2.1. Adsorption Properties of Levodopa
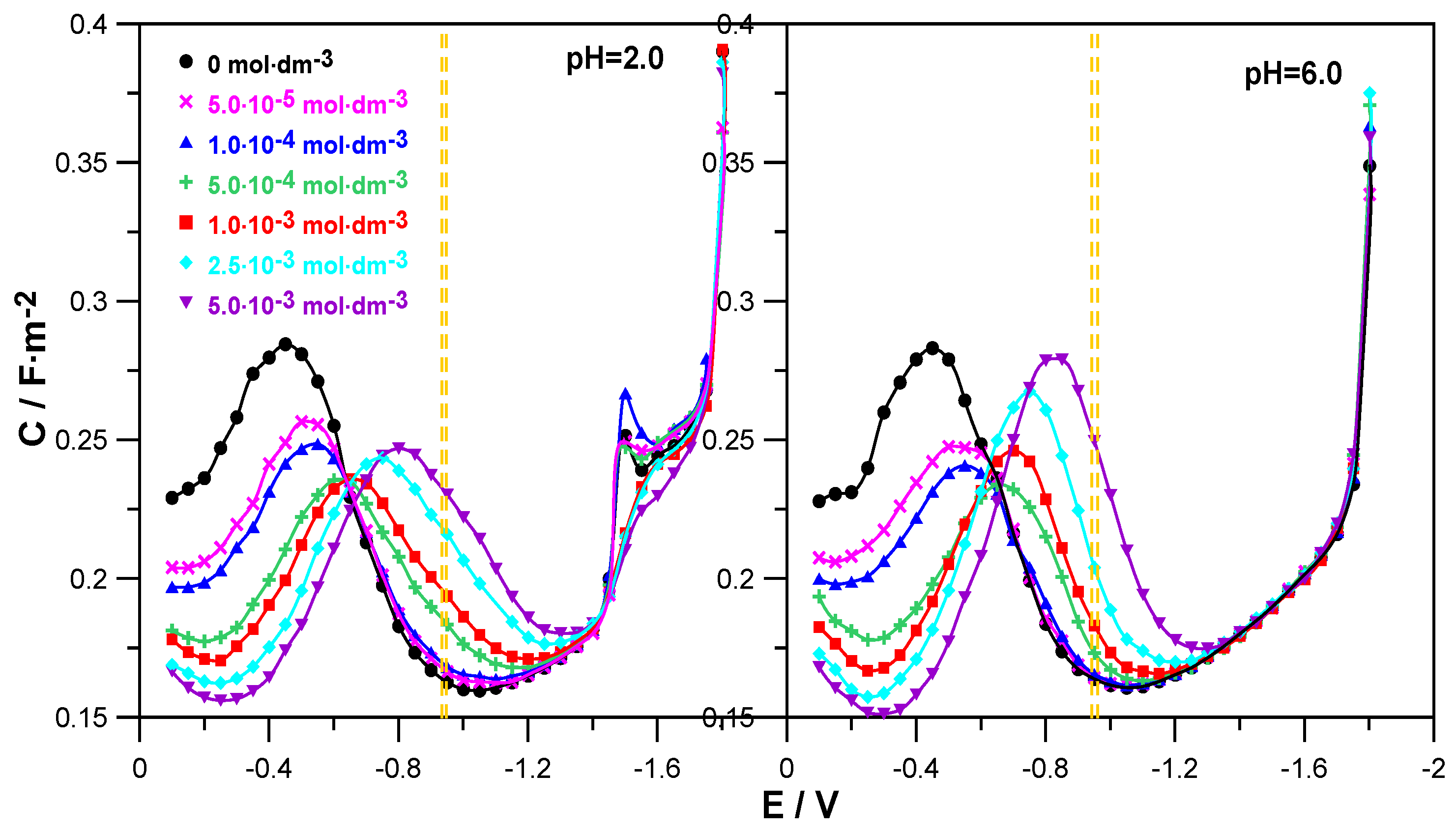
Figure 2 shows the changes in the differential capacitance of the double layer as a function of the electrode potential for the adsorption of L-DOPA on mercury from 1.0 mol·dm
−3 NaClO
4 at pH = 2.0 and 6.0.
The comparison of the C = f(E) curves obtained in the presence of the drug in solutions at both pH = 2.0 and pH = 6.0 with the differential capacitance curve of the supporting electrolyte distinguished two areas of L-DOPA adsorption potentials. In the range of less negative potentials from E = −0.1 V to E ≈ −0.6 V, the presence of L-DOPA in the NaClO4 solution caused a clear decrease in differential capacitance in relation to that recorded for the supporting electrolyte. The higher the concentration of the organic compound, the greater it was and indicated an increase in the adsorption of L-DOPA on mercury. It is worth noticing that this effect was a bit clearer in the case of solutions with a pH = 6. This indicates a slightly stronger adsorption of L-DOPA at pH = 6.0. In the range of potentials −0.6 V to −1.4 V, the opposite situation was observed. An increase in the drug concentration caused a growth in the differential capacitance. This regularity applied to both solutions with pH = 2.0 and 6.0. Then, in the range of the most negative potentials E < −1.4 V, the C = f(E) curves converged, which indicated a lack of L-DOPA adsorption on mercury in this electrode potential range.
In the range of Zn
2+ ion reduction potentials (
Figure 2, the area marked with yellow dashed lines), the use of L-DOPA at concentrations of c ≤ 1.0 × 10
−4 mol·dm
−3 caused a slight increase in the differential capacitance in relation to the capacity of NaClO
4. However, in the range of higher L-DOPA concentrations c ≥ 5.0 × 10
−4 mol·dm
−3, this increase was clear and grew with increasing amounts of the drug. Nevertheless, this was not the range of strong adsorption of L-DOPA on the mercury electrode but could rather be called the area of labile adsorption. In this way, one of the conditions of the cap-pair rule was met.
In addition, the analysis of the changes in the differential capacitance curves shown for the solution at pH = 2.0 enabled us to notice that at the potential E = −1.5 V for NaClO
4, a small peak was formed, the height of which increased in the presence of the smallest amounts of L-DOPA (c ≤ 1.0 × 10
−4 mol·dm
−3). Further growth of the drug concentration caused a gradual extinction of this peak. The peak observed in the solution at pH = 2.0 was probably related to the electrochemical reduction of perchlorate ions [
19].
2.2. Kinetics and Mechanism of Electroreduction of Zn2+ Ions in the Presence of Levodopa
Levodopa is an organic compound that is characterized by labile adsorption on the mercury surface in the range of Zn
2+ reduction potentials (
Section 2.1). It can therefore affect the rate of the aforementioned electrode process. The SWV voltammetry method allows for the qualitative assessment of whether and how the drug changes the kinetics of zinc electrodeposition on mercury.
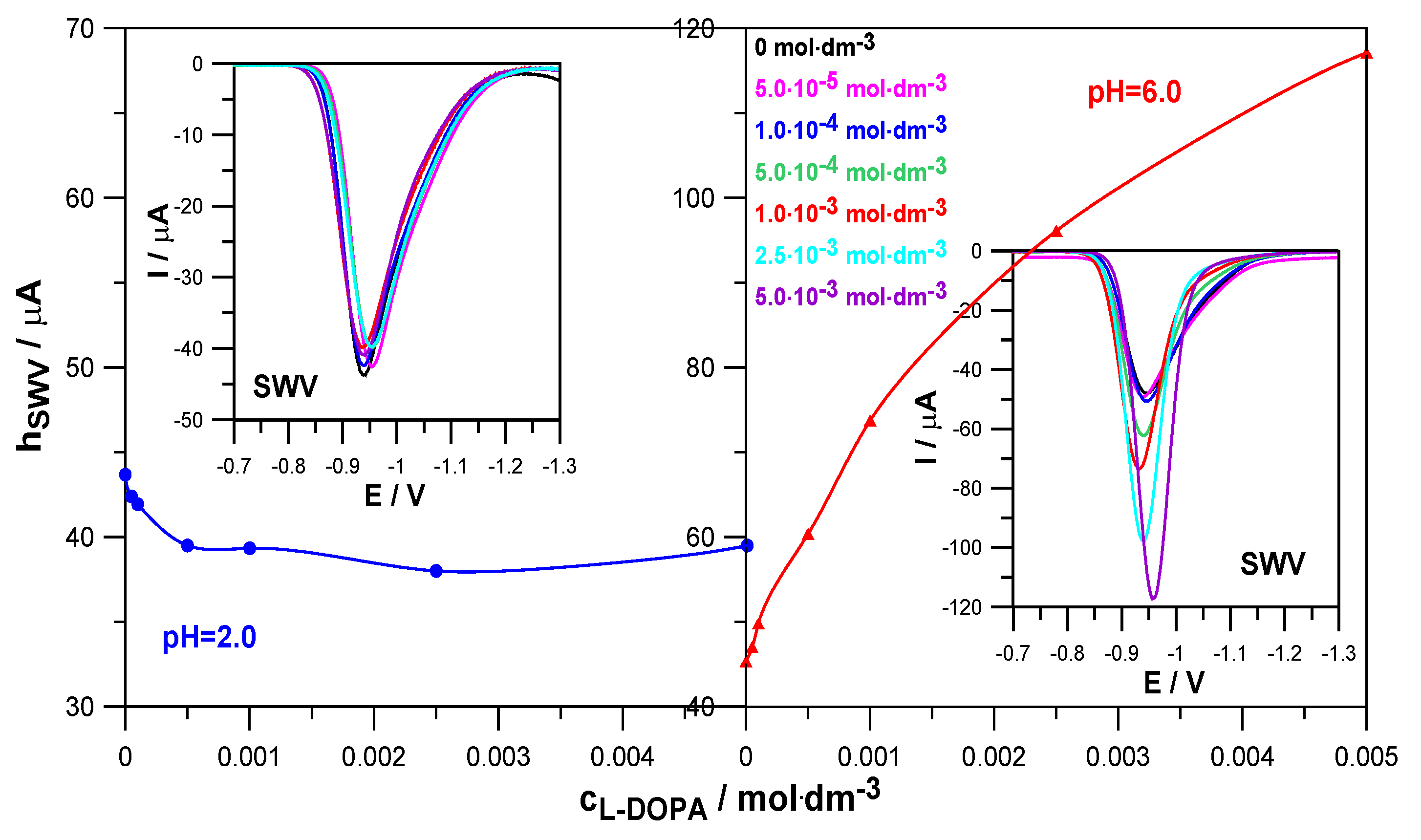
Figure 3 shows how an increase in L-DOPA concentration affects the height of SWV peaks in depolarizer solutions at pH = 2.0 and pH = 6.0.
In a solution with pH = 2.0, the presence of levodopa slightly reduced the height of the SWV peaks, compared to the height of the peak corresponding to the reference solution (Zn
2+ in the absence of levodopa). This indicates a slight inhibitory effect of this compound on the rate of the electroreduction of Zn
2+ ions. In a solution with pH = 6.0, a clear increase in the height of the SWV peaks and a decrease in the width of the peaks in the middle of their height were observed due to the increase in the concentration of levodopa. This proves its catalytic abilities on the kinetics of the analyzed electrode reaction [
20,
21]. Such different behavior in solutions with pH 2.0 and 6.0 probably resulted from the different forms in which levodopa occurred, depending on the pH.
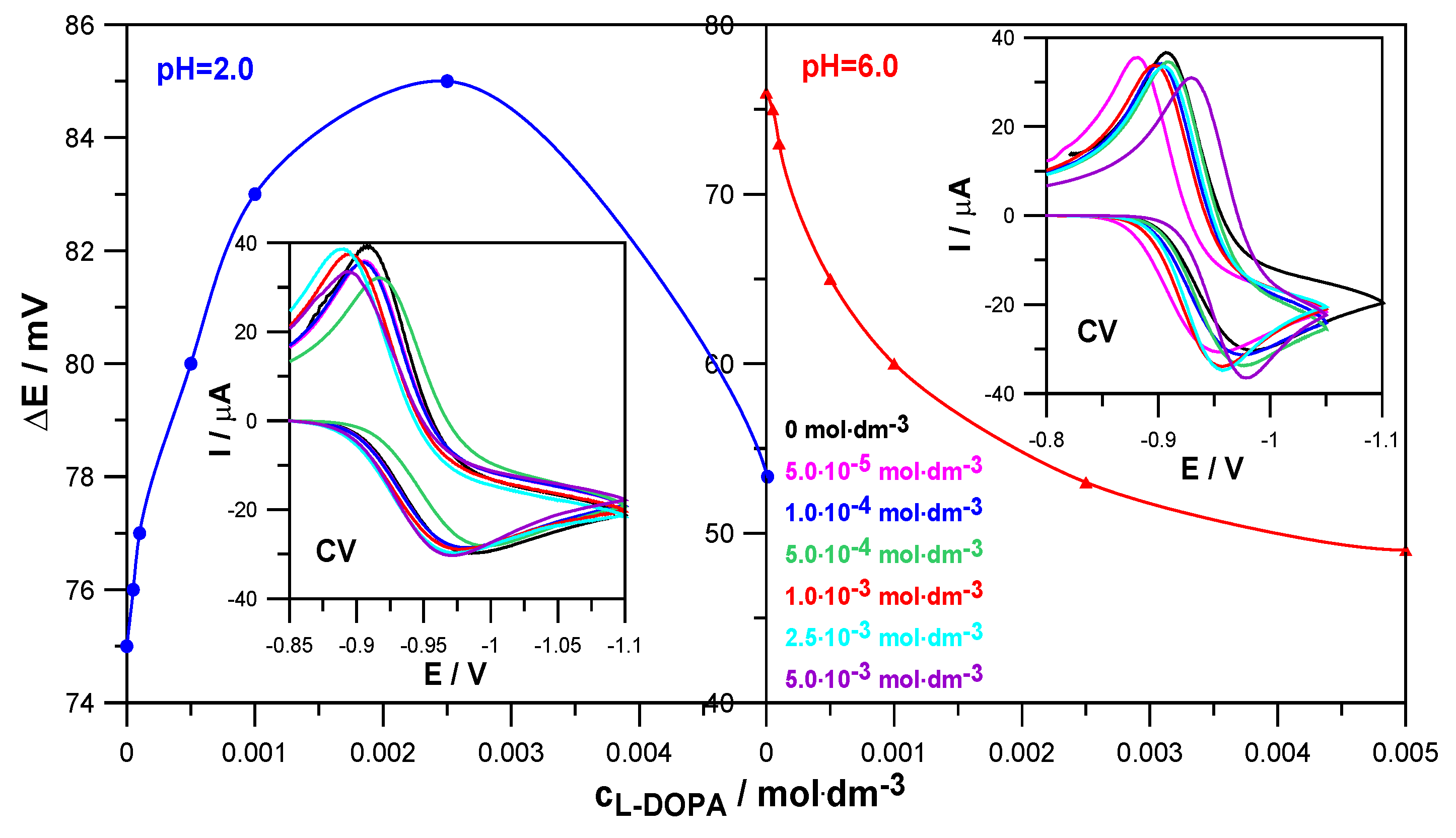
Similar conclusions were obtained based on the results of CV voltammetry measurements (
Figure 4). The values of the difference between the anodic and cathodic peak potentials, ∆E = 75 mV at pH = 2.0 and ∆E = 76 mV at pH = 6.0, obtained for the electroreduction of Zn
2+ in 1.0 mol·dm
−3 NaClO
4 in the absence of the drug indicate the quasi-reversible nature of this process. On the other hand, an increase in the concentration of levodopa resulted in a slight increase in the ∆E values in solutions with pH = 2.0 and a significant decrease in solutions with pH = 6.0. Such trends of changes confirm earlier conclusions about a slight slowing down of the electrodeposition of zinc on mercury by L-DOPA in solutions with a lower pH and an acceleration in solutions with a higher pH [
22]. It can be seen that the catalytic effect of the drug is much more pronounced than its inhibitory abilities.
In addition, CV voltammograms allowed us to determine the values of reversible half-wave potentials and formal potentials of the electroreduction of Zn
2+ ions in the analyzed systems [
23]. Since their values changed only slightly, it can be concluded that stable Zn
2+-levodopa complexes were not formed in solutions of pH = 2.0 and pH = 6.0 [
24].
The electrochemical impedance spectroscopy EIS method helps to determine the kinetics of electrode reactions and the characteristics of the electrode/electrolyte solution interface.
Figure 5 shows the EIS spectra recorded at formal potentials for the electroreduction of Zn
2+ ions in NaClO
4 at pH = 2.0 and pH = 6.0. Based on them, the values of the minimum charge-transfer resistance R
a(min) were determined, which, as a function of L-DOPA concentration, are also shown in
Figure 5. The decrease in the R
a(min) value in the presence of levodopa in solutions at pH = 6.0 indicates a clear catalytic effect of this compound on the electroreduction of Zn
2+. Different behavior is shown by the dependence R
a(min) = f(c
L-DOPA) obtained in a solution at pH = 2.0. The increase in the values of the minimum charge-transfer resistance caused by the increase in the drug concentration indicates the increasing difficulty of depolarization of Zn
2+ ions on the mercury surface [
25].
In order to precisely determine the effect of levodopa adsorption on the mercury electrode on the kinetics and mechanism of zinc electrodeposition, depending on the acidity of the solution, the electrode reaction rate constants k
f were determined for the analyzed systems as a function of the electrode potential. The following parameters were used for these calculations: charge-transfer resistance, reversible potential of the half-wave electrode reaction, and diffusion coefficients of zinc ions in the analyzed solutions. The details and calculation methodology were described in another paper [
24].
The obtained Tafel dependencies for solutions with different pHs are presented in
Figure 6.
Based on them, it can be seen that lnk
f = f(E − E
0f) was not linear and was characterized by a change in slope with the changes in electrode potential and levodopa concentration. This applied to both solutions with pH = 2.0 and pH = 6.0, although in a less acidic solution, this behavior was much more pronounced. This indicates the stepwise nature of the electroreduction of Zn
2+ ions in 1.0 mol·dm
−3 NaClO
4 and in solutions containing L-DOPA. This stepwise nature probably resulted from subsequent reactions related to the exchange of charge between the electrode and the depolarizer ions but also from the preceding chemical reactions related to the dehydration of Zn
2+ and Zn
+ aquaions, as well as the reaction of the formation of an active complex: depolarizer ions–organic substance adsorbed on the electrode surface. The stages related to water loss and the formation of active complexes in the multistage electroreduction of Zn
2+ ions occurred much faster than the charge transfer stages [
26,
27]. Based on a simple theoretical model, it can be assumed that the electroreduction of Zn
2+ on the mercury electrode, in particular in solutions with pH = 6.0, was carried out in two stages of transferring successive electrons [
28]. Of course, these stages were preceded by the above-mentioned fast chemical reactions.
During the analysis of
Figure 6, it can also be seen that in a solution with pH = 2.0, an increase in the concentration of L-DOPA caused the k
f values to decrease in a significant part of the potential range, compared to those obtained in the absence of the drug (black curve). The higher the concentration of L-DOPA was, the greater this decrease was. The inhibitory effect of levodopa on the electroreduction of zinc ions resulted from the blocking of the electrode surface by the L-DOPA adsorbed on it. It can be assumed that the adsorption of levodopa was facilitated by its cationic form, in which it occurred next to the zwitterion form in a solution with pH = 2.0. As a result, blocking the electrode led to an increase in the overpotential and made it difficult for positively charged zinc aquaions to approach the electrode surface [
21]. Different and definitely more pronounced changes in the kinetics of the electroreduction of Zn
2+ ions were caused by the adsorption of zwitterions in the form of which L-DOPA occurred in a solution at pH = 6.0. In the entire potential range analyzed, levodopa catalyzed the electrode reaction. The acceleration effect was greater the higher the drug concentration was. It can be assumed that the diffusion of depolarizer ions to the electrode was facilitated by the attractive interaction of the acetate group of levodopa adsorbed on the electrode surface. It is likely that in specific conditions prevailing on the electrode, the presence of Zn
2+ ions, which were in the immediate vicinity of the adsorption layer, promoted the transfer of H
+ ions from the NH
3+ group to the CH
3COO
− ion, thus unblocking the free electron pair of the nitrogen atom in the NH
2 group. Thanks to this, it was possible to create an active complex on the mercury surface: zinc ions–levodopa, mediating and facilitating the exchange of charge during the electrode process.
The measures of the catalytic/inhibitory capacity of the analyzed organic substances for the subsequent stages of the electrode process were the standard stepwise rate constants k
s1 and k
s2 [
25]. The change in their values with the increase in the L-DOPA concentration is shown in
Figure 7.
As can be seen, in a solution with pH = 2.0, the presence of L-DOPA inhibited both the first and second stages of Zn
2+ electroreduction. In a solution with pH = 6.0, both stages of the electrode reaction were accelerated. What made the influence of L-DOPA on the kinetics in both solutions similar was the fact that in both the lower and higher pH solutions, the first stage was slower and determined the speed of the entire electrode process. As already mentioned, it can be assumed that the first stage was associated with the exchange of the first electron between hydrated Zn
2+ ions and the mercury electrode. The second stage involved the exchange of another electron between the less hydrated Zn
+ ions and mercury. As is known, water molecules, both those from the hydration sphere of the depolarizer ions and those adsorbed on the electrode surface, constitute a significant barrier to zinc depolarization. The higher hydration of the Zn
2+ aquaion compared to the Zn
+ aquaion would explain the slower rate of the Zn
2+ + e → Zn
+ reaction than the rate of the Zn
+ + e → Zn(Hg) reaction [
23].
Investigating the inhibitory effect of levodopa on the rate of the first and second stages of the electroreduction of zinc ions on mercury in a solution with pH = 2.0 (
Figure 7), it can be seen that it was definitely smaller than its catalytic effect in a solution with pH = 6.0. The introduction of levodopa to a solution with a lower pH caused a slight decrease in the values of k
s1 (1.1-fold) and k
s2 (1.3-fold). In turn, the presence of the drug in a solution of NaClO
4 with pH = 6.0 caused a significant increase in the values of the standard rate constants of both stages. It should be noted here that both in the case of changes in the values of k
s1 = f(c
L-DOPA) and k
s2 = f(c
L-DOPA), two areas of linearity of these dependencies can be distinguished. Their occurrence proves the formation of various unstable active complexes on the mercury surface, which, according to a bridging model, facilitated the transfer of electrons across the inner layer [
29]. They were formed when the hydration sphere of Zn
2+ ions was partially degraded and therefore had a different composition. Further considering the change in the values of standard rate constants with the increasing concentration of L-DOPA in a solution of pH = 6.0, it can be seen that its influence on the transfer of the first electron was greater than on the transfer of the second electron. The values of k
s1 increased 7.5-fold at the highest drug concentration, and the values of k
s2 increased almost 5-fold. This meant that active complexes of depolarizer ions with the accelerating substance were already formed before the exchange of the first electron and were also formed before the exchange of the next electron [
30].
2.3. Molecular Dynamics Simulations
There is no doubt that experimental research is a basic tool in the hands of scientists. However, as in every case, as a complementary, it is worth reaching for theoretical methods. They often allow for a more detailed explanation of observed phenomena and sometimes point to new directions of studies.
To expand the knowledge of investigated systems, theoretical investigations of levodopa aqueous solutions were performed by means of molecular dynamics simulations using Gromacs 2024.04 suite [
31]. Calculations were conducted by applying General Amber Force Field (gaff), and the partial atomic charges were calculated using the RESP procedure [
32].
In the experimental part of this paper, we investigated levodopa solutions at pH = 2.0 and pH = 6.0. Frequently, the pH solution affects the degree of dissociation of dissolved electrolytes. This was also the case here. Frazao et al. [
6] showed the pH-dependent population of L-DOPA protonated states. The published research results showed that at pH = 6.0, levodopa existed only in the zwitterion dissociated form (see
Figure 1b), whereas at pH = 2.0, it was present in the solution in two states, with a population of 1:1 (
Figure 1a,b) [
6].
Initially, simulations were performed in a box cube with an edge length of 27 nm. We prepared a few boxes corresponding to different pH and levodopa concentrations: 0 M, 5.0 × 10−3 mol·dm−3, and 5.0 × 10−4 mol·dm−3 for pH = 2.0 and pH = 6.0. The selection of L-DOPA concentrations was determined by its ion number in the simulation box. In the case of its lowest concentration of 5.0 × 10−4 mol·dm−3, the applied box with an average volume of 19.683 nm3 included only four L-DOPA ions. If one wants to simulate solutions with its lower concentrations, the simulation box should be significantly enlarged, which will cause a high increase in simulation times.
Taking into account the research published by Frazao et al. [
6], systems with pH = 6.0 included only L-DOPA state (b), the “zwitterion anion”, whereas systems with pH = 2.0 were prepared by using equal numbers of both L-DOPA states, as shown in
Figure 1.
Simulation systems reflected the composition of solutions used in the experiment. Therefore, besides L-DOPA ions, boxes included adequate amounts of tip3p water, ClO4−, NO3−, Zn2+, and Na+ ions. Calculations were performed in the isothermal–isobaric ensemble at T = 298.15 K and p = 1 bar applying the Nose–Hoover thermostat and the Parrinello–Rahman barostat. For long-range electrostatic interactions, we used the PME algorithm. Lennard-Jones and columbic cut-off distances were set to 1.2 nm. Equations of motion were solved using the Verlet leap-frog algorithm with a time step of 1 fs. Each simulation was initially equilibrated for 20 ps and then run for 2.5 ns.
One of the most common and useful tools to analyze simulation data is the radial distribution function g(r) (RDF).
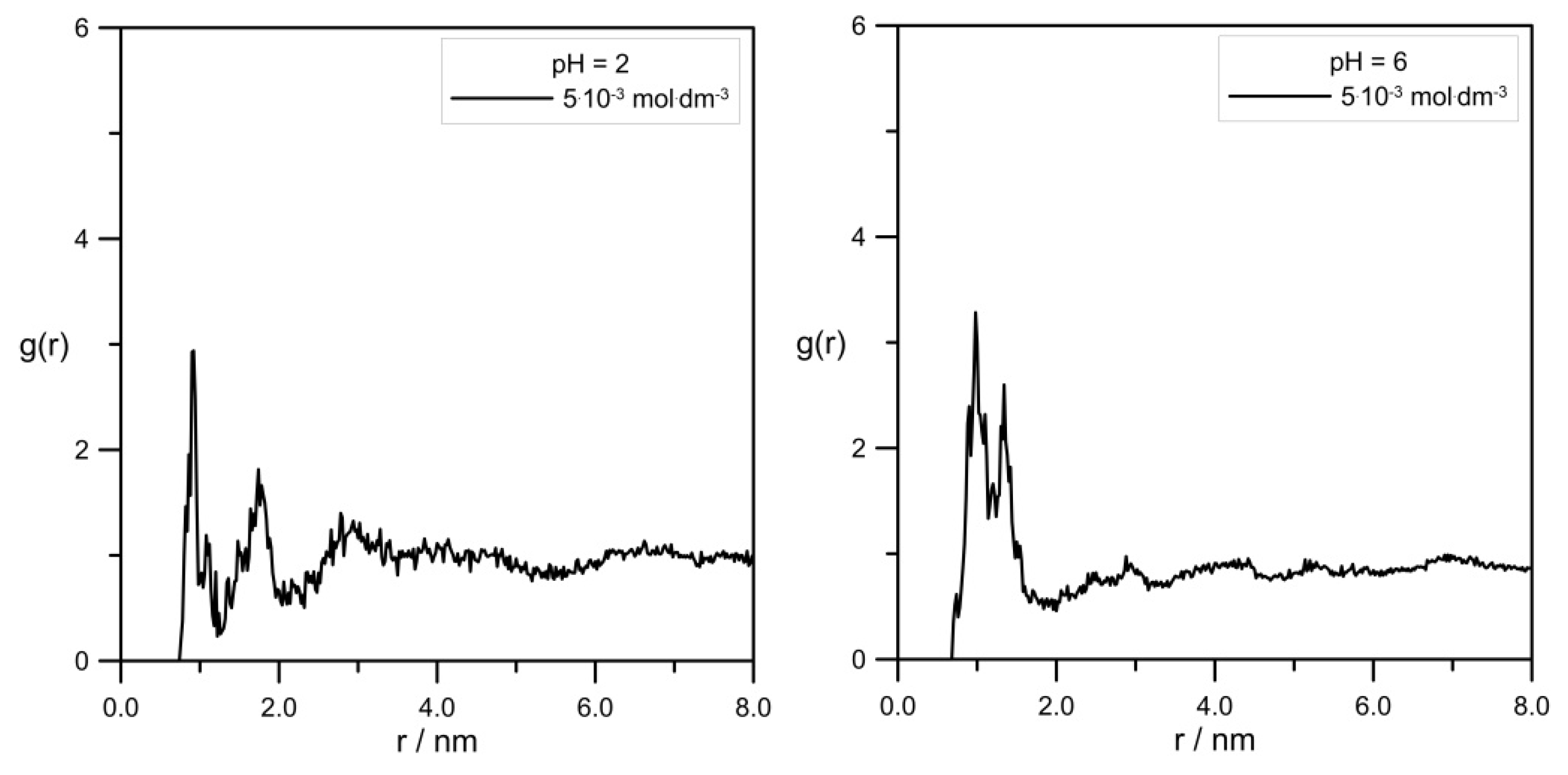
Figure 8 shows the comparison of RDFs calculated for the pair Zn
2+/tip3p water for systems with varied levodopa concentrations at pH = 2.0 and pH = 6.0.
Figure 9 shows a very interesting effect of L-DOPA on the solvation sphere of Zn
2+ ions. First of all, at the acidic pH = 2.0, the highest L-DOPA concentration had no effect on the number of water molecules in the zinc hydration sphere. In the case of pH = 6, this effect was visible: the increase in the levodopa concentration caused a decrease in the number of tip3p water in the zinc hydration sphere. What is more, pH and L-DOPA did not affect the radius of the hydration sphere. With the increasing distance between Zn
2+ and tip3p water, RDFs were at both pHs identical, and they were alike for 0 mol·dm
−3 and 5.0 × 10
−3 mol·dm
−3 levodopa concentrations as well.
The influence of L-DOPA on the mobility of zinc ions could be reflected by its mean square displacement (MSD). MSD is the measure of particle travel following a random walk plotted as the function of time. In addition to that, MSD is related to self-diffusivity D of a particle by the following formula:
where r is the displacement, and d is the dimension of a system. Strictly speaking, the diffusion coefficient can be determined from the tangent of the MSD slope divided, in our case, by six.
Figure 9 and
Table 1 show MSD curves determined for simulated systems and calculated zinc diffusion coefficients.
The values of Zn
2+ diffusion coefficients were in agreement with the conclusion drawn using RDFs, as shown in
Figure 8. In general, the hydration level of the zinc cation should affect its mobility in an aqueous solution. Because at pH = 2.0, the cationic L-DOPA form did not affect Zn
2+, the hydration number (tip3p water molecules in its hydration sphere), the value of the Zn
2+ diffusion coefficient was approximately unchanged (see
Table 1). At pH = 6.0, the number of water molecules in the Zn
2+ hydration sphere decreased from 4.6 to 4.3, and the Zn
2+ diffusion coefficient value slightly increased. However, diffusion coefficients determined from MSD were burdened with error, as shown in
Table 1, and the above conclusion can be drawn only when Zn
2+/tip3p RDFs calculations were taken into account.
MD calculations enabled us to draw a more precise view of the average structure of simulated systems.
Figure 10 shows pair correlation functions determined for the L-DOPA/Zn
2+ pair.
First of all, it should be emphasized again that simulation systems contained a small amount of Zn
2+ and L-DOPA molecules, and RDF curve shapes shown in
Figure 10 were far from perfect. Nevertheless, it can be seen that the distance between L-DOPA and the Zn
2+ ions was affected to a small extent by pH and levodopa concentrations. The most valuable data was for the 5 × 10
−3 mol·dm
−3 concentration because in these simulation systems, we obtained better statistics (the larger number of L-DOPA molecules in comparison to 5 × 10
−4 mol·dm
−3).
Figure 10 shows, at pH = 2.0, two peaks at approx. 0.9 nm and 1.8 nm, which corresponded to two different ionic forms of L-DOPA. At pH = 6, we can observe one peak at about 1.1 nm. It can be suspected that the protonated L-DOPA (
Figure 1a) was closer to Zn
2+ than its zwitterion form (
Figure 1b). The simultaneous presence of protonated -NH
3+ and dissociated -COO
− groups caused a serious change in charge density in the L-DOPA molecule. As a result, the protonated L-DOPA form attracted the zinc cation more strongly than its zwitterion form. This thesis confirmed the charge distribution of both levodopa states, as shown in
Figure 11. Moreover, a slight decrease in the hydration number of the Zn
2+ at pH = 6 also made it easier to get levodopa closer to the zinc cation.
Figure 12 demonstrates the comparison of pair correlation functions for the L-DOPA/tip3p pair.
The radial distribution functions in
Figure 12 were almost identical for all simulation systems. It indicated the lack of influence of pH and levodopa concentrations on the average mutual arrangement of levodopa and water. Minimal differences in L-DOPA/tip3p RDF shapes at pH = 6.0 were probably caused by the presence of the zwitterion form of the levodopa ion.