. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, K.C.; Hai, F.I.; Kang, J.; Price, W.E.; Guo, W.; Ngo, H.H.; Nghiem, L.D. The Fate of Pharmaceuticals, Steroid Hormones, Phytoestrogens, UV-Filters and Pesticides during MBR Treatment. Bioresour. Technol. 2013, 144, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Arias, C.; Brix, H.; Bayona, J.M. Preliminary Screening of Small-Scale Domestic Wastewater Treatment Systems for Removal of Pharmaceutical and Personal Care Products. Water Res. 2009, 43, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Diaz, O.; Schultze-Nobre, L.; Moeder, M.; Nivala, J.; Kuschk, P.; Koeser, H. Removal of Selected Organic Micropollutants in Planted and Unplanted Pilot-Scale Horizontal Flow Constructed Wetlands under Conditions of High Organic Load. Ecol. Eng. 2014, 71, 234–245. [Google Scholar] [CrossRef]
- Park, N.; Vanderford, B.J.; Snyder, S.A.; Sarp, S.; Kim, S.D.; Cho, J. Effective Controls of Micropollutants Included in Wastewater Effluent Using Constructed Wetlands under Anoxic Condition. Ecol. Eng. 2009, 35, 418–423. [Google Scholar] [CrossRef]
- Matamoros, V.; Nguyen, L.X.; Arias, C.A.; Salvadó, V.; Brix, H. Evaluation of Aquatic Plants for Removing Polar Microcontaminants: A Microcosm Experiment. Chemosphere 2012, 88, 1257–1264. [Google Scholar] [CrossRef]
- Francini, A.; Mariotti, L.; Di Gregorio, S.; Sebastiani, L.; Andreucci, A. Removal of Micro-Pollutants from Urban Wastewater by Constructed Wetlands with Phragmites Australis and Salix Matsudana. Environ. Sci. Pollut. Res. 2018, 25, 36474–36484. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic Ecotoxicity of Pharmaceuticals Including the Assessment of Combination Effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Sampaio, C.F.; Gravato, C.; De Oliveira, D.P.; Dorta, D.J. Deleterious Effects of Benzotriazoles on Zebrafish Development and Neurotransmission: 5-Chloro-Benzotriazole versus 1H-Benzotriazole. Sci. Total Environ. 2024, 912, 168741. [Google Scholar] [CrossRef]
- Giraudo, M.; Douville, M.; Cottin, G.; Houde, M. Transcriptomic, Cellular and Life-History Responses of Daphnia Magna Chronically Exposed to Benzotriazoles: Endocrine-Disrupting Potential and Molting Effects. PLoS ONE 2017, 12, e0171763. [Google Scholar] [CrossRef]
- Gatidou, G.; Anastopoulou, P.; Aloupi, M.; Stasinakis, A.S. Growth Inhibition and Fate of Benzotriazoles in Chlorella Sorokiniana Cultures. Sci. Total Environ. 2019, 663, 580–586. [Google Scholar] [CrossRef]
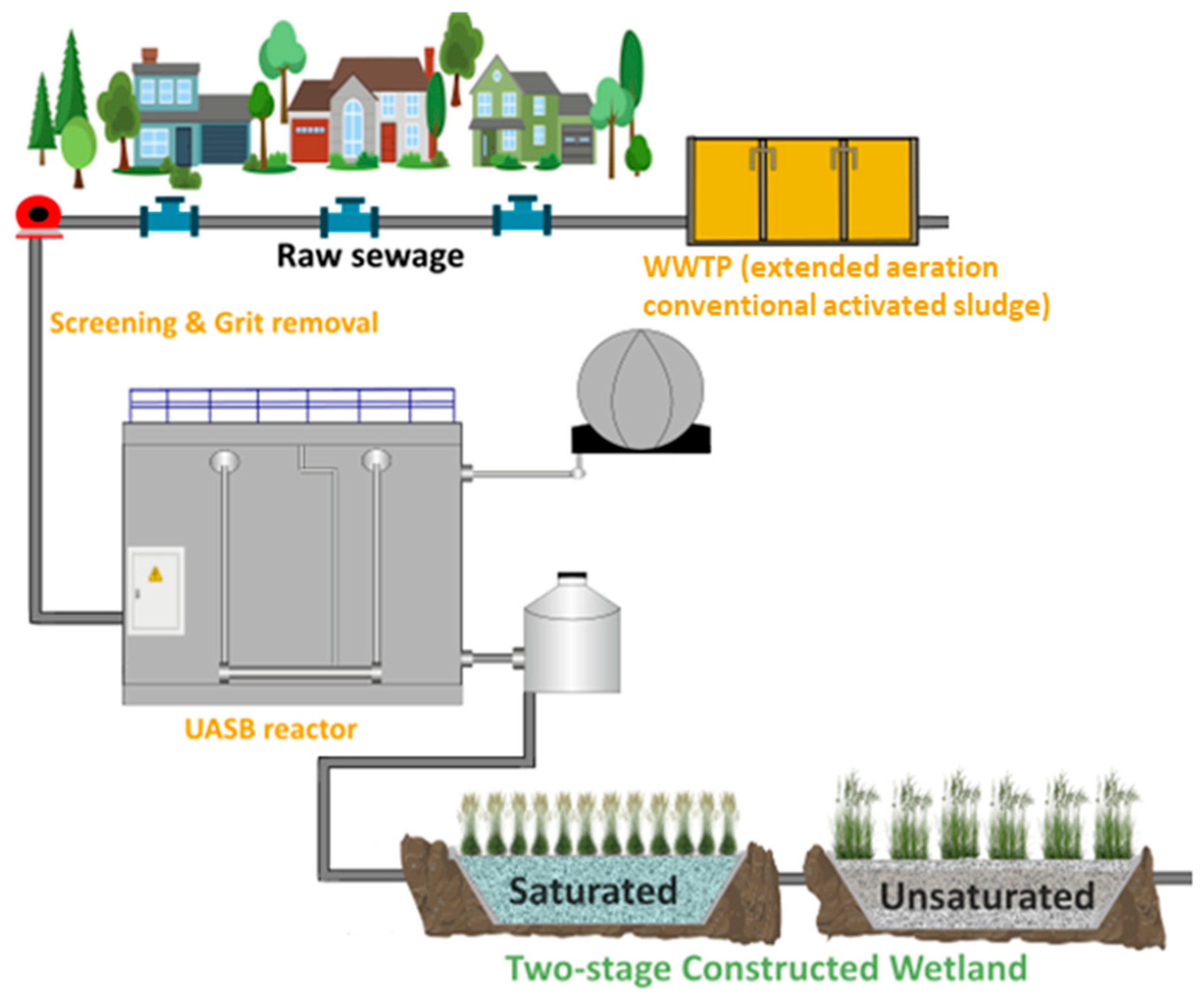
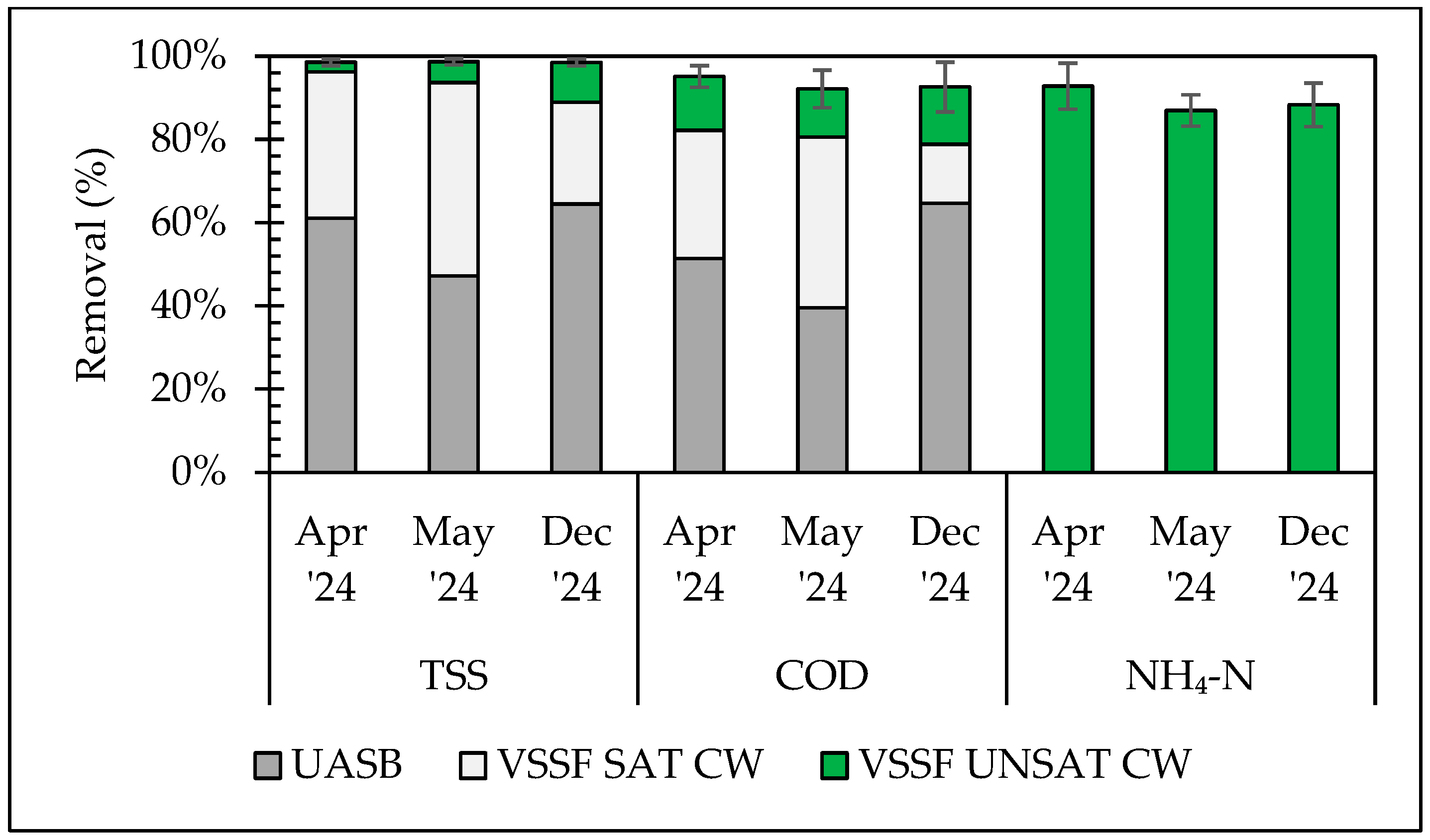
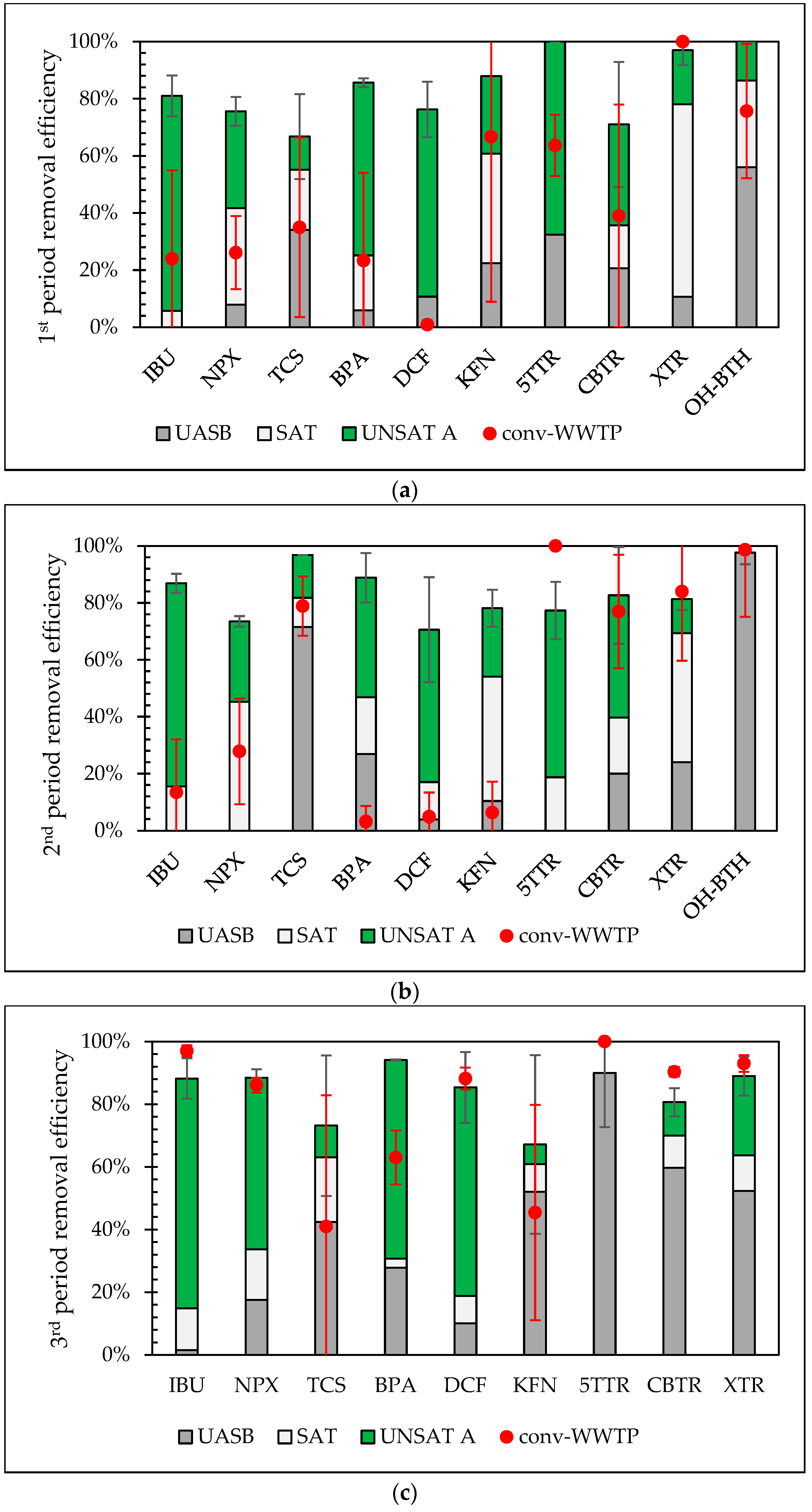
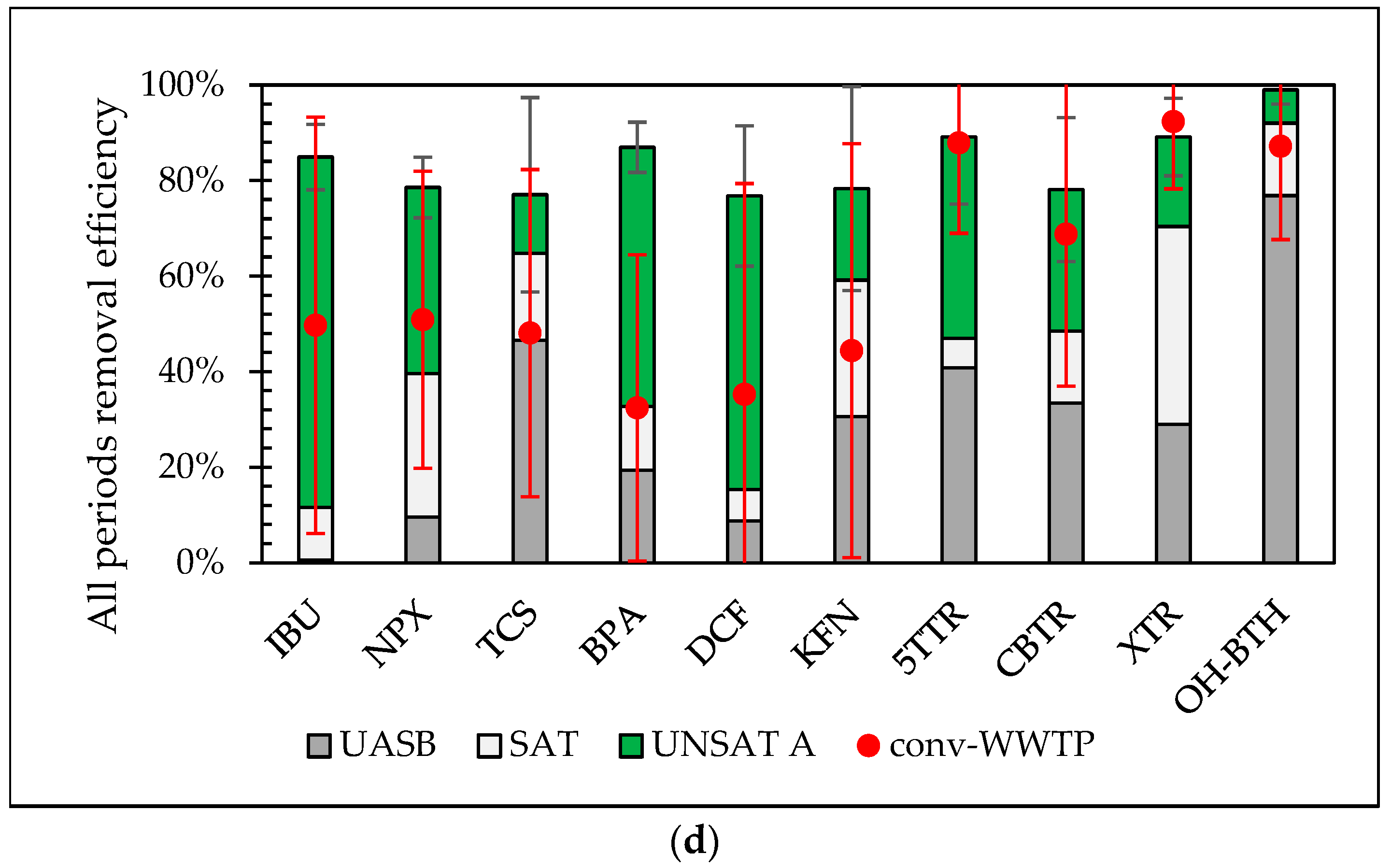
| Period | 1st (April) | 2nd (May) | 3rd (December) | |
|---|---|---|---|---|
| Parameter | ||||
| Samples (n) | 8 | 9 | 8 | |
| EC (μS/cm) | 1303 ± 124 | 1434 ± 73 | 1370 ± 274 | |
| Turb (NTU) | 230 ± 63 | 309 ± 72 | 214 ± 118 | |
| pH | 7.8 ± 0.1 | 7.7 ± 0.1 | 7.4 ± 0.2 | |
| TSS (mg/L) | 329 ± 107 | 407 ± 105 | 302 ± 122 | |
| VSS (mg/L) | 279 ± 89 | 355 ± 79 | 235 ± 111 | |
| BOD5 (mg/L) | 308 ± 70 | 353 ± 47 | 447 ± n/a | |
| tCOD (mg/L) | 621 ± 179 | 748 ± 248 | 666 ± 274 | |
| sCOD (mg/L) | 124 ± 59 | 133 ± 32 | 172 ± 85 | |
| NH4-N (mg/L) | 51.1 ± 10.0 | 65.3 ± 9.7 | 60.7 ± 1.4 | |
| NO3-N (mg/L) | n.d. 1 | n.d. | n.d. | |
| TP (mg/L) | 8.5 ± 2.5 | 11.1 ± 2.4 | 6.6 ± n/a | |
| PO4-P (mg/L) | 5.8 ± 1.1 | 7.4 ± 0.8 | 5.6 ± 1.7 | |
| Period | 1st (April) | 2nd (May) | 3rd (December) | |
|---|---|---|---|---|
| Parameter | ||||
| Τ (°C) | 15.9 ± 1.4 | 19.3 ± 1.7 | 16.8 ± 0.7 | |
| Qpilot (m3/d) | 41.2 ± 3.6 | 59.2 ± 0.2 | 76.1 ± 6.6 | |
| HRTUASB (h) | 24.0 ± 1.8 | 16.6 ± 0.1 | 12.8 ± 1.0 | |
| OLRUASB (kg COD/m3-d) | 0.6 ± 0.2 | 1.1 ± 0.4 | 1.2 ± 0.5 | |
| HRTVSSF SAT CW (d) | 3.1 ± 0.2 | 2.1 ± 0 | 1.7 ± 0.2 | |
| SLRVSSF SAT CW (g TSS/m2-d) | 25 ± 13 | 48 ± 10 | 34 ± 23 | |
| OLRVSSF SAT CW (g COD/m2-d) | 51 ± 20 | 98 ± 13 | 74 ± 29 | |
| Resting periodVSSF UNSAT CW (h) | 7.8 ± 0.7 | 5.3 ± 0 | 4 ± 0.4 | |
| SLRVSSF UNSAT CW (g TSS/m2-d) | 0.8 ± 0.4 | 2.7 ± 1 | 4.6 ± 3.6 | |
| OLRVSSF UNSAT CW (g COD/m2-d) | 7.4 ± 2.3 | 12.6 ± 1.5 | 16.5 ± 5.3 | |
| NLRVSSF UNSAT CW (g NH₄-N/m2-d) | 3.7 ± 0.9 | 6.8 ± 0.7 | 7.4 ± 1.1 | |
| Qconv-WWTP (m3/d) | 63.4 ± 5.8 | 25.1 ± 4.2 | 10.5 ± 3.2 | |
| Compound | Average | SD | Min | Max | Frequency of Detection | Average Mass Loads |
|---|---|---|---|---|---|---|
| Unit | (ng/L) | (%) | (mg/d/1000 inh) | |||
| IBU | 6595 | 1841 | 4558 | 9296 | 100 | 1055 |
| NPX | 5267 | 1325 | 2734 | 6813 | 100 | 843 |
| TCS | 444 | 166 | 281 | 780 | 100 | - |
| BPA | 652 | 125 | 498 | 809 | 100 | - |
| DCF | 5462 | 1840 | 3225 | 7697 | 100 | 874 |
| KFN | 745 | 523 | 282 | 2067 | 100 | 119 |
| 5TTR | 3249 | 1558 | 1127 | 5830 | 100 | - |
| CBTR | 8608 | 7504 | 2675 | 23,085 | 100 | - |
| XTR | 5681 | 4726 | 2010 | 13,290 | 100 | - |
| OH-BTH | 7392 | 6283 | n.d. | 16,698 | 67 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barka, E.; Koukoura, A.; Statiris, E.; Seintos, T.; Stasinakis, A.S.; Mamais, D.; Malamis, S.; Noutsopoulos, C. The Fate of Contaminants of Emerging Concern in an Upflow Anaerobic Sludge Blanket Reactor Coupled with Constructed Wetlands for Decentralized Domestic Wastewater Treatment. Molecules 2025, 30, 2671. https://doi.org/10.3390/molecules30132671
Barka E, Koukoura A, Statiris E, Seintos T, Stasinakis AS, Mamais D, Malamis S, Noutsopoulos C. The Fate of Contaminants of Emerging Concern in an Upflow Anaerobic Sludge Blanket Reactor Coupled with Constructed Wetlands for Decentralized Domestic Wastewater Treatment. Molecules. 2025; 30(13):2671. https://doi.org/10.3390/molecules30132671
Chicago/Turabian StyleBarka, Evridiki, Asimina Koukoura, Evangelos Statiris, Taxiarchis Seintos, Athanasios S. Stasinakis, Daniel Mamais, Simos Malamis, and Constantinos Noutsopoulos. 2025. "The Fate of Contaminants of Emerging Concern in an Upflow Anaerobic Sludge Blanket Reactor Coupled with Constructed Wetlands for Decentralized Domestic Wastewater Treatment" Molecules 30, no. 13: 2671. https://doi.org/10.3390/molecules30132671
APA StyleBarka, E., Koukoura, A., Statiris, E., Seintos, T., Stasinakis, A. S., Mamais, D., Malamis, S., & Noutsopoulos, C. (2025). The Fate of Contaminants of Emerging Concern in an Upflow Anaerobic Sludge Blanket Reactor Coupled with Constructed Wetlands for Decentralized Domestic Wastewater Treatment. Molecules, 30(13), 2671. https://doi.org/10.3390/molecules30132671












