Pressure Dependence of Structural Behavior in the Polymorphs of Fe(PM–BiA)2(NCS)2
Abstract
1. Introduction
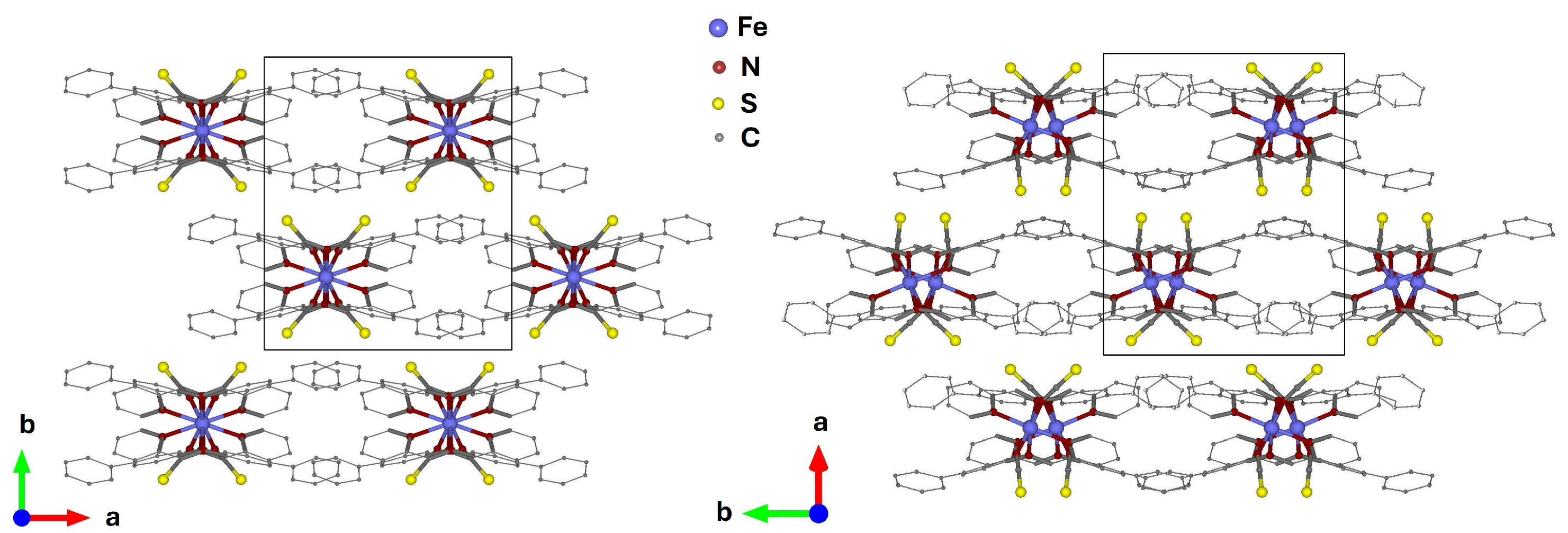
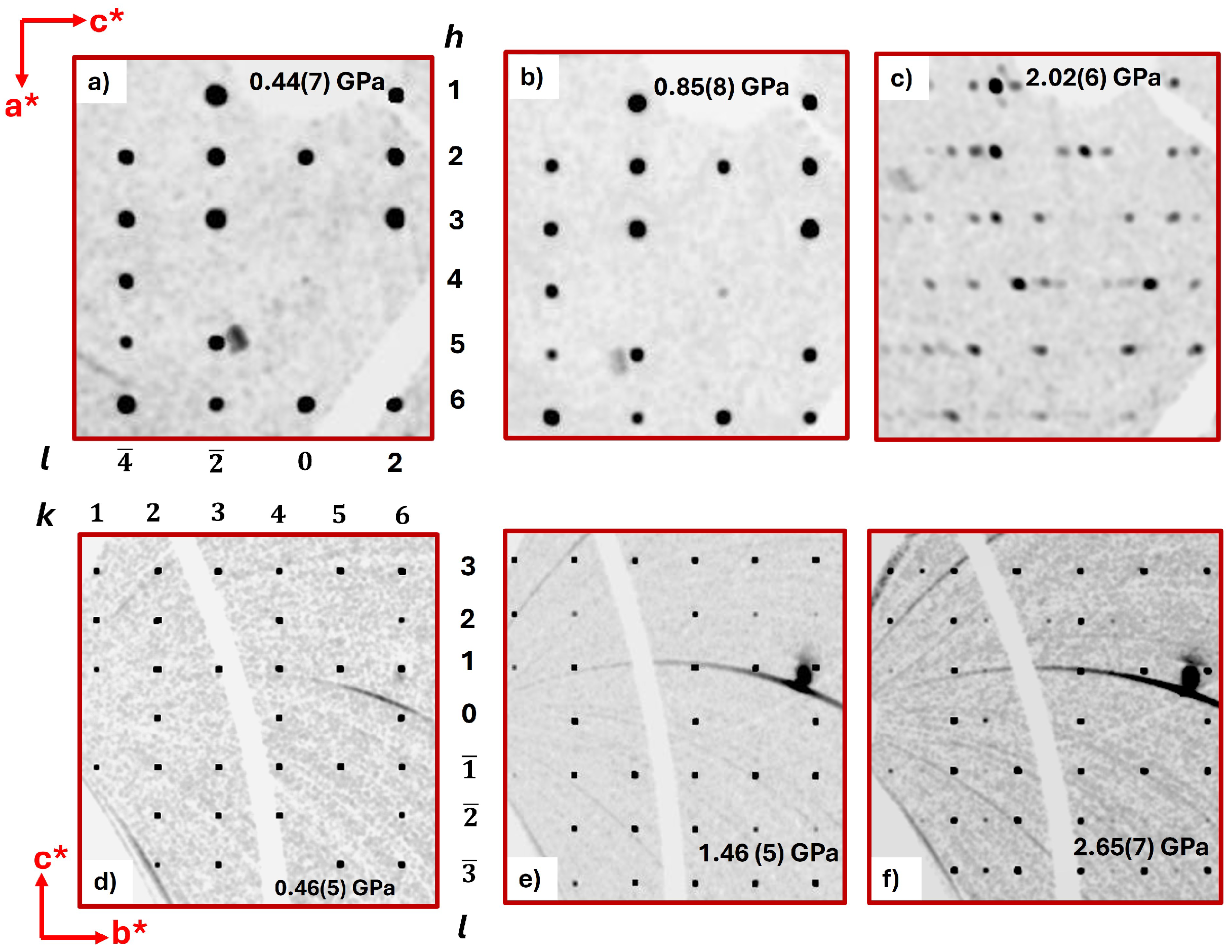
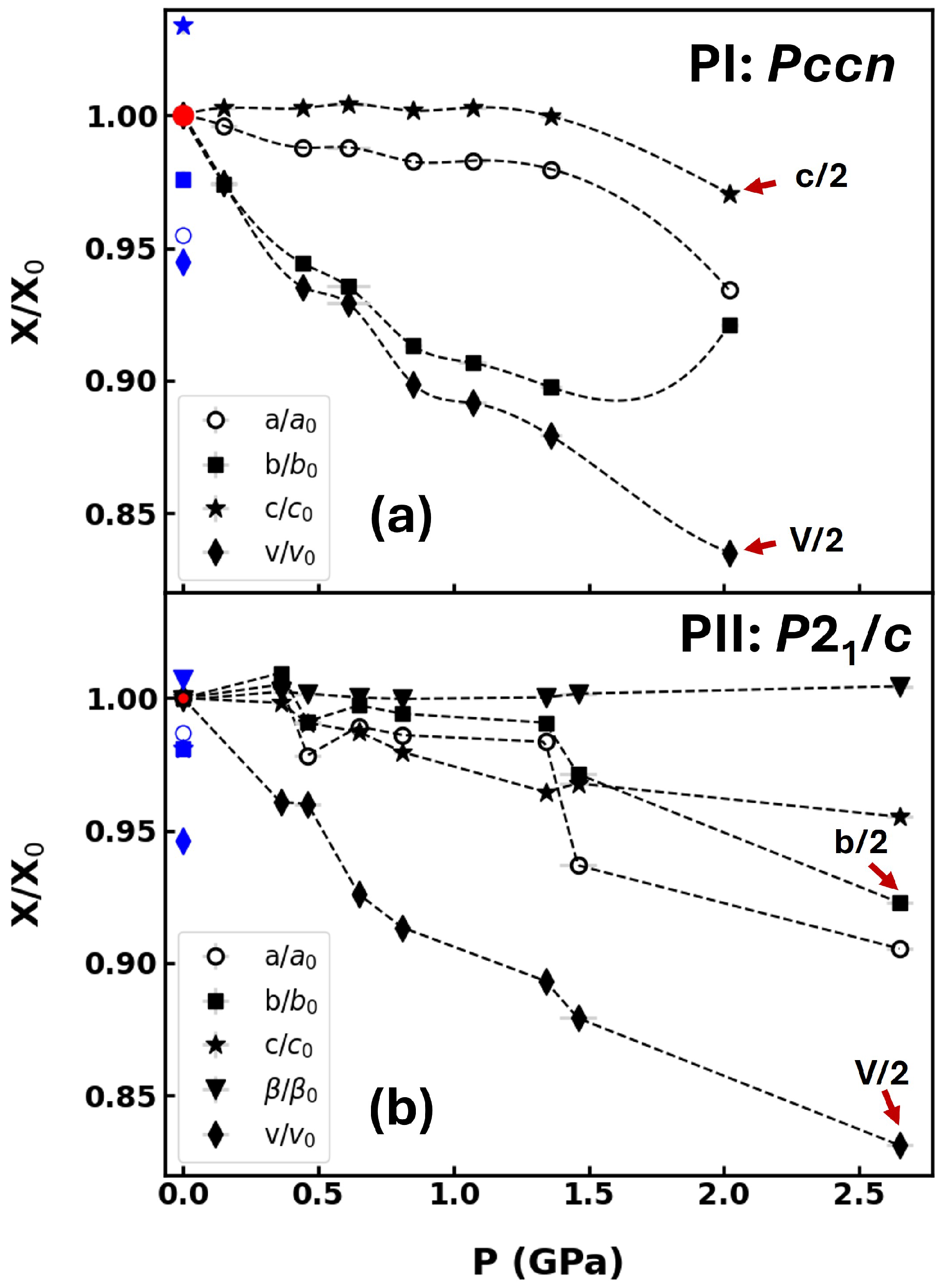
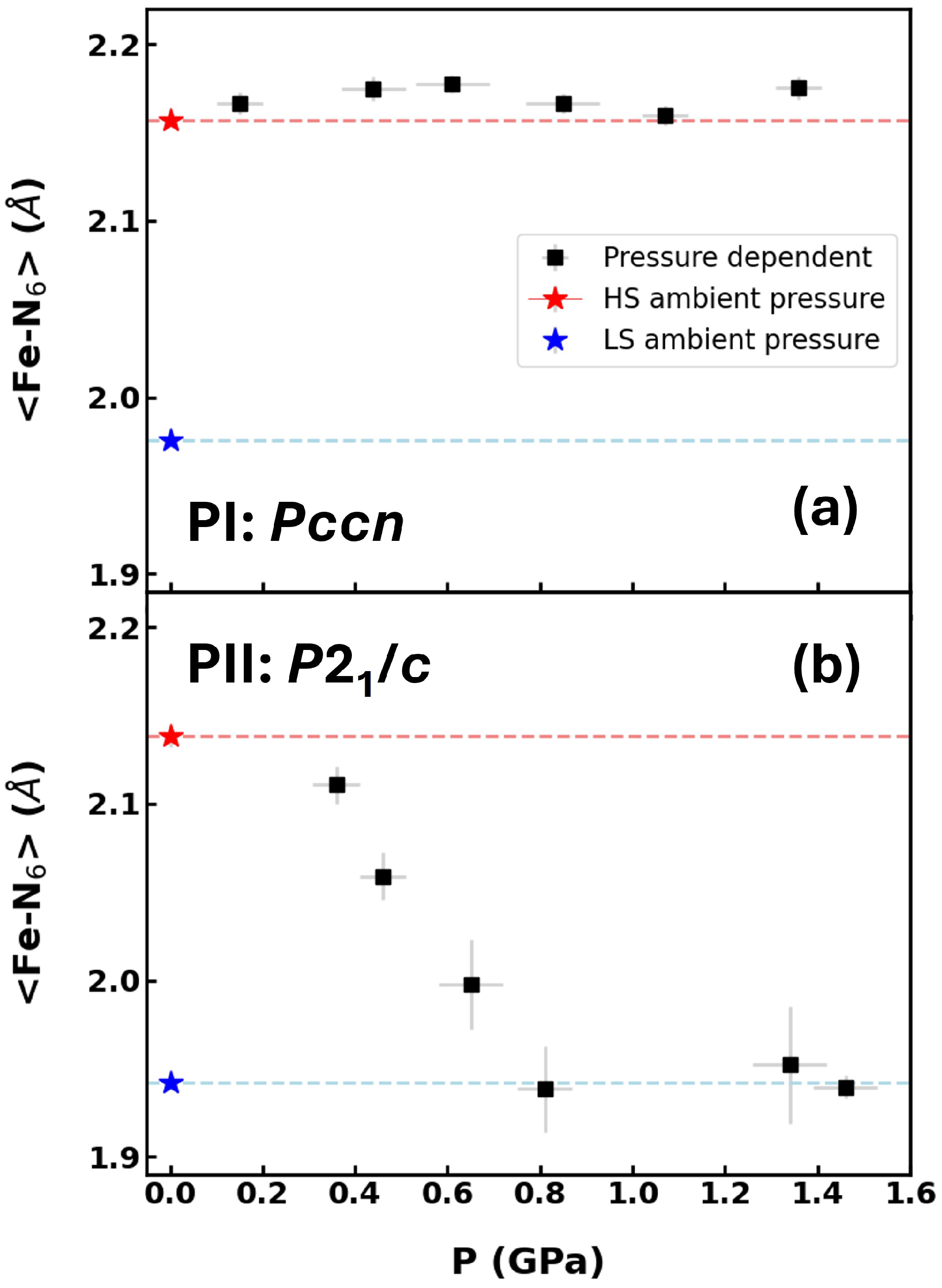
2. Results and Discussion
2.1. Synchrotron X-Ray Diffraction
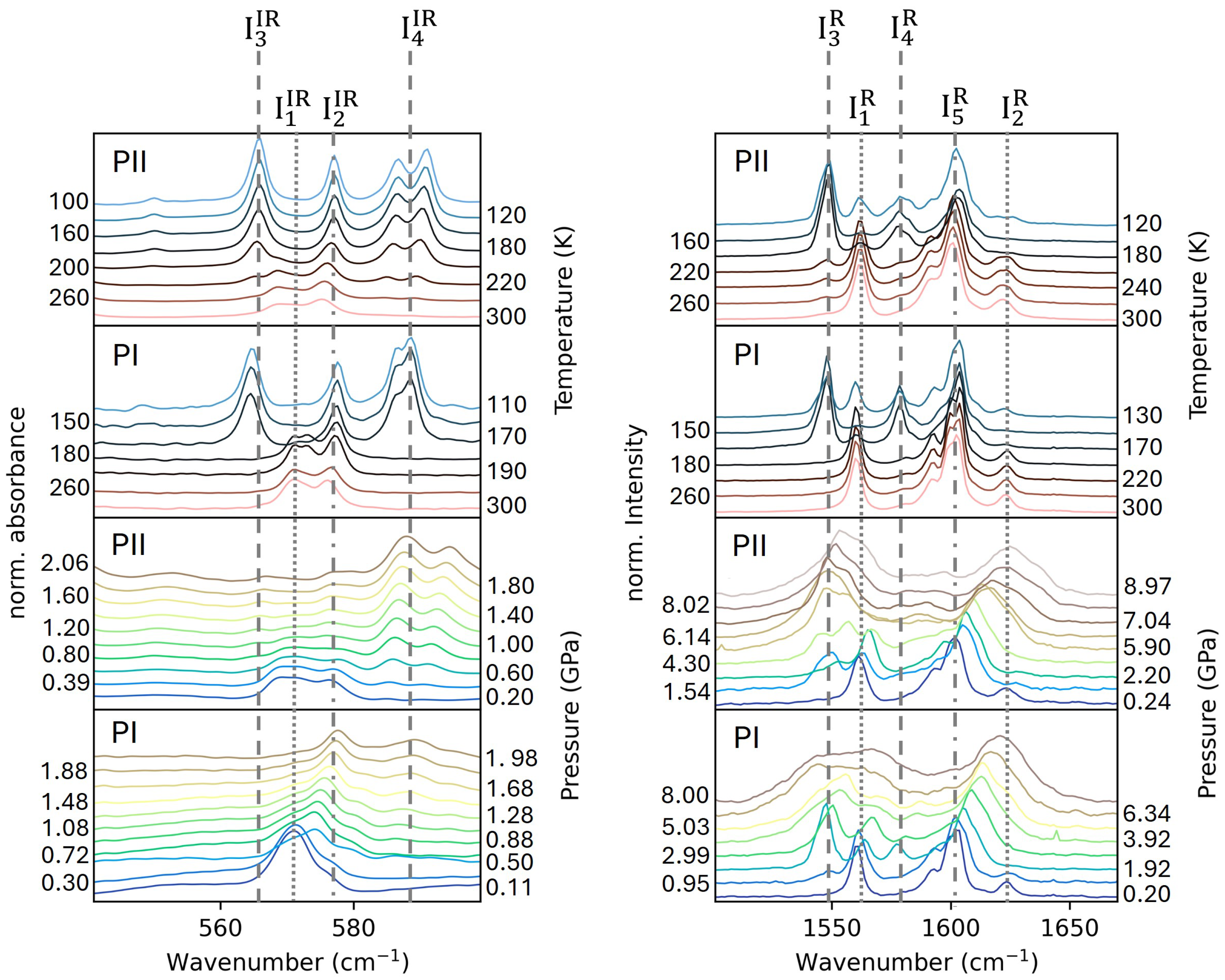
2.2. Vibrational Spectroscopy
3. Materials and Methods
3.1. Single-Crystal X-Ray Diffraction
3.2. Infrared Spectroscopy
3.3. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirillo, L.; Greco, A.; Masselli, C. Cooling through barocaloric effect: A review of the state of the art up to 2022. Therm. Sci. Eng. Prog. 2022, 33, 101380. [Google Scholar] [CrossRef]
- Ribeiro, P.O.; Alho, B.P.; Nobrega, E.P.; de Sousa, V.S.R.; Carvalho, A.M.G.; von Ranke, P.J. Theoretical investigation of the barocaloric effect in spin-crossover systems upon first- and second-order phase transition conversion. J. Appl. Phys. 2023, 133, 125104. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Bibik, Y.S.; Gural’skiy, I.A.; Zatovsky, I.V.; Liu, Z.; Li, Q.; Li, B.; Levchenko, G.; Liu, B. Colossal barocaloric effect of the spin-crossover compound Fe(pz)2(BH3CN)2 near room temperature. Appl. Phys. Lett. 2024, 124, 122202. [Google Scholar] [CrossRef]
- Seo, J.; Braun, J.D.; Dev, V.M.; Mason, J.A. Driving Barocaloric Effects in a Molecular Spin-Crossover Complex at Low Pressures. J. Am. Chem. Soc. 2022, 144, 6493–6503. [Google Scholar] [CrossRef] [PubMed]
- Sandeman, K.G.; Halcrow, M.A. Spin crossover complexes for barocaloric cooling. In Barocaloric Effects in the Solid State: Materials and Methods; IOP Publishing: Bristol, UK, 2023. [Google Scholar] [CrossRef]
- Gütlich, P.; Goodwin, H.A. Spin Crossover—An Overall Perspective. In Spin Crossover in Transition Metal Compounds I; Gütlich, P., Goodwin, H.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–47. [Google Scholar] [CrossRef]
- von Ranke, P.J.; Alho, B.P.; da Silva, P.H.S.; Ribas, R.M.; Nobrega, E.P.; de Sousa, V.S.R.; Carvalho, A.M.G.; Ribeiro, P.O. Refrigeration through Barocaloric Effect Using the Spin Crossover Complex Fe[H2B(pz)2]2(bipy). Phys. Status Solidi (b) 2021, 258, 2100108. [Google Scholar] [CrossRef]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Muñoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalton Trans. 2005, 12, 2062–2079. [Google Scholar] [CrossRef]
- Ksenofontov, V.; Levchenko, G.; Spiering, H.; Gütlich, P.; Létard, J.F.; Bouhedja, Y.; Kahn, O. Spin crossover behavior under pressure of Fe(PM-L)2(NCS)2 compounds with substituted 2′-pyridylmethylene 4-anilino ligands. Chem. Phys. Lett. 1998, 294, 545–553. [Google Scholar] [CrossRef]
- Decurtins, S.; Gütlich, P.; Köhler, C.P.; Spiering, H.; Hauser, A. Light-induced excited spin state trapping in a transition-metal complex: The hexa-1-propyltetrazole-iron (II) tetrafluoroborate spin-crossover system. Chem. Phys. Lett. 1984, 105, 1–4. [Google Scholar] [CrossRef]
- McGravey, J.J.; Lawthers, I. Photochemically-induced perturbation of the 1A ⇌ 5T equilibrium in Fe11 complexes by pulsed laser irradiation in the metal-to-ligand charge-transfer absorption band. J. Chem. Soc. Chem. Commun. 1982, 906–907. [Google Scholar] [CrossRef]
- Bonhommeau, S.; Molnár, G.; Goiran, M.; Boukheddaden, K.; Bousseksou, A. Unified dynamical description of pulsed magnetic field and pressure effects on the spin crossover phenomenon. Phys. Rev. 2006, 74, 064424. [Google Scholar] [CrossRef]
- Lefter, C.; Tan, R.; Dugay, J.; Tricard, S.; Molnár, G.; Salmon, L.; Carrey, J.; Nicolazzi, W.; Rotaru, A.; Bousseksou, A. Unidirectional electric field-induced spin-state switching in spin crossover based microelectronic devices. Chem. Phys. Lett. 2016, 644, 138–141. [Google Scholar] [CrossRef]
- Ohba, M.; Yoneda, K.; Agustí, G.; Muñoz, M.; Gaspar, A.; Real, J.; Yamasaki, M.; Ando, H.; Nakao, Y.; Sakaki, S.; et al. Bidirectional Chemo-Switching of Spin State in a Microporous Framework. Angew. Chem. Int. Ed. 2009, 48, 4767–4771. [Google Scholar] [CrossRef] [PubMed]
- Cobo, S.; Molnár, G.; Real, J.A.; Bousseksou, A. Multilayer Sequential Assembly of Thin Films That Display Room-Temperature Spin Crossover with Hysteresis. Angew. Chem. Int. Ed. 2006, 45, 5786–5789. [Google Scholar] [CrossRef]
- Kamilya, S.; Dey, B.; Kaushik, K.; Shukla, S.; Mehta, S.; Mondal, A. Realm of Spin State Switching Materials: Toward Realization of Molecular and Nanoscale Devices. Chem. Mater. 2024, 36, 4889–4915. [Google Scholar] [CrossRef]
- Handzlik, G.; Dziubek, K.F.; Hanfland, M.; Pinkowicz, D. Simultaneous manipulation of iron(ii) spin crossover and LIESST behaviour using pressure, temperature and light. Dalton Trans. 2024, 53, 7677–7681. [Google Scholar] [CrossRef]
- Wolny, J.A.; Diller, R.; Schünemann, V. Vibrational Spectroscopy of Mono- and Polynuclear Spin-Crossover Systems. Eur. J. Inorg. Chem. 2012, 2012, 2635–2648. [Google Scholar] [CrossRef]
- Shahed, H.; Sharma, N.; Angst, M.; Voigt, J.; Persson, J.; Prakash, P.; Tornroos, K.W.; Chernyshov, D.; Gildenast, H.; Ohl, M.; et al. Structural insight into the cooperativity of spin crossover compounds. Acta Crystallogr. Sect. 2023, 79, 354–367. [Google Scholar] [CrossRef]
- Nicolazzi, W.; Bousseksou, A. Thermodynamical aspects of the spin crossover phenomenon. Comptes Rendus Chim. 2018, 21, 1060–1074. [Google Scholar] [CrossRef]
- Köhler, C.P.; Jakobi, R.; Meissner, E.; Wiehl, L.; Spiering, H.; Gütlich, P. Nature of the phase transition in spin crossover compounds. J. Phys. Chem. Solids 1990, 51, 239–247. [Google Scholar] [CrossRef]
- Birchall, L.T.; Shepherd, H.J. 10.04—Synchrotron diffraction studies on spin crossover materials. In Comprehensive Inorganic Chemistry III, 3rd ed.; Reedijk, J., Poeppelmeier, K.R., Eds.; Elsevier: Oxford, UK, 2023; pp. 86–107. [Google Scholar] [CrossRef]
- Marchivie, M.; Guionneau, P.; Letard, J.F.; Chasseau, D. Towards direct correlations between spin-crossover and structural features in iron(II) complexes. Acta Crystallogr. Sect. 2003, 59, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Buron-Le Cointe, M.; Hébert, J.; Baldé, C.; Moisan, N.; Toupet, L.; Guionneau, P.; Létard, J.F.; Freysz, E.; Cailleau, H.; Collet, E. Intermolecular control of thermoswitching and photoswitching phenomena in two spin-crossover polymorphs. Phys. Rev. B 2012, 85, 064114. [Google Scholar] [CrossRef]
- Tailleur, E.; Marchivie, M.; Negrier, P.; Denux, D.; Massip, S.; Mondieig, D.; Chastanet, G.; Guionneau, P. Using polymorphism to master the spin crossover mechanism in [Fe(PM-PeA)2(NCSe)2]. CrystEngComm 2019, 21, 6246–6251. [Google Scholar] [CrossRef]
- Legrand, V.; Pechev, S.; Létard, J.F.; Guionneau, P. Synergy between polymorphism, pressure, spin-crossover and temperature in [Fe(PM-BiA)2(NCS)2]: A neutron powder diffraction investigation. Phys. Chem. Chem. Phys. 2013, 15, 13872–13880. [Google Scholar] [CrossRef]
- Legrand, V.; Le Gac, F.; Guionneau, P.; Letard, J.F. Neutron powder diffraction studies of two spin transition FeII complexes under pressure. J. Appl. Crystallogr. 2008, 41, 637–640. [Google Scholar] [CrossRef]
- Rotaru, A.; Varret, F.; Codjovi, E.; Boukheddaden, K.; Linares, J.; Stancu, A.; Guionneau, P.; Létard, J.F. Hydrostatic pressure investigation of the spin crossover compound [Fe(PM–BiA)2(NCS)2] polymorph I using reflectance detection. J. Appl. Phys. 2009, 106, 053515. [Google Scholar] [CrossRef]
- Marbeuf, A.; Matar, S.F.; Négrier, P.; Kabalan, L.; Létard, J.F.; Guionneau, P. Molecular dynamics of spin crossover: The (P,T) phase diagram of [Fe(PM-BIA)2(NCS)2]. Chem. Phys. 2013, 420, 25–34. [Google Scholar] [CrossRef]
- Matar, S.F.; Guionneau, P.; Chastanet, G. Multiscale experimental and theoretical investigations of spin crossover Fe(II) complexes: Examples of [Fe(phen)2(NCS)2] and [Fe(PM-BiA)2(NCS)2]. Int. J. Mol. Sci. 2015, 16, 4007–4027. [Google Scholar] [CrossRef]
- Shepherd, H.J.; Rosa, P.; Vendier, L.; Casati, N.; Létard, J.F.; Bousseksou, A.; Guionneau, P.; Molnár, G. High-pressure spin-crossover in a dinuclear Fe(ii) complex. Phys. Chem. Chem. Phys. 2012, 14, 5265–5271. [Google Scholar] [CrossRef]
- Molnár, G.; Mikolasek, M.; Ridier, K.; Fahs, A.; Nicolazzi, W.; Bousseksou, A. Molecular Spin Crossover Materials: Review of the Lattice Dynamical Properties. Ann. Der Phys. 2019, 531, 1900076. [Google Scholar] [CrossRef]
- Kazuo, N. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley and Sons: New York, NY, USA, 1986; Volume 1. [Google Scholar]
- Lagaron, J.M. The factor group splitting phenomenon: A vibrational spectroscopy approach to assess polymer crystallinity and crystalline density. Macromol. Symp. 2002, 184, 19–36. [Google Scholar] [CrossRef]
- Celeste, A.; Borondics, F.; Capitani, C. Hydrostaticity of pressure-transmitting media for high pressure infrared spectroscopy. High Press. Res. 2019, 39, 608–618. [Google Scholar] [CrossRef]
- Spiering, H.; Kohlhaas, T.; Romstedt, H.; Hauser, A.; Bruns-Yilmaz, C.; Kusz, J.; Gütlich, P. Correlations of the distribution of spin states in spin crossover compounds. Coord. Chem. Rev. 1999, 190–192, 629–647. [Google Scholar] [CrossRef]
- Kabalan, L. Ab Initio Modeling of Magnetic Properties, Spectroscopic and Switching of Molecular Complexes Approches. Ph.D. Thesis, University of Bordeaux, Bordeaux, France, 2010. [Google Scholar]
- Marchivie, M.; Guionneau, P.; Letard, J.F.; Chasseau, D. Photo-induced spin-transition: The role of the iron(II) environment distortion. Acta Crystallogr. Sect. 2005, 61, 25–28. [Google Scholar] [CrossRef]
- Létard, J.F. Photomagnetism of iron(ii) spin crossover complexes—The T(LIESST) approach. J. Mater. Chem. 2006, 16, 2550–2559. [Google Scholar] [CrossRef]
- Hayami, S.; Kawajiri, R.; Juhász, G.; Kawahara, T.; Hashiguchi, K.; Sato, O.; Inoue, K.; Maeda, Y. Study of Intermolecular Interaction for the Spin-Crossover Iron(II) Compounds. Bull. Chem. Soc. Jpn. 2003, 76, 1207–1213. [Google Scholar] [CrossRef]
- Chastanet, G.; Desplanches, C.; Baldé, C.; Rosa, P.; Marchivie, M.; Guionneau, P. A critical review of the T(LIESST) temperature in spin crossover materials—What it is and what it is not. Chem. Squared 2018, 2, 2. [Google Scholar] [CrossRef]
- Craig, G.A.; Costa, J.S.; Roubeau, O.; Teat, S.J.; Shepherd, H.J.; Lopes, M.; Molnár, G.; Bousseksou, A.; Aromí, G. High-temperature photo-induced switching and pressure-induced transition in a cooperative molecular spin-crossover material. Dalton Trans. 2014, 43, 729–737. [Google Scholar] [CrossRef]
- Commins, P.; Desta, I.T.; Karothu, D.P.; Panda, M.K.; Naumov, P. Crystals on the move: Mechanical effects in dynamic solids. Chem. Commun. 2016, 52, 13941–13954. [Google Scholar] [CrossRef]
- Hagiwara, H.; Konomura, S. Thermosalience coupled to abrupt spin crossover with dynamic ligand motion in an iron(ii) molecular crystal. CrystEngComm 2022, 24, 4224–4234. [Google Scholar] [CrossRef]
- Tolkiehn, M.; Schulze-Ritter, H.; Drube, W.; Paulmann, C.; Berghäuser, A.; Ropers, D. Beamline P24—Chemical Crystallography; Technical Report DESY, DESY: Hamburg, Germany, 2014. [Google Scholar]
- Dyadkin, V.; Pattison, P.; Dmitriev, V.; Chernyshov, D. A new multipurpose diffractometer PILATUS@SNBL. J. Synchrotron Radiat. 2016, 23, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Boehler, R. New diamond cell for single-crystal x-ray diffraction. Rev. Sci. Instrum. 2006, 77, 115103. [Google Scholar] [CrossRef]
- Cheng, Y.; Brenk, J.; Friedrich, B.; Perßon, J.; Maraytta, N.; Gibson, J.S.K.L.; Korte-Kerzel, S.; Roth, G.; Su, Y.; Zhu, F.; et al. Ni–Cr–Al Alloy for neutron scattering at high pressures. Mater. Sci. Technol. 2020, 36, 949–954. [Google Scholar] [CrossRef]
- Cheng, Y. A Diamond Anvil Cell for Combined X-Ray and Neutron Single- Crystal Studies. Master’s Thesis, RWTH Aachen University, Aachen, Germany, 2018. [Google Scholar]
- Syassen, K. Ruby under pressure. High Press. Res. 2008, 28, 75–126. [Google Scholar] [CrossRef]
- Shen, G.; Wang, Y.; Dewaele, A.; Wu, C.; Fratanduono, D.E.; Eggert, J.; Klotz, S.; Dziubek, K.F.; Loubeyre, P.; Fat’yanov, O.V.; et al. Toward an international practical pressure scale: A proposal for an IPPS ruby gauge (IPPS-Ruby2020). High Press. Res. 2020, 40, 299–314. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent: Santa Clara, CA, USA, 2014. [Google Scholar]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. FüR Krist.-Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Reibel, Y.; Rubaldo, L.; Bonnouvrier, G.; Verdet, S.; Billon-Lanfrey, D.; Destéfanis, G.; Mollard, L.; Baylet, J.; Rothman, J.; Druart, G.; et al. Latest developments in advanced MCT infrared cooled detectors. In Proceedings Volume 8185, Electro-Optical and Infrared Systems: Technology and Applications VIII; SPIE Press: Bellingham, WA, USA, 2011. [Google Scholar]
- Bruker Optik GmbH OPUS Spectroscopic Software: Reference Manual; Bruker Optik: Ettlingen, Germany, 2004.
- Dumas, P.; Polack, F.; Lagarde, B.; Chubar, O.; Giorgetta, J.L.; Lefrançois, S. Synchrotron infrared microscopy at the French Synchrotron Facility SOLEIL. Infrared Phys. Technol. 2006, 49, 152–160. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. OMNIC; Thermo Fisher Scientific: Waltham, MA, USA, 2021. [Google Scholar]
- Zhao, J.; Ross, N.L. Non-hydrostatic behavior of KBr as a pressure medium in diamond anvil cells up to 5.63 GPa. J. Phys. Condens. Matter 2015, 27, 185402. [Google Scholar] [CrossRef]
- Bachmann, K.; Peisl, H. Elastic distortions and interactions of point defects in KBr. J. Phys. Chem. Solids 1970, 31, 1525–1529. [Google Scholar] [CrossRef]
- Yoshikawa, M. Raman and Infrared (IR) Spectroscopy. In Advanced Optical Spectroscopy Techniques for Semiconductors: Raman, Infrared, and Cathodoluminescence Spectroscopy; Springer International Publishing: Cham, Switzerland, 2023; pp. 3–25. [Google Scholar] [CrossRef]
- Piermarini, G.J.; Block, S.; Barnett, J. Hydrostatic limits in liquids and solids to 100 kbar. J. Appl. Phys. 1973, 44, 5377–5382. [Google Scholar] [CrossRef]
- Plyler, E.K. Infrared spectra of methanol, ethanol, and n-propanol. J. Res. Natl. Bur. Stand. 1952, 48, 281–286. [Google Scholar] [CrossRef]
- Chernyshov, D.; Hostettler, M.; Törnroos, K.W.; Bürgi, H.B. Ordering Phenomena and Phase Transitions in a Spin-Crossover Compound—Uncovering the Nature of the Intermediate Phase of [Fe(2-pic)3]Cl2· EtOH. Angew. Chem. Int. Ed. 2003, 42, 3825–3830. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, P.; Shahed, H.; Qi, J.; Grzechnik, A.; Angst, M.; Voigt, J.; Perßon, J.; Cheng, Y.; Gasharova, B.; Mathis, Y.-L.; et al. Pressure Dependence of Structural Behavior in the Polymorphs of Fe(PM–BiA)2(NCS)2. Molecules 2025, 30, 2651. https://doi.org/10.3390/molecules30122651
Prakash P, Shahed H, Qi J, Grzechnik A, Angst M, Voigt J, Perßon J, Cheng Y, Gasharova B, Mathis Y-L, et al. Pressure Dependence of Structural Behavior in the Polymorphs of Fe(PM–BiA)2(NCS)2. Molecules. 2025; 30(12):2651. https://doi.org/10.3390/molecules30122651
Chicago/Turabian StylePrakash, Pulkit, Hend Shahed, Ji Qi, Andrzej Grzechnik, Manuel Angst, Jörg Voigt, Jörg Perßon, Yao Cheng, Biliana Gasharova, Yves-Laurent Mathis, and et al. 2025. "Pressure Dependence of Structural Behavior in the Polymorphs of Fe(PM–BiA)2(NCS)2" Molecules 30, no. 12: 2651. https://doi.org/10.3390/molecules30122651
APA StylePrakash, P., Shahed, H., Qi, J., Grzechnik, A., Angst, M., Voigt, J., Perßon, J., Cheng, Y., Gasharova, B., Mathis, Y.-L., Capitani, F., Paulmann, C., McMonagle, C., Chernyshov, D., & Friese, K. (2025). Pressure Dependence of Structural Behavior in the Polymorphs of Fe(PM–BiA)2(NCS)2. Molecules, 30(12), 2651. https://doi.org/10.3390/molecules30122651






