Mediterranean Basin Erica Species: Traditional Uses, Phytochemistry and Pharmacological Properties
Abstract
1. Introduction
2. Characteristic Features and Geographical Distribution of Erica spp. in the Mediterranean Basin Region
3. Traditional Uses of Erica Species
4. Chemical Constituents of Erica Species of the Mediterranean Basin
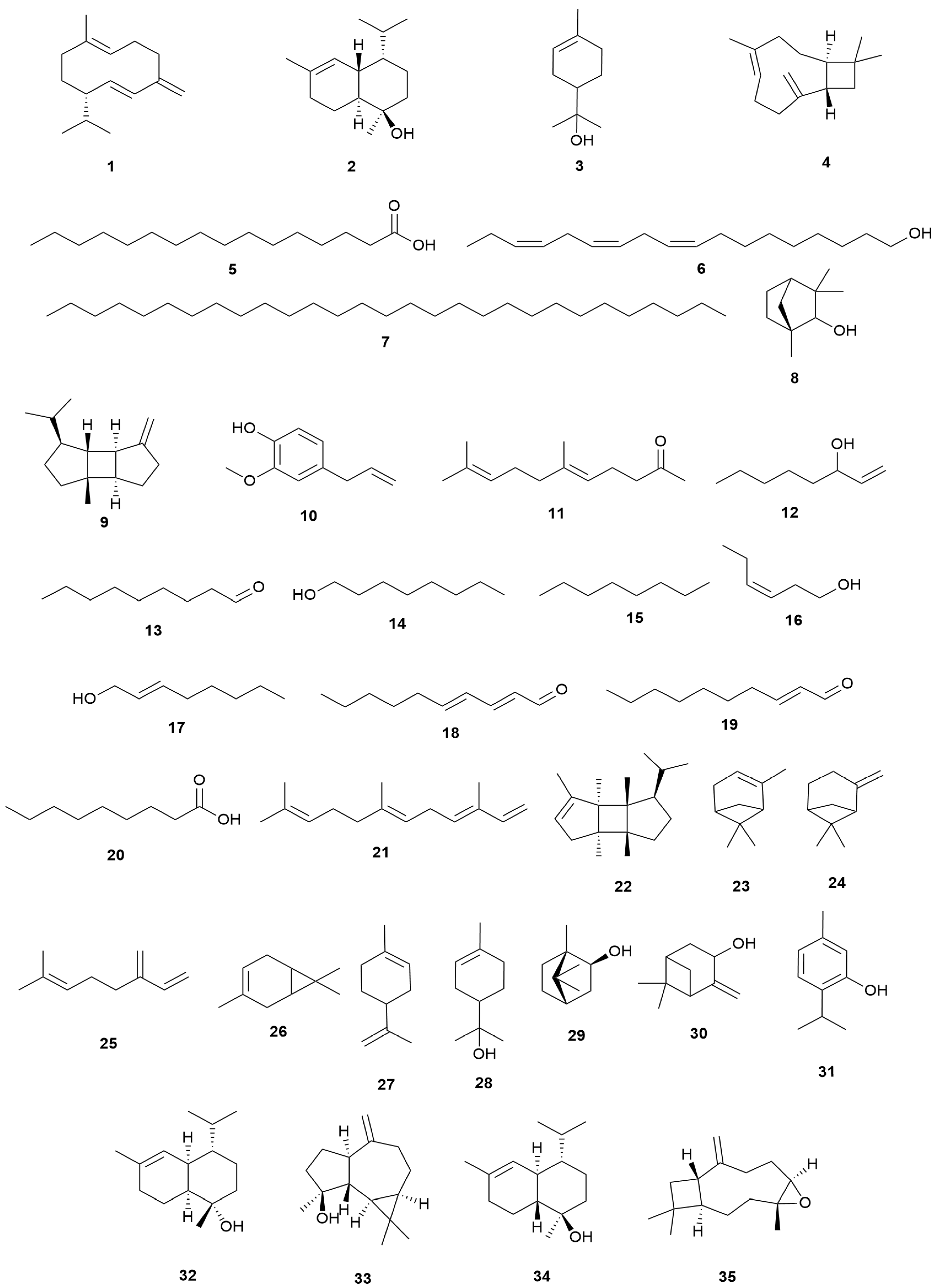
4.1. Essential Oil Constituents
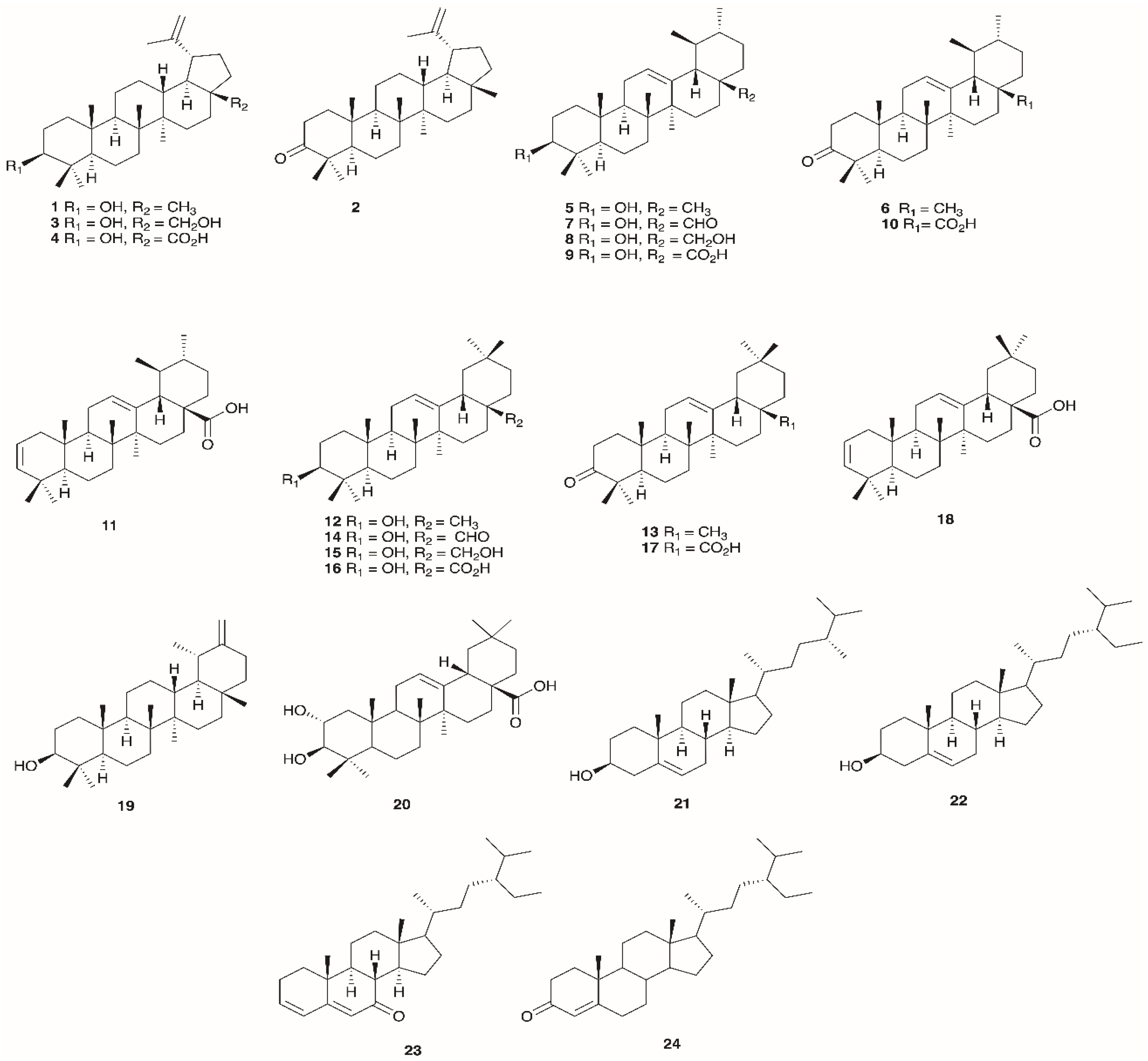
4.2. Triterpenoids
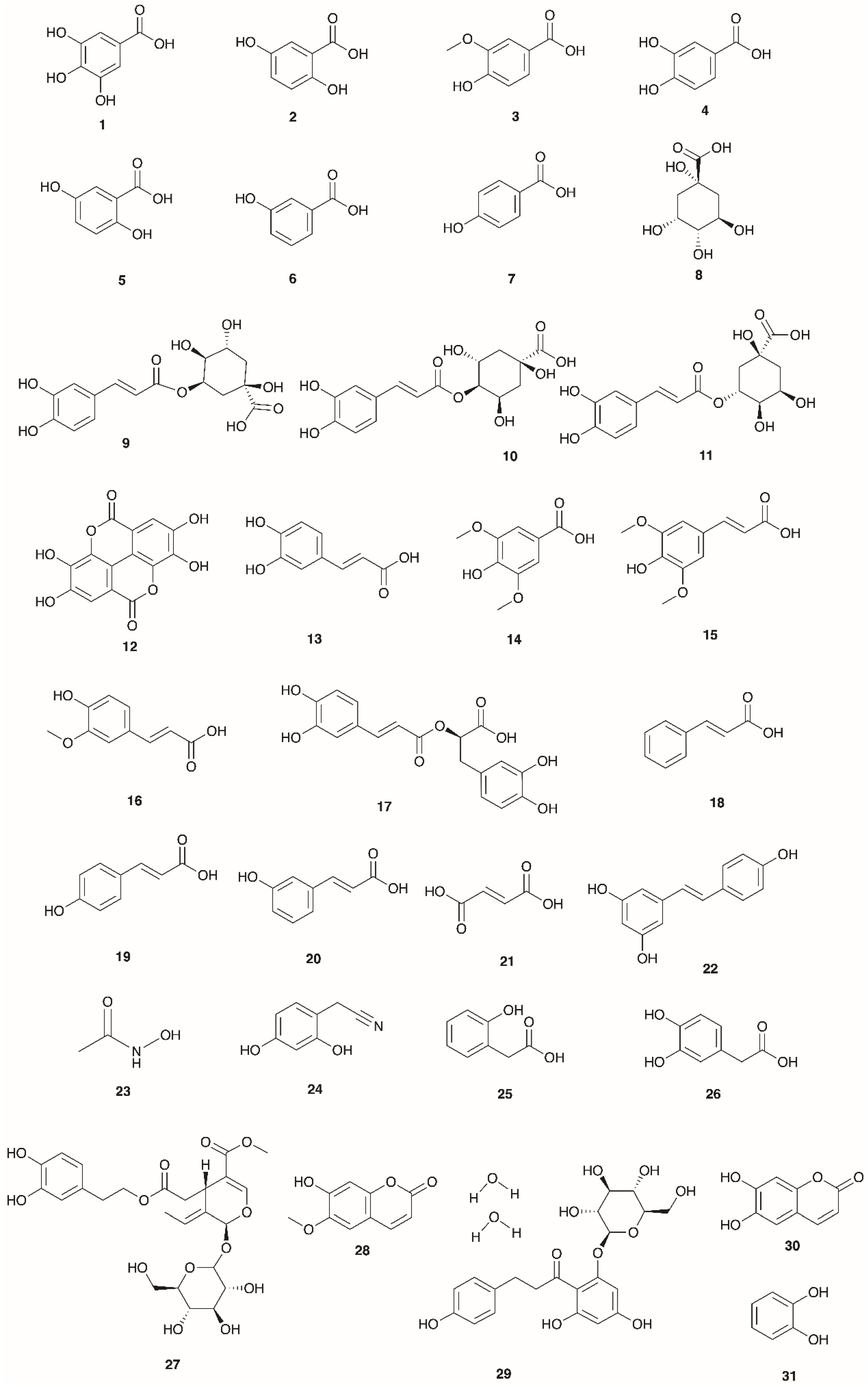
4.3. Phenolic Acids and Esters
4.4. Phenylpropanoid Glucosides
4.5. Flavonoids and Flavonoid Glycosides
4.6. Catechins
4.7. Anthocyanidins
5. Biological Activities
5.1. Anti-Inflammatory Activity
5.2. Analgesic Activity
5.3. Antioxidant Activity
5.4. Antibacterial Activity
5.5. Antiviral Activity
5.6. Melanogenesis Stimulation
5.7. Anti-Hyperlipidemia
5.8. Acetylcholinesterase Inhibition
5.9. Anti-Urolithiatic Activity
5.10. Diuretic Effect
5.11. Antifungal Activity
5.12. Antileishmanial Activity
5.13. Hair-Growth-Promoting Activity
6. Toxicity of Erica Species
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Fatima, B.; Anteur Djame, L.; Mohammed, B. The study groups to Erica arborea phytoecologique in the north–west Algerian: Case of the forest bissa. Plant Arch. 2017, 17, 1478–1482. [Google Scholar]
- Kron, K.A.; Chase, M.W. Systematics of the Ericaceae, Empetraceae, Epacridaceae and related taxa based upon rbcL sequence data. Ann. Mol. Bot. Gard. 1993, 80, 735–741. [Google Scholar] [CrossRef]
- McGuire, A.F.; Kron, K.A. Phylogenetic relationships of European and African ericas. Int. J. Plant Sci. 2005, 166, 311–318. [Google Scholar] [CrossRef]
- Ojeda, F. Biogeography of seeder and resprouter Erica species in the Cape Floristic Region—Where are the resprouters? Biol. J. Linn. Soc. 1998, 63, 331–347. [Google Scholar] [CrossRef]
- Adu-Amankwaah, F.; Mpundu, H.V.; Nyambo, K.; Strauss, P.; Tapfuma, K.I.; Tshililo, N.; Badejo, M.V.; Mabasa, L.; Mavumengwana, V.; Baatjies, L. Phytochemical and Pharmacological Review of Erica Genus (L.) Ericaceae Plants. Phytomedicine Plus 2024, 5, 100697. [Google Scholar] [CrossRef]
- Nelson, E.C. Hardy Heathers from the Northern Hemisphere; Kew Publishing: Richmond, UK, 2011. [Google Scholar]
- Amroun, D.; Hamoudi, M.; Khennouf, S.; Boutefnouchet, S.; Harzallah, D.; Amrane, M.; Dahamna, S. In-vivo anti-inflammatory activity and safety assessment of the aqueous extract of Algerian Erica arborea L.(Ericaceae) aerial parts. J. Ethnopharmacol. 2021, 271, 113881. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Senkardes, I.; Dogan, A.; Seebaluck-Sandoram, R.; Rengasamy, K.R.; Sinan, K.I. Chemical composition and bio-functional perspectives of Erica arborea L. extracts obtained by different extraction techniques: Innovative insights. Ind. Crops Prod. 2019, 142, 111843. [Google Scholar] [CrossRef]
- Szkudlarz, P. Taxonomy of South African ericas (Erica L.) and differentiation of their seeds. Biodivers. Res. Conserv. 2006, 2, 25–30. [Google Scholar] [CrossRef]
- Oliver, E. Erica, A Remarkable Genus. Veld Flora 1972, 58, 57. [Google Scholar]
- Gadouche, L.; Alsoufi, A.S.M.; Pacholska, D.; Skotarek, A.; Pączkowski, C.; Szakiel, A. Triterpenoid and steroid content of lipophilic extracts of selected medicinal plants of the Mediterranean region. Molecules 2023, 28, 697. [Google Scholar] [CrossRef]
- Köroğlu, A.; Kendir, G. The leaf anatomy of some Erica taxa native to Turkey. Turk. J. Bot. 2012, 36, 253–262. [Google Scholar] [CrossRef]
- Nazemiyeh, H.; Bahadori, F.; Delazar, A.; Ay, M.; Topcu, G.; Kolak, U.; Nahar, L.; Auzie, A.; Sarker, S. Tricetin 4′-O-α-L-rhamnopyranoside: A new flavonoid from the aerial parts of Erica arborea. Chem. Nat. Compd. 2008, 44, 174–177. [Google Scholar] [CrossRef]
- Nazemiyeh, H.; Bahadori, F.; Delazar, A.; Ay, M.; Topçu, G.; Nahar, L.; Majinda, R.R.; Sarker, S.D. Antioxidant phenolic compounds from the leaves of Erica arborea (Ericaceae). Nat. Prod. Res. 2008, 22, 1385–1392. [Google Scholar] [CrossRef]
- Guendouze-Bouchefa, N.; Madani, K.; Chibane, M.; Boulekbache-Makhlouf, L.; Hauchard, D.; Kiendrebeogo, M.; Stévigny, C.; Okusa, P.N.; Duez, P. Phenolic compounds, antioxidant and antibacterial activities of three Ericaceae from Algeria. Ind. Crops Prod. 2015, 70, 459–466. [Google Scholar] [CrossRef]
- Tlili, N.; Sarikurkcu, C. Bioactive compounds profile, enzyme inhibitory and antioxidant activities of water extracts from five selected medicinal plants. Ind. Crops Prod. 2020, 151, 112448. [Google Scholar] [CrossRef]
- Márquez-García, B.; Fernández, M.Á.; Córdoba, F. Phenolics composition in Erica sp. differentially exposed to metal pollution in the Iberian Southwestern Pyritic Belt. Bioresour. Technol. 2009, 100, 446–451. [Google Scholar] [CrossRef]
- Yüksel, A.K.; Dikici, E.; Yüksel, M.; Işik, M.; Tozoğlu, F.; Köksal, E. Phytochemicals analysis and some bioactive properties of Erica manipuliflora Salisb. (EMS); antibacterial, antiradical and anti-lipid peroxidation. Iran. J. Pharm. Res. IJPR 2021, 20, 422. [Google Scholar] [PubMed]
- Ayuso Gonzalez, M.; Reyes Ruiz, M.; Toro Sainz, M. Antimicrobial activity of isolated triterpenic compounds from Erica andevalensis Cabezudo-Ribera. Anal. Real Acad. Farm. 1991, 57, 419–423. [Google Scholar]
- Crowden, R.; Jarman, S. Anthocyanins in the genus Erica. Phytochemistry 1976, 15, 1796–1797. [Google Scholar] [CrossRef]
- Bessah, R.; Benyoussef, E.-H. Essential oil composition of Erica arborea L. leaves from Algeria. J. Essent. Oil Bear. Plants 2014, 17, 931–935. [Google Scholar] [CrossRef]
- Sen, B.; Gurdal, B.; Estep, A.S.; Tabanca, N.; Kurkcuoglu, M.; Goger, F.; Gul, Z.; Bardakci, H.; Becnel, J.; Mat, A. The insecticidal activities of Erica manipuliflora Salisb. Extracts in the flowering and fruiting periods and their evaluation in term of chemical profiles of active extracts. Ind. Crops Prod. 2022, 187, 115380. [Google Scholar] [CrossRef]
- Amari, S.; Karbab, A.; Charef, N.; Arrar, L.; Mubarak, M.S. Anti-urolithiatic, antibacterial, anti-inflammatory and analgesic effects of Erica arborea flowers and leaves hydromethanolic extracts: An ethnopharmacological study. Saudi J. Biol. Sci. 2023, 30, 103785. [Google Scholar] [CrossRef]
- Feás, X.; Estevinho, M.L. A survey of the in vitro antifungal activity of heather (Erica sp.) organic honey. J. Med. Food 2011, 14, 1284–1288. [Google Scholar] [CrossRef]
- Bozkurt, A.E.; Terzioğlu, S. The aromatic-medicinal plant taxa of pure scots pine stands in Sürmene-Camburnu (Trabzon). Int. J. Second. Metab. 2017, 4, 517–529. [Google Scholar] [CrossRef][Green Version]
- Sargin, S.A. Plants used against obesity in Turkish folk medicine: A review. J. Ethnopharmacol. 2021, 270, 113841. [Google Scholar] [CrossRef]
- Capasso, F.; Grandolini, G.; Izzo, A.A. Fitoterapia: Impiego Razionale Delle Droghe Vegetali; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Bhat, M.N.; Singh, B.; Surmal, O.; Singh, B.; Shivgotra, V.; Musarella, C.M. Ethnobotany of the Himalayas: Safeguarding medical practices and traditional uses of Kashmir regions. Biology 2021, 10, 851. [Google Scholar] [CrossRef]
- Maruca, G.; Spampinato, G.; Turiano, D.; Laghetti, G.; Musarella, C.M. Ethnobotanical notes about medicinal and useful plants of the Reventino Massif tradition (Calabria region, Southern Italy). Genet. Resour. Crop Evol. 2019, 66, 1027–1040. [Google Scholar] [CrossRef]
- Tavilla, G.; Crisafulli, A.; Ranno, V.; Picone, R.M.; Redouan, F.Z.; del Galdo, G.G. First contribution to the ethnobotanical knowledge in the Peloritani Mounts (NE Sicily). Res. J. Ecol. Environ. Sci. 2022, 2, 1–34. [Google Scholar] [CrossRef]
- Heywood, V. The Mediterranean region a major centre of plant diversity. In Wild Food and Non-Food Plants: Information Networking; CIHEAM-IAMC: Chania, Greece, 1999; pp. 5–13. [Google Scholar]
- Quezel, P. Definition of the Mediterranean region and the origin of its flora. In Plant Conservation in the Mediterranean Area; Gomez-Campo, C., Ed.; Geobotany 7; W. Junk: Dordrecht, the Netherlands, 1985; pp. 9–24. [Google Scholar]
- Greuter, W. Botanical diversity, endemism, rarity, and extinction in the Mediterranean area: An analysis based on the published volumes of Med-Checklist. Bot. Chron. 1991, 10, 63–79. [Google Scholar]
- González-Tejero, M.; Casares-Porcel, M.; Sánchez-Rojas, C.; Ramiro-Gutiérrez, J.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.; Censorii, E.; De Pasquale, C.; Della, A. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Güneş, S.; Savran, A.; Paksoy, M.Y.; Koşar, M.; Çakılcıoğlu, U. Ethnopharmacological survey of medicinal plants in Karaisalı and its surrounding (Adana-Turkey). J. Herb. Med. 2017, 8, 68–75. [Google Scholar] [CrossRef]
- Gürdal, B.; Kültür, Ş. An ethnobotanical study of medicinal plants in Marmaris (Muğla, Turkey). J. Ethnopharmacol. 2013, 146, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Sağıroğlu, M.; Dalgıç, S.; Toksoy, S. Medicinal plants used in Dalaman (Muğla), Turkey. J. Med. Plants Res. 2013, 7, 2053–2066. [Google Scholar]
- Kalankan, G.; Özkan, Z.C.; Akbulut, S. Medicinal and aromatic wild plants and traditional usage of them in Mount Ida (Balıkesir/Turkey). J. Appl. Biol. Sci. 2015, 9, 25–33. [Google Scholar]
- Polat, R.; Satıl, F. An ethnobotanical survey of medicinal plants in Edremit Gulf (Balıkesir–Turkey). J. Ethnopharmacol. 2012, 139, 626–641. [Google Scholar] [CrossRef]
- Tuzlacı, E.; Aymaz, P.E. Turkish folk medicinal plants, part IV: Gönen (Balıkesir). Fitoterapia 2001, 72, 323–343. [Google Scholar] [CrossRef]
- Genç, G.E.; Özhatay, N. An ethnobotanical study in Çatalca (European part of Istanbul) II. Turk. J. Pharm. Sci. 2006, 3, 73–89. [Google Scholar]
- Erarslan, Z.B.; Kültür, Ş. Ethnoveterinary medicine in Turkey: A comprehensive review. Turk. J. Vet. Anim. Sci. 2019, 43, 55–582. [Google Scholar] [CrossRef]
- Alan, S.Ç.; Rahşan, Ö. Medicinal plants used in wound treatment in veterinary folklore in Turkey: A literature review. Kafkas Üniversitesi Vet. Fakültesi Derg. 2021, 27, 663–673. [Google Scholar]
- Gürbüz, İ.; Özkan, A.M.G.; Akaydin, G.; Salihoğlu, E.; Günbatan, T.; Demirci, F.; Yeşilada, E. Folk medicine in Düzce province (Turkey). Turk. J. Bot. 2019, 43, 769–784. [Google Scholar] [CrossRef]
- Kızılarslan, Ç. An Ethnobotanical Survey in the South Part of İzmit Gulf. Unpublished MSc Dissertation, Department of Pharmaceutical Botany, İstanbul University, İstanbul, Turkey, 2008. [Google Scholar]
- Şen, G.; Akbulut, S.; Karaköse, M. Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye). Open Chem. 2022, 20, 873–911. [Google Scholar] [CrossRef]
- Güven, K.; Bekler, F.M.; Yalaz, S.; Güven, R.G.; Aksu, M.D.; İpekçı, M.; Tuşar, F.R.; Polat, N. Evaluation of antibacterial effects of some traditional plants against pathogen microorganisms. Banat. J. Biotechnol. 2020, 11, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Baytop, T. Therapy with medicinal plants in Turkey (past and present). Publ. Istanb. Univ. 1999, 312, 2–3. [Google Scholar]
- Akkol, E.K.; Yeşilada, E.; Güvenç, A. Valuation of anti-inflammatory and antinociceptive activities of Erica species native to Turkey. J. Ethnopharmacol. 2008, 116, 251–257. [Google Scholar] [CrossRef]
- Palabaş-Uzun, S.; Şimşir-Bozdağ, R. Ethnobotanical study on traded medicinal plants and herbal market analyses in Gaziantep/Turkey. Appl. Ecol. Environ. Res. 2022, 20, 3955–3976. [Google Scholar] [CrossRef]
- Sargin, S.A.; Selvi, S.; López, V. Ethnomedicinal plants of sarigöl district (manisa), Turkey. J. Ethnopharmacol. 2015, 171, 64–84. [Google Scholar] [CrossRef]
- Fakir, H.; Korkmaz, M.; Güller, B. Medicinal plant diversity of western Mediterranean region in Turkey. J. Appl. Biol. Sci. 2009, 3, 33–43. [Google Scholar]
- Güzel, Y.; Güzelşemme, M.; Miski, M. Ethnobotany of medicinal plants used in Antakya: A multicultural district in Hatay Province of Turkey. J. Ethnopharmacol. 2015, 174, 118–152. [Google Scholar] [CrossRef]
- Obón, C.; Rivera, D.; Fonollá, E.; Alcaraz, F.; Attieh, L. A comparison study on traditional mixtures of herbal teas used in Eastern Mediterranean area. Front. Pharmacol. 2021, 12, 632692. [Google Scholar] [CrossRef]
- Celik, S.; Karabacak, E.; Uysal, I. Plants have been collected from Mythological Kazdağı (Mt. Ida) National Park, West Turkey by Turkmens and their folk, cultural and social uses. Eur. J. Sci. Res. 2008, 19, 835–843. [Google Scholar]
- Kültür, Ş.; Gürdal, B.; Sari, A.; Melikoğlu, G. Traditional herbal remedies used in kidney diseases in Turkey: An overview. Turk. J. Bot. 2021, 45, 269–287. [Google Scholar] [CrossRef]
- Sargın, S.A.; Akçicek, E.; Selvi, S. An ethnobotanical study of medicinal plants used by the local people of Alaşehir (Manisa) in Turkey. J. Ethnopharmacol. 2013, 150, 860–874. [Google Scholar] [CrossRef]
- Olcay, B.; Kültür, Ş. Medicinal plants used in traditional treatment of hypertension in Turkey. Int. J. Sci. Technol. Res. 2020, 6, 80–95. [Google Scholar]
- Ozturk, M.; Altay, V.; Latiff, A.; Shareef, S.; Shaheen, F.; Iqbal Choudhry, M. Potential medicinal plants used in the hypertension in Turkey, Pakistan, and Malaysia. In Plant and Human Health, Volume 1: Ethnobotany and Physiology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 595–618. [Google Scholar]
- Akbulut, S.; Yilmaz, D. Ethnobotanical knowledge on the plants used by people on the Datca Peninsula (Mulga, Turkey). Appl. Ecol. Environ. Res. 2022, 20, 1887–1910. [Google Scholar] [CrossRef]
- Göç, F.; Erel, E.; Sarı, A. Plants used in traditional treatment for boils in Turkey. Int. J. Tradit. Complement. Med. Res. 2021, 2, 49–61. [Google Scholar]
- Erarslan, Z.B.; Ecevit-genç, G.; Kültür, Ş. Medicinal plants traditionally used to treat skin diseases in Turkey–eczema, psoriasis, vitiligo. J. Fac. Pharm. Ank. Univ. 2020, 44, 137–166. [Google Scholar]
- Karahan, F.; Büşra, K. An Ethnobotanical Study in Ceylanlı Village (Kırıkhan/Hatay-Türkiye). Commagene J. Biol. 2022, 6, 218–231. [Google Scholar] [CrossRef]
- Akbulut, S.; Bayramoglu, M.M. The trade and use of some medical and aromatic herbs in Turkey. Stud. Ethno-Med. 2013, 7, 67–77. [Google Scholar] [CrossRef]
- Şimşek, A.; Toptan, Ö.Ü.M.; Özenoğlu, A.; Tekinşen, K.K.; Bayraktar, M.; Uysal, F.F.; Tekin, M.; Bayraktar, N.; Telli, Ö.Ü.A.E.; Güneş, Ö.Ü.A.E. Tip Bilimlerinde Farkli Bakişlar. 2019. Available online: https://iksadyayinevi.com/home/tip-bilimlerinde-farkli-bakislar/ (accessed on 6 June 2025).
- Nelly, A.; Annick, D.-D.; Frederic, D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334. [Google Scholar]
- Abou-Chaar, C.I. Medicinal plants of Lebanon. Archaeol. Hist. Leban. 2004, 19, 70–85. [Google Scholar]
- Khatib, C.; Nattouf, A.; Agha, M.I.H. Ethnobotanical survey of medicinal herbs in the Western region in Syria (Latakia and Tartus). 2021; in review. [Google Scholar] [CrossRef]
- Ozturk, M.; Altay, V.; Gonenç, T. Herbal from high mountains in the East Mediterranean. In Drug Discovery from Herbs–Approaches and Applications; DAYA Publishing House: New Delhi, India, 2017; pp. 327–367. [Google Scholar]
- Benarba, B.; Belabid, L.; Righi, K.; Amine Bekkar, A.; Elouissi, M.; Khaldi, A.; Hamimed, A. Ethnobotanical study of medicinal plants used by traditional healers in Mascara (North West of Algeria). J. Ethnopharmacol. 2015, 175, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Senouci, F.; Ababou, A.; Chouieb, M. Ethnobotanical survey of the medicinal plants used in the Southern Mediterranean. Case study: The region of Bissa (Northeastern Dahra Mountains, Algeria). Pharmacogn. J. 2019, 11, 647–659. [Google Scholar] [CrossRef]
- Eddaikra, N.; Boudjelal, A.; Amine Sbabdji, M.; Eddaikra, A.; Boudrissa, A.; Mounir Bouhenna, M.; Chemat, S.; Harrat, Z. Leishmanicidal and cytotoxic activity of Algerian medicinal plants on Leishmania major and Leishmania infantum. J. Med. Microbiol. Infect. Dis. 2019, 7, 66–71. [Google Scholar] [CrossRef]
- Amari, S. Evaluation of the Anti-Urolithiatic, Antibacterial, Anti-Inflammatory and Analgesic Activities of the Hydro-Methanolic Extracts from Different Parts of Erica arborea L. Doctoral Dissertation, Dépôt Institutionnel de l’Université Ferhat ABBAS, Setif, Algeria, 2023. [Google Scholar]
- Taibi, K.; Abderrahim, L.A.; Boussaid, M.; Taibi, F.; Achir, M.; Souana, K.; Benaissa, T.; Farhi, K.H.; Naamani, F.Z.; Said, K.N. Unraveling the ethnopharmacological potential of medicinal plants used in Algerian traditional medicine for urinary diseases. Eur. J. Integr. Med. 2021, 44, 101339. [Google Scholar] [CrossRef]
- Meddour, R.; Sahar, O.; Ouyessad, M. Ethnobotanical survey on medicinal plants in the Djurdjura National Park and its influence area, Algeria. Ethnobot. Res. Appl. 2020, 20, 1–25. [Google Scholar]
- Hadj-Said, D.; Bouazza, B. Medicinal plants used for the treatment of respiratory diseases in Kabylia, north of Algeria: An ethnomedicinal survey. J. Herb. Med. 2023, 40, 100685. [Google Scholar] [CrossRef]
- Senouci, F.; Ababou, A.; Senouci, S.; Bouzada, N. Traditional medicinal plants applied for the treatment of gastrointestinal diseases in Chlef, Algeria. Egypt. J. Bot. 2023, 63, 419–429. [Google Scholar] [CrossRef]
- Meddour, R.; Meddour-Sahar, O. Medicinal plants and their traditional uses in Kabylia (Tizi Ouzou, Algeria). Arab. J. Med. Aromat. Plants 2015, 1, 137–151. [Google Scholar]
- Yaici, K.; Dahamna, S.; Moualek, I.; Habold, D.; Houali, K. Évaluation de la teneur des composés phénoliques, des propriétés antioxydantes et antimicrobiennes de l’espèce Erica arborea L.(Ericaceae) dans la médecine traditionnelle du Tell sétifien dans l’Est Algérien. Phytothérapie 2021, 19, 226–234. [Google Scholar] [CrossRef]
- Skeffington, M.S.; Scott, N. Were the five rare heathers of the west of Ireland introduced through human activity? An ecological, genetic, biogeographical and historical assessment. Br. Ir. Bot. 2023, 5, 221–251. [Google Scholar] [CrossRef]
- Sadki, C.; Hacht, B.; Souliman, A.; Atmani, F. Acute diuretic activity of aqueous Erica multiflora flowers and Cynodon dactylon rhizomes extracts in rats. J. Ethnopharmacol. 2010, 128, 352–356. [Google Scholar] [CrossRef]
- Ben Sassi, A.; Harzallah-Skhiri, F.; Bourgougnon, N.; Aouni, M. Antiviral activity of some Tunisian medicinal plants against Herpes simplex virus type 1. Nat. Prod. Res. 2008, 22, 53–65. [Google Scholar] [CrossRef]
- Kawano, M.; Han, J.; Kchouk, M.E.; Isoda, H. Hair growth regulation by the extract of aromatic plant Erica multiflora. J. Nat. Med. 2009, 63, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Fatma, G.; Sami, B.H.A.; Ahmed, L. Investigation of extracts from Tunisian ethnomedicinal plants as antioxidants, cytotoxins, and antimicrobials. Biomed. Environ. Sci. 2017, 30, 811–824. [Google Scholar]
- Harnafi, H.; el Houda Bouanani, N.; Aziz, M.; Caid, H.S.; Ghalim, N.; Amrani, S. The hypolipidaemic activity of aqueous Erica multiflora flowers extract in Triton WR-1339 induced hyperlipidaemic rats: A comparison with fenofibrate. J. Ethnopharmacol. 2007, 109, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Bekkai, D.; Oulad El Majdoub, Y.; Bekkai, H.; Cacciola, F.; Miceli, N.; Taviano, M.F.; Cavò, E.; Errabii, T.; Laganà Vinci, R.; Mondello, L. Determination of the Phenolic Profile by Liquid Chromatography, Evaluation of Antioxidant Activity and Toxicity of Moroccan Erica multiflora, Erica scoparia, and Calluna vulgaris (Ericaceae). Molecules 2022, 27, 3979. [Google Scholar] [CrossRef]
- Noureddine, B.; Mostafa, E.; Mandal, S.C. Ethnobotanical, pharmacological, phytochemical, and clinical investigations on Moroccan medicinal plants traditionally used for the management of renal dysfunctions. J. Ethnopharmacol. 2022, 292, 115178. [Google Scholar] [CrossRef]
- Chaachouay, N.; Azeroual, A.; Douira, A.; Zidane, L. Ethnoveterinary practices of medicinal plants among the Zemmour and Zayane tribes, Middle Atlas, Morocco. S. Afr. J. Bot. 2022, 151, 826–840. [Google Scholar] [CrossRef]
- Bachar, M.; ElYacoubi, H.; Zidane, L.; Rochdi, A. Ethnomedicinal and traditional phytotherapeutic plants used in Bouhachem Natural Regional Park (Rif of Morocco): Case of Bni-Leit and Al-Oued districts. J. Pharm. Pharmacogn. Res. 2021, 9, 284–312. [Google Scholar] [CrossRef]
- Carrera, C.; Aliaño-González, M.J.; Rodríguez-López, J.; Ferreiro-González, M.; Ojeda-Copete, F.; Barbero, G.F.; Palma, M. Optimization of an ultrasound-assisted extraction method for the analysis of major anthocyanin content in Erica australis flowers. Molecules 2021, 26, 2884. [Google Scholar] [CrossRef]
- Fennane, M.; Rejdali, M. Aromatic and medicinal plants of Morocco: Richness, diversity and threats. Bull. L’institut Sci. Rabat 2016, 38, 27–42. [Google Scholar]
- Rios, J.; Recio, M.; Villar, A. Antimicrobial activity of selected plants employed in the Spanish Mediterranean area. J. Ethnopharmacol. 1987, 21, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Benítez, G.; González-Tejero, M.; Molero-Mesa, J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): Ethnopharmacological synthesis. J. Ethnopharmacol. 2010, 129, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.À.; Parada, M.; Selga, A.; Valles, J. Studies on pharmaceutical ethnobotany in the regions of L’Alt Empordà and Les Guilleries (Catalonia, Iberian Peninsula). J. Ethnopharmacol. 1999, 68, 145–168. [Google Scholar] [CrossRef]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef]
- Brussell, D.E. Medicinal plants of mt. Pelion, greece. Econ. Bot. 2004, 58, S174–S202. [Google Scholar] [CrossRef]
- Viegi, L.; Pieroni, A.; Guarrera, P.M.; Vangelisti, R. A review of plants used in folk veterinary medicine in Italy as basis for a databank. J. Ethnopharmacol. 2003, 89, 221–244. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M. Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef]
- Antonone, R.; De Simone, F.; Morrica, P.; Ramundo, E. Traditional phytotherapy in the Roccamonfina volcanic group, Campania, Southern Italy. J. Ethnopharmacol. 1988, 22, 295–306. [Google Scholar] [CrossRef]
- Pieroni, A.; Howard, P.; Volpato, G.; Santoro, R. Natural remedies and nutraceuticals used in ethnoveterinary practices in inland southern Italy. Vet. Res. Commun. 2004, 28, 55–80. [Google Scholar] [CrossRef]
- La Rosa, A.; Cornara, L.; Saitta, A.; Salam, A.M.; Grammatico, S.; Caputo, M.; La Mantia, T.; Quave, C.L. Ethnobotany of the Aegadian Islands: Safeguarding biocultural refugia in the Mediterranean. J. Ethnobiol. Ethnomedicine 2021, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Caruana, U.; Attard, E. An ethno botanical survey of medicinal plants used in the Island of Gozo. Stud. Ethno-Med. 2016, 10, 269–281. [Google Scholar] [CrossRef][Green Version]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical survey of traditionally used plants in human therapy of east, north and north-east Bosnia and Herzegovina. J. Ethnopharmacol. 2011, 133, 1051–1076. [Google Scholar] [CrossRef] [PubMed]
- Gunther, R.T. The Greek Herbal of Dioscorides; Hafner Pub. Co.,: New York, NY, USA, 1959. [Google Scholar]
- Kus, C.; Tas, M.; Kucukaydin, S.; Tel-Cayan, G.; Duru, M.E. Chemical analysis and in vitro antioxidant and anticholinesterase activities of essential oils and extracts from different parts of Erica manipuliflora. J. Res. Pharm. 2019, 23, 1098–1105. [Google Scholar]
- Dias, P.; Martins, A.; Figueiredo, A.C.; Rauter, A.P. Flower Colour and Essential oil composition in Erica australis L. Grown in Portugal. J. Essent. Oil Bear. Plants 2016, 19, 1013–1018. [Google Scholar] [CrossRef]
- Llusia, J.; Penuelas, J.; Alessio, G.A.; Estiarte, M. Contrasting species-specific, compound-specific, seasonal, and interannual responses of foliar isoprenoid emissions to experimental drought in a Mediterranean shrubland. Int. J. Plant Sci. 2008, 169, 637–645. [Google Scholar] [CrossRef]
- Mitic, V.D.; Ilic, M.D.; Stankov-Jovanovic, V.P.; Stojanovic, G.S.; Dimitrijevic, M.V. Essential oil composition of Erica spiculifolia Salisb-first report. Nat. Prod. Res. 2018, 32, 222–224. [Google Scholar] [CrossRef]
- Omuzbuken, B.; Kacar, A.; Avunduk, S. The Antifungal Activity of Erica manipuliflora Salisb. Extracts and Secondary Metabolite: Ursolic Acid. Mosc. Univ. Chem. Bull. 2021, 76, 227–229. [Google Scholar] [CrossRef]
- Martín-Cordero, C.; Reyes, M.; Ayuso, M.J.; Toro, V. Cytotoxic triterpenoids from Erica andevalensis. Z. Für Naturforschung C 2001, 56, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Villareal, M.O.; Han, J.; Matsuyama, K.; Sekii, Y.; Smaoui, A.; Shigemori, H.; Isoda, H. Lupenone from Erica multiflora leaf extract stimulates melanogenesis in B16 murine melanoma cells through the inhibition of ERK1/2 activation. Planta Medica 2013, 79, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.; Carvalho, I.S. Antioxidant activities, distribution of phenolics and free amino acids of Erica australis L. leaves and flowers collected in Algarve, Portugal. Nat. Prod. Res. 2013, 27, 1664–1667. [Google Scholar] [CrossRef]
- Ballester, A.; Albo, J.; Vieitez, E. The allelopathic potential of Erica scoparia L. Oecologia 1977, 30, 55–61. Oecologia 1977, 30, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, A. Phenolic inhibitors in Erica australis L. and in associated soil. J. Chem. Ecol. 1980, 6, 593–596. [Google Scholar] [CrossRef]
- Márquez-García, B.; Fernández-Recamales, M.; Córdoba, F. Effects of cadmium on phenolic composition and antioxidant activities of Erica andevalensis. J. Bot. 2012, 2012, 936950. [Google Scholar] [CrossRef]
- Khlifi, R.; Dhaouefi, Z.; Toumia, I.B.; Lahmar, A.; Sioud, F.; Bouhajeb, R.; Bellalah, A.; Chekir-Ghedira, L. Erica multiflora extract rich in quercetin-3-O-glucoside and kaempferol-3-O-glucoside alleviates high fat and fructose diet-induced fatty liver disease by modulating metabolic and inflammatory pathways in Wistar rats. J. Nutr. Biochem. 2020, 86, 108490. [Google Scholar] [CrossRef]
- Ballester, A.; Verwey, A.; Overeem, J. 2-Hydroxyphenyl acetic acid and 2, 4-dihydroxyphenyl acetonitrile from Erica scoparia: Phytochemical report. Phytochemistry 1975, 14, 1667–1668. [Google Scholar] [CrossRef]
- Demirkiran, O.; Topçu, G.; Bahadori, F.; Ay, M.; Nazemiyeh, H.; Choudhary, I. Two new phenylpropanoid glycosides from the leaves and flowers of Erica arborea. Helv. Chim. Acta 2010, 93, 77–83. [Google Scholar] [CrossRef]
- Gournelis, D.C. Flavonoids of Erica verticillata. J. Nat. Prod. 1995, 58, 1065–1069. [Google Scholar] [CrossRef]
- Dias, P.; Falé, P.L.; Martins, A.; Rauter, A.P. Digestibility and bioavailability of the active components of Erica australis L. aqueous extracts and their therapeutic potential as acetylcholinesterase inhibitors. Evid.-Based Complement. Altern. Med. 2015, 2015, 854373. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Carvalho, R. Profiling of Polyphenol Composition and Antiradical Capacity of Erica cinerea. Antioxidants 2017, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Martin, C.; Lastra, C.A.d.l.; Trujillo, J.; Toro, M.; Ayuso, M. Antiulcerogenicity of the flavonoid fraction from Erica andevalensis Cabezudo-Rivera. Z. Für Naturforschung C 1996, 51, 563–569. [Google Scholar] [CrossRef]
- Kacar, A.; Avunduk, S.; Omuzbuken, B.; Aykin, E. Biocidal activities of a triterpenoid saponin and flavonoid extracts from the Erica manipuliflora Salisb. against microfouling bacteria. Int. J. Agric. For. Life Sci. 2018, 2, 40–46. [Google Scholar]
- Amezouar, F.; Badri, W.; Hsaine, M.; Bourhim, N.; Fougrach, H. Évaluation des activités antioxydante et anti-inflammatoire de Erica arborea L. du Maroc. Pathol. Biol. 2013, 61, 254–258. [Google Scholar] [CrossRef]
- Nayebi, A.M.; Nazemieh, H.; Omidbakhsh, R.; Çobanoglu, S. Analgesic effect of the methanol extract of Erica arborea (L.) in mice using formalin test. DARU J. Pharm. Sci. 2008, 16, 229–232. [Google Scholar]
- Amari, S.; Ahlem, K.; Arrar, L.; Noureddine, C. Fractionation, Phytochemical Screening and Antioxidant Activity of Different Sub-Fractions from Leaves and Flowers of Erica arborea L. Turk. J. Agric.-Food Sci. Technol. 2023, 11, 830–837. [Google Scholar]
- Kıvçak, B.; Erdoğan, T.F.; Gönenç, T.; Pabuçcuoğlu, A.; Oran, E.; Kahraman, F.; Öztürk, T. Erıca bocquetıı p. F. Stevens And Erıca arborea L.’nın Antioksidan, Antimikrobiyal Ve Sitotoksik Aktiviteler. Gümüşhane Üniversitesi Sağlık Bilim. Derg. 2013, 2, 52–65. [Google Scholar]
- Nefzi, K.; Ben Jemaa, M.; Baraket, M.; Dakhlaoui, S.; Msaada, K.; Nasr, Z. In Vitro Antioxidant, Antibacterial and Mechanisms of Action of Ethanolic Extracts of Five Tunisian Plants against Bacteria. Appl. Sci. 2022, 12, 5038. [Google Scholar] [CrossRef]
- Nunes, R.; Anastácio, A.; Carvalho, I.S. Antioxidant and Free Radical Scavenging Activities of Different Plant Parts from Two E rica Species. J. Food Qual. 2012, 35, 307–314. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Tlas, L.T.; Chamaa, I.; Fandi, J.; Al Haddad, E. In-vitro antibacterial activity and chemical composition of syrian Erica manipuliflora essential oil. Bull. Pharm. Sci. Assiut Univ. 2021, 44, 63–71. [Google Scholar] [CrossRef]
- Rios, J.-L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J., 3rd; Kannel, W.; Wolf, P.A.; Cupples, L.; D’agostino, R. The relative importance of selected risk factors for various manifestations of cardiovascular disease among men and women from 35 to 64 years old: 30 years of follow-up in the Framingham Study. Circulation 1987, 75 Pt 2, V65–V73. [Google Scholar]
- La Rosa, J.; Hunninghake, D.; Bush, D. The cholesterol fact: A summary of the evidence relating dietary fats, serum cholesterol and CHD. A joint statement by the American Heart Association and the National Heart–Lung and Blood Institute. Circulation 1990, 81, 1721–1733. [Google Scholar] [CrossRef]
- Schneider, L.S. A critical review of cholinesterase inhibitors as a treatment modality in Alzheimer’s disease. Dialogues Clin. Neurosci. 2000, 2, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Halpin, C.M.; Reilly, C.; Walsh, J.J. Nature’s anti-alzheimer’s drug: Isolation and structure elucidation of galantamine from Leucojum aestivum. J. Chem. Educ. 2010, 87, 1242–1243. [Google Scholar] [CrossRef]
- Mills, C.; Cleary, B.V.; Walsh, J.J.; Gilmer, J.F. Inhibition of acetylcholinesterase by tea tree oil. J. Pharm. Pharmacol. 2004, 56, 375–379. [Google Scholar] [CrossRef]
- Perry, N.; Court, G.; Bidet, N.; Court, J.; Perry, E. European herbs with cholinergic activities: Potential in dementia therapy. Int. J. Geriatr. Psychiatry 1996, 11, 1063–1069. [Google Scholar] [CrossRef]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.-G. Kidney stones. Nat. Rev. Dis. Primers 2016, 2, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, Ö. Antibacterial and antifungal activity of ethanolic extracts from eleven spice plants. Biologia 2006, 61, 275–278. [Google Scholar] [CrossRef]
- Heo, Y.A. Birch Bark Extract: A Review in Epidermolysis Bullosa. Drugs 2023, 83, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
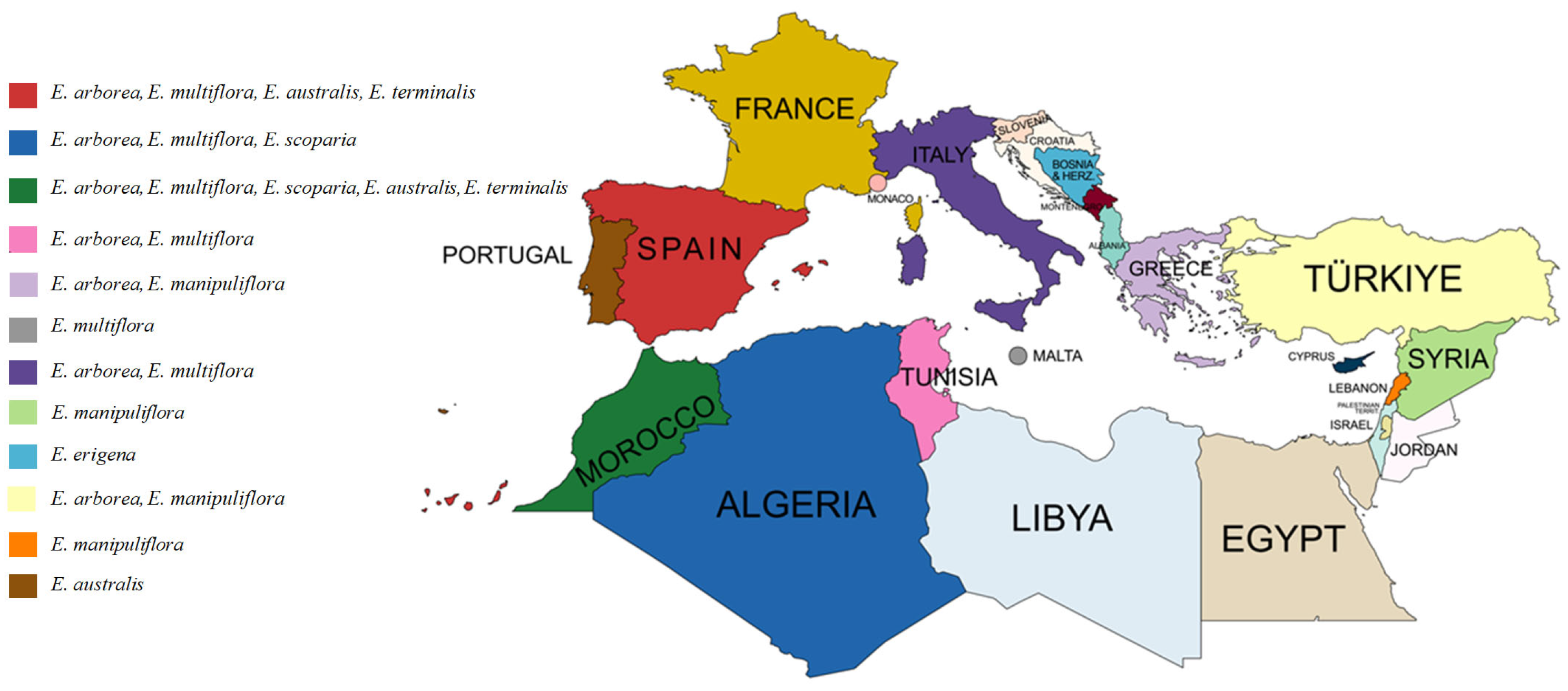

| Species | Height | Leaf Morphology | Flower Morphology | Growing Regions in the Mediterranean Basin |
|---|---|---|---|---|
| E. arborea (Tree heath) | To 7 m | Leaves arranged in whorls of 3, linear, 5–7 mm in length | White or very pale pink, terminal on short leafy shoots in umbels of 2–4 | Widely distributed in the region across southern Europe, northern Africa and to the east in countries including Turkey, Lebanon and Syria |
| E. multiflora (Many-flowered heath) | To 2.5 m | Thick, leathery leaves arranged in whorls of 3–5, linear, 10–15 mm in length and 1–1.5 mm broad | White to pink in axillary clusters of 1–4 | Europe: eastern Spain and the Balearic Islands, southern France (including the northern tip of Corsica), Italy (including Lampedusa, Sardinia and Sicily), Malta and Gozo, southern coastal Croatia, Albania and north-west Greece. North Africa: Algeria, Morocco, Tunisia and Libya |
| E. scoparia, E. scoparia subsp. scoparia (Besom heath) | 1 to 4 m | Leaves arranged in whorls of 3 or 4, linear, 4–10 mm in length | Inflorescences are numerous and crowded on shoots; individual inflorescence are umbels of 1–3 greenish flowers, rarely tinged with red, on very reduced lateral branchlets | Western Mediterranean basin. Europe: Portugal, Spain including the Balearic Islands, southern and south-western France including Corsica, north-western Italy and Sardinia. North Africa: Morocco, Algeria and Tunisia |
| E. manipuliflora (Whorled heath) | To 4 m | Leathery leaves arranged in whorls of 3 or 4, 3–9 mm in length | Inflorescences composed of several to many axillary umbels of 1–5 flowers on very short shoots, in varying shades of mauve, pink or rarely white | Italy, southern Croatia, Montenegro, Albania, Greece including Crete and the Ionian and Aegean islands, Turkey, Northern Cyprus, Syria and Lebanon |
| E. australis (Southern heath) | To 2.5 m | Leaves arranged in whorls of 4, linear in shape, to 7 mm in length | The inflorescences are terminal on leafy lateral shoots, flowers in 4 s, sometimes with subsidiary whorls, in varying shades of pale pink to lilac-link and sometimes white | Western Iberian Peninsula, in regions of Portugal and Spain, as well as in northern Morocco |
| E. terminalis (Corsican heath) | To 2–3 m | Leaves arranged in whorls of 4–5, lanceolate to linear, to 9 mm in length | Inflorescences are a single terminal umbel, or a compound inflorescence of several umbels on leafy lateral shoots, generally in pink to purple | Southwestern and southern Europe: Spain, Corsica and Italy including Sardinia. North Africa: Morocco |
| E. sicula. subsp. sicula (Sicilian heath) | To 0.6 m | Leaves arranged in whorls of 4 to 5, spreading or ascending, linear 3–13 mm in length | Inflorescences with 2–8 flowers in terminal umbels on main or axillary shoots in pale to deep pink, sometimes white | Italy (specifically Sicily), Libya, Turkey (specifically Anatolia), and areas of Cyprus, Lebanon and Libya |
| E. sicula subsp. bocquetii (Bocquet’s heath) | To 0.25 m often spreading to form hummocks | Leaves arranged in whorls of 3 to 4, spreading or ascending, linear, 3–6 mm in length | Flowers 2–3, rarely solitary, in umbel, terminal on main or axillary shoots in pale to deep pink | Western Asia: Turkey (Anatolia only) above 1000 m altitude |
| E. spiculifolia (Balkan heath, Spike heath) | To 15 cm | Arranged in irregular whorls of 2 to 6 or spirally arranged, linear-lanceolate, 4–6 mm in length, although the leaves found in inflorescences can be longer, reaching up to 9 mm | The inflorescences typically consist of a terminal raceme with 8–40 flowers, in bright pink to red-pink, very rarely white | South-eastern Europe: Bosnia and Herzegovina, Montenegro, Macedonia, Albania and Greece. Western Asia: northern Turkey |
| E. umbellata (Dwarf Spanish heath) | To 0.6 m | Leaves arranged in whorls of 3, linear, small at 2–5 mm length and 0.5 mm in width | Inflorescences are terminal umbels of 1–6 flowers, in pink to purple, occasionally white | Spain and Portugal and northern Morocco |
| E. andevalensis | To 2 m | Arranged in whorls of 4 to 5, with young shoot internodes ~1.5 mm long, while older shoot internodes range from 5 to 7.5 mm long, ovate, ~5 mm in length and to 2.5 mm in width | Inflorescences are terminal and umbellate in dark pink, rarely white | South-western Iberian Peninsula only, in regions of Spain and Portugal |
| E. lusitanica (Spanish heath, Portuguese heath) | To 4.5 m | Leaves arranged in whorls of 4 (sometimes in 3 s), linear with edges parallel or lanceolate and narrowing slightly to tip, 7 mm in length and 0.5 mm in width | Inflorescences are numerous and crowded towards ends of shoots, 1–4 flowers in each terminal umbel at tip of short, leafy lateral shoots, in white, often tinged pink in the bud | Iberian Peninsula: Small pockets widely scattered in southern and western Portugal and south-western Spain |
| Plant Species (Local Name) | Region | Plant Part(s) | Preparation | Uses/Treatment | Reference(s) |
|---|---|---|---|---|---|
| Western Asia | |||||
| Turkey | |||||
| E. manipuliflora Salisb. | Turkey | Flowers, branches and leaves | Decoction/Infusion | Obesity | [27] |
| E. manipuliflora Salisb.(Püren) | Karaisalı | Branches and flowers | Infusion | Weight loss | [36] |
| E. manipuliflora Salisb. (Piren, Funda) | Marmaris, Muğla | Leaves | Infusion | Weight loss and as a diuretic | [37] |
| E. manipuliflora Salisb. (Püren and Funda) | Dalaman, Muğla | Leaves and flowers | Decoction | Weight loss and for diabetes treatment | [38] |
| E. arborea (Funda) | Mount Ida (Balıkesir) | Leaves | Infusion | Weight loss | [39] |
| E. arborea (Briar, Tree heath) | Turkey | Leaves and seeds | Infusion | For treatment of obesity | [27] |
| E. arborea (Püren, Piren) | Edremit Bay (Balıkesir) | Flowers and branches | Infusion | Asthma | [40] |
| E. arborea (Funda) | Gönen, Balıkesir | Flowering branches | Decoction | Diuretic | [41] |
| E. arborea (Funda, Piren, Süpürge otu, Süpürge çalısı) | Çatalca | Fruit | Externally | Foot wounds and mouth sores | [42,43,44] |
| E. arborea (Funda, Piren, Süpürge otu, Süpürge çalısı) | Çatalca | Fruit | Internally | Foot and mouth disease in animals | [42] |
| E. arborea (Çalısüpürgesi, pirançalısı) | Düzce province | Flowers | Infusion | Sooth itching in anal fissure | [45] |
| E. arborea (Süpürge) | South part of İzmit Gulf | Aerial parts | Decoction | Hypertension | [46] |
| E. arborea (Funda) | Kastamonu province | Leaves | Infusion | Inflammation, urinary tract infection and kidney stones | [47] |
| E. arborea | Turkey | Leaves and flowers | Not defined | Constipation, diuretic, hypertension, renal lithiasis, inflammation, sooth itching in anal fissure, urinary tract infection, kidney stones, renal fluid flow, poor eyesight, snakebites, stomach problems, sleeping disorders, mouth sores, poor circulation, colds, gout, lumbago, muscular aches, motion sickness, hangover cure | [15,48,49] |
| E. arborea | Turkey | Leaves | A glass of 5% decoction or infusion | Edema | [50] |
| E. arborea (Funda/Tree heath) | Sourced in Gaziantep herbal markets, Turkey | Leaves and shoots | Infusion | Urinary and respiratory disorders | [51] |
| E. arborea | Turkey | Flower tips | Decoction | Renal lithiasis, diuretic and a urinary antiseptic | [52] |
| E. manipuliflora Salisb. (Süpürge) | Western region of Turkey | Shoots | Infusion | Diuretic | [53] |
| E. manipuliflora Salisb. (Acram) | In the district of Antakya | Flowering parts | Not defined | Anthelmintic | [54,55] |
| E. manipuliflora Salisb. (Funda) | Kazdağı National Park, West Turkey | Leaves | Not defined | Urinary tract infection and appetite suppressant | [56] |
| E. manipuliflora Salisb. (Püren, Pürenotu, Süpürgeotu, Sükürteotu) | Turkey | Flowers and branches | Decoction Internal/drink one glass 3 times a day for 4–8 weeks | Kidney stones | [57] |
| E. manipuliflora Salisb. (Püren, Pürenotu, Süpürgeotu, Sükürteotu and Funda) | Alaşehir (Manisa) | Flowers, branches and leaves | Decoction (one glass 3 times daily) or infusion | Diabetes, hypertension, constipation, arthritis, obesity, nephralgia, gastrointestinal diseases, diuretic, ureter infection, sedative and kidney stones | [28,37,39,40,41,54,57,58,59,60,61,62] |
| E. manipuliflora Salisb. (Funda, Püren) | Turkey | Flowers and leaves | Decoction | Hypertension | [59,60] |
| E. manipuliflora Salisb. (Piren, Püren) | Datça Peninsula, South-west Turkey | Flowers | Infusion | Sedative | [61] |
| E. manipuliflora Salisb. (Funda, Süpürge out and Püren) | Turkey | Aerial parts | External as ointment with olive oil | Boils | [62] |
| E. manipuliflora Salisb. (Funda, Süpürge out, Püren) | Turkey | Fruit, flowers and branches | As ointment with olive oil | Eczema | [63] |
| E. manipuliflora Salisb. (Püren) | Ceylanlı village of Kırıkhan district of Hatay area | Stems | Not defined | Diuretic, constipation, arthritis and weight loss | [64] |
| E. manipuliflora Salisb.and E. arborea | Turkey | Aerial parts | Infusion | Constipation, urethritis and diuretic effects | [65] |
| E. manipuliflora Salisb. (Püren) | Antakya | Flowers | Infusion | Anthelmintic properties | [66] |
| Lebanon | |||||
| E. manipuliflora Salisb. (Khalanj laqui, Shantaf) | Lebanon | Flowers and twigs | Decoction | Rheumatism and antineuralgic | [67] |
| E. manipuliflora Salisb. | Lebanon | Flowers | Not defined | Sedative | [68] |
| Syria | |||||
| E. manipuliflora Salisb. (Ajram) | Western Region (Latakia and Tartus) | Flowers | Decoction | Sedative, diuretic, gout and urinary tract infection, while the heather honey of the plant is commonly used as a tonic, expectorant, to treat rheumatism asthma, dysmenorrhea and arthritis, as a laxative, disinfectant for the respiratory tract, urinary tract infections, acute nephritis, relieving nerve pain, depression, treating insomnia, bladder and prostate pain and enlargement | [69] |
| Syria, Lebanon, Turkey, Cyprus | |||||
| E. manipuliflora Salisb. | Syria, Lebanon, Turkey, Cyprus | Flowers, leaves, branches and shoots | Infusion/Decoction and boiled | Urethritis, arthritis, weight loss, diuretic, constipation | [70] |
| North Africa | |||||
| Algeria | |||||
| E. arborea (Khlenj) | Algeria | Aerial parts and stems | Oral, infusion or decoction | Diuretic, anti-inflammatory, astringent, antiulcer and antimicrobial agent, treat hypertension, kidney inflammations, urolithiasis, renal lithiasis, pinworm infection, urinary infections, stomachache and prostate diseases | [8,71,72,73,74,75] |
| E. arborea (Bouhadad, khlenj) | Tadergount, Derguina-Bejaia, North of Algeria | Flowers, leaves and aerial parts | External/Internal | Kidney stones, eczema, urinary and gastric diseases, inflammation, microbial infections and snakebites | [74] |
| E. arborea (Elkhlilanj) | Algeria | Aerial parts | Infusion/Decoction | Lithiasis and urinary infections | [75] |
| E. arborea (Akhlendj) | The Djurdjura National Park | Flowers | Infusion | Physical weakness and anxiety | [76] |
| E. arborea (Axlenǧ) | Kabylia region | Leaves/Roots | Decoction, Cataplasm | Rheumatism | [77] |
| E. arborea (Elkhlilanj) | The region of Chlef | Stems | Infusion | Gastrointestinal illnesses including pinworm infection and stomachache | [78] |
| E. arborea (Akheloundj) | Kabylia area (North Algeria) | Flowers | Internal | Urinary stone | [79] |
| E. arborea (Akheloundj) | Kabylia area (North Algeria) | Flowers | External | Freckles | [79] |
| E. arborea | The Setifian Tell, East Algeria | Flowers | Infusion | Acute and chronic urinary infection | [80] |
| E. arborea (Akhlenj) | Djurdjura Biosphere Reserve | Flowers | Decoction | Indigestion and nervousness | [81] |
| Tunisia | |||||
| E. multiflora | Kalaa Sghira | Aerial parts | Not defined | Diuretic, urinary infections, tranquilizing, astringent and prostate cancer | [51,82,83] |
| Morocco | |||||
| E. multiflora | Morocco | Not defined | Not defined | Diuretic | [82] |
| E. multiflora (Khlenj) | Morocco | Not defined | Not defined | Hypertension, inflammation, hyperlipidemia and atherosclerosis | [84,85,86] |
| E. scoparia and E. multiflora | Northern Morocco | Not defined | Infusion | Analgesic and anti-inflammatory activities | [87] |
| E. multiflora | Northern Morocco | Not defined | Infusion | Liver function repair effects and antilithiatic actions | [88] |
| E. terminalis Salisb. (El Khalanj) | Zemmour and Zayane | Whole plant | Decoction or oral | Veterinary use for lameness | [89] |
| E. arborea (Khlenj) | Bni-Leit and Al-Oued districts, a part of the Natural Regional Park of Bouhachem | Seeds | Decoction or local application | Headaches and sexual diseases | [90] |
| E. australis | Morocco | Not defined | Infusion | Diuretic, antiseptic and to treat infected wounds | [91] |
| Southern European countries | |||||
| Spain | |||||
| E. multiflora (Brezo o Erica) | Spain | Aerial parts | Not defined | Wound healing | [92,93] |
| E. terminalis Salisb. | Western part of Granada (southern Spain) | Flowers | Decoction | Urinary infections | [94] |
| E. scoparia (Bruc) | L’Alt Empordà and Les Guilleries, located in North East Catalonia | Floral tops | Infusion | Antiemetic and antispasmodic | [95] |
| Portugal | |||||
| E. australis | In Vilar de Perdizes | Flower | Not defined | Prostate, bladder and kidney disease | [96] |
| Greece | |||||
| E. arborea | Mt. Pelion | Leaves and stems | Decoction | Rheumatism, anemia, cystitis, diarrhea, diuretic and acne | [97] |
| E. manipuliflora Salisb. (Sousora) | Mt. Pelion | Leaves, flowers and stems | Decoction | Urinary tract diseases and treat prostate | [97] |
| Italy | |||||
| E. arborea (Ulece) | Peninsula Sorrentina, Campania, Southern Italy | Not defined | Not defined | Nervous system disorders in folk veterinary medicine | [98] |
| E. arborea (Urxa and Socche) | Eastern Riviera (Liguria) | Not defined | Not defined | Mouth infections | [99] |
| E. arborea | Roccamonfina region in Campania, Southern Italy | Flowers | Decoction | Prostatic cystitis | [100] |
| E. arborea | Inland Southern Italy | Stems | Not defined | Sedative in veterinary medicine | [101,102] |
| Malta | |||||
| E. multiflora (Xkattapietra) | Gozo, Malta | Aerial parts | Decoction | Urinary tract disorders | [103] |
| Bosnia and Herzegovina | |||||
| E. erigena R.Ross (Erika) | Middle, southern and western Bosnia and Herzegovina | Aerial parts | Not defined | Renal disorders | [104] |
| No. | Compound | Species | Location | Plant Part(s) | Identification | Reference |
|---|---|---|---|---|---|---|
| 1 | Germacrene-D | E. arborea | Algeria | Leaves | GC/MS | [22] |
| E. manipuliflora | Turkey | Aerial parts | GC/MS | [106] | ||
| 2 | τ-Cadinol | E. manipuliflora | Turkey | Aerial parts | GC/MS | [106] |
| 3 | α-Terpineol | |||||
| 4 | β-Caryophyllene | E. manipuliflora | Turkey | Aerial parts | GC/MS | [106] |
| E. arborea | Algeria | Leaves | GC/MS | [22] | ||
| 5 | Palmitic acid | E. arborea | Algeria | Leaves | GC/MS | [22] |
| 6 | (Z,Z,Z)-9,12,15-Octadecatrien-1-ol | |||||
| 7 | Nonacosane | |||||
| 8 | β-Fenchyl alcohol | |||||
| 9 | β-Bourbonene | |||||
| 10 | Eugenol | |||||
| 11 | Geranylacetone | E. arborea | Algeria | Leaves | GC/MS | [22] |
| E. australis | Portugal | Flowering aerial parts | GC/MS | [107] | ||
| 12 | 1-Octen-3-ol | E. australis | Portugal | Flowering aerial parts | GC/MS | [107] |
| 13 | n-Nonanal | |||||
| 14 | n-Octanol | |||||
| 15 | n-Heptanol | |||||
| 16 | cis-3-Hexen-1-ol | |||||
| 17 | 2-Octen-1-ol | |||||
| 18 | 2-trans, 4-trans-Decadienal | |||||
| 19 | 2-trans-Decenal | |||||
| 20 | Nonanoic acid | |||||
| 21 | trans, trans-α-Farnesene | |||||
| 22 | cis-Bourbonene | |||||
| 23 | α-Pinene | E. multiflora | Spain | Foliar emissions | GC/MS | [108] |
| 24 | β-Pinene | |||||
| 25 | β-Myrcene | |||||
| 26 | A3-Carene | |||||
| 27 | Limonene | |||||
| 28 | α-Terpineol | E. spiculifolia Salisb. | Bulgaria | Aerial parts | GC/MS | [109] |
| 29 | endo-Borneol | |||||
| 30 | Pinocarveol | |||||
| 31 | Thymol | |||||
| 32 | τ-Murrolol | |||||
| 33 | Spathulenol | |||||
| 34 | α-Cadinol | |||||
| 35 | Caryophyllene oxide | E. spiculifolia Salisb. | Bulgaria | Aerial parts | GC/MS | [109] |
| E. manipuliflora | Turkey | Aerial parts | GC/MS | [106] |
| No. | Compound | Species | Location | Plant Part(s) | Identification | Reference |
|---|---|---|---|---|---|---|
| 1 | Lupeol | E. arborea | Algeria | Aerial parts | GC-MS | [12] |
| 2 | Lupenone | E. arborea | Algeria | Aerial parts | GC-MS | [12] |
| E. multiflora | Tunisia | Leaves | HPLC | [112] | ||
| 3 | Betulin | E. arborea | Algeria | Aerial parts | GC-MS | [12] |
| 4 | Betulinic acid | |||||
| 5 | α-Amyrin | E. arborea | Algeria | Aerial parts | GC-MS | [12] |
| E. andevalensis | Spain | Aerial parts | IR, MS, NMR | [111] | ||
| 6 | α-Amyrenone | E. arborea | Algeria | Aerial parts | GC-MS | [12] |
| 7 | Ursolic aldehyde | |||||
| 8 | Uvaol | |||||
| 9 | Ursolic acid | E. arborea | Algeria | Aerial part | GC-MS | [12] |
| E. manipuliflora | Turkey | Aerial parts | NMR and MS | [110] | ||
| E. andevalensis | Spain | Aerial parts | IR, MS, NMR | [111] | ||
| 10 | 3-Oxoursolic acid | E. arborea | Algeria | Aerial parts | GC-MS | [12] |
| 11 | Ursa-2,12-dien-28-oic acid | |||||
| 12 | β-Amyrin | |||||
| 13 | β-Amyrenone | |||||
| 14 | Oleanolic aldehyde | |||||
| 15 | Erythrodiol | |||||
| 16 | Oleanolic acid | |||||
| 17 | 3-Oxooleanolic acid | |||||
| 18 | Olean-2,12-dien-28-oic acid | |||||
| 19 | Taraxasterol | |||||
| 20 | Maslinic acid | |||||
| 21 | Campesterol | |||||
| 22 | Sitosterol | |||||
| 23 | Tremulone | |||||
| 24 | Sitostenone |
| No. | Compound | Species | Location | Plant Part(s) | Identification | Reference |
|---|---|---|---|---|---|---|
| 1 | Gallic acid | E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] |
| E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] | ||
| E. multiflora | Tunisia | Aerial parts | HPLC | [85] | ||
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| 2 | Gentisic acid | E. scoparia | Spain | Leaves | TLC | [114] |
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| E. australis | Spain | Flowers, stems and roots | TLC | [115] | ||
| 3 | Vanillic acid | E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] |
| E. arborea | Turkey | Leaves | HPLC-LTQ OrbiTrap MS | [9] | ||
| E. multiflora | Tunisia | Aerial parts | HPLC | [85] | ||
| E. australis | Spain | Leaves, stems and roots | TLC | [115] | ||
| E. scoparia | Spain | Leaves | TLC | [114] | ||
| E. andevalensis | Spain | Leaves | HPLC | [18] | ||
| E. australis | Spain | Leaves | HPLC | [18] | ||
| E. arborea | Spain | Leaves | HPLC | [18] | ||
| 4 | 3,4-Dihydroxybenzoic acid | E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] |
| E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] | ||
| E. scoparia | Spain | Leaves | TLC | [114] | ||
| E. arborea | Turkey | Leaves | HPLC-LTQ OrbiTrap MS | [9] | ||
| 5 | 2,5-Dihydroxybenzoic acid | E. arborea | Turkey | Leaves | HPLC-LTQ OrbiTrap MS | [9] |
| E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] | ||
| 6 | 3-Hydroxybenzoic acid | E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] |
| 7 | 4-Hydroxybenzoic acid | E. arborea | Turkey | Leaves | HPLC-LTQ OrbiTrap MS | [9] |
| E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] | ||
| E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] | ||
| E. australis | Spain | Leaves, stems, roots and flowers | TLC | [115] | ||
| 8 | Quinic acid | E. multiflora | Tunisia | Leaves | LC–MS/MS | [117] |
| 9 | 5-O-Caffeoylquinic acid | E. arborea | Turkey | Leaves | HPLC-LTQ OrbiTrap MS | [9] |
| 10 | 4-O-Caffeoylquinic acid | E. multiflora | Morocco | Aerial parts | LC–DAD/ESI–MS | [87] |
| 11 | 3-O-Caffeoylquinic acid (Chlorogenic acid) | E. multiflora | Tunisia | Leaves | LC–MS/MS | [117] |
| E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] | ||
| E. arborea | Turkey | Leaves | HPLC-LTQ OrbiTrap MS | [9] | ||
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| 12 | Ellagic acid | E. andevalensis | Spain | Leaves | HPLC | [18] |
| E. australis | Spain | Leaves | HPLC | [18] | ||
| E. arborea | Spain | Leaves | HPLC | [18] | ||
| 13 | Caffeic acid | E. arborea | Turkey | Leaves | HPLC-LTQ OrbiTrap MS | [9] |
| E. arborea | Spain | Leaves | HPLC | [18] | ||
| E. multiflora | Algeria | Flowered aerial parts | HPLC–DAD–ESI-MS | [16] | ||
| E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] | ||
| E. scoparia | Spain | Leaves | TLC | [114] | ||
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| E. australis | Spain | Roots | TLC | [115] | ||
| E. andevalensis | Spain | Leaves | HPLC | [18] | ||
| E. australis | Spain | Leaves | HPLC | [18] | ||
| 14 | Syringic acid | E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] |
| E. scoparia | Spain | Leaves | TLC | [114] | ||
| 15 | Sinapic acid | E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] |
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| E. australis | Spain | Roots | TLC | [115] | ||
| 16 | Ferulic acid | E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] |
| E. scoparia | Spain | Leaves | TLC | [114] | ||
| E. australis | Spain | Leaves, stems, roots and flowers | TLC | [115] | ||
| 17 | Rosmarinic acid | E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] |
| 18 | Cinnamic acid | E. australis | Portugal | Leaves and flowers | HPLC | [113] |
| E. andevalensis | Spain | Seeds | HPLC | [116] | ||
| E. andevalensis | Spain | Leaves | HPLC | [18] | ||
| 19 | p-Coumaric acid | E. arborea | Turkey | Leaves | HPLC-LTQ OrbiTrap MS | [9] |
| E. multiflora | Algeria | Flowered aerial parts | HPLC–DAD–ESI-MS | [16] | ||
| E. scoparia | Spain | Leaves | TLC | [114] | ||
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| E. australis | Spain | Leaves, flowers and roots | TLC | [115] | ||
| E. australis | Spain | Leaves | HPLC | [18] | ||
| E. andevalensis | Spain | Leaves | HPLC | [18] | ||
| E. andevalensis | Spain | Seeds | HPLC | [116] | ||
| 20 | m-Coumaric acid | E. australis | Spain | Leaves | HPLC | [18] |
| E. arborea | Spain | Leaves | HPLC | [18] | ||
| E. andevalensis | Spain | Seeds | HPLC | [116] | ||
| 21 | Fumaric acid | E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] |
| 22 | Resveratrol | E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] |
| 23 | Acetohydroxamic acid | E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] |
| 24 | 2,4-Dihydroxy-phenyl acetonitrile | E. scoparia | Spain | Leaves | NMR | [118] |
| 25 | 2-Hydroxyphenyl acetic acid | E. scoparia | Spain | Leaves | NMR | [118] |
| 26 | 3,4-Dihydroxyphenyl acetic acid | E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] |
| 27 | Oleuropein | E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] |
| 28 | Scopoletin | E. australis | Spain | Leaves, flowers, stems and roots | TLC | [115] |
| 29 | Phloridzin dihydrate | E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] |
| 30 | Aesculetin | E. australis | Spain | Leaves, flowers, stems and roots | TLC | [115] |
| 31 | Pyrocatechol | E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] |
| No. | Compound | Species | Location | Plant Part(s) | Identification | Reference |
|---|---|---|---|---|---|---|
| 1 | Ericarborin | E. arborea | Turkey | Leaves | NMR | [15] |
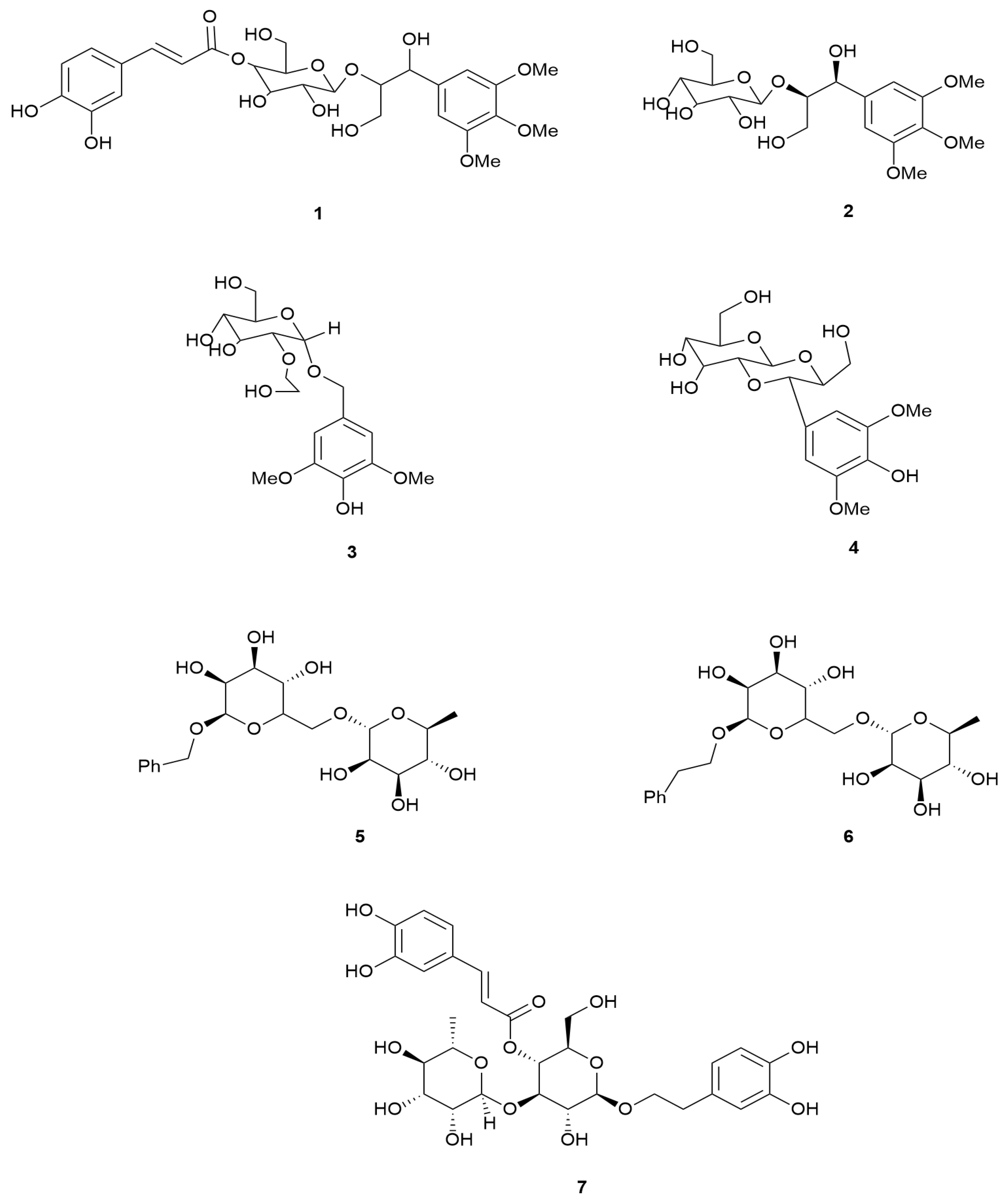
| 2 | 1,2-Erythro-1-(3,4,5-trimethoxyphenyl)-2-(β-D-glucopyranosyloxy) propan-1,3-diol | E. arborea | Turkey | Leaves and Flowers | NMR and MS | [118] |
| 3 | Ericarboside | |||||
| 4 | Ficuscarpanoside B | |||||
| 5 | Benzylrutinoside | |||||
| 6 | Phenethylrutinoside | |||||
| 7 | Verbascoside | E. arborea | Turkey | Not defined | LC–ESI-MS/MS | [17] |
| No. | Compound | Species | Location | Plant Part(s) | Identification | Reference(s) |
|---|---|---|---|---|---|---|
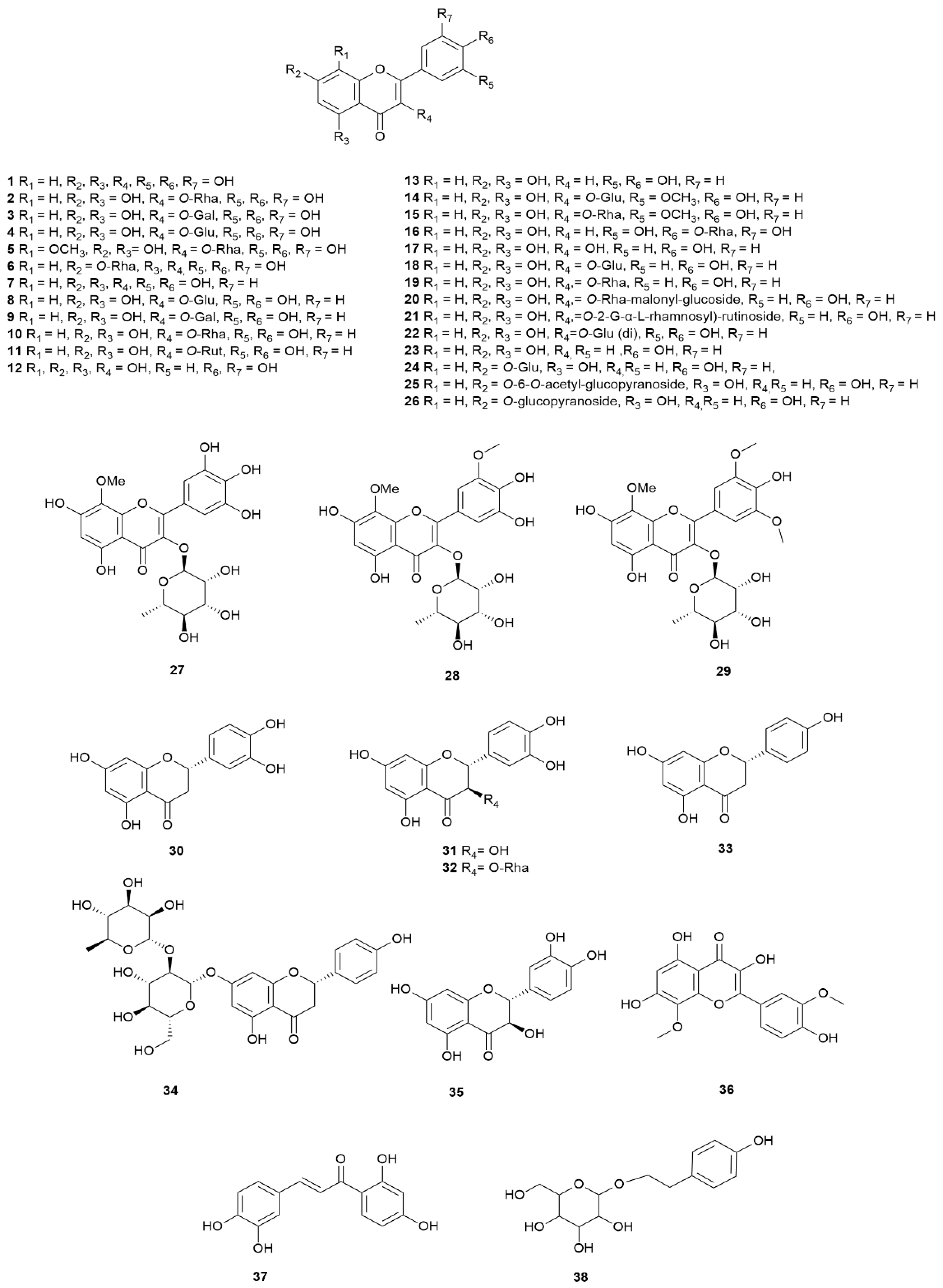
| 1 | Myricetin | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] | ||
| E. manipuliflora | Greece | Aerial parts | NMR | [120] | ||
| E. andevalensis | Spain | Leaves | HPLC | [18] | ||
| E. australis | Spain | Leaves | HPLC | [18] | ||
| E. arborea | Spain | Leaves | HPLC | [18] | ||
| E. multiflora | Tunisia | Leaves | LC–MS/MS | [117] | ||
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| E. australis | Spain | Flowers and roots | TLC | [115] | ||
| 2 | Myricetin 3-O-rhamnoside | E. scoparia | Morocco | Leaves | LC–DAD/ESI–MS | [87] |
| E. australis | Portugal | Flowering aerial parts | HPLC-DAD and HPLC-ESI-MS | [121] | ||
| 3 | Myricetin 3-O-galactoside | E. andevalensis | Spain | Flowering tops | IR, MS, NMR | [122] |
| E. andevalensis | Spain | Flowering tops | IR, MS, NMR | [123] | ||
| 4 | Myricetin 3-O-glucoside | E. multiflora | Tunisia | Leaves | LC–MS/MS | [117] |
| E. australis | Portugal | Flowering aerial parts | HPLC-DAD and HPLC-ESI-MS | [121] | ||
| 5 | 8-Methoxy-myricetin 3-O-rhamnoside | E. arborea | Turkey | Leaves | HPLC-LTQ OrbiTrap MS | [9] |
| E. scoparia | Morocco | Aerial parts | LC–DAD/ESI–MS | [87] | ||
| 6 | Myricetin 7-O-rhamnoside | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| 7 | Quercetin | E. australis | Spain | Leaves, flowers and roots | TLC | [115] |
| E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] | ||
| E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] | ||
| E. multiflora | Algeria | Flowered aerial parts | HPLC–DAD–ESI-MS | [16] | ||
| E. multiflora | Morocco | Aerial parts and leaves | LC–DAD/ESI–MS | [87] | ||
| E. multiflora | Tunisia | Aerial parts | HPLC | [85] | ||
| E. manipuliflora | Greece | Aerial parts | NMR | [120] | ||
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| 8 | Quercetin 3-O-β-D-glucopyranoside | E. arborea | Turkey | Leaves | NMR | [15] |
| E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] | ||
| E. multiflora | Tunisia | Leaves | LC–MS/MS | [117] | ||
| 9 | Quercetin 3-O-galactoside (Hyperoside) | E. arborea | Turkey | Not defined | LC–ESI–MS/MS | [17] |
| 10 | Quercetin 3-O-α-L-rhamnopyranoside | E. arborea | Turkey | Leaves | NMR | [15] |
| E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] | ||
| E. australis | Portugal | Flowering aerial parts | HPLC-DAD and HPLC-ESI-MS | [121] | ||
| 11 | Quercetin 3-O-rutinoside | E. multiflora | Tunisia | Leaves | LC–MS/MS | [117] |
| 12 | Gossypetin | E. australis | Portugal | Flowering aerial parts | HPLC-DAD and HPLC-ESI-MS | [121] |
| 13 | Luteolin | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] | ||
| 14 | Isorhamnetin 3-O- glucoside | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| 15 | Isorhamnetin 3-O-α-L-rhamnopyranoside | E. arborea | Turkey | Aerial parts | UV, MS, and NMR | [14] |
| 16 | Tricetin 4′-O-α-L-rhamnopyranoside | E. arborea | Turkey | Aerial parts | UV, MS, and NMR | [14] |
| 17 | Kaempferol | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| E. multiflora | Tunisia | Aerial parts | HPLC | [85] | ||
| E. multiflora | Algeria | Flowered aerial parts | HPLC–DAD–ESI-MS | [16] | ||
| E. andevalensis | Spain | Leaves | HPLC | [18] | ||
| E. australis | Spain | Leaves | HPLC | [18] | ||
| E. arborea | Spain | Leaves | HPLC | [18] | ||
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| E. australis | Spain | Leaves, flowers and roots | TLC | [115] | ||
| 18 | Kaempferol 3-O-glucoside | E. arborea | Algeria | Leaves and flowers | HPLC-MS | [24,74,122] |
| E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] | ||
| E. multiflora | Tunisia | Leaves | LC–MS/MS | [117] | ||
| 19 | Kaempferol 3-O- rhamnoside | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| E. australis | Portugal | Flowering aerial parts | HPLC-DAD and HPLC-ESI-MS | [121] | ||
| 20 | Kaempferol 3-O-rhamnoside-malonyl-glucoside | E. multiflora | Tunisia | Leaves | LC–MS/MS | [117] |
| 21 | Kaempferol 3-O-2G-α-L-rhamnosyl-rutinoside | E. multiflora | Tunisia | Leaves | LC–MS/MS | [117] |
| 22 | Rutin | E. multiflora | Morocco | Aerial parts | LC–DAD/ESI–MS | [87] |
| E. multiflora | Tunisia | Aerial parts | HPLC | [85] | ||
| E. andevalensis | Spain | Leaves | HPLC | [18] | ||
| E. andevalensis | Spain | Seeds | HPLC | [116] | ||
| E. australis | Spain | Leaves | HPLC | [18] | ||
| E. arborea | Spain | Leaves | HPLC | [18] | ||
| 23 | Apigenin | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| E. multiflora | Tunisia | Aerial parts | HPLC | [85] | ||
| 24 | Apigenin 7-O-glucoside | E. arborea | Turkey | Leaves | NMR | [15] |
| E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] | ||
| E. multiflora | Tunisia | Leaves | LC–MS/MS | [117] | ||
| 25 | Apigenin 7-O-β-D-(6-O-acetyl-glucopyranoside) | E. arborea | Turkey | Leaves | NMR | [15] |
| 26 | Apigenin 7-O-D-glucopyranoside | E. arborea | Turkey | Leaves | NMR | [15] |
| 27 | 3,5,7,3′,4′,5′-Hexahydroxy-8-methoxyflavone-3-O-L-rhamnopyranoside | E. manipuliflora | Greece | Aerial parts | NMR | [120] |
| 28 | 3,5,7,3′,4′-Pentahydroxy- 8,5′-dimethoxyflavone-3-O-α-L-rhamnopyranoside | |||||
| 29 | 3,5,7,4′-Tetrahydroxy-8,3′,5′-trimethoxyflavone-3-O-α-L-rhamnopyranoside | |||||
| 30 | Eriodictyol | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| 31 | Taxifolin | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| 32 | Taxifolin 3-O-rhamnoside | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| 33 | Naringenin | E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] |
| E. multiflora | Tunisia | Aerial parts | HPLC | [85] | ||
| 34 | Naringin | E. multiflora | Algeria | Flowered aerial parts | HPLC–DAD–ESI-MS | [16] |
| 35 | Aromodedrin | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| 36 | Limocitrin | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| 37 | Butein | E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] |
| 38 | Phenylethanoid glycosides | E. manipuliflora | Turkey | Aerial parts | TLC | [124] |
| No. | Compound | Species | Location | Plant Part(s) | Identification | Reference(s) |
|---|---|---|---|---|---|---|
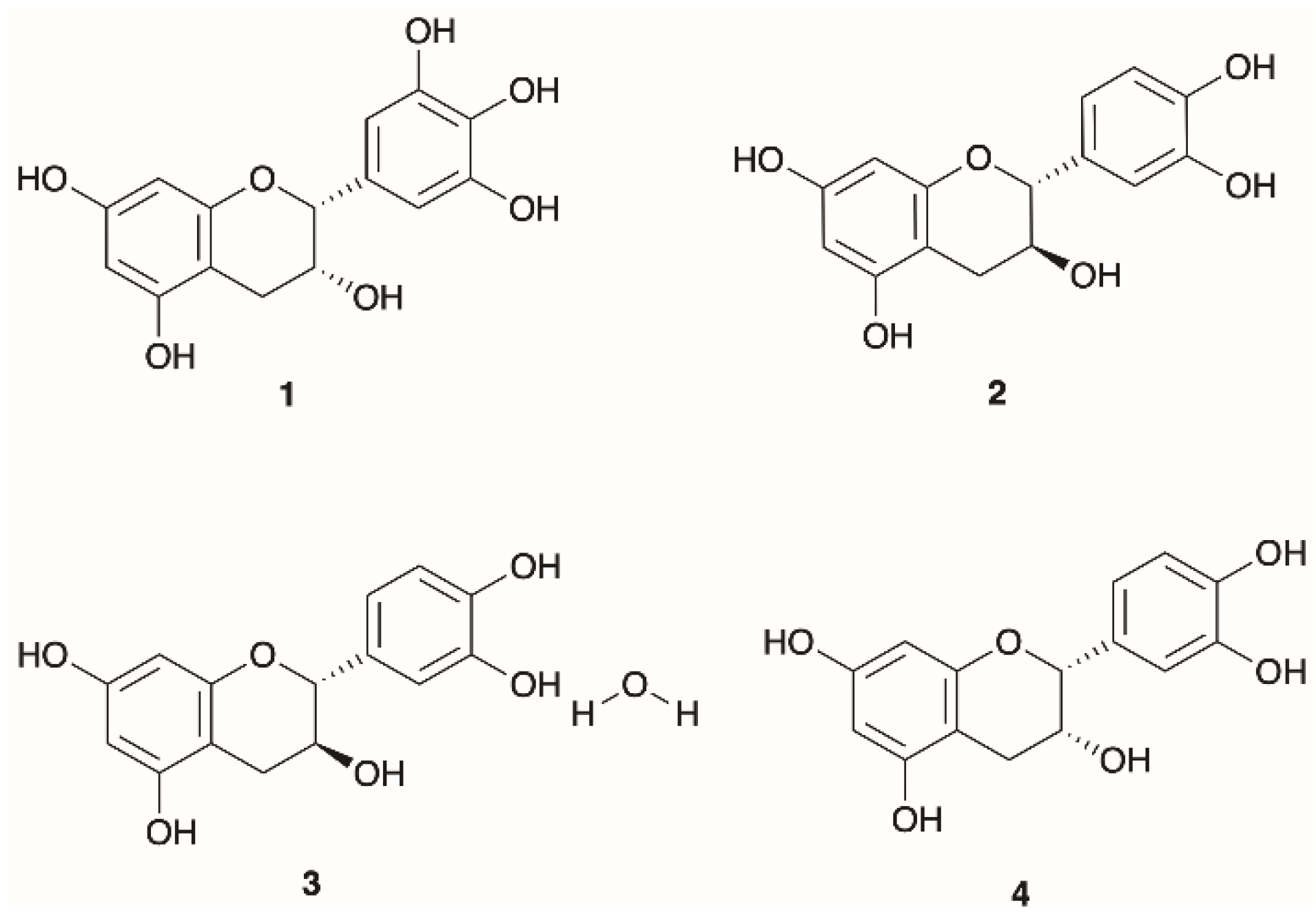
| 1 | Epigallocatechin | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| 2 | Catechin | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| E. multiflora | Tunisia | Aerial parts | HPLC | [85] | ||
| E. andevalensis | Spain | Leaves | HPLC | [18] | ||
| E. australis | Spain | Leaves | HPLC | [18] | ||
| E. arborea | Spain | Leaves | HPLC | [18] | ||
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| E. australis, E. arborea | Spain | Leaves | HPLC | [18] | ||
| 3 | Catechin hydrate | E. manipuliflora | Turkey | Aerial parts | LC-MS/MS | [19] |
| 4 | Epicatechin | E. arborea | Turkey | Leaves | HPLC-LTQ Orbitrap MS | [9] |
| E. multiflora | Tunisia | Aerial parts | HPLC | [85] | ||
| E. andevalensis | Spain | Leaves | HPLC | [18] | ||
| E. australis | Spain | Leaves | HPLC | [18] | ||
| E. arborea | Spain | Leaves | HPLC | [18] | ||
| E. arborea | Algeria | Leaves and flowers | HPLC-MS | [24,74] | ||
| E. australis | Portugal | Leaves and flowers | HPLC | [113] | ||
| E. andevalensis | Spain | Seeds | HPLC | [116] |
| No. | Compound | Species | Location | Plant Parts | Identification | Reference |
|---|---|---|---|---|---|---|
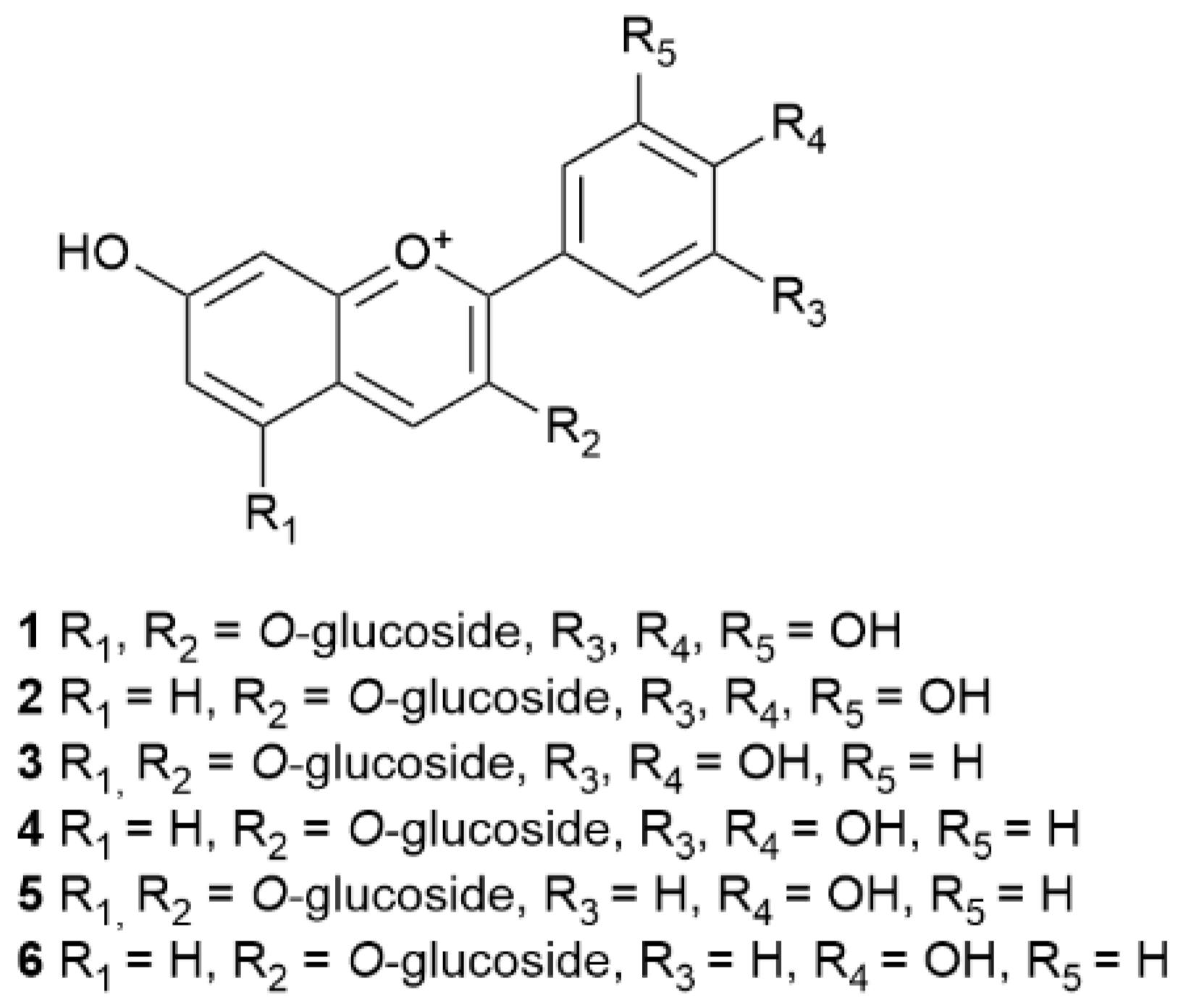
| 1 | Delphinidin 3-5-O-diglucoside | E. australis | Portugal | Leaves and flowers | HPLC | [113] |
| 2 | Delphinidin 3-O-glucoside | |||||
| 3 | Cyanidin 3,5-O-diglucoside | |||||
| 4 | Cyanidin 3-O-glucoside | |||||
| 5 | Pelargonidin 3,5-O-diglucoside | |||||
| 6 | Pelargonidin 3-O-glucoside |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabal, K.A.; Pigott, M.; Sheridan, H.; Walsh, J.J. Mediterranean Basin Erica Species: Traditional Uses, Phytochemistry and Pharmacological Properties. Molecules 2025, 30, 2616. https://doi.org/10.3390/molecules30122616
Jabal KA, Pigott M, Sheridan H, Walsh JJ. Mediterranean Basin Erica Species: Traditional Uses, Phytochemistry and Pharmacological Properties. Molecules. 2025; 30(12):2616. https://doi.org/10.3390/molecules30122616
Chicago/Turabian StyleJabal, Khadijah A., Maria Pigott, Helen Sheridan, and John J. Walsh. 2025. "Mediterranean Basin Erica Species: Traditional Uses, Phytochemistry and Pharmacological Properties" Molecules 30, no. 12: 2616. https://doi.org/10.3390/molecules30122616
APA StyleJabal, K. A., Pigott, M., Sheridan, H., & Walsh, J. J. (2025). Mediterranean Basin Erica Species: Traditional Uses, Phytochemistry and Pharmacological Properties. Molecules, 30(12), 2616. https://doi.org/10.3390/molecules30122616







