3.3. Characterization Data of Synthesized Compounds
Characterization data for the starting materials
4b–
e,
5a–
k, and
6a–
q, with their precursors reported in the
Supplementary Materials.
3.3.1. Characterization Data of Final Compounds 1b–g and 2a–k
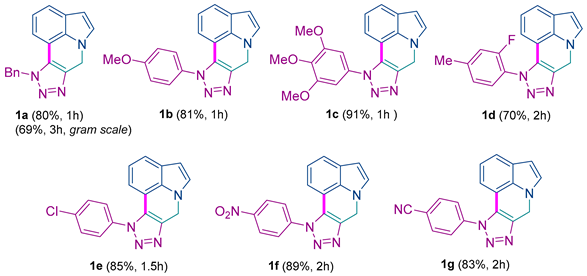
10-(4-methoxyphenyl)-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 1b: 81% yield; brown solid; mp: 238–240 °C; IR (neat): 3115, 2980, 1365, 911, 709 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.45 (d, J = 8.8 Hz, 2H), 7.40 (d, J = 8.3 Hz, 1H), 7.11 (d, J = 3.1 Hz, 1H), 7.03 (d, J = 8.8 Hz, 2H), 6.76 (t, J = 7.7 Hz, 1H), 6.59 (d, J = 7.3 Hz, 1H), 6.48 (d, J = 3.1 Hz, 1H), 5.69 (s, 2H), 3.85 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 161.0 (C), 139.1 (C), 133.6 (C), 129.9 (C), 129.8 (C), 127.4 (CH), 126.8 (CH), 125.9 (C), 122.5 (CH), 119.9 (CH), 114.8 (CH), 114.2 (CH), 109.3 (C), 103.7 (CH), 55.7 (CH3), 44.6 (CH2). HRMS: m/z [M + H]+ calcd for C18H15N4O: 303.1240; found: 303.1233.
10-(3,4,5-trimethoxyphenyl)-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 1c: 91% yield; yellow solid; mp: 240–242 °C; IR (neat): 3109, 2991, 1344, 924, 715 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.40 (d, J = 7.9 Hz, 1H), 7.08 (d, J = 2.9 Hz, 1H), 6.84–6.72 (m, 4H), 6.46 (d, J = 2.9 Hz, 1H), 5.63 (s, 2H), 3.88 (s, 3H), 3.80 (s, 6H); 13C NMR (100.6 MHz) (CDCl3): δ 153.8 (C), 139.4 (C), 139.2 (C), 133.6 (C), 132.3 (C), 129.8 (C), 126.9 (CH), 126.0 (C), 122.7 (CH), 120.0 (CH), 114.4 (CH), 109.1 (C), 103.7 (CH), 103.6 (CH), 61.2 (CH3), 56.5 (CH3), 44.5 (CH2). HRMS: m/z [M + H]+ calcd for C20H19N4O3: 363.1452; found: 363.1466.
10-(2-fluoro-4-methylphenyl)-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 1d: 70% yield; brown solid; mp: 270–272 °C; IR (neat): 2877, 1537, 1194, 820, 722 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.46–7.36 (m, 2H), 7.21–7.10 (m, 3H), 6.77 (t, J = 7.7 Hz, 1H), 6.52–6.45 (m, 2H), 5.73 (s, 2H), 2.46 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 156.6 (d, JC-F = 253.0 Hz, C), 143.8 (d, JC-F = 7.7 Hz, C), 138.9 (C), 133.6 (C), 131.0 (C), 128.3 (CH), 126.8 (CH), 125.9 (C), 125.79 (d, JC-F = 3.8 Hz, CH), 122.7 (CH), 122.4 (d, JC-F = 12.4 Hz, C), 120.1 (CH), 117.6 (d, JC-F = 18.6 Hz, CH), 113.7 (CH), 109.0 (C), 103.7 (CH), 44.6 (CH2), 21.6 (CH3); 19F NMR (376.5 MHz) (CDCl3): δ −121.29 (m). HRMS: m/z [M + H]+ calcd for C18H14FN4: 305.1197; found: 305.1181.
10-(4-chlorophenyl)-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 1e: 85% yield; yellow solid; mp: 230–232 °C; IR (neat): 3059, 1508, 1198, 1087, 717 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.59–7.47 (m, 4H), 7.41 (d, J = 8.1 Hz, 1H), 7.11 (d, J = 3.2 Hz, 1H), 6.77 (t, J = 7.7 Hz, 1H), 6.61 (d, J = 7.3 Hz, 1H), 6.49 (d, J = 3.2 Hz, 1H), 5.68 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 139.4 (C), 136.5 (C), 135.5 (C), 133.6 (C), 130.0 (C), 129.8 (CH), 127.3 (CH), 126.9 (CH), 126.1 (C), 122.9 (CH), 120.0 (CH), 114.2 (CH), 108.9 (C), 103.8 (CH), 44.5 (CH2). HRMS: m/z [M + H]+ calcd for C17H12ClN4: 307.0745; found: 307.0730.
10-(4-nitrophenyl)-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 1f: 89% yield; yellow solid; mp: 246–248 °C; IR (neat): 3102, 1486, 1092, 815 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 8.51 (d, J = 8.8 Hz, 2H), 7.90 (d, J = 8.8 Hz, 2H), 7.53 (d, J = 8.0 Hz, 1H), 7.22 (d, J = 3.0 Hz, 1H), 6.87 (t, J = 7.7 Hz, 1H), 6.75 (d, J = 7.1 Hz, 1H), 6.59 (d, J = 2.6 Hz, 1H), 5.80 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 148.8 (C), 142.1 (C), 140.3 (C), 133.9 (C), 130.2 (C), 127.3 (CH), 127.0 (CH), 126.6 (C), 125.4 (CH), 123.6 (CH), 120.3 (CH), 114.6 (CH), 108.7 (C), 104.3 (CH), 44.8 (CH2). HRMS: m/z [M + H]+ calcd for C17H12N5O2: 318.0985; found: 318.0973.
4-(pyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinolin-10(7H)-yl)benzonitrile 1g: 83% yield; yellow solid; mp: 232–234 °C; IR (neat): 3048, 1517, 1185, 1076, 678 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.96 (d, J = 8.2 Hz, 2H), 7.84 (d, J = 8.2 Hz, 2H), 7.54 (d, J = 7.8 Hz, 1H), 7.22 (d, J = 3.2 Hz, 1H), 6.90 (t, J = 7.7 Hz, 1H), 6.75 (d, J = 7.3 Hz, 1H), 6.61 (d, J = 3.2 Hz, 1H), 5.79 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 140.5 (C), 140.0 (C), 133.8 (CH), 133.7 (C), 129.9 (C), 127.1 (CH), 126.7 (CH), 126.3 (C), 123.4 (CH), 120.0 (CH), 117.6 (C), 114.4 (C), 114.3 (CH) 108.6 (C), 104.0 (CH), 44.6 (CH2). HRMS: m/z [M + H]+ calcd for C18H12N5: 298.1087; found: 298.1099.
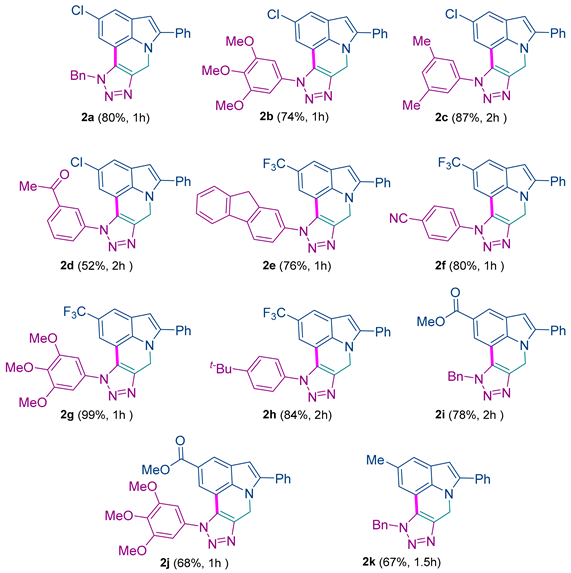
10-benzyl-2-chloro-5-phenyl-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 2a: 80% yield; white solid; mp: 240–242 °C; IR (neat): 3014, 1489, 1347, 1015, 985 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.63–7.21 (m, 10H), 7.16 (s, 1H), 6.91 (s, 1H), 6.43 (s, 1H), 5.77 (s, 2H), 5.54 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 142.0 (C), 140.8 (C), 134.2 (C), 132.9 (C), 131.4 (C), 129.2 (CH), 129.0 (CH), 128.8 (CH), 128.6 (CH), 128.5 (CH), 128.0 (C), 127.1 (C), 126.8 (CH), 126.0 (C), 121.0 (CH), 114.7 (CH), 110.3 (C), 102.9 (CH), 53.5 (CH2), 44.2 (CH2). HRMS: m/z [M + H]+ calcd for C24H18ClN4: 397.1214; found: 397.1225.
2-chloro-5-phenyl-10-(3,4,5-trimethoxyphenyl)-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 2b: 74% yield; brown solid; mp: 250–252 °C; IR (neat): 2924, 1596, 1414, 1228, 1127 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.53–7.48 (m, 2H), 7.45 (t, J = 7.5 Hz, 2H), 7.42–7.39 (m, 2H), 6.83 (s, 1H), 6.80 (s, 2H), 6.49 (s, 1H), 5.62 (s, 2H), 3.91 (s, 3H), 3.85 (s, 6H); 13C NMR (100.6 MHz) (CDCl3): δ 153.9 (C), 142.1 (C), 139.6 (C), 133.3 (C), 131.9 (C), 131.4 (C), 129.4 (C), 129.0 (CH), 128.9 (CH), 128.6 (CH), 127.9 (C), 127.3 (C), 125.9 (C), 121.4 (CH), 114.7 (CH), 110.4 (C), 103.3 (CH), 103.1 (CH), 61.2 (CH3), 56.5 (2CH3), 44.1(CH2); HRMS: m/z [M + H]+ calcd for C26H22ClN4O3: 473.1375; found: 473.1383.
2-chloro-10-(3,5-dimethylphenyl)-5-phenyl-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 2c: 87% yield; white solid; mp: 258–260 °C; IR (neat): 3179, 1380, 1259, 1170, 999 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.53–7.34 (m, 6H), 7.21–7.13 (m, 3H), 6.65 (d, J = 1.8 Hz, 1H), 6.46 (s, 1H), 5.60 (s, 2H), 2.38 (s, 6H); 13C NMR (100.6 MHz) (CDCl3): δ 142.0 (C), 140.0 (C), 139.8 (C), 136.4 (C), 133.2 (C), 132.2 (CH), 131.4 (C), 129.0 (CH), 128.8 (CH), 128.6 (CH), 128.4 (C), 127.2 (C), 125.8 (C), 123.4 (CH), 121.1 (CH), 114.6 (CH), 110.5 (C), 103.0 (CH), 44.1 (CH2), 21.3 (CH3); HRMS: m/z [M + H]+ calcd for C25H20ClN4: 411.1371; found: 411.1389.
1-(3-(2-chloro-5-phenylpyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinolin-10(7H)-yl)phenyl)ethan-1-one 2d: 52% yield; yellow solid; mp: 250–252 °C; IR (neat): 3185, 1734, 1495, 1107, 662 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 8.24–8.14 (m, 2H), 7.81–7.70 (m, 2H), 7.52–7.38 (m, 6H), 6.56 (d, J = 1.8 Hz, 1H), 6.48 (s, 1H), 5.62 (s, 2H), 2.61 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 196.2 (C), 142.2 (C), 140.4 (C), 138.6 (C), 137.0 (C), 133.2 (C), 131.3 (C), 130.3 (CH), 130.2 (CH), 129.9 (CH), 129.0 (CH), 128.9 (CH), 128.7 (C), 128.59 (C), 128.56 (CH), 127.4 (C), 125.8 (C), 125.7 (CH), 121.6 (CH), 114.3 (CH), 110.1 (C), 103.1 (CH), 44.1 (CH2), 26.8 (CH3); HRMS: m/z [M + H]+ calcd for C25H18ClN4O: 425.1164; found: 425.1152.
10-(9H-fluoren-2-yl)-5-phenyl-2-(trifluoromethyl)-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 2e: 76% yield; brown solid; mp: 280–282 °C; IR (neat): 2921, 1487, 1294, 1109, 735 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.94 (d, J = 8.1 Hz, 1H), 7.84 (d, J = 7.5 Hz, 1H), 7.77–7.70 (m, 2H), 7.61–7.50 (m, 4H), 7.48–7.30 (m, 5H), 7.00 (s, 1H), 6.62 (s, 1H), 5.67 (s, 2H), 3.97 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 144.8 (C), 144.1 (C), 143.8 (C), 142.6 (C), 140.2 (C), 140.1 (C), 136.0 (C), 134.7 (C), 131.2 (C), 129.1 (CH), 128.64 (CH), 128.55 (C), 127.9 (CH), 127.2 (CH), 125.7 (C), 125.35 (CH), 125.34 (CH), 124.4 (CH), 124.7 (q, JC-F = 273.0 Hz, C), 122.9 (q, JC-F = 31.3 Hz, C), 122.5 (CH), 120.72 (CH), 120.68 (CH), 119.7 (q, JC-F = 4.4 Hz, CH), 110.8 (q, JC-F = 4.4 Hz, CH), 110.0 (C), 104.2 (CH), 44.2 (CH2), 37.0 (CH2); 19F NMR (376.5 MHz) (CDCl3): δ −60.92 (s); HRMS: m/z [M + H]+ calcd for C31H20F3N4: 505.1635; found: 505.1647.
4-(5-phenyl-2-(trifluoromethyl)pyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinolin-10(7H)-yl)benzonitrile 2f: 80% yield; white solid; mp: 224–226 °C; IR (neat): 3176, 2284, 1240, 789, 655 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.84 (s, 1H), 7.67–7.52 (m, 9H), 7.00 (s, 1H), 6.73 (s, 1H), 5.75 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): 142.7 (C), 140.3 (C), 136.9 (C), 135.9 (C), 135.0 (C), 131.1 (C), 130.1 (CH), 129.1 (CH), 129.1 (CH), 128.6 (CH), 127.0 (CH), 125.8 (C), 124.5 (q, JC-F = 273.0 Hz, C), 122.9 (q, JC-F = 31.8 Hz, C), 120.0 (q, JC-F = 4.3 Hz, CH), 110.6 (q, JC-F = 4.3 Hz, CH), 109.6 (C), 104.3 (CH), 44.2 (CH2); 19F NMR (376.5 MHz) (CDCl3): δ −60.93 (s); HRMS: m/z [M + H]+ calcd for C25H15F3N5: 442.1274; found: 442.1283.
5-phenyl-2-(trifluoromethyl)-10-(3,4,5-trimethoxyphenyl)-7,10-dihydropyrrolo[3,2,1ij][1,2,3]triazolo[4,5-c]quinoline 2g: 99% yield; white solid; mp: 212–214 °C; IR (neat): 3125, 1602, 1336, 1126, 816 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.91 (s, 1H), 7.65 (s, 1H), 7.49 (s, 4H), 7.40 (s, 1H), 6.83 (s, 2H), 6.74 (s, 1H), 5.99 (s, 2H), 3.91 (s, 6H), 3.87 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 153.9 (C), 146.7 (C), 145.8 (C), 138.4 (C), 135.2 (C), 132.7 (C), 131.3 (C), 131.1 (C), 129.8 (CH), 129.2 (CH), 128.8 (CH), 124.0 (CH), 124.0 (q, JC-F = 273.0 Hz, C), 124.1 (q, JC-F = 31.8 Hz, C), 124.0 (q, JC-F = 3.6 Hz, CH), 119.9 (CH), 117.7 (q, JC-F = 3.6 Hz, CH), 105.0 (CH), 104.2 (C), 98.5 (CH), 61.0 (CH3), 56.4 (CH3), 41.5 (CH2); 19F NMR (376.5 MHz) (CDCl3): δ −60.78 (s); HRMS: m/z [M + H]+ calcd for C27H22F3N4O3: 507.1638; found: 507.1645.
10-(4-(tert-butyl)phenyl)-5-phenyl-2-(trifluoromethyl)-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 2h: 84% yield; orange solid; mp: 230–232 °C; IR (neat): 3152, 2978, 1325, 1254, 595 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.91 (s, 1H), 7.65 (s, 1H), 7.57–7.52 (m, 2H), 7.51–7.46 (m, 6H), 7.37 (s, 1H), 6.73 (s, 1H), 6.00 (s, 2H), 1.35 (s, 9H); 13C NMR (100.6 MHz) (CDCl3): δ 154.4 (C), 142.5 (C), 139.9 (C), 136.0 (C), 133.8 (C), 131.2 (C), 129.1 (CH), 128.71 (C), 128.62 (CH), 126.8 (CH), 125.67 (C), 125.45 (CH), 124.7 (q, JC-F = 273.2 Hz, C) 122.8 (q, JC-F = 31.5 Hz, C), 120.5 (d, JC-F = 4.0 Hz, C), 119.6 (q, JC-F = 3.7 Hz, CH), 110.6 (q, JC-F = 3.7 Hz, CH), 109.8 (C), 104.1 (CH), 44.2 (CH2), 35.1 (C), 31.3 (CH3); 19F NMR (376.5 MHz) (CDCl3): δ −60.93 (s); HRMS: m/z [M + H]+ calcd for C28H24F3N4: 473.1948; found: 473.1961.
methyl 10-benzyl-5-phenyl-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline-2-carboxylate 2i: 78% yield; white solid; mp: 242–244 °C; IR (neat): 3042, 1768, 1225, 1015, 966 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 8.15 (s, 1H), 7.74 (s, 1H), 7.50–7.34 (m, 5H), 7.33–7.16 (m, 5H), 6.56 (s, 1H), 5.83 (s, 2H), 5.56 (s, 2H), 3.85 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 167.4 (C), 161.4 (C), 142.1 (C), 140.4 (C), 136.8 (C), 134.3 (C), 131.3 (C), 129.1 (CH), 129.0 (CH), 128.9 (CH), 128.6 (CH), 128.5 (CH), 127.1 (CH), 125.7 (C), 124.8 (CH), 122.6 (C), 115.5 (CH), 109.1 (C), 104.6 (CH), 53.6 (CH2), 52.1 (CH3), 44.3 (CH2); HRMS: m/z [M + H]+ calcd for C26H21N4O2: 421.1659; found: 421.1677.
methyl 5-phenyl-10-(3,4,5-trimethoxyphenyl)-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo [4,5-c]quinoline-2-carboxylate 2j: 68% yield; white solid; mp: 242–244 °C; IR (neat): 3108, 2897, 1759, 1047, 987 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 8.34 (s, 1H), 8.12 (s, 1H), 7.48 (s, 4H), 7.42 (s, 1H), 6.83 (s, 2H), 6.74 (s, 1H), 5.99 (s, 2H), 3.96 (s, 3H), 3.90 (s, 6H), 3.87 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 166.7 (C), 153.9 (C), 147.0 (C), 145.2 (C), 138.4 (C), 136.2 (C), 132.7 (C), 131.4 (C), 131.2 (C), 129.8 (CH), 129.1 (CH), 128.8 (CH), 128.6 (CH), 123.8 (C), 122.7 (CH), 119.9 (CH), 105.5 (CH), 103.7 (C), 98.5 (CH), 61.1 (CH3), 56.5 (CH3), 52.2 (CH3), 41.6 (CH2); HRMS: m/z [M + H]+ calcd for C28H25N4O5: 497.1819; found: 497.1826.
10-benzyl-2-methyl-5-phenyl-7,10-dihydropyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinoline 2k: 67% yield; white solid; mp: 250–252 °C; IR (neat): 3314, 3111, 1487, 1019, 971 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.65–7.42 (m, 5H), 7.42–7.22 (m, 6H), 6.91 (s, 1H), 6.53 (s, 1H), 5.91 (s, 2H), 5.67 (s, 2H), 2.37 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 140.9 (C), 140.5 (C), 134.7 (C), 133.3 (C), 132.0 (C), 129.7 (C), 129.1 (CH), 129.0 (CH), 128.9 (CH), 128.5 (CH), 128.41 (CH), 128.36 (CH), 126.8 (CH), 126.6 (C), 121.5 (CH), 116.0 (CH), 109.1 (C), 102.8 (CH), 53.4 (CH2), 44.1 (CH2), 21.7 (CH3); HRMS: m/z [M + H]+ calcd for C25H21N4: 377.1761; found: 377.1749.
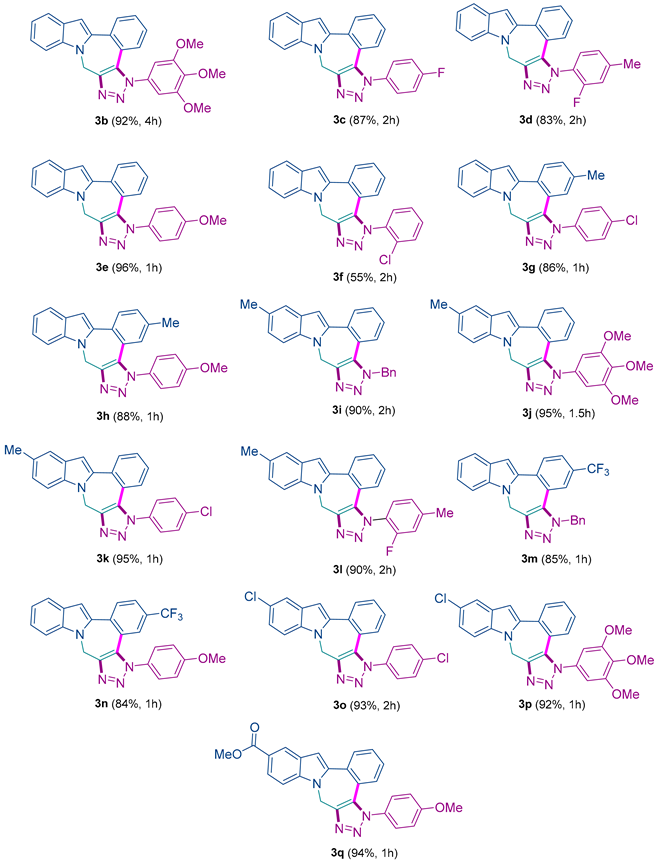
3.3.2. Characterization Data of Final Compounds 3b–k
5-(3,4,5-trimethoxyphenyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino [1,2-a]indole 3b: 92% yield; yellow solid; mp: 192–194 °C; IR (neat): 1506, 1460, 1230, 1032, 748 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.81 (d, J = 7.8 Hz, 1H), 7.56 (d, J = 7.8 Hz, 1H), 7.49 (d, J = 8.4 Hz, 1H), 7.36 (t, J = 7.6 Hz, 1H), 7.22–7.15 (m, 2H), 7.05–6.99 (m, 2H), 6.77 (s, 1H), 6.53 (s, 2H), 5.33 (s, 2H), 3.80 (s, 3H), 3.65 (s, 6H); 13C NMR (100.6 MHz) (CDCl3): δ 153.8 (C), 144.6 (C), 138.9 (C), 138.5 (C), 136.9 (C), 133.7 (C), 132.2 (C), 132.1 (C), 131.6 (CH), 129.62 (CH), 128.6 (CH), 127.7 (CH), 127.6 (C), 122.7 (C), 122.6 (CH), 120.8 (CH), 120.2 (CH), 109.3 (CH), 103.9 (CH), 102.82 (CH), 61.10 (CH3), 56.40 (CH3), 39.4 (CH2). HRMS: m/z [M + H]+ calcd for C26H23N4O3: 439.1765; found: 439.1774.
5-(4-fluorophenyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino [1,2-a]indole 3c: 87% yield; white solid; mp: 243–245 °C; IR (neat): 1596, 1457, 1237, 1032, 762, 669 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.80 (d, J = 7.9 Hz, 1H), 7.57 (d, J = 7.9 Hz, 1H), 7.49 (d, J = 7.34 Hz, 1H), 7.38–7.30 (m, 3H), 7.21 (t, J = 7.5 Hz, 1H), 7.14 (t, J = 7.1 Hz, 1H), 7.10–7.03 (m, 3H), 6.87 (d, J = 7.8 Hz, 1H), 6.76 (s, 1H), 5.34 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 162.9 (d, JC-F = 254 Hz, C), 144.8 (C), 138.5 (C), 137.0 (C), 133.9 (C), 132.8 (d, JC-F = 3.1 Hz, C), 132.5 (C), 131.8 (CH), 129.7 (CH), 128.5 (CH), 127.8 (C), 127.7 (CH), 127.1 (d, JC-F = 8.7 Hz, CH), 122.7 (C), 122.7 (CH), 121.0 (CH), 120.2 (CH), 116.8 (d, JC-F = 22.6 Hz, CH), 109.3 (CH), 104.0 (CH), 39.5 (CH2); 19F NMR (376.5 MHz) (CDCl3): δ −110.36 (m). HRMS: m/z [M + H]+ calcd for C28H25N4O5: 497.1819; found: 497.1827.
5-(2-fluoro-4-methylphenyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino [1,2-a]indole 3d: 83% yield; orange solid; mp: 255–257 °C; IR (neat): 1506, 1456, 1230, 1019, 989 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.78 (d, J = 7.8 Hz, 1H), 7.56 (d, J = 7.8 Hz, 1H), 7.49 (d, J = 8.3 Hz, 1H), 7.34–7.26 (m, 2H), 7.20 (t, J = 7.4 Hz, 1H), 7.10 (td, J1 = 7.9 Hz, J2 = 0.8 Hz, 1H), 7.03 (t, J = 7.5 Hz, 1H), 6.99 (d, J = 8.0 Hz, 1H), 6.92–6.89 (m, 2H), 6.74 (s, 1H), 5.34 (s, 2H), 2.32 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 155.6 (d, JC-F = 254 Hz, C), 143.8 (C), 143.1 (d, JC-F = 7.41 Hz, C), 138.6 (C), 137.0 (C), 135.3 (C), 132.2 (C), 131.7 (CH), 129.7 (CH), 127.9 (C), 127.8 (d, JC-F = 8.1 Hz, CH), 126.9 (CH), 125.9 (d, JC-F = 3.54 Hz, CH), 122.9 (C), 122.5 (CH), 122.2 (d, JC-F = 12.56 Hz, C), 120.9 (CH), 120.1 (CH), 117.6 (d, JC-F = 18.30Hz, CH), 109.3 (CH), 104.0 (CH), 39.4 (CH2), 21.5 (CH3); 19F NMR (376.5 MHz) (CDCl3): δ −121.03 (bs). HRMS: m/z [M + H]+ calcd for C24H18FN4: 381.1510; found: 381.1531.
5-(4-methoxyphenyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino[1,2-a]indole 3e: 96% yield; white solid; mp: 211–213 °C; IR (neat): 1509, 1458, 1250, 1032, 832 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.81 (d, J1 = 8.2 Hz, J2 = 0.6 Hz 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.51 (d, J = 8.3 Hz, 1H), 7.36 (td, J1 = 7.7 Hz, J2 = 0.7 Hz, 1H), 7.27–7.20 (m, 3H), 7.13 (td, J1 = 7.5 Hz, J2 = 1.1 Hz, 1H), 7.06 (t, J = 7.5 Hz, 1H), 6.93–6.87 (m, 3H), 6.76 (s, 1H), 5.36 (s, 2H), 3.78 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): 160.4 (C), 144.6 (C), 138.7 (C), 137.0 (C), 133.8 (C), 132.4 (C), 131.7 (CH), 129.7 (C), 129. (CH), 128.5 (CH), 127.9 (C), 127.7 (CH), 126.6 (CH), 123.1 (C), 122.6 (CH), 120.9 (CH), 120.2 (CH), 114.8 (CH), 109.4 (CH), 103.9 (CH), 55.7 (CH3), 39.5 (CH2). HRMS: m/z [M + H]+ calcd for C24H19N4O: 379.1553; found: 379.1565.
5-(2-chlorophenyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino[1,2-a]indole 3f: 55% yield; white solid; mp: 210–212 °C; IR (neat): 1508, 1457, 1125, 1037, 764 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.83 (dd, J1= 7.85 Hz, J2= 0.54 Hz, 1H), 7.60 (d, J = 7.86 Hz, 1H), 7.54 (d, J = 8.40 Hz, 1H), 7.50–7.35 (m, 5H), 7.25 (td, J1= 15.39 Hz, J2= 7.67 Hz, J3= 0.92 Hz, 1H), 7.13–7.06 (m, 2H), 6.82 (dd, J1= 8.05 Hz, J2= 0.53 Hz, 1H), 6.77 (s, 1H), 5.49–5.34 (m, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 14.72 (C), 138.64 (C), 137.13 (C), 135.56 (C), 134.78 (C), 132.36 (C), 131.81 (CH), 131.71 (CH), 131.61 (C), 131.05 (CH), 129.78 (CH), 129.21 (CH), 128.22 (CH), 127.97 (C), 127.95 (CH), 127.02 (CH), 122.96 (C), 122.65 (CH), 121.04 (CH), 120.20 (CH), 109.43 (CH), 104.13 (CH), 39.54 (CH2). HRMS: m/z [M + H]+ calcd for C23H16ClN4: 383.1058; found: 383.1073.
5-(4-chlorophenyl)-3-methyl-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino [1,2-a]indole 3g: 86% yield; yellow solid; mp: 281–283 °C; IR (neat): 1501, 1460, 1228, 989, 750 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.63 (s, 1H), 7.57 (d, J = 7.8 Hz, 1H), 7.49 (d, J = 8.3 Hz, 1H), 7.37–7.35 (m, 2H), 7.29–7.27 (m, 2H), 7.23–7.19 (m, 1H), 7.05 (t, J = 7.4 Hz, 1H), 6.97 (dd, J1 = 7.9 Hz, J2 = 0.9 Hz, 1H), 6.76 (d, J = 7.8 Hz, 2H), 5.33 (s, 2H), 2.36 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 144.7 (C), 140.0 (C), 138.6 (C), 136.9 (C), 135.6 (C), 135.3 (C), 133.9 (C), 132.4 (C), 132.3 (CH), 129.9 (CH), 128.8 (CH), 128.5 (CH), 127.8 (C), 126.3 (CH), 122.6 (CH), 120.9 (CH), 120.2 (CH), 119.9 (C), 109.3 (CH), 103.8 (CH), 39.4 (CH2), 21.5 (CH3). HRMS: m/z [M + H]+ calcd for C24H18ClN4: 397.1214; found: 397.1203.
5-(4-methoxyphenyl)-3-methyl-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino [1,2-a]indole 3h: 88% yield; yellow solid; mp: 237–238 °C; IR (neat): 1513, 1555, 1034, 742, 670 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.63 (s, 1H), 7.57 (d, J = 7.8 Hz, 1H), 7.50 (d, J = 8.3 Hz, 1H), 7.26–7.20 (m, 3H), 7.05 (t, J = 7.4, 1H), 6.95 (dd, J1 = 7.9 Hz, J2 = 1.1 Hz, 1H), 6.91–6.87 (m, 2H), 6.80 (d, J = 8.05 Hz, 1H), 6.76 (s, 1H), 5.33 (s, 2H), 3.78 (s, 3H), 2.35 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 160.4 (C), 144.2 (C), 139.7 (C), 138.9 (C), 136.9 (C), 133.9 (C), 132.3 (C), 132.1 (CH), 129.8 (C), 128.7 (CH), 128.4 (CH), 127.9 (C), 126.5 (CH), 122.5 (CH), 120.9 (CH), 120.4 (C), 120.1 (CH), 114.8 (CH), 109.4 (CH), 103.7 (CH), 55.7 (CH3), 39.6 (CH2), 21.5 (CH3). HRMS: m/z [M + H]+ calcd for C25H21N4O: 393.1710; found: 393.1722.
5-benzyl-12-methyl-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino[1,2-a] indole 3i: 90% yield; white solid; mp: 163–165 °C; IR (neat): 1510, 1452, 1032, 762, 738 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.80 (d, J = 7.9 Hz, 1H), 7.43–7.37 (m, 2H), 7.33 (s, 1H), 7.3–7.24 (m, 5H), 7.14–7.12 (m, 2H), 7.03 (dd, J1 = 8.5 Hz, J2 = 1.3 Hz, 1H), 6.61 (s, 1H), 5.59 (s, 2H), 5.29 (s, 2H), 2.37 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 144.3 (C), 138.4 (C), 135.5 (C), 134.6 (C), 132.7 (C), 131.9 (CH), 129.8 (CH), 129.3 (C), 129.2 (CH), 128.4 (CH), 128.1 (C), 128.0 (CH), 127.7 (CH), 126.8 (CH), 124.3 (CH), 122.9 (C), 120.5 (CH), 109.1 (CH), 103.3 (CH), 52.5 (CH2), 39.6 (CH2), 21.5 (CH3). HRMS: m/z [M + H]+ calcd for C25H21N4: 377.1761; found: 377.1778.
12-methyl-5-(3,4,5-trimethoxyphenyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6] azepino[1,2-a]indole 3j: 95% yield; white solid; mp: 234–236 °C; IR (neat): 1596, 1450, 1224, 1024, 1031 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ (dd, J1 =7.9 Hz, J2 = 0.78 Hz, 1H), 7.51–7.46 (m, 3H), 7.29–7.25 (m, 1H), 7.16 (dd, J1 = 8.5 Hz, J2 = 1.2 Hz, 1H), 7.10 (dd, J1 = 7.9 Hz, J2 = 0.8 Hz, 1H), 6.81 (s, 1H), 6.65 (s, 2H), 5.44 (s, 2H), 3.93 (s, 3H), 3.78 (s, 6H), 2.49 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ153.8 (C), 144.7 (C), 138.9 (C), 138.6 (C), 135.5 (C), 133.8 (C), 132.4 (C), 132.3 (C), 131.6 (CH), 129.6 (C), 129.5 (CH), 128.7 (CH), 128.1 (C), 127.5 (CH), 124.5 (CH), 122.7 (C), 120.4 (CH), 109.1 (CH), 103.4 (CH), 102.9 (CH), 61.2 (CH3), 56.5 (CH3), 39.6 (CH2), 21.5 (CH3). HRMS: m/z [M + H]+ calcd for C27H25N4O3: 453.1921; found: 453.1907.
5-(4-chlorophenyl)-12-methyl-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6] azepino [1,2-a]indole 3k: 95% yield; white solid; mp: 215–217 °C; IR (neat): 1499, 1456, 1120, 1041, 789 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ (dd, J1 = 7.9 Hz, J2 = 0.7 Hz, 1H), 7.39–7.36 (m, 5H), 7.30–7.28 (m, 2H), 7.17–7.13 (m, 1H), 7.05 (dd, J1= 8.5 Hz, J2 = 1.1 Hz, 1H), 6.88 (dd, J1 = 7.8 Hz, J2 = 0.8 Hz, 1H), 6.68 (s, 1H), 5.32 (s, 2H), 2.37 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 145.1 (C), 138.4 (C), 135.7 (C), 135.6 (C), 135.3 (C), 133.8 (C), 132.6 (C), 131.8 (CH), 130.0 (CH), 129.8 (CH), 129.5 (C), 128.6 (CH), 128.1 (C), 127.7 (CH), 126.4 (CH), 124.5 (CH), 122.6 (C), 120.5 (CH), 109.0 (CH), 103.5 (CH), 39.6 (CH2), 21.5 (CH3). HRMS: m/z [M + H]+ calcd for C24H18ClN4: 397.1214; found: 397.1228.
5-(2-fluoro-4-methylphenyl)-12-methyl-5,8-dihydrobenzo[3,4][1,2,3]triazolo [4′,5′:5,6]azepino[1,2-a]indole 3l: 90% yield; white solid; mp: 231–233 °C; IR (neat): 1499, 1448, 1338, 1053, 767 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.78 (dd, J1 = 8.0 Hz, J2 = 0.8 Hz, 1H), 7.39–7.27 (m, 4H), 7.12–7.08 (m, 1H), 7.05–6.99 (m, 2H), 6.94–6.89 (m, 2H), 6.66 (s, 1H), 5.33 (s, 2H), 2.36 (s, 3H), 2.34 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 155.7 (JC-F = 253.0 Hz, C), 143.9 (C), 143.14 (JC-F = 7.5 Hz, C), 138.6 (C), 135.5 (C), 135.3 (C), 132.i4 (C), 131.7 (CH), 129.6 (CH), 129.4 (C), 128.2 (C), 127.8 (JC-F = 10.2 Hz, CH), 126.9 (CH), 125.9 (JC-F = 3.16 Hz, CH), 124.3 (CH), 122.9 (C), 122.3 (JC-F = 12.5 Hz, C), 120.5 (CH), 117.6 (JC-F = 18.8 Hz, CH), 109.0 (CH), 103.5 (CH3), 39.5 (CH2), 21.5 (CH3); 19F NMR (376.5 MHz) (CDCl3): δ −121.01 (bs). HRMS: m/z [M + H]+ calcd for C25H20FN4: 395.1666; found: 395.1681.
5-benzyl-3-(trifluoromethyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino [1,2-a]indole 3m: 85% yield; gray solid; mp: 227–229 °C; IR (neat): 1455, 1338, 1124, 1031, 738 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.90 (d, J = 8.2 Hz, 1H), 7.62 (dd, J1 = 8.3 Hz, J2 = 1.2 Hz, 1H), 7.57 (d, J = 8.06 Hz, 1H), 7.54–7.47 (m, 2H), 7.33–7.23 (m, 4H), 7.21 (m, 2H), 7.09–7.05 (m, 1H), 6.67 (s, 1H), 5.58 (s, 2H), 5.33 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 144.6 (C), 137.4 (C), 137.0 (C), 135.6 (C), 134.8 (C), 133.4 (C), 132.3 (CH), 131.2 (C), 130.0 (JC-F = 33.0 Hz) (C), 129.4 (CH), 128.8 (CH), 127.7 (C), 127.1 (CH), 126.2 (JC-F = 3.5 Hz, CH), 125.1 (JC-F = 3.6 Hz, CH), 123.8 (JC-F = 272.0 Hz, C), 123.4 (CH), 121.3 (CH), 120.5 (CH), 109.5 (CH), 105.2 (CH), 53.1 (CH2), 39.5 (CH2); 19F NMR (376.5 MHz) (CDCl3): δ −62.64 (s). HRMS: m/z [M + H]+ calcd for C25H18F3N4: 431.1478; found: 431.1485.
5-(4-methoxyphenyl)-3-(trifluoromethyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo [4′,5′:5,6]azepino[1,2-a]indole 3n: 84% yield; yellow solid; mp: 260–262 °C; IR (neat): 1513, 1336, 1296, 1251, 1123 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.93 (d, J = 8.3 Hz, 1H), 7.62–7.57 (m, 2H), 7.53 (d, J = 8.4 Hz, 1H), 7.29–7.22 (m, 3H), 7.14–7.07 (m, 2H), 6.95–6.90 (m, 2H), 6.85 (s, 1H), 5.40 (s, 2H), 3.79 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 160.8 (C), 144.8 (C), 137.4 (C), 137.2 (C), 135.5 (C), 132.76 (C), 132.0 (CH), 129.6 (q, JC-F = 33.0 Hz, C), 129.1 (C), 127.8 (C), 126.6 (CH), 125.8 (q, JC-F = 3.5 Hz, CH), 125.5 (q, JC-F = 3.8 Hz, CH), 123.5 (C), 123.4 (CH), 123.2 (q, JC-F = 270.3 Hz), 121.3 (CH), 120.6 (CH), 115.09 (CH), 109.53 (CH), 105.41 (CH), 55.8 (CH3), 39.6 (CH2); 19F NMR (376.5 MHz) (CDCl3): δ −62.24 (s). HRMS: m/z [M + H]+ calcd for C25H18F3N4O: 447.1427; found: 447.1411.
12-chloro-5-(4-chlorophenyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino [1,2-a]indole 3o: 93% yield; yellow solid; mp: 273–275 °C; IR (neat): 1497, 1333, 992, 760, 539 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.81 (d, J1 = 7.9 Hz, 1H), 7.55 (d, J = 1.8 Hz, 1H), 7.43–7.38 (m, 4H), 7.4–7.28 (m, 2H), 7.20–7.16 (m, 2H), 6.91 (dd, J1 = 7.9 Hz, J2 = 0.9 Hz, 1H), 6.71 (s, 1H), 5.33 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 144.8 (C), 139.7 (C), 135.8 (C), 135.4 (C), 135.1 (C), 133.8 (C), 132.0 (C), 131.9 (CH), 130.0 (CH), 129.9 (CH), 128.7 (C), 128.6 (CH), 128.2 (CH), 126.3 (CH), 125.9 (C), 123.0 (CH), 122.7 (C), 120.3 (CH), 110.4 (CH), 103.5 (CH), 39.8 (CH3). HRMS: m/z [M + H]+ calcd for C23H15Cl2N4: 417.0668; found: 417.0653.
12-chloro-5-(3,4,5-trimethoxyphenyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6] azepino[1,2-a]indole 3p: 92% yield; yellow solid; mp: 232–234 °C; IR (neat): 1605, 1461, 1125, 826, 774 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.81 (dd, J1 = 7.9 Hz, J2 = 0.7 Hz, 1H), 7.54 (d, J = 1.9 Hz, 1H), 7.43–7.38 (m, 2H); 7.24–7.14 (m, 2H); 7.02 (dd, J1 = 7.9 Hz, J2 = 0.7 Hz; 1H), 6.72 (s, 1H), 6.56 (s, 2H), 5.33 (s, 2H), 3.83 (s, 3H), 3.68 (s, 6H); 13C NMR (100.6 MHz) (CDCl3): δ 153.9 (C), 144.4 (C), 139.9 (C), 139.0 (C), 135.3 (C), 133.7 (C), 132.1 (C), 131.8 (C), 131.7 (CH), 129.8 (CH), 128.7 (CH), 128.7 (C), 128.0 (CH), 125.8 (C), 122.9 (CH), 122.8 (C), 120.2 (CH), 110.4 (CH), 103.4 (CH), 102.9 (CH), 61.2 (CH3), 56.5 (CH3), 39.8 (CH2). HRMS: m/z [M + H]+ calcd for C26H22ClN4O3: 473.1375; found: 473.1389.
methyl 5-(4-methoxyphenyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6] azepino[1,2-a]indole-12-carboxylate 3q: 94% yield; white solid; mp: 200–202 °C; IR (neat): 1703, 1508, 1243, 1053, 1032 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 8.34 (d, J = 1.1 Hz, 1H), 7.90 (dd, J1 = 8.8 Hz, J2 = 1.4 Hz, 1H), 7.79 (d, J = 7.9 Hz, 1H), 7.49 (d, J = 8.8 Hz, 1H), 7.35 (dt, J1 = 8.0 Hz, J2 = 1.1 Hz, 1H), 7.23 (d, J = 8.8 Hz, 2H), 7.15 (dt, J1 = 8.0 Hz, J2 = 1.1 Hz, 1H), 6.93–6.87 (m, 3H), 6.82 (s, 1H), 5.35 (s, 2H), 3.84 (s, 3H), 3.76 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 168.0 (C), 160.5 (C), 144.1 (C), 140.2 (C), 139.2 (C), 133.7 (C), 131.8 (CH), 131.7 (C), 129.6 (CH), 129.5 (C), 128.5 (CH), 128.1 (CH), 127.4 (C), 126.5 (CH), 124.1 (CH), 123.7 (CH), 123.2 (C), 122.1 (C), 114.8 (CH), 109.0 (CH), 105.1 (CH), 55.7 (CH3), 51.9 (CH3), 39.9 (CH2). HRMS: m/z [M + H]+ calcd for C26H21N4O3: 437.1608; found: 437.1621.
3.3.3. Characterization Data of Post-Synthetic Derivatives Compounds 22a and 23a–c
4-(4-(pyrrolo[3,2,1-ij][1,2,3]triazolo[4,5-c]quinolin-10(7H)-yl)phenyl)morpholine 22a: 72% yield; yellow solid; mp: 190–192 °C; IR (neat): 2876, 1582, 1236, 1132, 991 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.63–7.53 (m, 1H), 7.51–7.30 (m, 5H), 7.10 (d, J = 3.2 Hz, 1H), 7.02–6.94 (m, 2H), 6.75 (t, J = 7.6 Hz, 1H), 6.64 (d, J = 7.3 Hz, 1H), 6.47 (d, J = 3.2 Hz, 1H), 5.68 (s, 2H), 3.83 (t, J = 4.8 Hz, 4H), 3.22 (dd, J = 6.1, 3.6 Hz, 4H); 13C NMR (100.6 MHz) (CDCl3): δ 152.4 (C), 139.0 (C), 133.6 (C), 132.9 (C), 132.2 (CH), 132.1 (CH), 132.0 (CH), 131.9 (CH), 129.9 (C), 128.6 (CH), 128.5 (CH), 128.4 (C), 126.9 (CH), 126.8 (CH), 125.9 (C), 122.5 (CH), 119.9 (CH), 115.3 (CH), 114.3 (CH), 109.4 (C), 103.6 (CH), 66.7 (CH2), 48.5 (CH2), 44.6 (CH2). HRMS: m/z [M + H]+ calcd for C21H20N5O: 358.1662; found: 358.1654.
5-(4′-methoxy-[1,1′-biphenyl]-4-yl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6] azepino[1,2-a]indole 23a: 96% yield; yellow solid; mp: 248–250 °C; IR (neat): 1460, 1340, 1058, 763, 740 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.84 (d, J = 7.7 Hz, 1H), 7.61–7.57 (m, 3H), 7.53 (d, J = 8.6 Hz, 1H), 7.49 (d, J = 8.4 Hz, 2H), 7.40–7.38 (m, 3H), 7.24 (td, J1 = 8.2 Hz, J3 = 0.8 Hz, 1H), 7.18 -7.16 (m, 1H), 7.07 (t, J = 7.4 Hz, 1H), 7.00 (dd, J1 = 7.9 Hz, J2 = 0.9 Hz, 1H), 6.93 (d, J = 7.7 Hz, 2H), 6.79 (s, 1H), 5.39 (s, 2H), 3.79 (s, 3H);13C NMR (100.6 MHz) (CDCl3): δ 159.9 (C), 144.9 (C), 142.2 (C), 138.7 (C), 137.0 (C), 135.2 (C), 133.8 (C), 131.4 (C), 132.0 (C), 131.8 (CH), 129.6 (CH), 128.7 (CH), 128.3 (CH), 127.8 (CH), 125.4 (CH), 123.1 (C), 122.7 (CH), 121.0 (CH), 120.2 (CH), 114.6 (CH), 109.4 (CH), 103.9 (CH), 55.5 (CH3), 39.6 (CH2). HRMS: m/z [M + H]+ calcd for C30H23N4O: 455.1866; found: 455.1879.
5-([1,1′-biphenyl]-4-yl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino[1,2-a]indole 23b: 98% yield; white solid; mp: 220–222 °C; IR (neat): 1439, 1160, 1054, 995, 767 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.84 (d, J = 7.9 Hz, 1H), 7.63–7.59 (m, 3H), 7.55–7.51 (m, 3H), 7.43–7.37 (m, 5H), 7.33 (m, 1H), 7.23 (td, J1 = 7.7 Hz, J2 = 0.8 Hz, 1H), 7.18–7.14 (m, 1H), 7.07 (t, J = 7.5 Hz, 1H), 7.00 (dd, J1 = 7.9 Hz, J2 = 0.6 Hz, 1H), 6.80 (s, 1H), 5.38 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 144.8 (C), 142.5 (C), 139.5 (C), 138.6 (C), 136.9 (C), 135.7 (C), 133.7 (C), 132.4 (C), 131.7 (CH), 129.6 (CH), 129.0 (CH), 128.7 (CH), 128.2 (CH), 128.1 (CH), 127.8 (C), 127.7 (CH), 127.2 (CH), 125.3 (CH), 122.9 (C), 122.6 (CH), 120.9 (CH), 120.1 (CH), 109.3 (CH), 103.8 (CH), 39.5 (CH2). HRMS: m/z [M + H]+ calcd for C29H21N4: 425.1766; found: 425.1753.
5-(4-(thiophen-2-yl)phenyl)-5,8-dihydrobenzo[3,4][1,2,3]triazolo[4′,5′:5,6]azepino [1,2-a]indole 23c: 71% yield; gray solid; mp: 218–220 °C; IR (neat): 1458, 1319, 1032, 994, 728 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.83 (d, J = 8.0 Hz, 1H), 7.62–7.59 (m, 3H), 7.52 (d, J = 8.3, 1H), 7.46–7.44 (m, 1H), 7.39–7.32 (m, 5H), 7.23 (dt, J1 = 6.9 Hz, J2 = 0.8 Hz, 1H), 7.17–7.12 (m, 1H), 7.07 (t, J = 7.3 Hz, 1H), 6.97 (d, J = 7.86 Hz, 1H), 6.79 (s, 1H), 5.33 (s, 2H); 13C NMR (100.6 MHz) (CDCl3): δ 144.9 (C), 140.8 (C), 138.6 (C), 137.2 (C), 137.0 (C), 135.4 (C), 133.8 (C), 132.4 (C), 131.8 (CH), 129.6 (CH), 128.7 (CH), 127.9 (C), 127.8 (CH), 127.5 (CH), 127.0 (CH), 126.2 (CH), 126.0 (C),125.5 (CH), 122.7 (CH), 121.6 (CH), 121.0 (CH), 120.2 (CH), 109.4 (CH), 103.9 (CH), 39.6 (CH2). HRMS: m/z [M + H]+ calcd for C27H19N4S: 431.1330; found: 431.1346.