Synthesis and Properties of 1H-Pyrrolo[3′,2′:3,4]fluoreno[9,1-gh]quinolines and 7H-Pyrrolo[2′,3′,4′:4,10]anthra[1,9-fg]quinolines
Abstract
1. Introduction
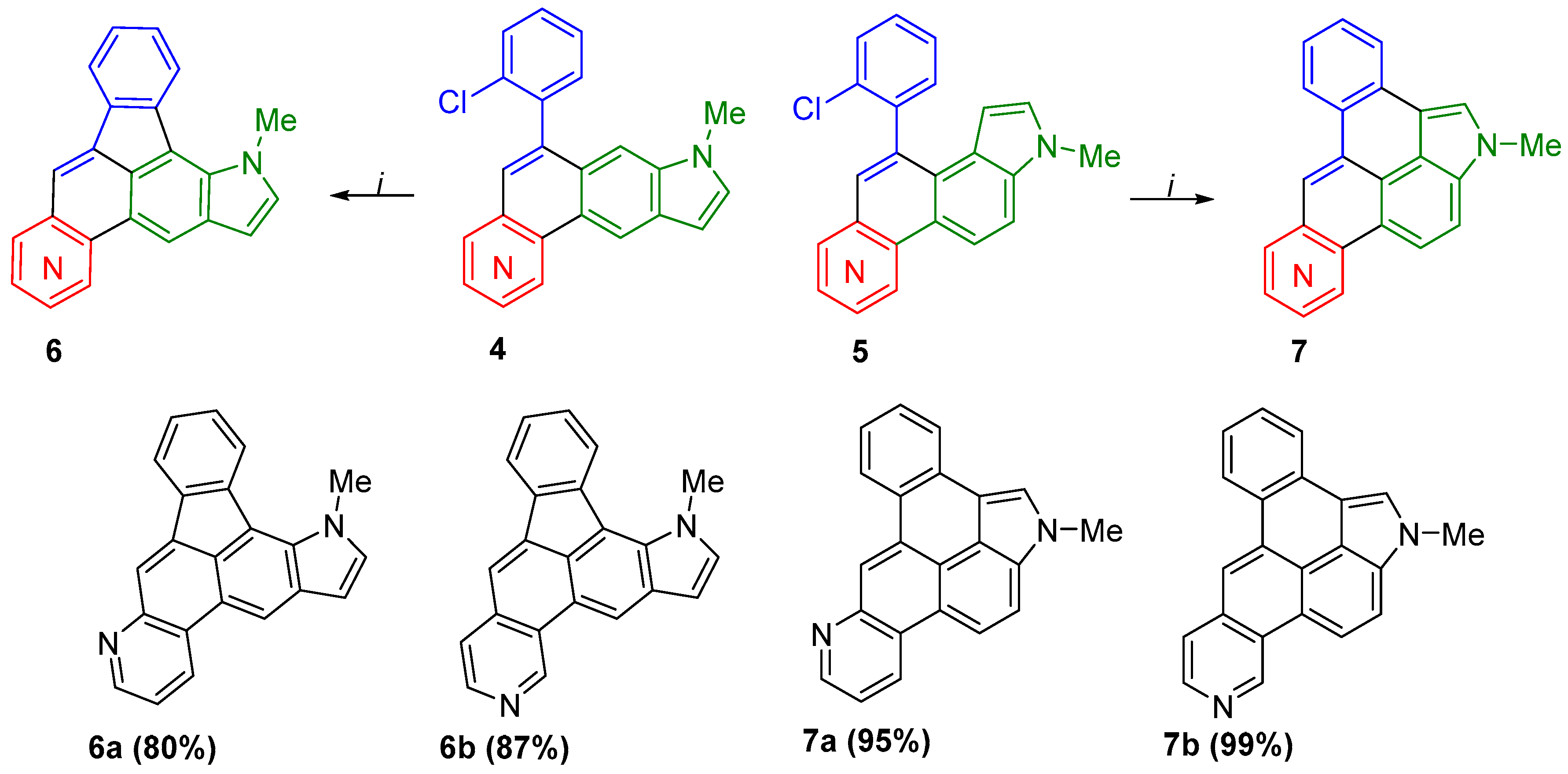
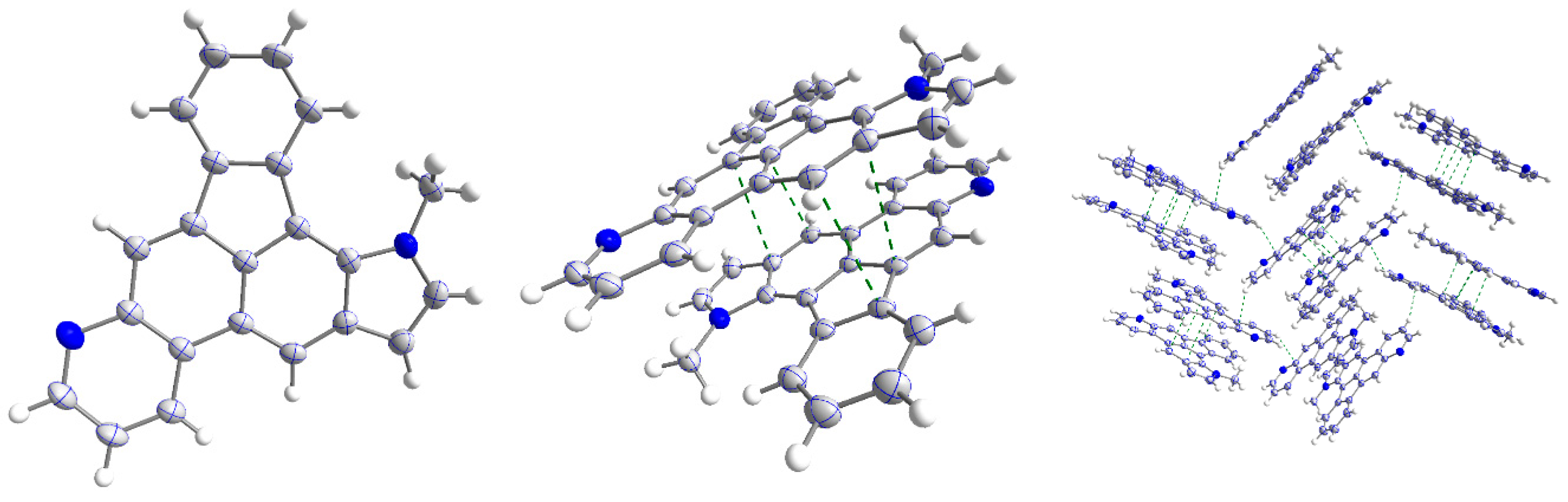
2. Results and Discussion
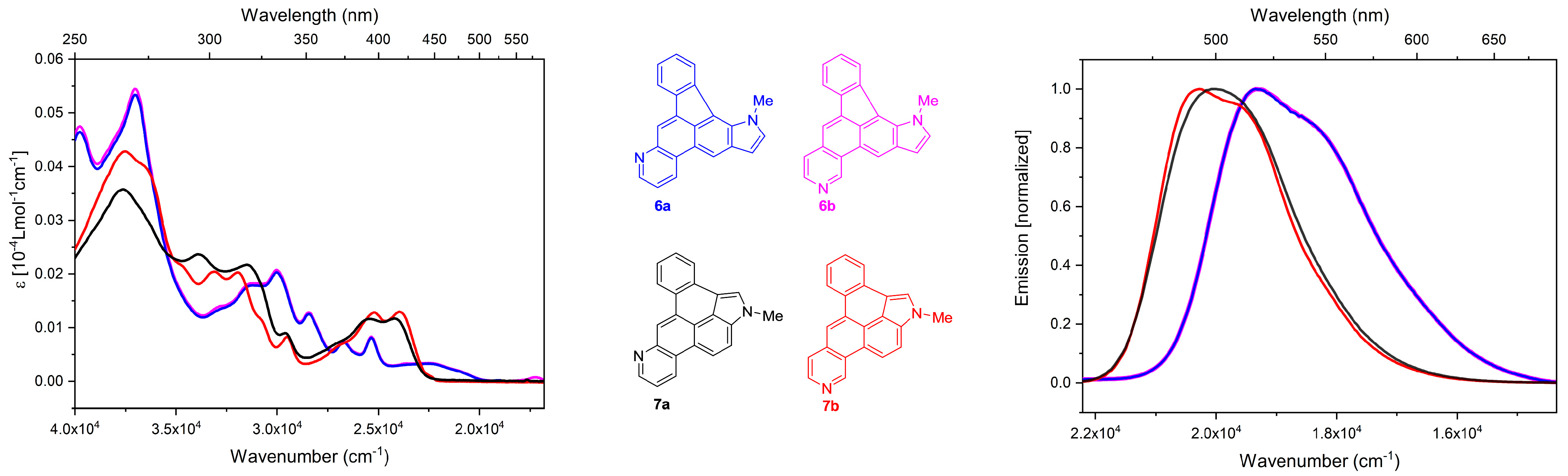
3. Physical Properties
4. Experimental Section
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, B.; Dong, Q.; Li, F.; Wang, T.; Qiu, X.; Zhu, T. A Systematic Review of Polycyclic Aromatic Hydrocarbon Derivatives: Occurrences, Levels, Biotransformation, Exposure Biomarkers, and Toxicity. Environ. Sci. Technol. 2023, 57, 15314–15335. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Lin, C.; Huang, R.-J.; Duan, J.; Zhong, H.; Xu, W. Polycyclic aromatic hydrocarbons from cooking emissions. Sci. Total Environ. 2022, 818, 151700. [Google Scholar] [CrossRef]
- Keyte, I.J.; Harrison, R.M.; Lammel, G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons—A review. Chem. Soc. Rev. 2013, 42, 9333–9391. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Y.; Xie, Z.; Zhen, Y.; Hu, W.; Dong, H. Polycyclic aromatic hydrocarbon-based organic semiconductors: Ring-closing synthesis and optoelectronic properties. J. Mater. Chem. C 2022, 10, 2411–2430. [Google Scholar] [CrossRef]
- Goyal, H.; Kumar, P.; Gupta, R. Polycyclic Aromatic Hydrocarbon-based Soft Materials: Applications in Fluorescent Detection, Gelation, AIEE and Mechanochromism. Chem. Asian J. 2023, 18, e202300355. [Google Scholar] [CrossRef]
- Ball, M.; Zhong, Y.; Wu, Y.; Schenck, C.; Ng, F.; Steigerwald, M.; Xiao, S.; Nuckolls, C. Contorted polycyclic aromatics. Acc. Chem. Res. 2015, 48, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Narita, A.; Wang, X.-Y.; Feng, X.; Müllen, K. New advances in nanographene chemistry. Chem. Soc. Rev. 2015, 44, 6616–6643. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Y.; Colella, N.S.; Liang, Y.; Brédas, J.-L.; Houk, K.N.; Briseno, A.L. Unconventional, chemically stable, and soluble two-dimensional angular polycyclic aromatic hydrocarbons: From molecular design to device applications. Acc. Chem. Res. 2015, 48, 500–509. [Google Scholar] [CrossRef]
- Kilaru, S.; Gade, R.; bhongiri, Y.; Tripathi, A.; Chetti, P.; Pola, S. Organic materials based on hetero polycyclic aromatic hydrocarbons for organic thin-film transistor applications. Mater. Sci. Semicond. Process. 2022, 147, 106730. [Google Scholar] [CrossRef]
- Wu, J. Polycyclic Aromatic Compounds for Organic Field-Effect Transistors:Molecular Design and Syntheses; Bentham Science Publishers: Sharjah, United Arab Emirates, 2007. [Google Scholar]
- Bauri, J.; Choudhary, R.B.; Mandal, G. Recent advances in efficient emissive materials-based OLED applications: A review. J. Mater. Sci. 2021, 56, 18837–18866. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, L. Polycyclic Aromatic Hydrocarbon Derivatives toward Ideal Electron-Transporting Materials for Organic Light-Emitting Diodes. J. Phys. Chem. Lett. 2019, 10, 2528–2537. [Google Scholar] [CrossRef]
- Oner, S.; Bryce, M.R. A review of fused-ring carbazole derivatives as emitter and/or host materials in organic light emitting diode (OLED) applications. Mater. Chem. Front. 2023, 7, 4304–4338. [Google Scholar] [CrossRef]
- Aumaitre, C.; Morin, J.-F. Polycyclic Aromatic Hydrocarbons as Potential Building Blocks for Organic Solar Cells. Chem. Rec. 2019, 19, 1142–1154. [Google Scholar] [CrossRef]
- Yao, H.; Wang, J.; Xu, Y.; Zhang, S.; Hou, J. Recent Progress in Chlorinated Organic Photovoltaic Materials. Acc. Chem. Res. 2020, 53, 822–832. [Google Scholar] [CrossRef]
- Gu, X.; Luhman, W.A.; Yagodkin, E.; Holmes, R.J.; Douglas, C.J. Diarylindenotetracenes via a selective cross-coupling/C-H functionalization: Electron donors for organic photovoltaic cells. Org. Lett. 2012, 14, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Borissov, A.; Maurya, Y.K.; Moshniaha, L.; Wong, W.-S.; Żyła-Karwowska, M.; Stępień, M. Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds. Chem. Rev. 2022, 122, 565–788. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Ahrens, L.; Brosius, V.; Freudenberg, J.; Bunz, U.H.F. Unusual stabilization of larger acenes and heteroacenes. J. Mater. Chem. C 2019, 7, 14011–14034. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X. Bottom-Up Synthesis of Nitrogen-Doped Polycyclic Aromatic Hydrocarbons. Synlett 2020, 31, 211–222. [Google Scholar] [CrossRef]
- Hou, X.-Q.; Sun, Y.-T.; Liu, L.; Wang, S.-T.; Geng, R.-L.; Shao, X.-F. Bowl-shaped conjugated polycycles. Chin. Chem. Lett. 2016, 27, 1166–1174. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Siegel, J.S. Aromatic molecular-bowl hydrocarbons: Synthetic derivatives, their structures, and physical properties. Chem. Rev. 2006, 106, 4843–4867. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Yu, S.-Q.; Liang, J.-Q.; Huang, X.; Gong, H.-Y. Nitrogen-containing polycyclic aromatic hydrocarbons (PAHs) with bowl-shaped structures: Synthesis, architecture, and applications. Org. Chem. Front. 2025, 12, 1340–1354. [Google Scholar] [CrossRef]
- Wegner, H.A.; Reisch, H.; Rauch, K.; Demeter, A.; Zachariasse, K.A.; de Meijere, A.; Scott, L.T. Oligoindenopyrenes: A new class of polycyclic aromatics. J. Org. Chem. 2006, 71, 9080–9087, Correction in J. Org. Chem. 2007, 72, 1870. https://doi.org/10.1021/jo070008s. [Google Scholar] [CrossRef]
- Ausekle, E.; Ehlers, P.; Villinger, A.; Langer, P. Dibenzoindolo1,8naphthyridines: Synthesis and Characterization of X-Shaped Aza4,6helicenes. Chem. Eur. J. 2024, 30, e202303225. [Google Scholar] [CrossRef]
- Radolko, J.; Tuan, H.M.; Molenda, R.; Villinger, A.; Ehlers, P.; Langer, P. N-doped and Bowl-Shaped Azabenzo3,4azulenofluorene: Synthesis and Characterization of Bridged Aza-Triarylamines. Chem. Eur. J. 2024, 30, e202403004. [Google Scholar] [CrossRef] [PubMed]
- Spruner von Mertz, F.; Polkaehn, J.; Villinger, A.; Ehlers, P.; Langer, P. π-Expanded and N-Doped Fluoranthenes. J. Org. Chem. 2025, 90, 1024–1035. [Google Scholar] [CrossRef]
- Khomutetckaia, A.; Hildebrandt, N.; Ehlers, P.; Villinger, A.; Langer, P. Synthesis and Properties of Diindeno[1,2,3-cd:1′,2′,3′- mn ]pyrene and Two of Its Aza-Analogs. Eur. J. Org. Chem. 2024, 27, e202301101. [Google Scholar] [CrossRef]
- Lee, R.G.M.; Coleman, P.; Jones, J.L.; Jones, K.C.; Lohmann, R. Emission factors and importance of PCDD/Fs, PCBs, PCNs, PAHs and PM10 from the domestic burning of coal and wood in the U.K. Environ. Sci. Technol. 2005, 39, 1436–1447. [Google Scholar] [CrossRef]
- Schiedt, B. Über Dinaphthoperylen, zugleich ein Beitrag zur Chemie des Chrysens. Ber. dtsch. Chem. Ges. A/B 1938, 71, 1248–1253. [Google Scholar] [CrossRef]
- Clar, E. Neue Synthesen von Benzologen des Pyrens und Benzanthrens (Aromatische Kohlenwasserstoffe, XLI. Mitteil). Ber. dtsch. Chem. Ges. A/B 1943, 76, 609–621. [Google Scholar] [CrossRef]
- Zinke, A.; Zimmer, W. Untersuchungen über Perylen und seine Derivate. Monatsh. Chem. 1950, 81, 783–785. [Google Scholar] [CrossRef]
- Halton, B. Acephenanthrylenes from flash vacuum thermolysis of diarylmethylidenecycloproparenes. Tetrahedron Lett. 2006, 47, 1077–1079. [Google Scholar] [CrossRef]
- Cho, B.P.; Harvey, R.G. Polycyclic fluoranthene hydrocarbons. 2. A new general synthesis. J. Org. Chem. 1987, 52, 5668–5678. [Google Scholar] [CrossRef]
- Clar, E.; Stewart, D.G. 147. Aromatic hydrocarbons. Part LIX. 1: 2–3: 4-Dibenzpyrene. J. Chem. Soc. 1951; 687–690. [Google Scholar] [CrossRef]
- Lavit-Lamy, D.; Buu-Hoï, N.P. The true nature of “dibenzo[a,l]pyrene” and its known derivatives. Chem. Commun. 1966, 92–94. [Google Scholar] [CrossRef]
- Zander, M. Synthese von 1.2;3.4-Dibenzpyren und 1.2;4.5;8.9-Tribenzpyren. Chem. Ber. 1959, 92, 2749–2751. [Google Scholar] [CrossRef]
- Khomutetckaia, A.; Ehlers, P.; Villinger, A.; Langer, P. Synthesis and Properties of Azadibenzo[a,e]pyrenes. Eur. J. Org. Chem. 2024, 27, e202301299. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Bredas, J.-L. Mind the gap! Mater. Horiz. 2014, 1, 17–19. [Google Scholar] [CrossRef]
- Stanger, A. NICS—Past and Present. Eur. J. Org. Chem. 2020, 2020, 3120–3127. [Google Scholar] [CrossRef]
- Gershoni-Poranne, R.; Stanger, A. Magnetic criteria of aromaticity. Chem. Soc. Rev. 2015, 44, 6597–6615. [Google Scholar] [CrossRef]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.v.R. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar] [CrossRef] [PubMed]
- Paenurk, E.; Gershoni-Poranne, R. Simple and efficient visualization of aromaticity: Bond currents calculated from NICS values. Phys. Chem. Chem. Phys. 2022, 24, 8631–8644. [Google Scholar] [CrossRef] [PubMed]



| 6a | 6b | 7a | 7b | |
|---|---|---|---|---|
| λabs1 [nm] | 446 | 446 | 414 | 418 |
| (ε1 [a]) | 0.15 | 0.15 | 0.28 | 0.31 |
| λabs2 [nm] | 395 | 395 | 392 | 397 |
| (ε2 [a]) | 0.22 | 0.22 | 0.28 | 0.30 |
| λabs3 [nm] | 374 | 374 | 339 | 393 |
| (ε3 [a]) | 0.21 | 0.21 | 0.23 | 0.22 |
| λem1 [nm] | 519 | 519 | 500 | 494 |
| λem2 [nm] | 541 sh | 541 sh | - | 509 sh |
| φ [%] [b] | 10 | 10 | 48 | 34 |
| UV/vis | DFT | |||
|---|---|---|---|---|
| Egopt. (eV) (a) | HOMODFT (eV) (b) | LUMODFT (eV) (b) | ΔEgDFT (eV) | |
| 6a | 2.62 | −5.41 | −2.05 | 3.36 |
| 6b | 2.62 | −5.49 | −2.19 | 3.30 |
| 7a | 2.90 | −5.24 | −1.74 | 3.50 |
| 7b | 2.90 | −5.35 | −1.85 | 3.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khomutetckaia, A.; Ehlers, P.; Villinger, A.; Langer, P. Synthesis and Properties of 1H-Pyrrolo[3′,2′:3,4]fluoreno[9,1-gh]quinolines and 7H-Pyrrolo[2′,3′,4′:4,10]anthra[1,9-fg]quinolines. Molecules 2025, 30, 2615. https://doi.org/10.3390/molecules30122615
Khomutetckaia A, Ehlers P, Villinger A, Langer P. Synthesis and Properties of 1H-Pyrrolo[3′,2′:3,4]fluoreno[9,1-gh]quinolines and 7H-Pyrrolo[2′,3′,4′:4,10]anthra[1,9-fg]quinolines. Molecules. 2025; 30(12):2615. https://doi.org/10.3390/molecules30122615
Chicago/Turabian StyleKhomutetckaia, Aleksandra, Peter Ehlers, Alexander Villinger, and Peter Langer. 2025. "Synthesis and Properties of 1H-Pyrrolo[3′,2′:3,4]fluoreno[9,1-gh]quinolines and 7H-Pyrrolo[2′,3′,4′:4,10]anthra[1,9-fg]quinolines" Molecules 30, no. 12: 2615. https://doi.org/10.3390/molecules30122615
APA StyleKhomutetckaia, A., Ehlers, P., Villinger, A., & Langer, P. (2025). Synthesis and Properties of 1H-Pyrrolo[3′,2′:3,4]fluoreno[9,1-gh]quinolines and 7H-Pyrrolo[2′,3′,4′:4,10]anthra[1,9-fg]quinolines. Molecules, 30(12), 2615. https://doi.org/10.3390/molecules30122615







