Simultaneous Detection of Polar and Nonpolar Molecules by Nano-ESI MS with Plasma Ignited by an Ozone Generator Power Supply
Abstract
:1. Introduction
2. Results
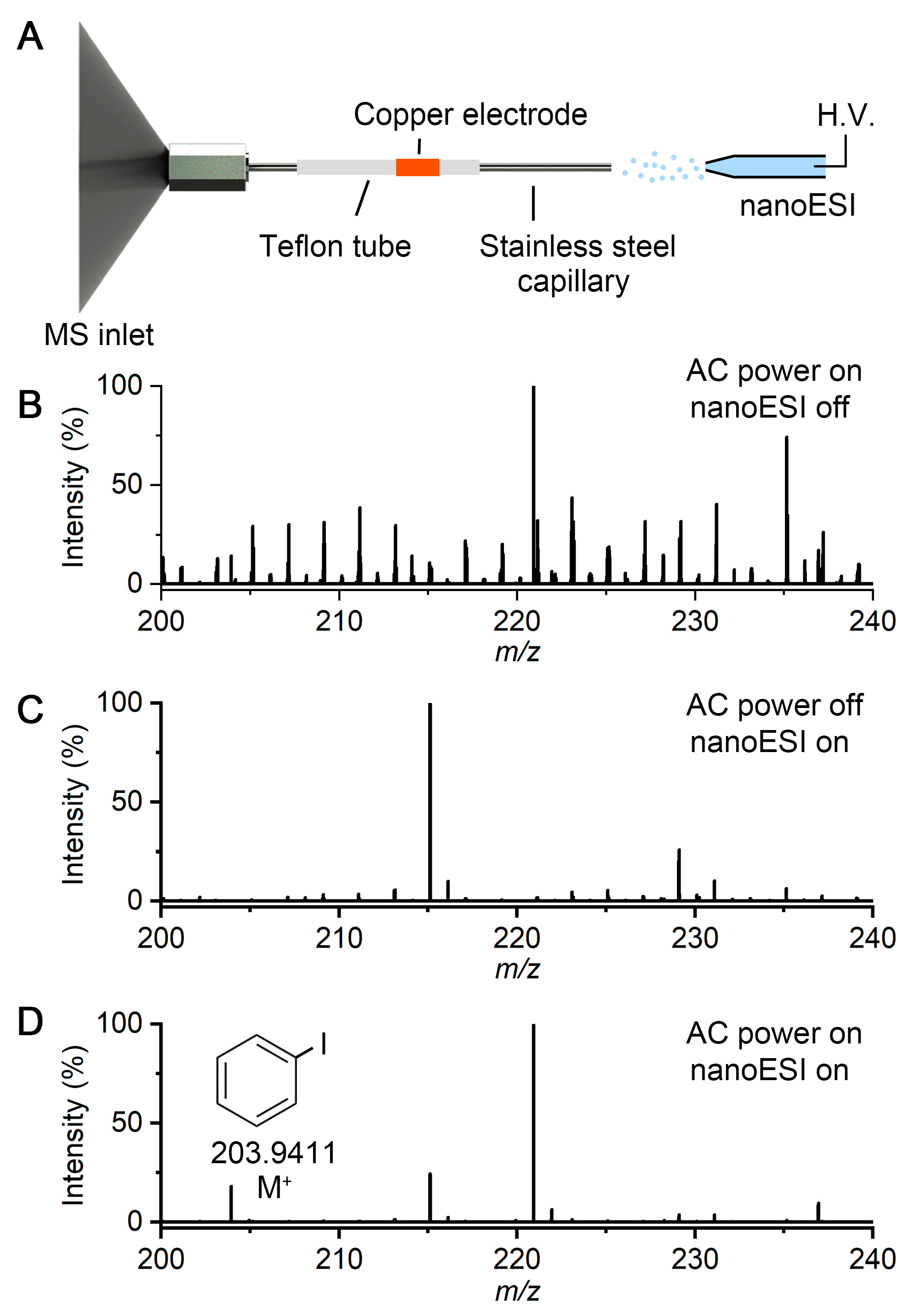
2.1. DBDI Source Powered by an Ozone Generator Power Supply
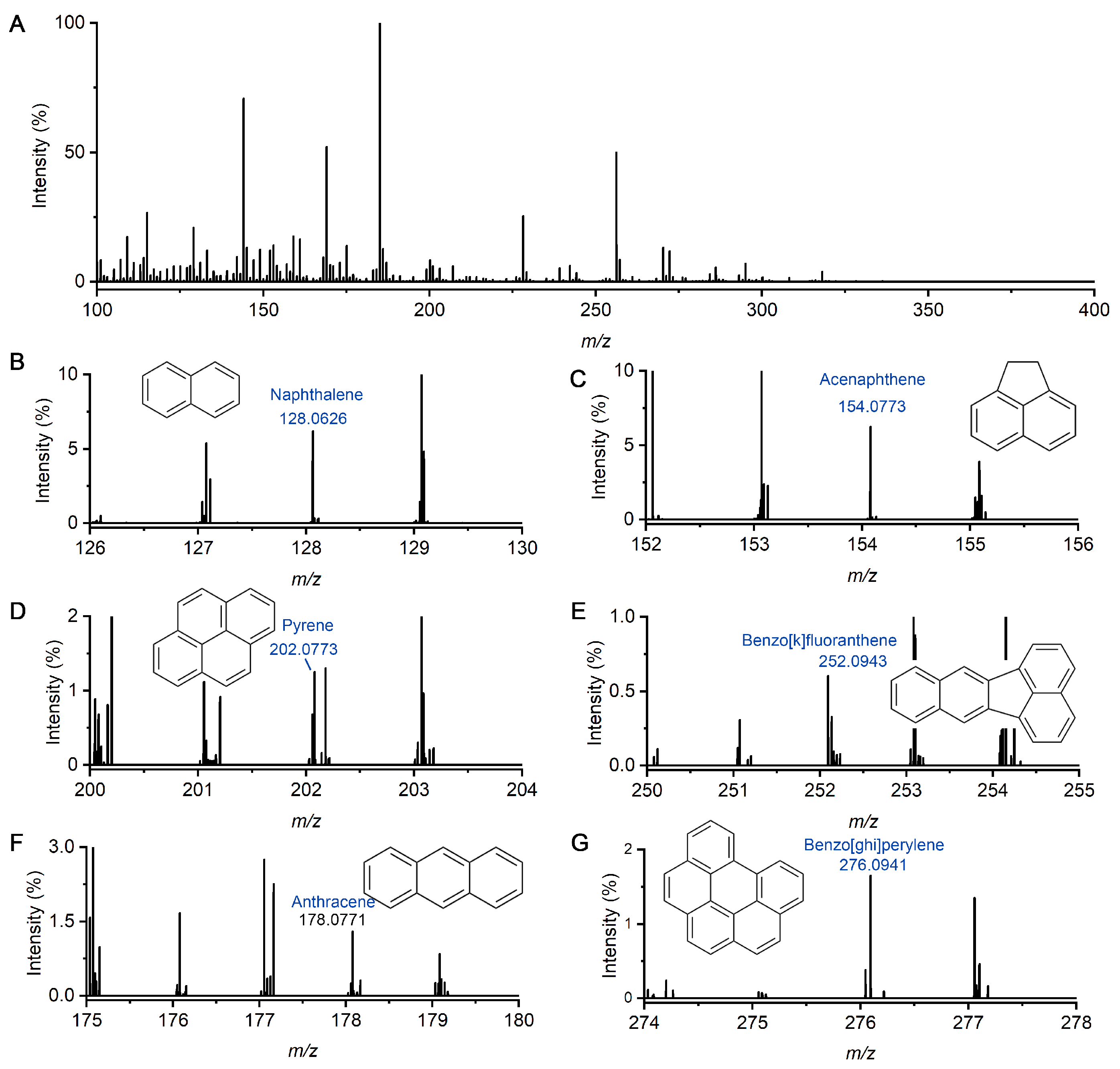
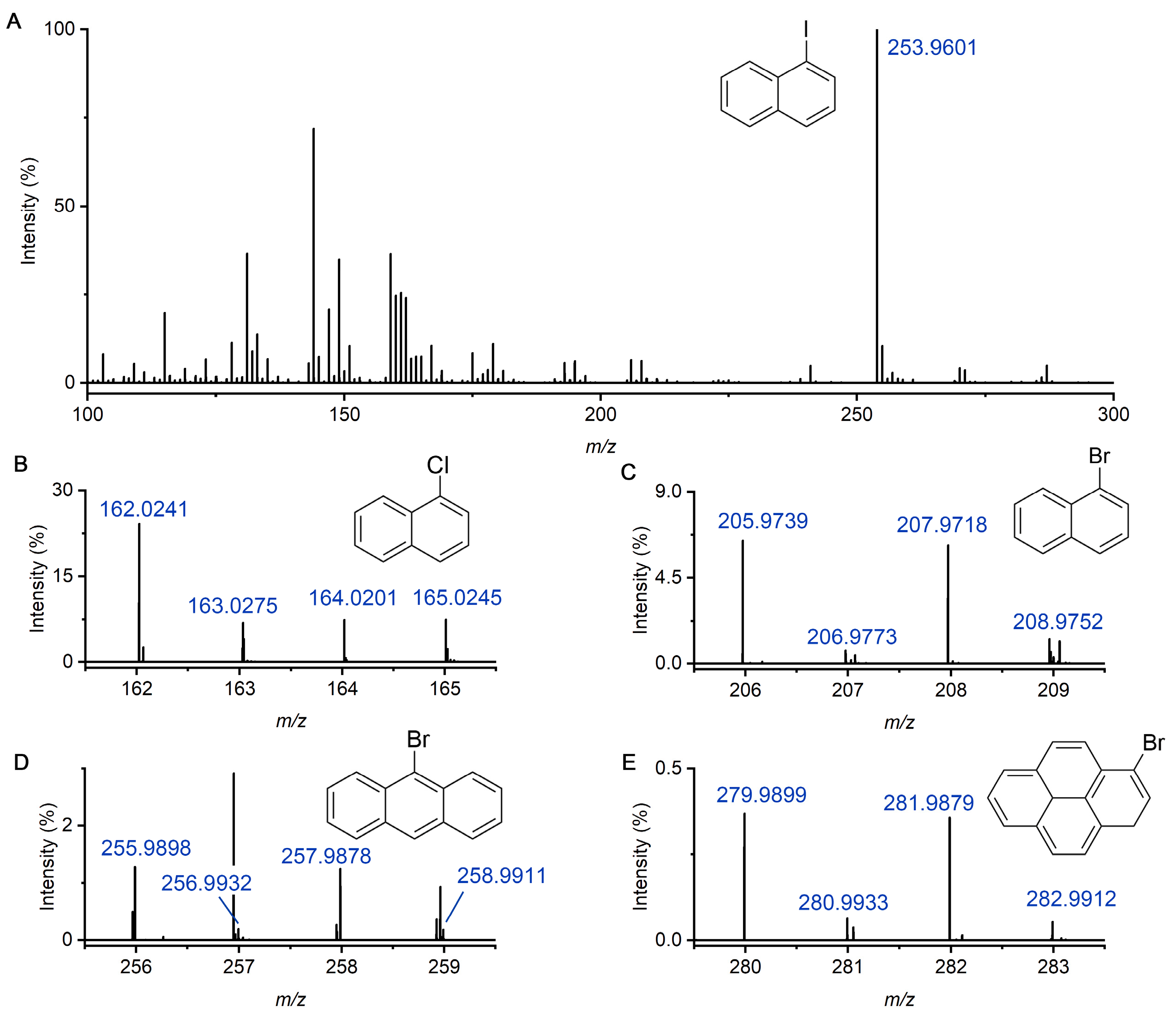
2.2. Detection of Multiple PAHs and HPAHs in Liquid Sample
2.3. Limit of Detection Evaluation
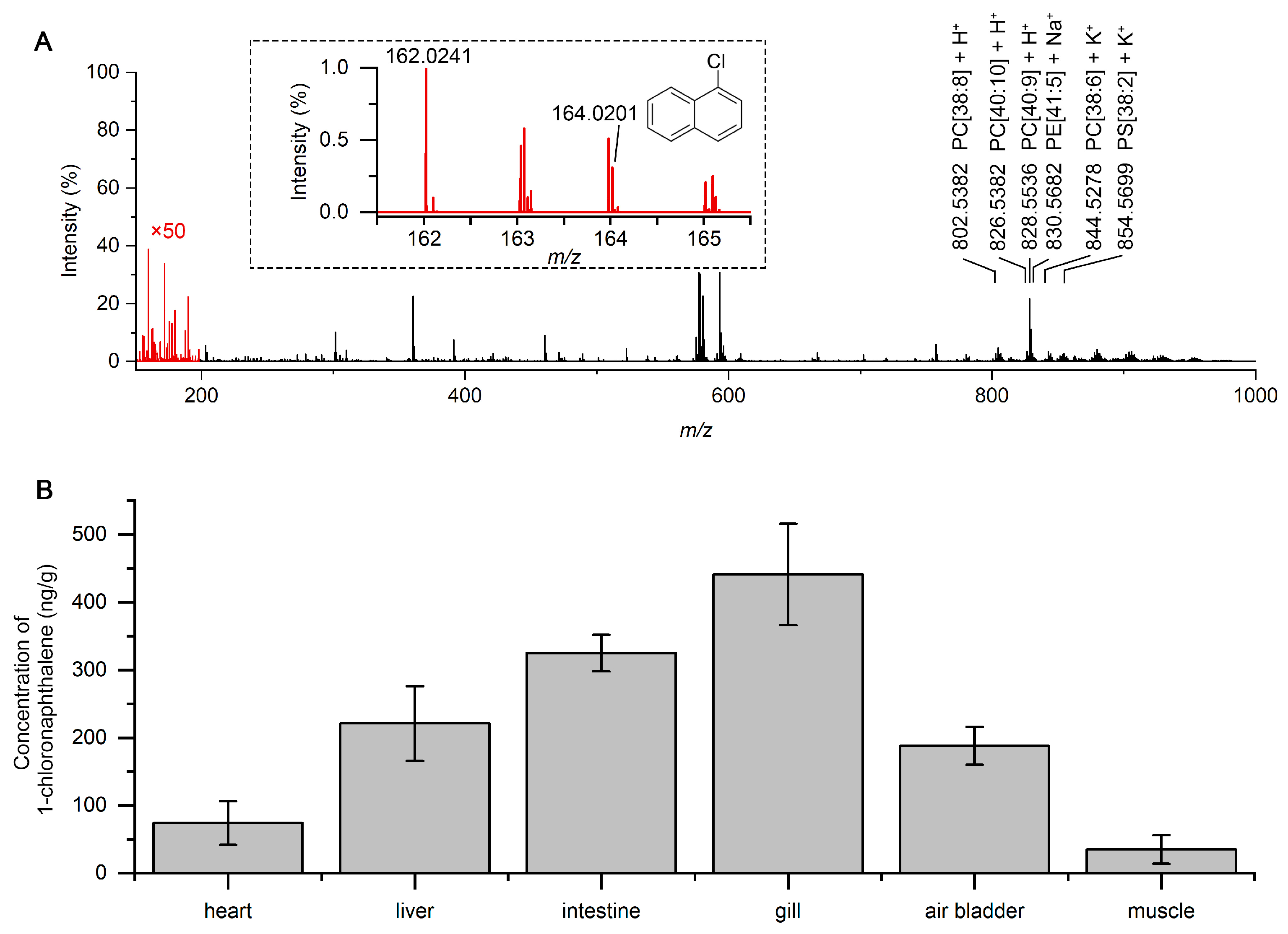
2.4. Simultaneous Detection of Low-Polarity HPAH and Polar Molecules in Fish
3. Discussion
4. Materials and Methods
4.1. Ionization Source Based on the Ozone Generator Power Supply
4.2. Chemicals
4.3. Sample Preparation
4.3.1. PAH and HPAH Standards
4.3.2. Fish Tissue Extraction
4.4. Safety Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Hoffmann, E.; Stroobant, V. Mass Spectrometry: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray Ionization for Mass Spectrometry of Large Biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization–principles and practice. Mass. Spectrom. Rev. 1990, 9, 37–70. [Google Scholar] [CrossRef]
- Prabhu, G.R.D.; Williams, E.R.; Wilm, M.; Urban, P.L. Mass spectrometry using electrospray ionization. Nat. Rev. Methods Primers 2023, 3, 23. [Google Scholar] [CrossRef]
- Zhu, G.; Shao, Y.; Liu, Y.; Pei, T.; Li, L.; Zhang, D.; Guo, G.; Wang, X. Single-cell metabolite analysis by electrospray ionization mass spectrometry. TrAC Trends Anal. Chem. 2021, 143, 116351. [Google Scholar] [CrossRef]
- Hsu, C.-C.; ElNaggar, M.S.; Peng, Y.; Fang, J.; Sanchez, L.M.; Mascuch, S.J.; Møller, K.A.; Alazzeh, E.K.; Pikula, J.; Quinn, R.A.; et al. Real-Time Metabolomics on Living Microorganisms Using Ambient Electrospray Ionization Flow-Probe. Anal. Chem. 2013, 85, 7014–7018. [Google Scholar] [CrossRef]
- Creydt, M.; Fischer, M. Plant Metabolomics: Maximizing Metabolome Coverage by Optimizing Mobile Phase Additives for Nontargeted Mass Spectrometry in Positive and Negative Electrospray Ionization Mode. Anal. Chem. 2017, 89, 10474–10486. [Google Scholar] [CrossRef]
- Lapainis, T.; Rubakhin, S.S.; Sweedler, J.V. Capillary Electrophoresis with Electrospray Ionization Mass Spectrometric Detection for Single-Cell Metabolomics. Anal. Chem. 2009, 81, 5858–5864. [Google Scholar] [CrossRef]
- Grouw, A.V.; Rainey, M.A.; Reid, O.K.; Ogle, M.M.; Moore, S.G.; Temenoff, J.S.; Fernández, F.M. Toward Machine Learning Electrospray Ionization Sensitivity Prediction for Semiquantitative Lipidomics in Stem Cells. J. Chem. Inf. Model. 2025, 65, 1826–1836. [Google Scholar] [CrossRef]
- Hu, A.; Wei, F.; Huang, F.; Xie, Y.; Wu, B.; Lv, X.; Chen, H. Comprehensive and High-Coverage Lipidomic Analysis of Oilseeds Based on Ultrahigh-Performance Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 8964–8980. [Google Scholar] [CrossRef]
- Gathungu, R.M.; Larrea, P.; Sniatynski, M.J.; Marur, V.R.; Bowden, J.A.; Koelmel, J.P.; Starke-Reed, P.; Hubbard, V.S.; Kristal, B.S. Optimization of Electrospray Ionization Source Parameters for Lipidomics To Reduce Misannotation of In-Source Fragments as Precursor Ions. Anal. Chem. 2018, 90, 13523–13532. [Google Scholar] [CrossRef]
- Chen, R.; Deng, J.; Fang, L.; Yao, Y.; Chen, B.; Wang, X.; Luan, T. Recent applications of ambient ionization mass spectrometry in environmental analysis. Trends Environ. Anal. Chem. 2017, 15, 1–11. [Google Scholar] [CrossRef]
- Zeng, J.; Yu, Z.; Mekic, M.; Liu, J.; Li, S.; Loisel, G.; Gao, W.; Gandolfo, A.; Zhou, Z.; Wang, X.; et al. Evolution of Indoor Cooking Emissions Captured by Using Secondary Electrospray Ionization High-Resolution Mass Spectrometry. Environ. Sci. Technol. Lett. 2020, 7, 76–81. [Google Scholar] [CrossRef]
- Rager, J.E.; Strynar, M.J.; Liang, S.; McMahen, R.L.; Richard, A.M.; Grulke, C.M.; Wambaugh, J.F.; Isaacs, K.K.; Judson, R.; Williams, A.J.; et al. Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environ. Int. 2016, 88, 269–280. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Sun, J.-L.; Zeng, H.; Ni, H.-G. Halogenated polycyclic aromatic hydrocarbons in the environment. Chemosphere 2013, 90, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Marín-Sáez, J.; Hernández-Mesa, M.; Cano-Sancho, G.; García-Campaña, A.M. Analytical challenges and opportunities in the study of endocrine disrupting chemicals within an exposomics framework. Talanta 2024, 279, 126616. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Jhang, S.-S.; Huang, M.-Z.; Shiea, J. Simultaneous Detection of Polar and Nonpolar Compounds by Ambient Mass Spectrometry with a Dual Electrospray and Atmospheric Pressure Chemical Ionization Source. Anal. Chem. 2015, 87, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Tiemann, O.; Hansen, H.J.; Rüger, C.P.; Zimmermann, R. Detailed Comparison of Xenon APPI (9.6/8.4 eV), Krypton APPI (10.6/10.0 eV), APCI, and APLI (266 nm) for Gas Chromatography High Resolution Mass Spectrometry of Standards and Complex Mixtures. J. Am. Soc. Mass. Spectrom. 2023, 34, 1632–1646. [Google Scholar] [CrossRef]
- Smith, D.R.; Robb, D.B.; Blades, M.W. Comparison of dopants for charge exchange ionization of nonpolar polycyclic aromatic hydrocarbons with reversed-phase LC-APPI-MS. J. Am. Soc. Mass. Spectrom. 2009, 20, 73–79. [Google Scholar] [CrossRef]
- Liu, H.; Han, X.; Gao, S.; Shi, F.; Tian, Y.; Hu, L.; Shi, J.; Jiang, G. Novel Laser-Assisted Electrospray Ionization Mass Spectrometry (Laser-Assisted ESI-MS): A Sensitive Method for Determining Tetrabromobisphenol A and Its Derivative. Anal. Chem. 2025, 97, 7622–7626. [Google Scholar] [CrossRef]
- Bierstedt, A.; You, Y.; van Wasen, S.; Bosc-Bierne, G.; Weller, M.; Riedel, J. Laser-Induced Microplasma as an Ambient Ionization Approach for the Mass-Spectrometric Analysis of Liquid Samples. Anal. Chem. 2019, 91, 5922–5928. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, J.; Wu, Y.; Liu, N.; Deng, J.; Luan, T. Nanospray Laser-Induced Plasma Ionization Mass Spectrometry for Sensitive and High-Coverage Analysis of Broad Polarity Organic Pollutants. Anal. Chem. 2024, 96, 14085–14089. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xie, J.; Lu, Q.; Xiao, X.; Wu, Y.; Liu, N.; Luo, L.; Luan, T.; Yang, Y. Nanospray Laser-Induced Plasma Ionization Mass Spectrometry for Rapid and Sensitive Analysis of Polycyclic Aromatic Hydrocarbons and Halogenated Derivatives. Anal. Chem. 2023, 95, 16791–16795. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lan, J.; Wu, R.; Begley, A.; Ge, W.; Zenobi, R. Hybrid Ionization Source Combining Nanoelectrospray and Dielectric Barrier Discharge Ionization for the Simultaneous Detection of Polar and Nonpolar Compounds in Single Cells. Anal. Chem. 2022, 94, 2873–2881. [Google Scholar] [CrossRef]
- Lu, Q.; Lin, R.; Du, C.; Meng, Y.; Yang, M.; Zenobi, R.; Hang, W. Metal Probe Microextraction Coupled to Dielectric Barrier Discharge Ionization–Mass Spectrometry for Detecting Drug Residues in Organisms. Anal. Chem. 2020, 92, 5921–5928. [Google Scholar] [CrossRef]
- Hagenhoff, S.; Franzke, J.; Hayen, H. Determination of Peroxide Explosive TATP and Related Compounds by Dielectric Barrier Discharge Ionization-Mass Spectrometry (DBDI-MS). Anal. Chem. 2017, 89, 4210–4215. [Google Scholar] [CrossRef]
- Lu, Q.; Guan, X.; You, X.; Zenobi, R. Identification of plasticizers using thermal desorption dielectric barrier discharge ionization mass spectrometry. Analyst 2025. [Google Scholar] [CrossRef]
- de Melo, A.P.Z.; Hoff, R.B.; Molognoni, L.; Kleemann, C.R.; de Oliveira, T.; de Oliveira, L.V.A.; Daguer, H.; Barreto, P.L.M. Determination of Polycyclic Aromatic Hydrocarbons in Seafood by PLE-LC-APCI-MS/MS and Preliminary Risk Assessment of the Northeast Brazil Oil Spill. Food Anal. Methods 2022, 15, 1826–1842. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Kang, X.; Wu, Z.; Yu, H.; Wu, R.a.; Yao, L.; Chen, J. Characterization of polycyclic aromatic hydrocarbons (PAHs) in atmospheric particles (PM2.5) in the North China Plain using positive-ion atmospheric pressure photoionization (APPI) coupled with fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS). Atmos. Environ. 2023, 314, 120090. [Google Scholar]
- Große Brinkhaus, S.; Thiäner, J.B.; Achten, C. Ultra-high sensitive PAH analysis of certified reference materials and environmental samples by GC-APLI-MS. Anal. Bioanal. Chem. 2017, 409, 2801–2812. [Google Scholar] [CrossRef]
- Hutzler, C.; Luch, A.; Filser, J.G. Analysis of carcinogenic polycyclic aromatic hydrocarbons in complex environmental mixtures by LC-APPI-MS/MS. Anal. Chim. Acta 2011, 702, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, W.; Shi, Z.; Li, J. A Sensitive Online SPE-LC–APCI–MS/MS Method for Simultaneous Determination of 17 Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbons in Atmospheric Particulate Matter. Chromatographia 2023, 86, 677–688. [Google Scholar] [CrossRef]
- Pérez, S.; Barceló, D. Determination of polycyclic aromatic hydrocarbons in sewage reference sludge by liquid chromatography-atmospheric-pressure chemical-ionization mass spectrometry. Chromatographia 2001, 53, 475–480. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Zhu, H.; Dong, J.; Cheng, P.; Zhou, Z. Spray-inlet microwave plasma torch and low temperature plasma ionization for ambient mass spectrometry of agrochemicals. Anal. Methods 2019, 11, 5421–5430. [Google Scholar] [CrossRef]
- Chen, W.; Zou, Y.; Mo, W.; Di, D.; Wang, B.; Wu, M.; Huang, Z.; Hu, B. Onsite Identification and Spatial Distribution of Air Pollutants Using a Drone-Based Solid-Phase Microextraction Array Coupled with Portable Gas Chromatography-Mass Spectrometry via Continuous-Airflow Sampling. Environ. Sci. Technol. 2022, 56, 17100–17107. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Q.; Deng, J.; Liu, X.; Hu, B. Portable Mass Spectrometry for On-site Detection of Hazardous Volatile Organic Compounds via Robotic Extractive Sampling. Anal. Chem. 2024, 96, 9325–9331. [Google Scholar] [CrossRef]

| Analyte | Method | Chromatography | LOD | Reference |
|---|---|---|---|---|
| PAHs | APLI | Yes | 50 pg/mL | [31] |
| PAHs | APPI | Yes | 1 ng/mL | [32] |
| PAH | APCI | Yes | 5 ng/mL | [29] |
| NPAHs | APCI | Yes | 0.4 ng/mL | [33] |
| PAHs | APCI | Yes | 0.7 ng/mL | [34] |
| PAHs | Laser-induced plasma | No | 22 ng/mL | [23] |
| Pyrimethamine | SPME-DBDI | No | 8 ng/mL | [26] |
| Chlorpyrifos | LTP | No | 1 ng/mL | [35] |
| PAHs & HPAHs | nanoESI-DBDI | No | 5–10 ng/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Meng, Y.; Zare, R.N. Simultaneous Detection of Polar and Nonpolar Molecules by Nano-ESI MS with Plasma Ignited by an Ozone Generator Power Supply. Molecules 2025, 30, 2546. https://doi.org/10.3390/molecules30122546
Tian Y, Meng Y, Zare RN. Simultaneous Detection of Polar and Nonpolar Molecules by Nano-ESI MS with Plasma Ignited by an Ozone Generator Power Supply. Molecules. 2025; 30(12):2546. https://doi.org/10.3390/molecules30122546
Chicago/Turabian StyleTian, Yunshuo, Yifan Meng, and Richard N. Zare. 2025. "Simultaneous Detection of Polar and Nonpolar Molecules by Nano-ESI MS with Plasma Ignited by an Ozone Generator Power Supply" Molecules 30, no. 12: 2546. https://doi.org/10.3390/molecules30122546
APA StyleTian, Y., Meng, Y., & Zare, R. N. (2025). Simultaneous Detection of Polar and Nonpolar Molecules by Nano-ESI MS with Plasma Ignited by an Ozone Generator Power Supply. Molecules, 30(12), 2546. https://doi.org/10.3390/molecules30122546


.png)



