Anti-Anxiety Effects of Essential Oil Microemulsion in Chronic Unpredictable Mild Stress-Induced Rats: Preparation, Characterization, and Mechanisms
Abstract
1. Introduction
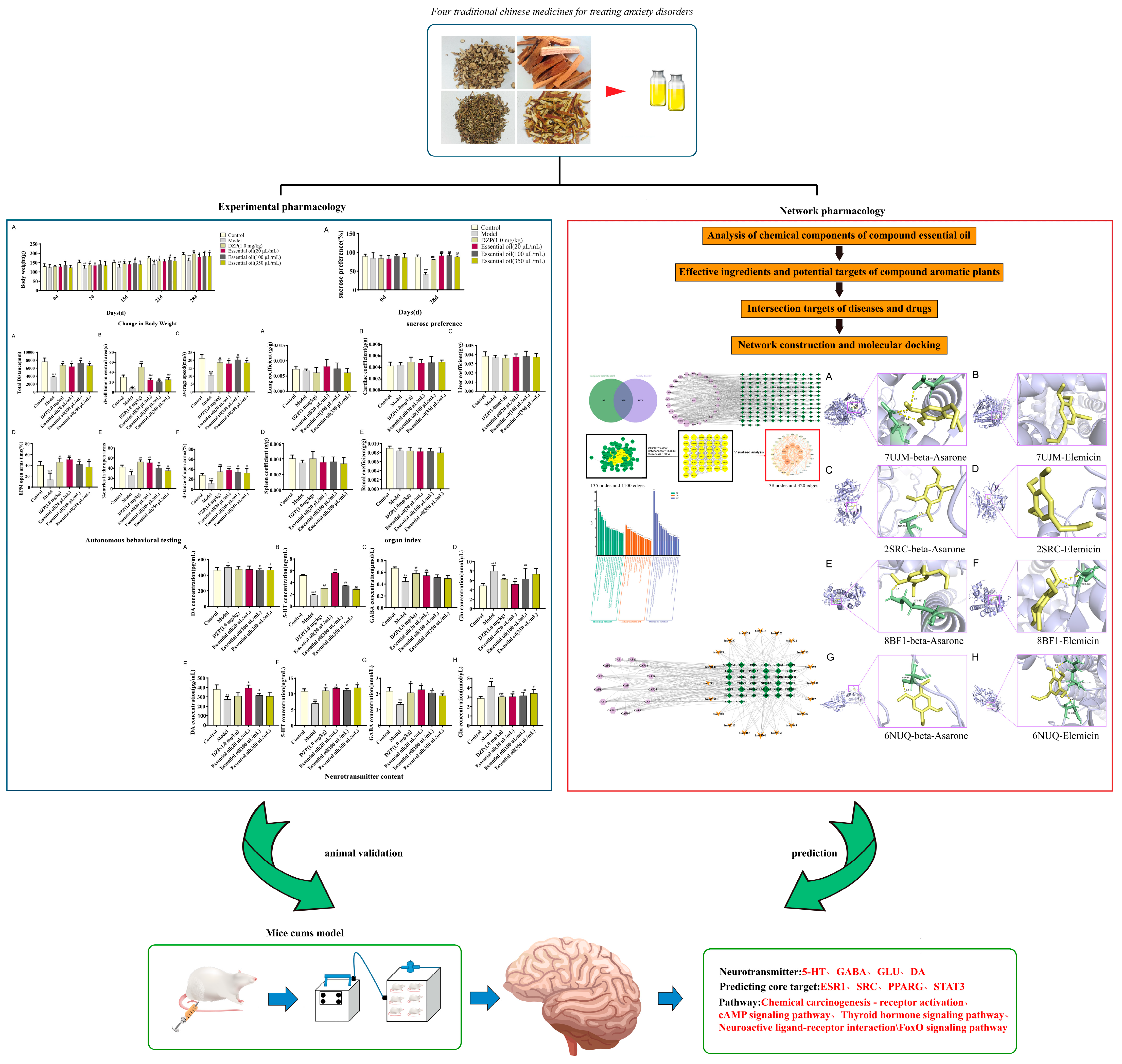
2. Results
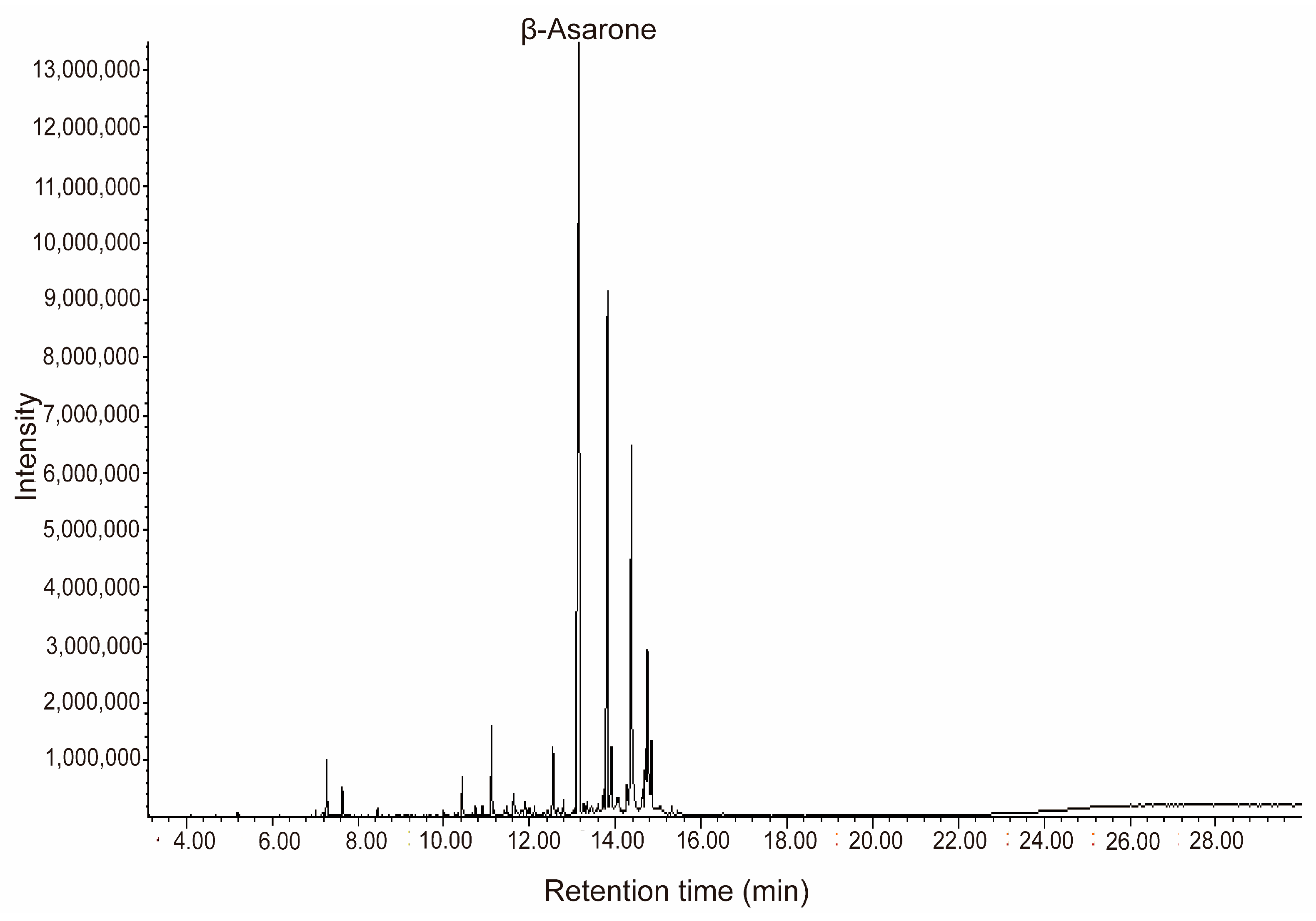
2.1. Chemical Composition Analysis of Essential Oils
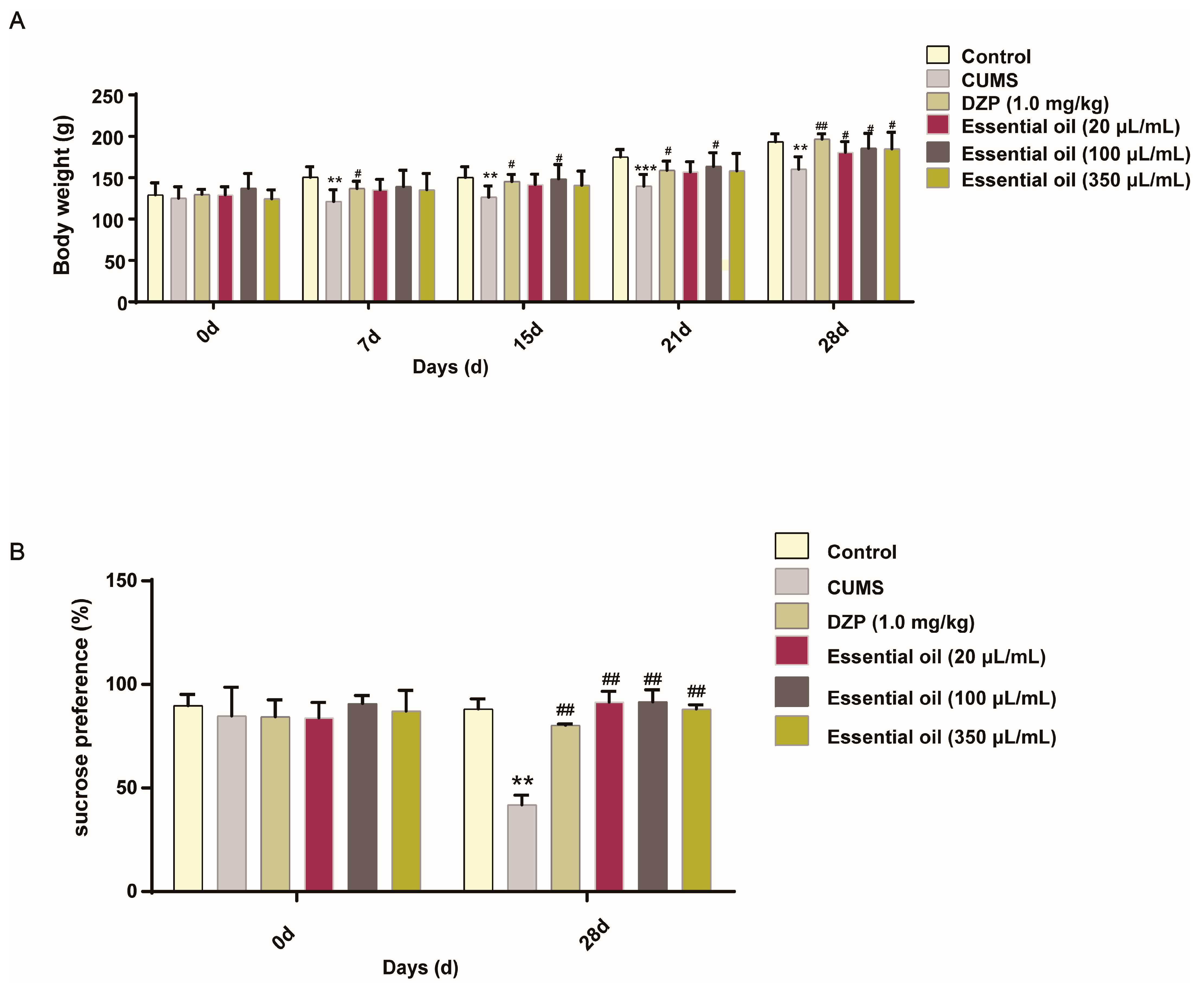
2.2. Pharmacodynamic Evaluation in CUMS-Induced Anxiety Model
2.2.1. Behavioral Assessments
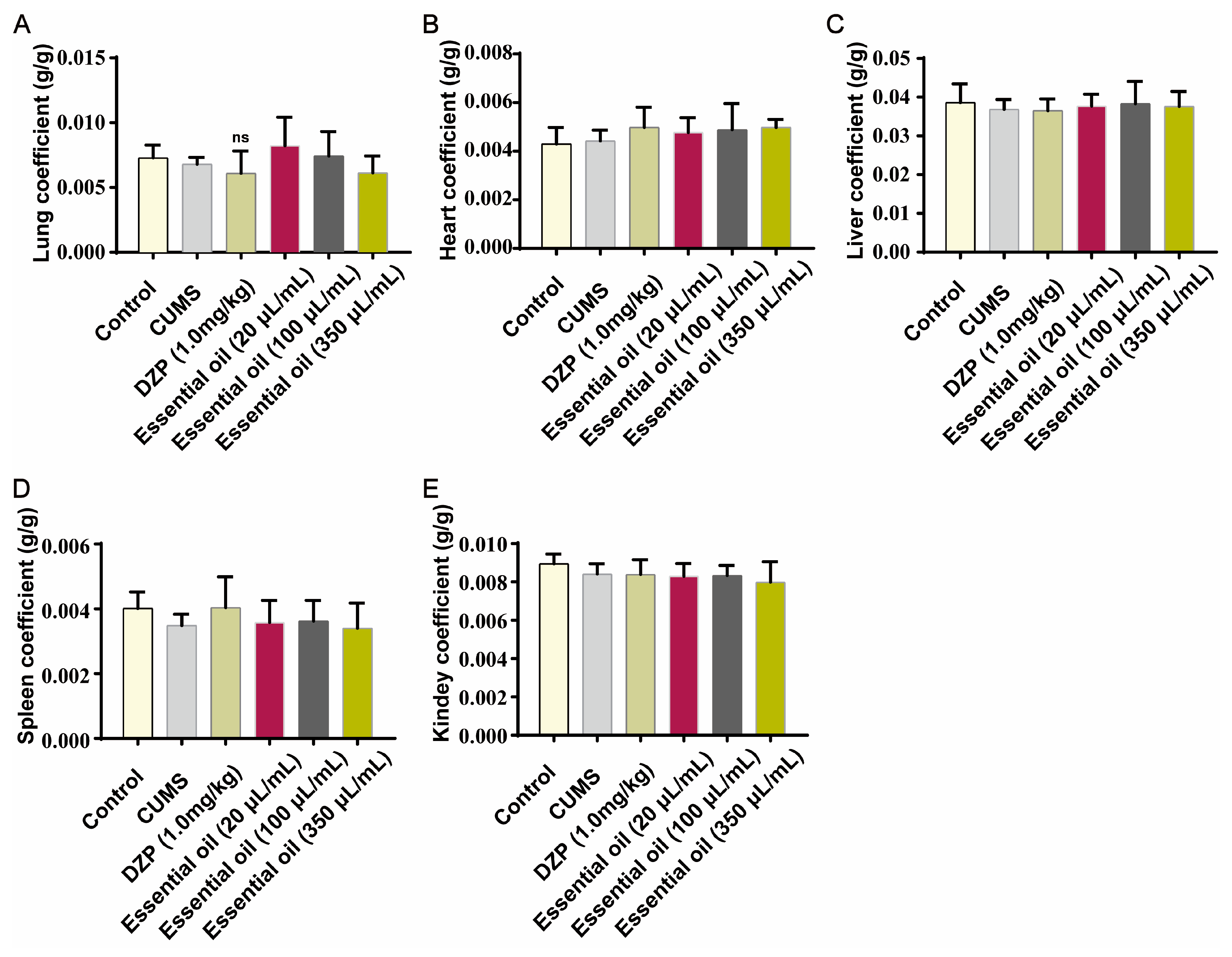
2.2.2. Safety Evaluation
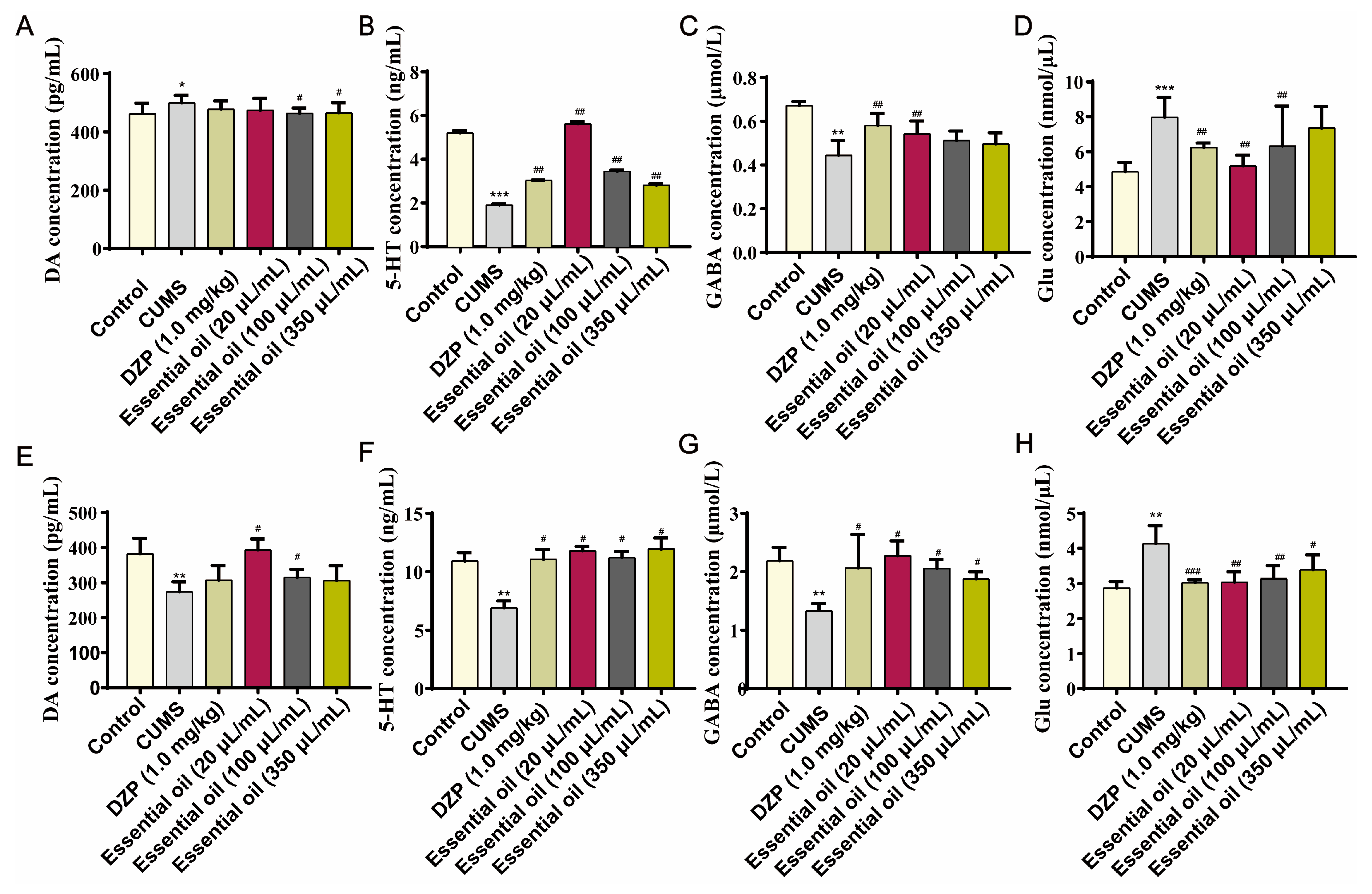
2.2.3. Neurotransmitter Modulation
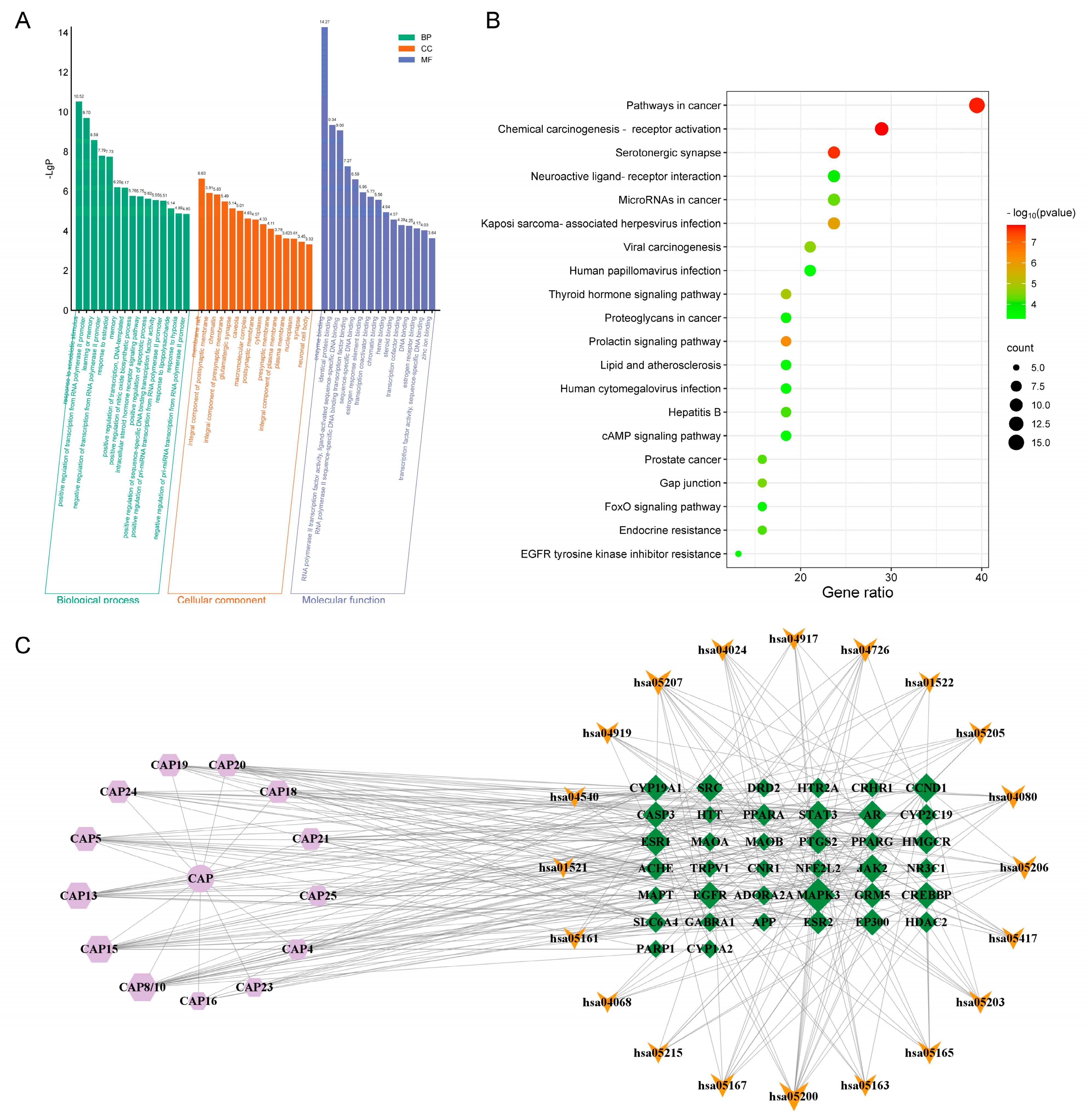
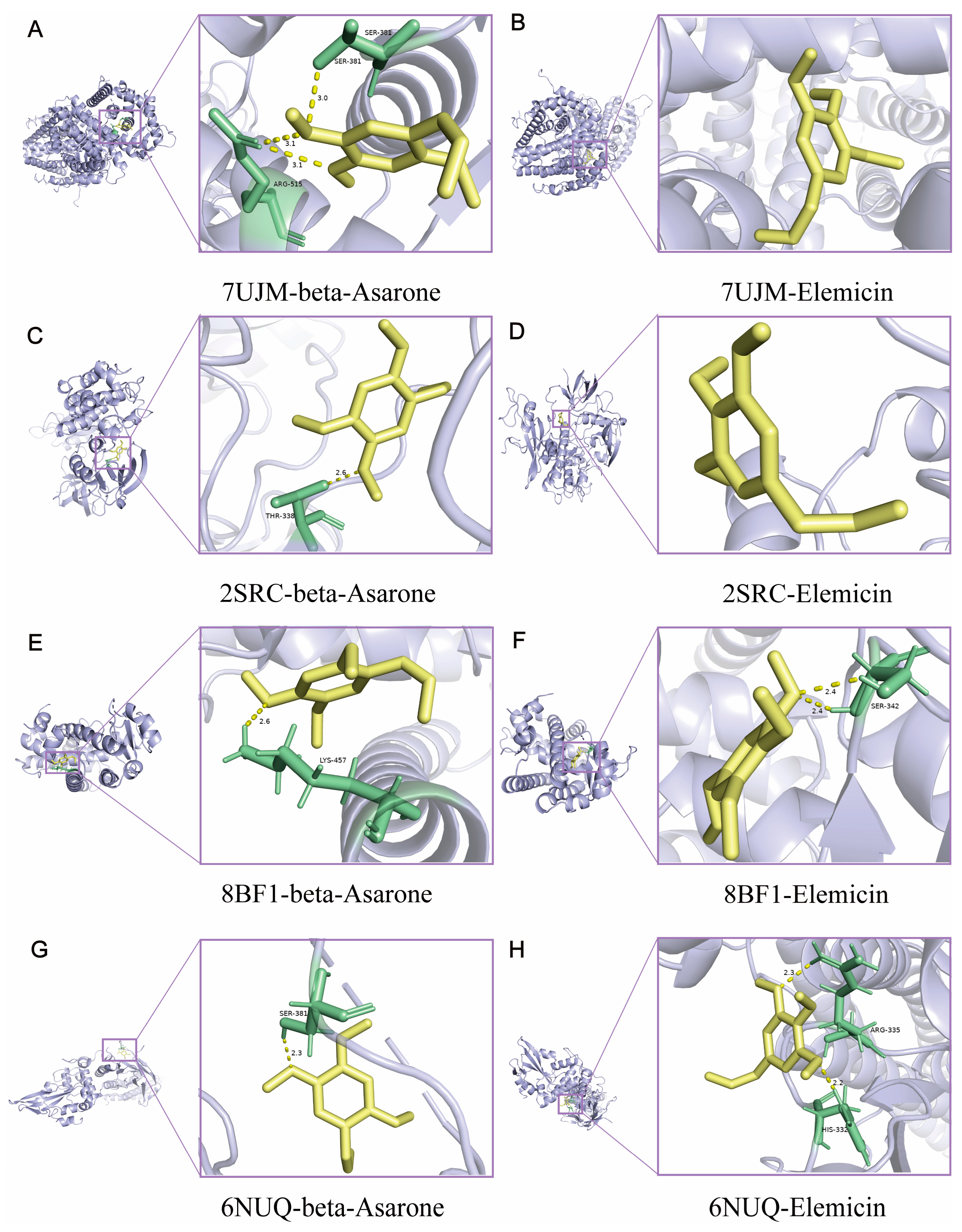
2.3. Network Pharmacological Analysis
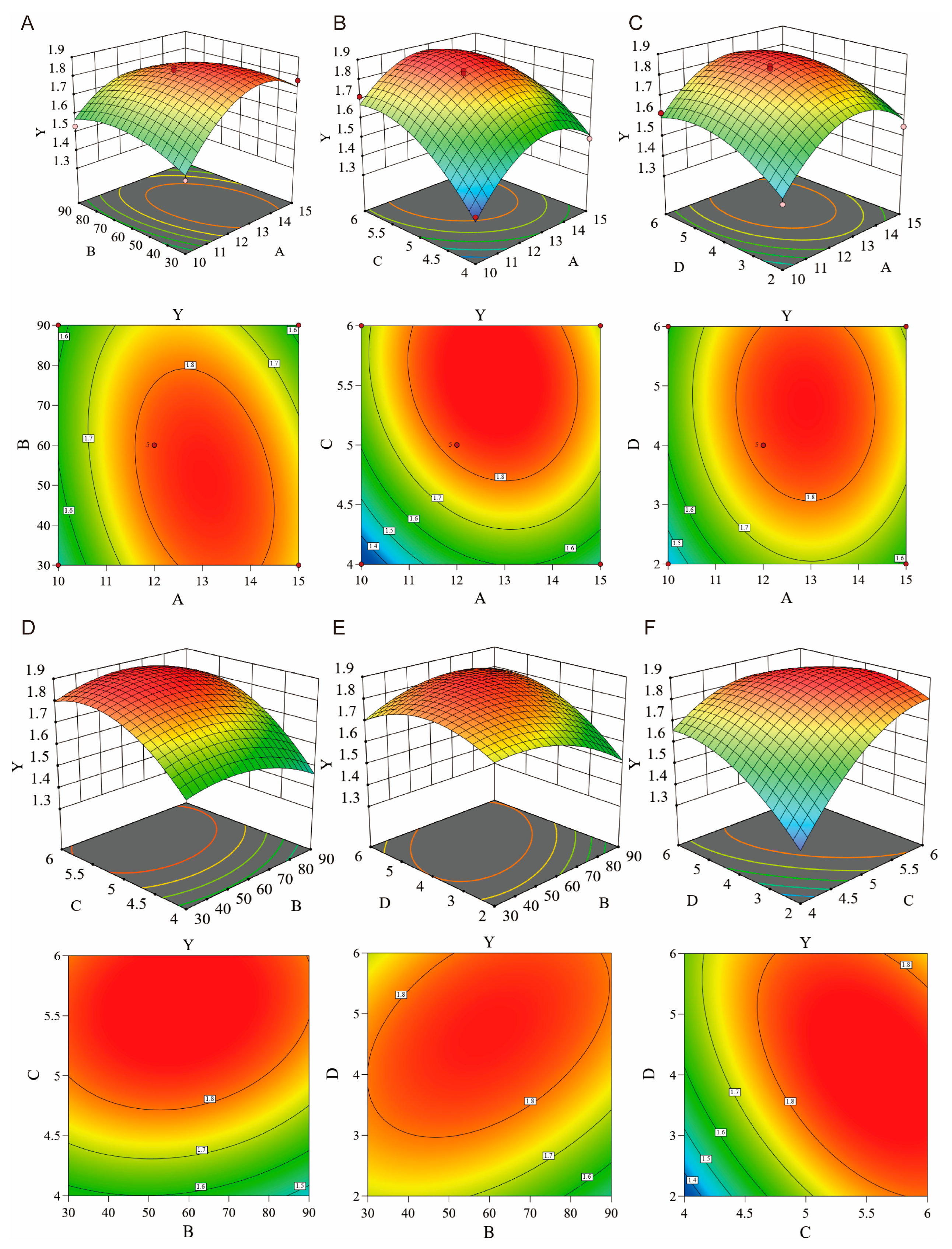
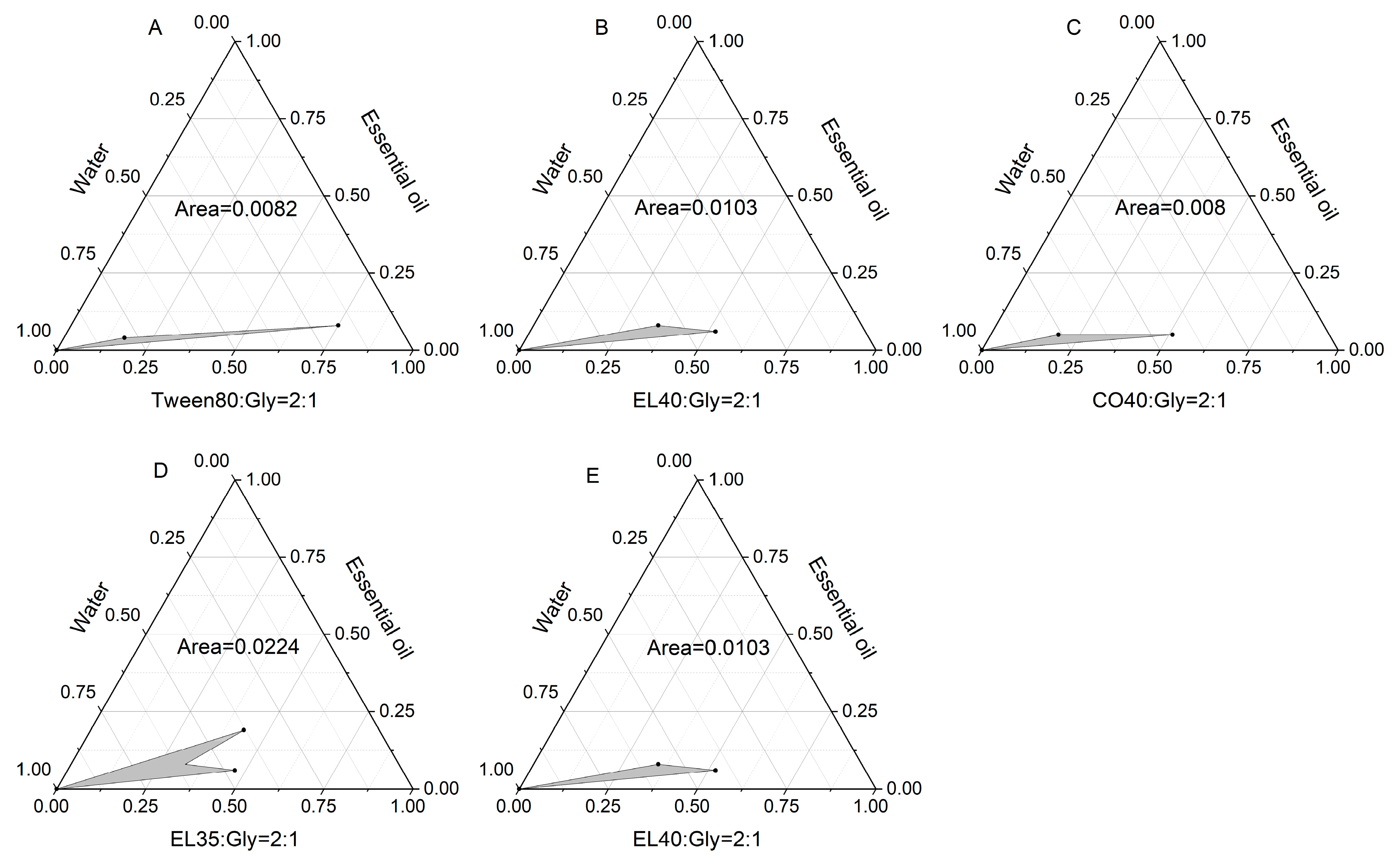
2.4. Preparation and Characterization of Essential Oil Microemulsion
2.4.1. Formulation Optimization
2.4.2. Physicochemical Properties
2.4.3. Stability Studies
3. Methods and Materials
3.1. Chemicals and Reagents
3.2. Animals
3.3. Equipment
3.4. Preparation of Essential Oil
3.5. GC-MS Analysis
3.6. CUMS Model Establishment
3.7. Organ Coefficient and Histopathology
3.8. Behavioral Evaluation
3.8.1. Sucrose Preference Test (SPT)
3.8.2. Open Field Test (OFT)
3.8.3. Elevated Plus Maze (EPM)
3.9. Neurotransmitter Measurement
3.10. Network Pharmacology
3.11. Molecular Docking
3.12. Microemulsion Preparation
3.13. Drawing of Pseudo Ternary Phase Diagram
3.14. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Crocq, M.A. A history of anxiety: From Hippocrates to DSM. Dialogues Clin. Neurosci. 2015, 17, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Baya, D.; Salinas-Perez, J.A.; Sanchez-Lopez, A.; Paino-Quesada, S.; Mendoza-Berjano, R. The Role of Developmental Assets in Gender Differences in Anxiety in Spanish Youth. Front. Psychiatry 2022, 13, 810326. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, R.; Liu, X. Curative effects of vanlafaxine hydrochloride in treating co-morbidity of depression and anxiety. Chin. J. Pract. Nerv. Dis. 2017, 20, 86–88. [Google Scholar]
- di Giacomo, E.; Colmegna, F.; Biagi, E.; Zappa, L.; Caslini, M.; Dakanalis, A.; UnimibPsychiatricResearch-Group; Massimo, C. Anxiety and Depression: A Key to Understanding the Complete Expression of Personality Disorders. J. Nerv. Ment. Dis. 2021, 209, 188–195. [Google Scholar] [CrossRef]
- Krause, K.R.; Chung, S.; Adewuya, A.O.; Albano, A.M.; Babins-Wagner, R.; Birkinshaw, L.; Brann, P.; Creswell, C.; Delaney, K.; Falissard, B.; et al. International consensus on a standard set of outcome measures for child and youth anxiety, depression, obsessive-compulsive disorder, and post-traumatic stress disorder. Lancet Psychiatry 2021, 8, 76–86. [Google Scholar] [CrossRef]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef]
- Starcevic, V.; Berle, D.; Milicevic, D.; Hannan, A.; Lamplugh, C.; Eslick, G.D. Pathological worry, anxiety disorders and the impact of co-occurrence with depressive and other anxiety disorders. J. Anxiety Disord. 2007, 21, 1016–1027. [Google Scholar] [CrossRef]
- Tully, P.J.; Harrison, N.J.; Cheung, P.; Cosh, S. Anxiety and Cardiovascular Disease Risk: A Review. Curr. Cardiol. Rep. 2016, 18, 120. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Gegechkori, V.; Morton, D.W. Anxiolytic Terpenoids and Aromatherapy for Anxiety and Depression. Rev. New Drug Targets Age-Relat. Disord. 2020, 1260, 283–296. [Google Scholar] [CrossRef]
- Altmann, H.; Stahl, S.T.; Gebara, M.A.; Lenze, E.J.; Mulsant, B.H.; Blumberger, D.M.; Reynolds, C.F.; Karp, J.F. Coprescribed Benzodiazepines in Older Adults Receiving Antidepressants for Anxiety and Depressive Disorders: Association with Treatment Outcomes. J. Clin. Psychiatry 2020, 81, 20m13283. [Google Scholar] [CrossRef]
- Purgato, M.; Gastaldon, C.; Papola, D.; Magni, L.R.; Rossi, G.; Barbui, C. Drug dose as mediator of treatment effect in antidepressant drug trials: The case of fluoxetine. Acta Psychiatr. Scand. 2015, 131, 408–416. [Google Scholar] [CrossRef]
- Jafari-Koulaee, A.; Elyasi, F.; Taraghi, Z.; Sadat Ilali, E.; Moosazadeh, M. A Systematic Review of the Effects of Aromatherapy with Lavender Essential Oil on Depression. Cent. Asian J. Glob. Health 2020, 9, e442. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Price, L. Aromatherapy for Health Professionals E-Book: Aromatherapy for Health Professionals E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Tapper, J. The Healing Intelligence of Essential Oils: The Science of Advanced Aromatherapy. Libr. J. 2011, 136, 101. [Google Scholar]
- Wang, Z.J.; Heinbockel, T. Essential Oils and Their Constituents Targeting the GABAergic System and Sodium Channels as Treatment of Neurological Diseases. Molecules 2018, 23, 1061. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R. Effects of essential oils on central nervous system: Focus on mental health. Phytother. Res. 2021, 35, 657–679. [Google Scholar] [CrossRef]
- Jafarizadeh-Malmiri, H.; Anarjan, N.; Berenjian, A. Developing three-component ginger-cinnamon-cardamom composite essential oil nanoemulsion as natural food preservatives. Environ. Res. 2022, 204 Pt B, 112133. [Google Scholar] [CrossRef]
- Enman, N.M.; Sabban, E.L.; McGonigle, P.; Van Bockstaele, E.J. Targeting the Neuropeptide Y System in Stress-related Psychiatric Disorders. Neurobiol. Stress 2015, 1, 33–43. [Google Scholar] [CrossRef]
- Rossi, C.; Chaves-López, C.; Mozina, S.S.; Di Mattia, C.; Scuota, S.; Luzzi, I.; Jenic, T.; Paparella, A.; Serio, A. Salmonella enterica adhesion: Effect of Cinnamomum zeylanicum essential oil on lettuce. LWT-Food Sci. Technol. 2019, 111, 16–22. [Google Scholar] [CrossRef]
- Amorim, D.; Amado, J.; Brito, I.; Fiuza, S.M.; Amorim, N.; Costeira, C.; Machado, J. Acupuncture and electroacupuncture for anxiety disorders: A systematic review of the clinical research. Complement. Ther. Clin. Pract. 2018, 31, 31–37. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.Y.; Cheng, Y.C.; Zhang, Y.W. Traditional Chinese Medicine in Depression Treatment: From Molecules to Systems. Front. Pharmacol. 2020, 11, 586. [Google Scholar] [CrossRef]
- Qi, Z.; Kelley, E. The WHO Traditional Medicine Strategy 2014–2023: A perspective. Science 2014, 346, S5–S6. [Google Scholar]
- Yang, X.; Shi, C.; Bao, T.; Zhang, Z. Editorial: Traditional Chinese medicine for depression and anxiety. Front. Psychiatry 2023, 14, 1217886. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Timberlake, M.A., II; Prall, K.; Dwivedi, Y. The recent progress in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 77, 99–109. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, F.; Liu, Q.; Li, X.; Xu, G.; Liu, G.; Zhang, Y.; Yang, X.; Yi, S.; Xu, F.; et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019, 364, 494–502. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Li, H.Y.; Qin, W.L.; Li, N.N.; Feng, S.X.; Wang, J.Q.; Zhang, Y.; Wang, T.Y.; Wang, C.L.; Cai, X.Y.; Sun, W.; et al. Effect of mindfulness on anxiety and depression in insomnia patients: A systematic review and meta-analysis. Front. Psychiatry 2023, 14, 1124344. [Google Scholar] [CrossRef]
- Setzer, W.N. Essential Oils and Anxiolytic Aromatherapy. Nat. Prod. Commun. 2009, 4, 1305–1316. [Google Scholar] [CrossRef]
- Ofori, H.; Hettiarachchi, D.; Sostaric, T.; Busetti, F.; Boyce, M.C. High-Performance Thin-Layer Chromatographic Fingerprinting of Sandalwood Essential Oils. JPC-J. Planar Chromatogr.-Mod. TLC 2019, 32, 205–210. [Google Scholar] [CrossRef]
- Zha, W.L.; An, T.Y.; Li, T.; Zhu, J.X.; Gao, K.; Sun, Z.J.; Xu, W.D.; Lin, P.C.; Zi, J.C. Reconstruction of the Biosynthetic Pathway of Santalols under Control of the GAL Regulatory System in Yeast. ACS Synth. Biol. 2020, 9, 449–456. [Google Scholar] [CrossRef]
- Nascimento, J.C.; Gonçalves, V.; Souza, B.R.S.; Nascimento, L.C.; Carvalho, B.M.R.; Nogueira, P.C.L.; Santos, J.P.S.; Borges, L.P.; Goes, T.C.; Souza, J.B.; et al. Effectiveness of aromatherapy with sweet orange oil (Citrus sinensis L.) in relieving pain and anxiety during labor. Explore 2025, 21, 103081. [Google Scholar] [CrossRef]
- Zhong, Y.; Zheng, Q.; Hu, P.Y.; Huang, X.Y.; Yang, M.; Ren, G.L.; Du, Q.; Luo, J.; Zhang, K.N.; Li, J.; et al. Sedative and hypnotic effects of compound Anshen essential oil inhalation for insomnia. BMC Complement. Altern. Med. 2019, 19, 306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Zhao, A.C.; Song, X.M.; Wei, L.L.; Fang, M.D.; Zheng, C.C.; Zhu, X.D. Sustainable fragrance release wax oil coating for wood substrate based on peppermint essential oil microcapsules. Ind. Crops Prod. 2024, 208, 117848. [Google Scholar] [CrossRef]
- Shi, X. Study on the prevention and treatment of epidemic febrile diseases with aromatic drugs based on the literature of the Ming and Qing Dynasties. 2019. [Google Scholar]
- Sun, L. Medicine Historiographic Research on Fragrant Mdeicines in Ming and Qing Dynasties. Doctor, 2015.
- Lu, Z. Nano-Aromatic Drugs for the Treatments of Neuropsychiatric Disorders. Doctor, 2021.
- Ru, J.L.; Li, P.; Wang, J.N.; Zhou, W.; Li, B.H.; Huang, C.; Li, P.D.; Guo, Z.H.; Tao, W.Y.; Yang, Y.F.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Wei, M.Q.; Li, H.; Li, Q.F.; Qiao, Y.; Ma, Q.; Xie, R.N.; Wang, R.; Liu, Y.; Wei, C.; Li, B.B.; et al. Based on Network Pharmacology to Explore the Molecular Targets and Mechanisms of Gegen Qinlian Decoction for the Treatment of Ulcerative Colitis. BioMed Res. Int. 2020, 2020, 5217405. [Google Scholar] [CrossRef]
- Limón, I.D.; Mendieta, L.; Díaz, A.; Chamorro, G.; Espinosa, B.; Zenteno, E.; Guevara, J. Neuroprotective effect of alpha-asarone on spatial memory and nitric oxide levels in rats injected with amyloid-beta (25–35). Neurosci. Lett. 2009, 453, 98–103. [Google Scholar] [CrossRef]
- Chang, J.; Yang, H.; Shan, X.; Zhao, L.; Li, Y.; Zhang, Z.; Abankwah, J.K.; Zhang, M.; Bian, Y.; Guo, Y. Bergamot essential oil improves CUMS-induced depression-like behaviour in rats by protecting the plasticity of hippocampal neurons. J. Cell Mol. Med. 2024, 28, e18178. [Google Scholar] [CrossRef]
- Rai, A.R.; Joy, T.; Poojari, M.; Pai, M.M.; Massand, A.; Murlimanju, B.V. Role of Acorus calamus in preventing depression, anxiety, and oxidative stress in long-term socially isolated rats. Vet. World 2023, 16, 1755–1764. [Google Scholar] [CrossRef]
- Zhang, N.; Yao, L. Anxiolytic Effect of Essential Oils and Their Constituents: A Review. J. Agric. Food Chem. 2019, 67, 13790–13808. [Google Scholar] [CrossRef]
- Winkelman, W.J. Aromatherapy, botanicals, and essential oils in acne. Clin. Dermatol. 2018, 36, 299–305. [Google Scholar] [CrossRef]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Dong, Z.; Milak, M.S.; Rashid, R.; Kegeles, L.S.; Javitt, D.C.; Lieberman, J.A.; John Mann, J. Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder. Transl. Psychiatry 2021, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.F.; Lau, D.; Sharma, S.; Fulton, S. Anxiety-like behavior in female mice is modulated by STAT3 signaling in midbrain dopamine neurons. Brain Behav. Immun. 2021, 95, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, B.; Ntim, M.; Zhang, X.; Na, X.Y.; Yuan, Y.H.; Wu, X.F.; Yang, J.Y.; Li, S. SRC-1 Deficiency Increases Susceptibility of Mice to Depressive-Like Behavior After Exposure to CUMS. Neurochem. Res. 2021, 46, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Rudko, O.I.; Tretiakov, A.V.; Naumova, E.A.; Klimov, E.A. Role of PPARs in Progression of Anxiety: Literature Analysis and Signaling Pathways Reconstruction. PPAR Res. 2020, 2020, 8859017. [Google Scholar] [CrossRef]




| Precentrifugation | Postcentrifugation | |||||
|---|---|---|---|---|---|---|
| Centrifugal Rate (rpm/min) | Particle Size (nm) | Zeta Potential (mv) | PDI | Particle Size (nm) | Zeta Potential (mv) | PDI |
| 2000 | 20.73 ± 0.14 | −0.77 | 0.16 ± 0.015 | 18.55 ± 0.64 | −0.67 | 0.16 ± 0.022 |
| 4000 | 20.47 ± 0.18 | −1.31 | 0.21 ± 0.0045 | 18.26 ± 0.15 | −0.99 | 0.20 ± 0.012 |
| 6000 | 20.09 ± 0.23 | −0.99 | 0.210 | 17.63 ± 0.19 | −0.95 | 0.15 ± 0.0085 |
| 8000 | 19.32 ± 0.25 | −1.21 | 0.17 ± 0.023 | 17.47 ± 0.095 | −0.91 | 0.15 ± 0.0035 |
| 10,000 | 22.43 ± 0.085 | −1.27 | 0.22 ± 0.014 | 17.88 ± 0.36 | −0.83 | 0.15 ± 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Jiang, P.; Hu, K.; Mei, D.; Jiao, Q.; Li, Y.; Deng, Y.; Wang, J.; Gao, R.; Chen, X.; et al. Anti-Anxiety Effects of Essential Oil Microemulsion in Chronic Unpredictable Mild Stress-Induced Rats: Preparation, Characterization, and Mechanisms. Molecules 2025, 30, 2652. https://doi.org/10.3390/molecules30122652
Tang W, Jiang P, Hu K, Mei D, Jiao Q, Li Y, Deng Y, Wang J, Gao R, Chen X, et al. Anti-Anxiety Effects of Essential Oil Microemulsion in Chronic Unpredictable Mild Stress-Induced Rats: Preparation, Characterization, and Mechanisms. Molecules. 2025; 30(12):2652. https://doi.org/10.3390/molecules30122652
Chicago/Turabian StyleTang, Wenxia, Pan Jiang, Ke Hu, Duo Mei, Qinghao Jiao, Yan Li, Yanping Deng, Jun Wang, Ran Gao, Xin Chen, and et al. 2025. "Anti-Anxiety Effects of Essential Oil Microemulsion in Chronic Unpredictable Mild Stress-Induced Rats: Preparation, Characterization, and Mechanisms" Molecules 30, no. 12: 2652. https://doi.org/10.3390/molecules30122652
APA StyleTang, W., Jiang, P., Hu, K., Mei, D., Jiao, Q., Li, Y., Deng, Y., Wang, J., Gao, R., Chen, X., & Yu, J. (2025). Anti-Anxiety Effects of Essential Oil Microemulsion in Chronic Unpredictable Mild Stress-Induced Rats: Preparation, Characterization, and Mechanisms. Molecules, 30(12), 2652. https://doi.org/10.3390/molecules30122652






