Hyperspectral Imaging Study of Wheat Grains Infected with Several Fusarium Fungal Species and Their Identification with PCA-Based Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Raman Spectroscopy Study
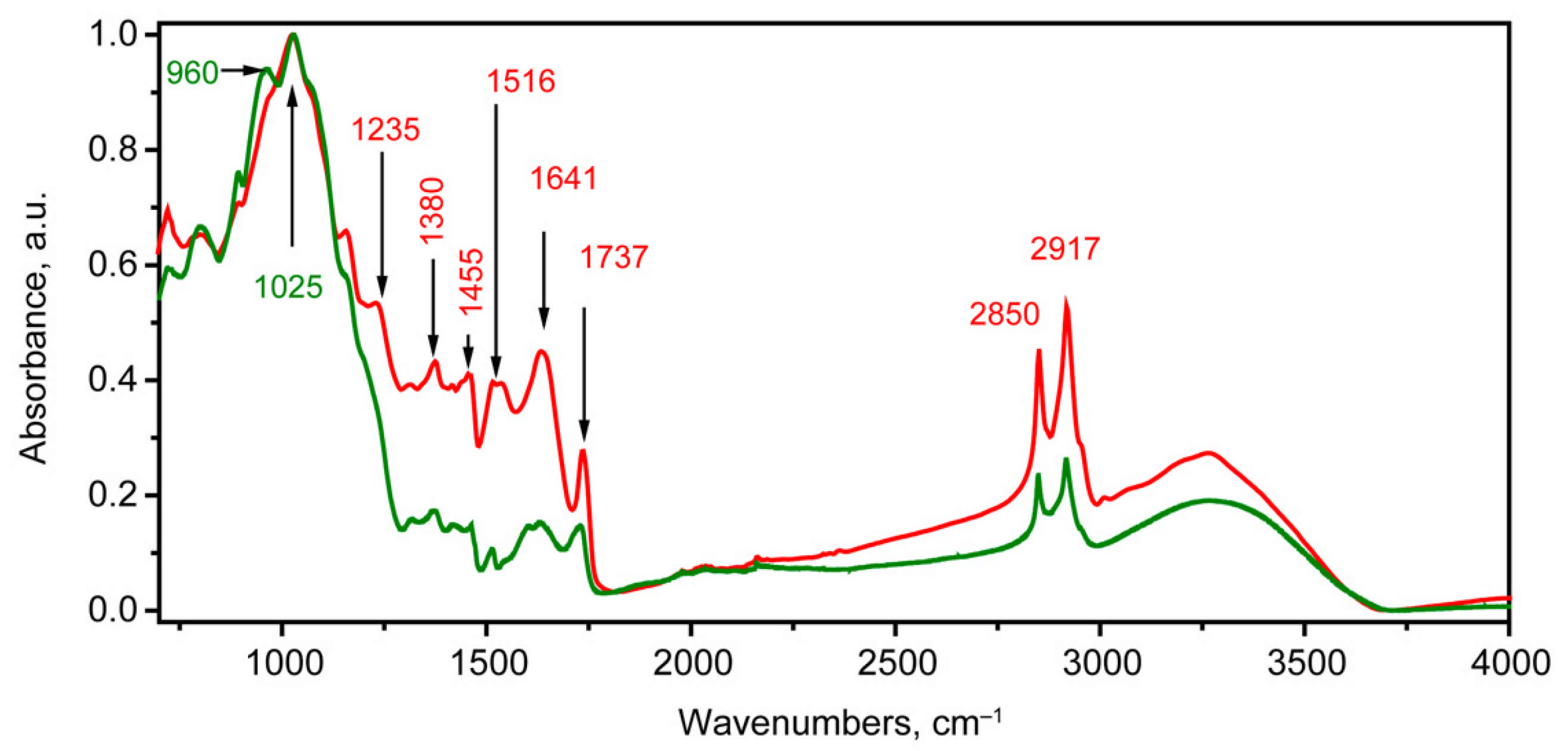
2.2. FTIR Spectroscopy Study
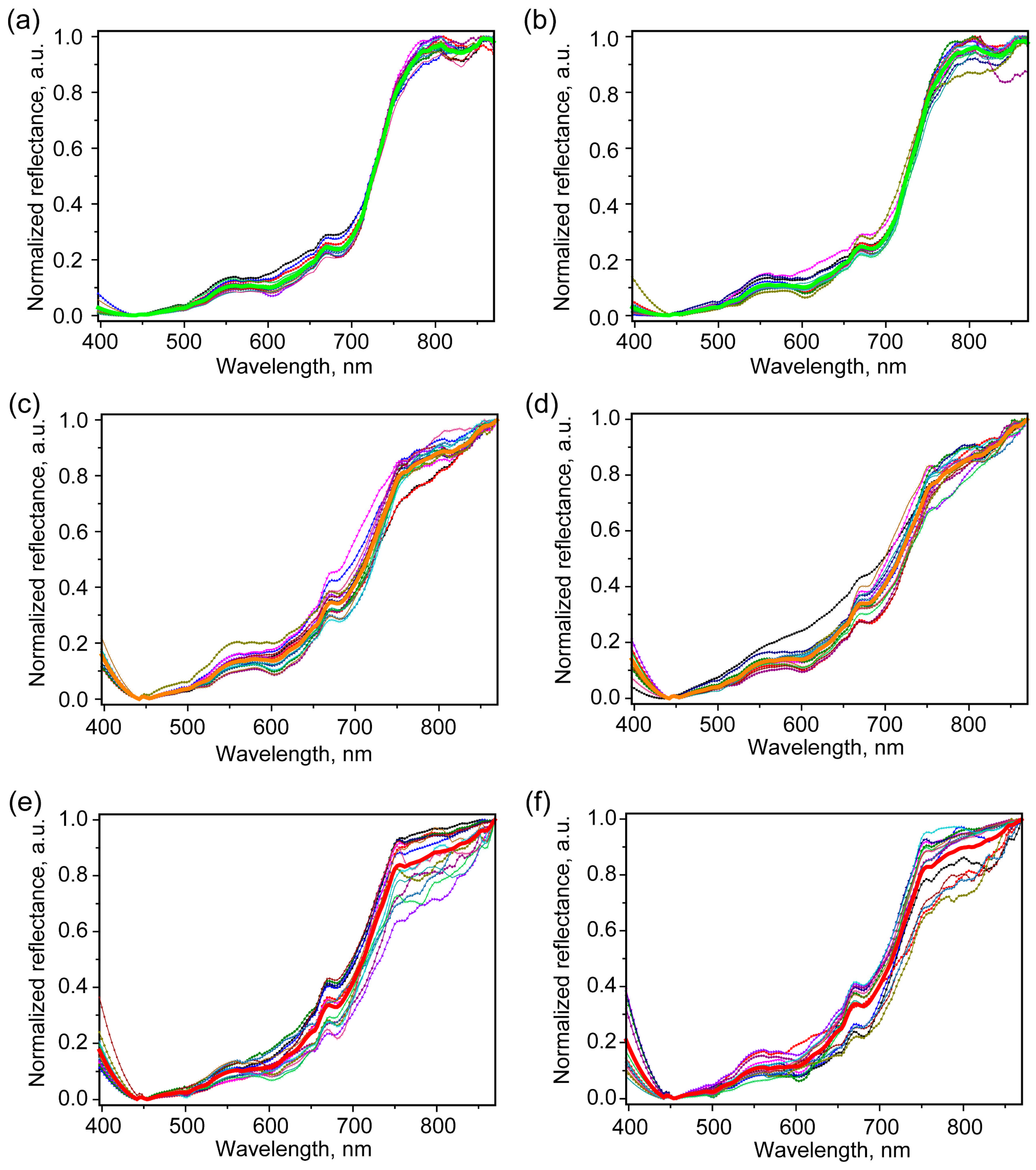
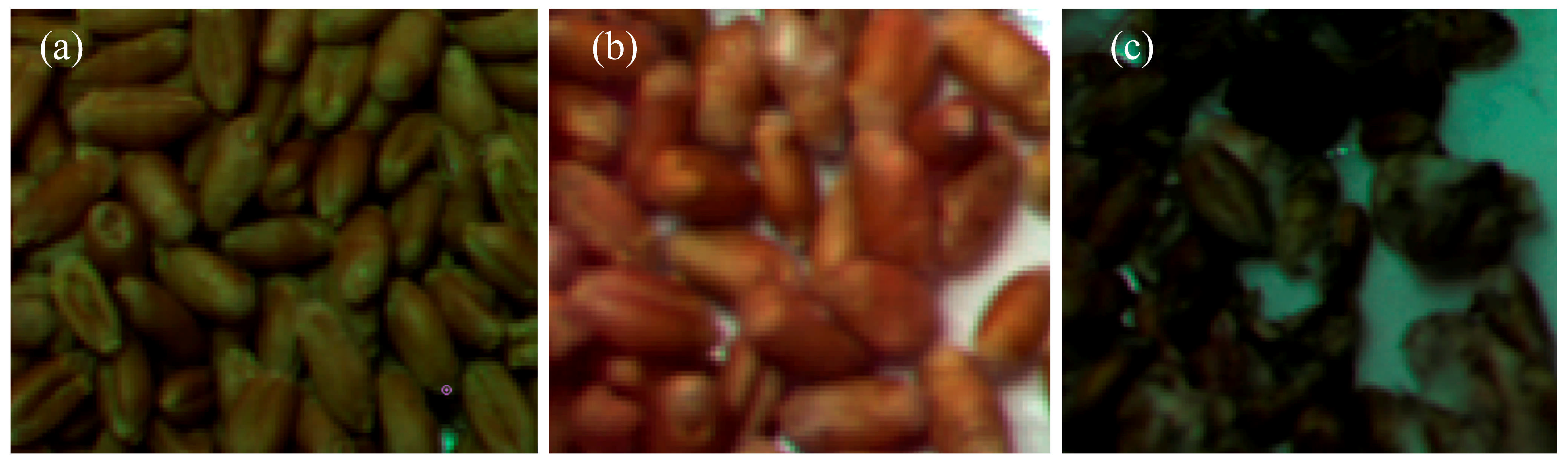
2.3. Hyperspectral Imaging Study
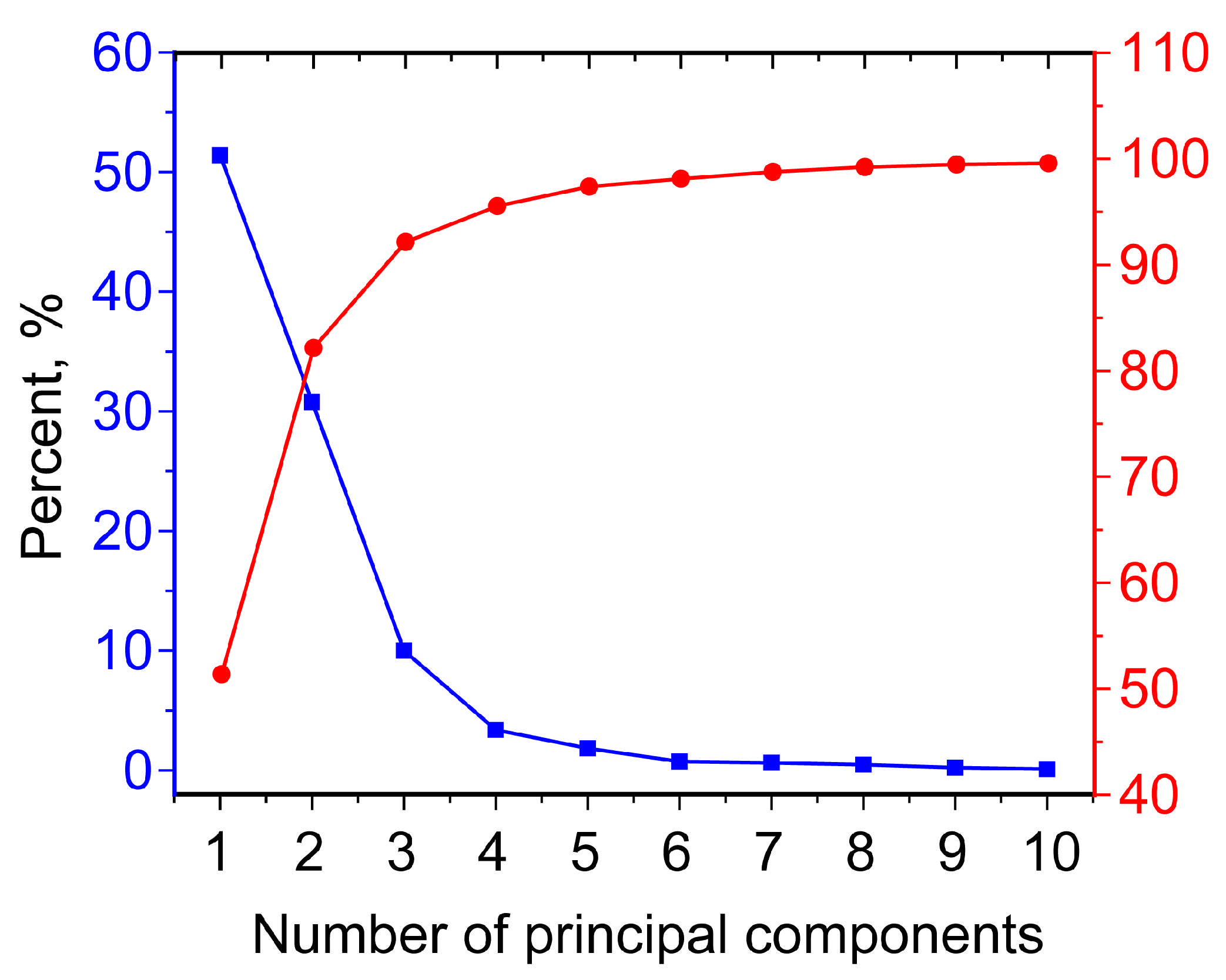
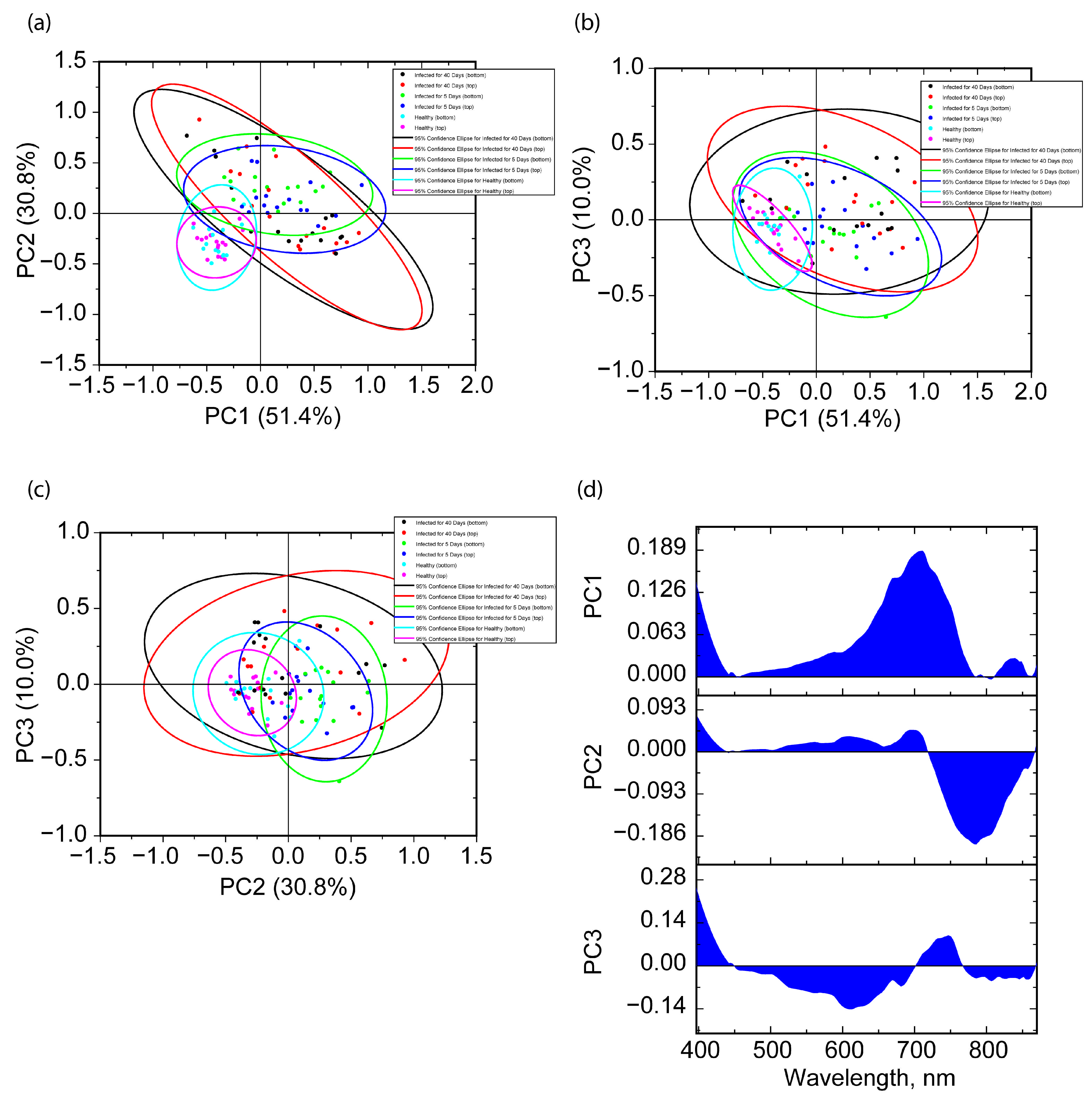
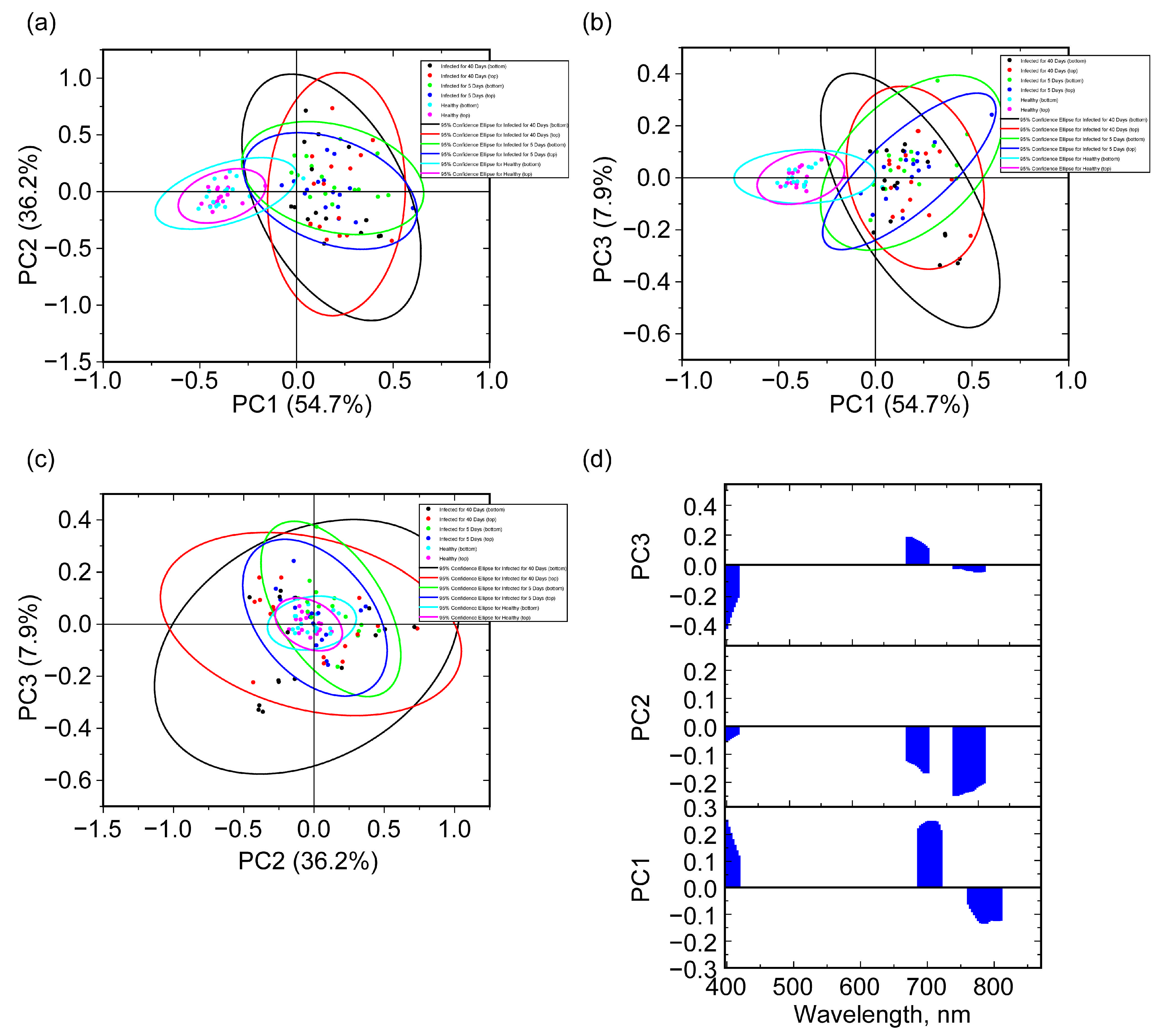
2.4. Hyperspectral Imaging Analysis
2.4.1. Full Spectral Range
2.4.2. Chemometric Analysis via Selected Spectral Bands
2.4.3. Chemometric Analysis via Selected Narrow Band Data
3. Materials and Methods
3.1. Grain Selection
3.2. Infected Grain Preparation and Selection
3.3. Spectroscopic Investigations
3.3.1. Raman Spectroscopy
3.3.2. FTIR Spectroscopy
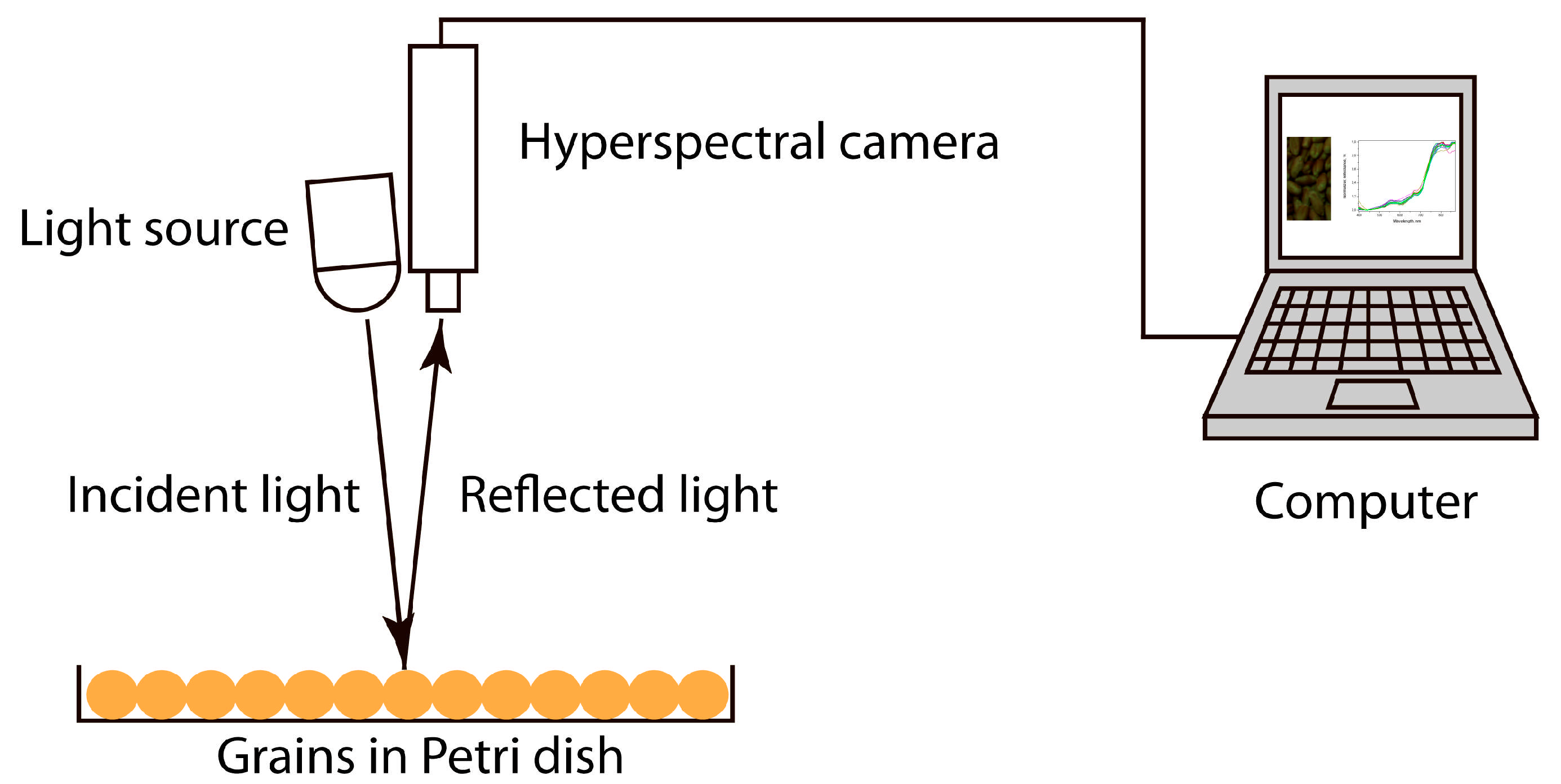
3.4. Hyperspectral Imaging Procedure
3.5. Data Preprocessing Procedure
Chemometric Approach Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reynolds, M.P.; Braun, H.-J. Wheat Improvement; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-90672-6. [Google Scholar]
- Inamdar, A.; Sakhare, S. Application of Color Sorter in Wheat Milling. Int. Food Res. J. 2014, 21, 2083–2089. [Google Scholar]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Global Wheat Production from 1990/1991 to 2024/2025 (in Million Metric Tons). Available online: https://www.statista.com/statistics/267268/production-of-wheat-worldwide-since-1990/#:~:text=in%20the%20marketing%20year%20of,to%20the%20previous%20marketing%20year (accessed on 29 May 2025).
- Whetton, R.L.; Waine, T.W.; Mouazen, A.M. Hyperspectral Measurements of Yellow Rust and Fusarium Head Blight in Cereal Crops: Part 2: On-Line Field Measurement. Biosyst. Eng. 2018, 167, 144–158. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, X.; He, H.-J.; Kamruzzaman, M. Advancements, Limitations and Challenges in Hyperspectral Imaging for Comprehensive Assessment of Wheat Quality: An up-to-Date Review. Food Chem. X 2024, 21, 101235. [Google Scholar] [CrossRef]
- Demissie, Y.T.; Sako, A.K. Investigation of Major Storage Fungal Pathogens Associated with Seeds of Some Crops. Middle East Res. J. Biol. Sci. 2023, 3, 37–44. [Google Scholar] [CrossRef]
- Acharya, P.; Ghimire, R.; Acosta-Martínez, V. Cover Crop-Mediated Soil Carbon Storage and Soil Health in Semi-Arid Irrigated Cropping Systems. Agric. Ecosyst. Environ. 2024, 361, 108813. [Google Scholar] [CrossRef]
- Rieker, M.E.G.; Lutz, M.A.; El-Hasan, A.; Thomas, S.; Voegele, R.T. Hyperspectral Imaging and Selected Biological Control Agents for the Management of Fusarium Head Blight in Spring Wheat. Plants 2023, 12, 3534. [Google Scholar] [CrossRef]
- Wang, X.; Polder, G.; Focker, M.; Liu, C. Sága, a Deep Learning Spectral Analysis Tool for Fungal Detection in Grains—A Case Study to Detect Fusarium in Winter Wheat. Toxins 2024, 16, 354. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Zhang, X.; Sun, Y.; Sun, H.; Wang, D.; Xu, X. Spectral Quantitative Analysis and Research of Fusarium Head Blight Infection Degree in Wheat Canopy Visible Areas. Agronomy 2023, 13, 933. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, L.; Huang, W.; Dong, Y.; Weng, S.; Zhao, J.; Ma, H.; Liu, L. Detection of Wheat Fusarium Head Blight Using UAV-Based Spectral and Image Feature Fusion. Front. Plant Sci. 2022, 13, 1004427. [Google Scholar] [CrossRef]
- Bao, W.; Huang, C.; Hu, G.; Su, B.; Yang, X. Detection of Fusarium Head Blight in Wheat Using UAV Remote Sensing Based on Parallel Channel Space Attention. Comput. Electron. Agric. 2024, 217, 108630. [Google Scholar] [CrossRef]
- Feng, G.; Gu, Y.; Wang, C.; Zhou, Y.; Huang, S.; Luo, B. Wheat Fusarium Head Blight Automatic Non-Destructive Detection Based on Multi-Scale Imaging: A Technical Perspective. Plants 2024, 13, 1722. [Google Scholar] [CrossRef] [PubMed]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J. Hyperspectral Imaging: A Review on UAV-Based Sensors, Data Processing and Applications for Agriculture and Forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Huang, W.; Du, X.; Ma, H. Monitoring Wheat Fusarium Head Blight Using Unmanned Aerial Vehicle Hyperspectral Imagery. Remote Sens. 2020, 12, 3811. [Google Scholar] [CrossRef]
- Guo, A.; Huang, W.; Dong, Y.; Ye, H.; Ma, H.; Liu, B.; Wu, W.; Ren, Y.; Ruan, C.; Geng, Y. Wheat Yellow Rust Detection Using UAV-Based Hyperspectral Technology. Remote Sens. 2021, 13, 123. [Google Scholar] [CrossRef]
- Alisaac, E.; Mahlein, A.-K. Fusarium Head Blight on Wheat: Biology, Modern Detection and Diagnosis and Integrated Disease Management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 Toxins: Toxicity, Occurrence and Analysis: A Review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef]
- Vejarano, R.; Siche, R.; Tesfaye, W. Evaluation of Biological Contaminants in Foods by Hyperspectral Imaging: A Review. Int. J. Food Prop. 2017, 20, 1264–1297. [Google Scholar] [CrossRef]
- Pankin, D.; Povolotckaia, A.; Borisov, E.; Povolotskiy, A.; Borzenko, S.; Gulyaev, A.; Gerasimenko, S.; Dorochov, A.; Khamuev, V.; Moskovskiy, M. Investigation of Spectroscopic Peculiarities of Ergot-Infected Winter Wheat Grains. Foods 2023, 12, 3426. [Google Scholar] [CrossRef]
- Egging, V.; Nguyen, J.; Kurouski, D. Detection and Identification of Fungal Infections in Intact Wheat and Sorghum Grain Using a Hand-Held Raman Spectrometer. Anal. Chem. 2018, 90, 8616–8621. [Google Scholar] [CrossRef]
- Trombete, F.; Barros, A.; Vieira, M.; Saldanha, T.; Venâncio, A.; Fraga, M. Simultaneous Determination of Deoxynivalenol, Deoxynivalenol-3-Glucoside and Nivalenol in Wheat Grains by HPLC-PDA with Immunoaffinity Column Cleanup. Food Anal. Methods 2016, 9, 2579–2586. [Google Scholar] [CrossRef]
- Pankin, D.; Povolotckaia, A.; Kalinichev, A.; Povolotskiy, A.; Borisov, E.; Moskovskiy, M.; Gulyaev, A.; Lavrov, A.; Izmailov, A. Complex Spectroscopic Study for Fusarium Genus Fungi Infection Diagnostics of “Zalp” Cultivar Oat. Agronomy 2021, 11, 2402. [Google Scholar] [CrossRef]
- Matveeva, T.A.; Sarimov, R.M.; Persidskaya, O.K.; Andreevskaya, V.M.; Semenova, N.A.; Gudkov, S.V. Application of Fluorescence Spectroscopy for Early Detection of Fungal Infection of Winter Wheat Grains. AgriEngineering 2024, 6, 3137–3158. [Google Scholar] [CrossRef]
- Fredlund, E.; Gidlund, A.; Pettersson, H.; Olsen, M.; Börjesson, T. Real-Time PCR Detection of Fusarium Species in Swedish Oats and Correlation to T-2 and HT-2 Toxin Content. World Mycotoxin J. 2010, 3, 77–88. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, G.; Zhang, H.; Jin, N.; Gu, C.; Weng, S.; Wang, Q.; Chen, Y. Integration of Spectroscopy and Image for Identifying Fusarium Damage in Wheat Kernels. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 236, 118344. [Google Scholar] [CrossRef]
- Liu, Y.; Delwiche, S.R.; Dong, Y. Feasibility of FT–Raman Spectroscopy for Rapid Screening for DON Toxin in Ground Wheat and Barley. Food Addit. Contam. Part A 2009, 26, 1396–1401. [Google Scholar] [CrossRef]
- Abramović, B.F.; Jajić, I.; Abramović, B.K.; Ćosić, J.; Jurić, V.B. Detection of Deoxynivalenol in Wheat by Fourier Transform Infrared Spectroscopy. Acta Chim. Slov. 2007, 54, 859–867. [Google Scholar]
- Alieva, R.; Sokolova, S.; Zhemchuzhina, N.; Pankin, D.; Povolotckaia, A.; Novikov, V.; Kuznetsov, S.; Gulyaev, A.; Moskovskiy, M.; Zavyalova, E. A Surface-Enhanced Raman Spectroscopy-Based Aptasensor for the Detection of Deoxynivalenol and T-2 Mycotoxins. Int. J. Mol. Sci. 2024, 25, 9534. [Google Scholar] [CrossRef]
- Peiris, K.H.S.; Bockus, W.W.; Dowell, F.E. Infrared Spectral Properties of Germ, Pericarp, and Endosperm Sections of Sound Wheat Kernels and Those Damaged by Fusarium graminearum. Appl. Spectrosc. 2012, 66, 1053–1060. [Google Scholar] [CrossRef]
- Saletnik, A.; Saletnik, B.; Zaguła, G.; Puchalski, C. Raman Spectroscopy for Plant Disease Detection in Next-Generation Agriculture. Sustainability 2024, 16, 5474. [Google Scholar] [CrossRef]
- Moskovskiy, M.N.; Belyakov, M.V.; Dorokhov, A.S.; Boyko, A.A.; Belousov, S.V.; Noy, O.V.; Gulyaev, A.A.; Akulov, S.I.; Povolotskaya, A.; Efremenkov, I.Y. Design of Device for Optical Luminescent Diagnostic of the Seeds Infected by Fusarium. Agriculture 2023, 13, 619. [Google Scholar] [CrossRef]
- Belyakov, M.V.; Moskovskiy, M.N.; Litvinov, M.A.; Lavrov, A.V.; Khamuev, V.G.; Efremenkov, I.Y.; Gerasimenko, S.A. Method of Optical Diagnostics of Grain Seeds Infected with Fusarium. Appl. Sci. 2022, 12, 4824. [Google Scholar] [CrossRef]
- Schlücker, S.; Szeghalmi, A.; Schmitt, M.; Popp, J.; Kiefer, W. Density Functional and Vibrational Spectroscopic Analysis of Β-carotene. J. Raman Spectrosc. 2003, 34, 413–419. [Google Scholar] [CrossRef]
- Novikov, V.S.; Kuzmin, V.V.; Darvin, M.E.; Lademann, J.; Sagitova, E.A.; Prokhorov, K.A.; Ustynyuk, L.Y.; Nikolaeva, G.Y. Relations between the Raman Spectra and Molecular Structure of Selected Carotenoids: DFT Study of α-Carotene, β-Carotene, γ-Carotene and Lycopene. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 270, 120755. [Google Scholar] [CrossRef]
- Pankin, D.; Povolotckaia, A.; Smirnov, M.; Borisov, E.; Gulyaev, A.; Dorochov, A.; Novikov, V.; Kuznetsov, S.; Noy, O.; Belousov, S.; et al. Theoretical Investigation of Anhydrofusarubin: Structural and Optical Properties. Crystals 2023, 13, 1556. [Google Scholar] [CrossRef]
- Moreira, L.M.; Lyon, J.P.; Lima, A.; Codognoto, L.; Machado, A.E.D.H.; Tiago, F.D.S.; Araújo, D.M.S.; Silva, E.L.; Hioka, N.; Rodrigues, M.R.; et al. Quinquangulin and Rubrofusarin: A Spectroscopy Study. Orbital Electron. J. Chem. 2017, 9, 290–298. [Google Scholar] [CrossRef]
- Martin, D.; Amado, A.M.; Gonzálvez, A.G.; Marques, M.P.M.; Batista de Carvalho, L.A.E.; Ureña, Á.G. FTIR Spectroscopy and DFT Calculations to Probe the Kinetics of β-Carotene Thermal Degradation. J. Phys. Chem. A 2019, 123, 5266–5273. [Google Scholar] [CrossRef]
- Pankin, D.; Povolotckaia, A.; Borisov, E.; Belyakov, M.; Borzenko, S.; Gulyaev, A.; Moskovskiy, M. Theoretical Modelling of Structure, Vibrational and UV–Vis Absorbance Spectra of Rubrofusarin Molecule. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 293, 122469. [Google Scholar] [CrossRef]
- Hussain, Z.; Rasool Bhat, H.; Naqvi, T.; Rana, K.M.; Ahmad Rizvi, M. Exploring Bikaverin as Metal Ion Biosensor: A Computational Approach. Acta Chim. Slov. 2019, 66, 351–359. [Google Scholar] [CrossRef]
- Povolotckaia, A.; Pankin, D.; Novikov, V.; Borisov, E.; Kuznetsov, S.; Dorokhov, A.; Gulyaev, A.; Zavyalova, E.; Alieva, R.; Akulov, S.; et al. Investigation of Structural and Spectral Peculiarities of Fusarium Sp. Indicator Pigment Bostrycoidin. Molecules 2024, 29, 4765. [Google Scholar] [CrossRef]
- Pishchalnikov, R.Y.; Chesalin, D.D.; Kurkov, V.A.; Shkirina, U.A.; Laptinskaya, P.K.; Novikov, V.S.; Kuznetsov, S.M.; Razjivin, A.P.; Moskovskiy, M.N.; Dorokhov, A.S.; et al. A Prototype Method for the Detection and Recognition of Pigments in the Environment Based on Optical Property Simulation. Plants 2023, 12, 4178. [Google Scholar] [CrossRef] [PubMed]
- Almoujahed, M.B.; Rangarajan, A.K.; Whetton, R.L.; Vincke, D.; Eylenbosch, D.; Vermeulen, P.; Mouazen, A.M. Non-Destructive Detection of Fusarium Head Blight in Wheat Kernels and Flour Using Visible near-Infrared and Mid-Infrared Spectroscopy. Chemom. Intell. Lab. Syst. 2024, 245, 105050. [Google Scholar] [CrossRef]
- Ajigboye, O.O.; Bousquet, L.; Murchie, E.H.; Ray, R.V. Chlorophyll Fluorescence Parameters Allow the Rapid Detection and Differentiation of Plant Responses in Three Different Wheat Pathosystems. Funct. Plant Biol. 2016, 43, 356. [Google Scholar] [CrossRef] [PubMed]
- Spanic, V.; Viljevac Vuletic, M.; Drezner, G.; Zdunic, Z.; Horvat, D. Performance Indices in Wheat Chlorophyll a Fluorescence and Protein Quality Influenced by FHB. Pathogens 2017, 6, 59. [Google Scholar] [CrossRef]
- Shahin, M.A.; Symons, S.J. Detection of Fusarium Damaged Kernels in Canada Western Red Spring Wheat Using Visible/near-Infrared Hyperspectral Imaging and Principal Component Analysis. Comput. Electron. Agric. 2011, 75, 107–112. [Google Scholar] [CrossRef]
- Weng, S.; Hu, X.; Wang, J.; Tang, L.; Li, P.; Zheng, S.; Zheng, L.; Huang, L.; Xin, Z. Advanced Application of Raman Spectroscopy and Surface-Enhanced Raman Spectroscopy in Plant Disease Diagnostics: A Review. J. Agric. Food Chem. 2021, 69, 2950–2964. [Google Scholar] [CrossRef]
- Liu, W.; He, L.; Xia, Y.; Ren, L.; Liu, C.; Zheng, L. Monitoring the Growth of Fusarium graminearum in Wheat Kernels Using Multispectral Imaging with Chemometric Methods. Anal. Methods 2022, 14, 106–113. [Google Scholar] [CrossRef]
- Weyer, L.G.; Lo, S.-C. Spectra-Structure Correlations in the Near-Infrared. In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Ropelewska, E.; Zapotoczny, P. Classification of Fusarium-Infected and Healthy Wheat Kernels Based on Features from Hyperspectral Images and Flatbed Scanner Images: A Comparative Analysis. Eur. Food Res. Technol. 2018, 244, 1453–1462. [Google Scholar] [CrossRef]
- Liang, K.; Liu, Q.X.; Xu, J.H.; Wang, Y.Q.; Okinda, C.S.; Shena, M.X. Determination and Visualization of Different Levels of Deoxynivalenol in Bulk Wheat Kernels by Hyperspectral Imaging. J. Appl. Spectrosc. 2018, 85, 953–961. [Google Scholar] [CrossRef]
- Hauptmann, A.; Hoelzl, G.; Mueller, M.; Bechtold-Peters, K.; Loerting, T. Raman Marker Bands for Secondary Structure Changes of Frozen Therapeutic Monoclonal Antibody Formulations During Thawing. J. Pharm. Sci. 2023, 112, 51–60. [Google Scholar] [CrossRef]
- Ghosal, S.; Macher, J.M.; Ahmed, K. Raman Microspectroscopy-Based Identification of Individual Fungal Spores as Potential Indicators of Indoor Contamination and Moisture-Related Building Damage. Environ. Sci. Technol. 2012, 46, 6088–6095. [Google Scholar] [CrossRef] [PubMed]
- Das, R.S.; Agrawal, Y.K. Raman Spectroscopy: Recent Advancements, Techniques and Applications. Vib. Spectrosc. 2011, 57, 163–176. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Wehling, B.; Moens, L.; Edwards, H.; De Reu, M.; Van Hooydonk, G. Analysis with Micro-Raman Spectroscopy of Natural Organic Binding Media and Varnishes Used in Art. Anal. Chim. Acta 2000, 407, 261–274. [Google Scholar] [CrossRef]
- Bock, P.; Nousiainen, P.; Elder, T.; Blaukopf, M.; Amer, H.; Zirbs, R.; Potthast, A.; Gierlinger, N. Infrared and Raman Spectra of Lignin Substructures: Dibenzodioxocin. J. Raman Spectrosc. 2020, 51, 422–431. [Google Scholar] [CrossRef]
- Agarwal, U.P. An Overview of Raman Spectroscopy as Applied to Lignocellulosic Materials. In Advances in Lignocellulosics Characterization; Chapter 9; TAPPI Press: Peachtree Corners, GA, USA, 1999. [Google Scholar]
- Edwards, H.G.M.; Farwell, D.W.; Webster, D. FT Raman Microscopy of Untreated Natural Plant Fibres. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53, 2383–2392. [Google Scholar] [CrossRef]
- Mayo, D.W.; Miller, F.A.; Hannah, R.W. Course Notes on the Interpretation of Infrared and Raman Spectra; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Cambaza, E. Comprehensive Description of Fusarium graminearum Pigments and Related Compounds. Foods 2018, 7, 165. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal Pigments and Their Prospects in Different Industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef]
- Kim, H.; Son, H.; Lee, Y.-W. Effects of Light on Secondary Metabolism and Fungal Development of Fusarium graminearum. J. Appl. Microbiol. 2014, 116, 380–389. [Google Scholar] [CrossRef]
- Pishchalnikov, R.Y.; Chesalin, D.D.; Kurkov, V.A.; Razjivin, A.P.; Gudkov, S.V.; Grishin, A.A.; Dorokhov, A.S.; Izmailov, A.Y. Classification of Fungal Pigments by Simulating Their Optical Properties Using Evolutionary Optimization. Mathematics 2024, 12, 3844. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Limón, M.C.; Avalos, J. HPLC Analysis of Carotenoids in Neurosporaxanthin-Producing Fungi. In Microbial Carotenoids. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; pp. 269–281. [Google Scholar]
- Solovchenko, A. Screening Pigments: General Questions. In Photoprotection in Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 9–31. [Google Scholar]
- Scott, J.K. Detection and Measurement of Carotenoids by UV/VIS Spectrophotometry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- James, R.L.; Cram, M.M. Forest Nursery Pests (Agriculture Handbook No. 680). 2012. Available online: https://research.fs.usda.gov/treesearch/54434 (accessed on 29 May 2025).
- Frisvad, J.C.; Thrane, U. Standardized High-Performance Liquid Chromatography of 182 Mycotoxins and Other Fungal Metabolites Based on Alkylphenone Retention Indices and UV—VIS Spectra (Diodearray Detection). J. Chromatogr. A 1987, 404, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Badr, A. Near Infra Red Spectroscopy. In Wide Spectra of Quality Control; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Workman, J., Jr.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780429119576. [Google Scholar]
- Available online: https://miratorg.ru/crops/en/catalog/semena/pshenitsa-ozimaya/timiryazevka-150/ (accessed on 29 May 2025).
- Prabhu, K.M.M. Window Functions and Their Applications in Signal Processing; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781315216386. [Google Scholar]
- Porter, C.D.; Tanner, D.B. Correction of Phase Errors in Fourier Spectroscopy. Int. J. Infrared Millim. Waves 1983, 4, 273–298. [Google Scholar] [CrossRef]
- Yao, H.; Lewis, D. Spectral Preprocessing and Calibration Techniques. In Hyperspectral Imaging for Food Quality Analysis and Control; Elsevier: Amsterdam, The Netherlands, 2010; pp. 45–78. ISBN 9780123747532. [Google Scholar]
- Demir, P.; Onde, S.; Severcan, F. Phylogeny of Cultivated and Wild Wheat Species Using ATR–FTIR Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Ropciuc, S. Botanical Authentication of Honeys Based on Raman Spectra. J. Food Meas. Charact. 2018, 12, 545–554. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Povolotckaia, A.; Pankin, D.; Gareev, M.; Serebrjakov, D.; Gulyaev, A.; Borisov, E.; Boyko, A.; Borzenko, S.; Belousov, S.; Noy, O.; et al. Hyperspectral Imaging Study of Wheat Grains Infected with Several Fusarium Fungal Species and Their Identification with PCA-Based Approach. Molecules 2025, 30, 2586. https://doi.org/10.3390/molecules30122586
Povolotckaia A, Pankin D, Gareev M, Serebrjakov D, Gulyaev A, Borisov E, Boyko A, Borzenko S, Belousov S, Noy O, et al. Hyperspectral Imaging Study of Wheat Grains Infected with Several Fusarium Fungal Species and Their Identification with PCA-Based Approach. Molecules. 2025; 30(12):2586. https://doi.org/10.3390/molecules30122586
Chicago/Turabian StylePovolotckaia, Anastasia, Dmitrii Pankin, Mikhail Gareev, Dmitrii Serebrjakov, Anatoliy Gulyaev, Evgenii Borisov, Andrey Boyko, Sergey Borzenko, Sergey Belousov, Oleg Noy, and et al. 2025. "Hyperspectral Imaging Study of Wheat Grains Infected with Several Fusarium Fungal Species and Their Identification with PCA-Based Approach" Molecules 30, no. 12: 2586. https://doi.org/10.3390/molecules30122586
APA StylePovolotckaia, A., Pankin, D., Gareev, M., Serebrjakov, D., Gulyaev, A., Borisov, E., Boyko, A., Borzenko, S., Belousov, S., Noy, O., & Moskovskiy, M. (2025). Hyperspectral Imaging Study of Wheat Grains Infected with Several Fusarium Fungal Species and Their Identification with PCA-Based Approach. Molecules, 30(12), 2586. https://doi.org/10.3390/molecules30122586







