Activation of Coke Fines Using CO2 and Steam: Optimization and Characterization of Carbon Sorbents
Abstract
1. Introduction
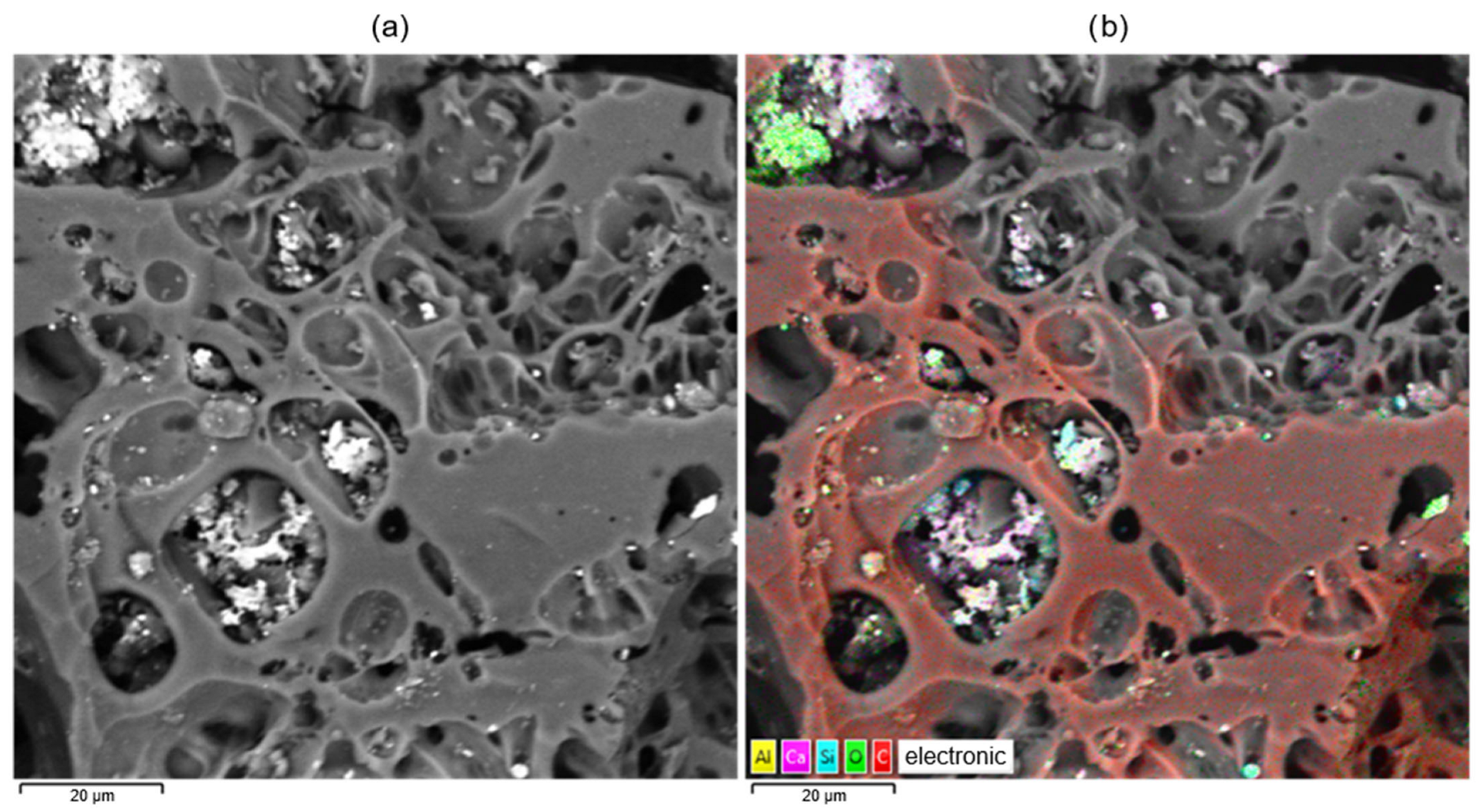
2. Results
3. Discussion
4. Materials and Methods
Checking the Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Naas, M.H.; Al-Zuhair, S.; Abu Alhaija, M. Removal of phenol from petroleum refinery wastewater through adsorption on date-pit activated carbon. Chem. Eng. J. 2010, 162, 997–1005. [Google Scholar] [CrossRef]
- Cho, D.-W.; Chon, C.-M.; Kim, Y.; Jeon, B.-H.; Schwartz, F.W.; Lee, E.-S.; Song, H. Adsorption of nitrate and Cr(VI) by cationic polymer-modified granular activated carbon. Chem. Eng. J. 2011, 175, 298–305. [Google Scholar] [CrossRef]
- Mansour, F.; Al-Hindi, M.; Yahfoufi, R.; Ayoub, G.M.; Ahmad, M.N. The use of activated carbon for the removal of pharmaceuticals from aqueous solutions: A review. Rev. Environ. Sci. Biotechnol. 2017, 16, 385–414. [Google Scholar] [CrossRef]
- Fröhlich, A.C.; dos Reis, G.S.; Pavan, F.A.; Lima, É.C.; Foletto, E.L.; Dotto, G.L. Improvement of activated carbon characteristics by sonication and its application for pharmaceutical contaminant adsorption. Environ. Sci. Pollut. Res. 2018, 25, 28574–28588. [Google Scholar] [CrossRef]
- Benammar, H.S.; Guergazi, S.; Youcef, S.; Youcef, L. Removal of Congo red and Naphthol blue black dyes from aqueous solution by adsorption on activated carbon: Characterization, kinetic and equilibrium in nonlinear models studies. Desalination Water Treat. 2021, 221, 396–405. [Google Scholar] [CrossRef]
- Toprak, A.; Kopac, T. Surface and hydrogen sorption characteristics of various activated carbons developed from Rat coal mine (Zonguldak) and anthracite. Chin. J. Chem. Eng. 2011, 19, 931–937. [Google Scholar] [CrossRef]
- Awadallah-F, A.; Al-Muhtaseb, S.A. Carbon dioxide sequestration and methane removal from exhaust gases using resorcinol–formaldehyde activated carbon xerogel. Adsorption 2013, 19, 967–977. [Google Scholar] [CrossRef]
- Giraldo, L.; Vargas, D.P.; Moreno-Piraján, J.C. Study of CO2 adsorption on chemically modified activated carbon with nitric acid and ammonium aqueous. Front. Chem. 2020, 8, 543452. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Rivera-Utrilla, J.; Carrasco-Marín, F.; López-Ramón, M.V. On the carbon dioxide and benzene adsorption on activated carbons to study their micropore structure. Langmuir 1997, 13, 5208–5210. [Google Scholar] [CrossRef]
- Iwanow, M.; Gärtner, T.; Sieber, V.; König, B. Activated carbon as catalyst support: Precursors, preparation, modification and characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [CrossRef]
- Rusanen, A.; Kupila, R.; Lappalainen, K.; Kärkkäinen, J.; Hu, T.; Lassi, U. Conversion of xylose to furfural over lignin-based activated carbon-supported iron catalysts. Catalysts 2020, 10, 821. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Bragg, D.; Oginni, O.; Levelle, R.; Singh, K.; Sivanandan, L.; Sabolsky, E.M. Performance of activated carbons synthesized from fruit dehydration biowastes for supercapacitor applications. Environ. Prog. Sustain. Energy 2018, 37, 1739–1753. [Google Scholar] [CrossRef]
- Lee, U.-W.; Yang, G.; Park, S.-J. Improvement of mesoporosity on supercapacitive performance of activated carbons derived from coffee grounds. Bull. Korean Chem. Soc. 2021, 42, e12251. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, H.; Zhang, Y.; Liu, Y.; Zhao, Y.; Liu, Z. Synergistic effect of CO2 and H2O co-activation of Zhundong coal at a low burn-off rate on high performance supercapacitor. Fuel 2023, 342, 127887. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Preparation of activated carbons from Spanish anthracite. II. Activation by NaOH. Carbon 2001, 39, 751–759. [Google Scholar] [CrossRef]
- Tsyntsarski, B.; Marinov, S.; Budinova, T.; Yardim, M.F.; Petrov, N. Synthesis and characterization of activated carbon from natural asphaltites. Fuel Process. Technol. 2013, 116, 346–349. [Google Scholar] [CrossRef]
- Hashemi Shahraki, Z.; Sharififard, H.; Lashanizadegan, A. Grape stalks biomass as raw material for activated carbon production: Synthesis, characterization and adsorption ability. Mater. Res. Express 2018, 5, 055603. [Google Scholar] [CrossRef]
- Blachnio, M.; Derylo-Marczewska, A.; Charmas, B.; Zienkiewicz-Strzalka, M.; Bogatyrov, V.; Galaburda, M. Activated carbon from agricultural wastes for adsorption of organic pollutants. Molecules 2020, 25, 5105. [Google Scholar] [CrossRef]
- Frikha, K.; Limousy, L.; Pons Claret, J.; Vaulot, C.; Florencio Pérez, K.; Corzo Garcia, B.; Bennici, S. Potential valorization of waste tires as activated carbon-based adsorbent for organic contaminants removal. Materials 2022, 15, 1099. [Google Scholar] [CrossRef]
- Sousa, J.C.; Mahamud, M.; Parra, J.B.; Pis, J.J.; Pajares, J.A. Effect of operation variables in the obtention of tailored active carbons from coals. Fuel Process. Technol. 1993, 36, 333–339. [Google Scholar] [CrossRef]
- Madadi Yeganeh, M.; Kaghazchi, T.; Soleimani, M. Effect of raw materials on properties of activated carbons. Chem. Eng. Technol. 2006, 29, 1247–1251. [Google Scholar] [CrossRef]
- Arenas, E.; Chejne, F. The effect of the activating agent and temperature on the porosity development of physically activated coal chars. Carbon 2004, 42, 2451–2455. [Google Scholar] [CrossRef]
- El Qada, E.N.; Allen, S.J.; Walker, G.M. Influence of preparation conditions on the characteristics of activated carbons produced in laboratory and pilot scale systems. Chem. Eng. J. 2008, 142, 1–13. [Google Scholar] [CrossRef]
- Ania, C.O.; Parra, J.B.; Pis, J.J. Effect of texture and surface chemistry on adsorptive capacities of activated carbons for phenolic compounds removal. Fuel Process. Technol. 2002, 77–78, 337–343. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Turov, V.V.; Kozynchenko, O.P.; Nikolaev, V.G.; Tennison, S.R.; Meikle, S.T.; Snezhkova, E.A.; Sidorenko, A.S.; Ehrburger-Dolle, F.; Morfin, I.; et al. Activation and structural and adsorption features of activated carbons with highly developed micro-, meso- and macroporosity. Adsorption 2011, 17, 453–460. [Google Scholar] [CrossRef]
- Lyubchik, S.B.; Benoit, R.; Béguin, F. Influence of chemical modification of anthracite on the porosity of the resulting activated carbons. Carbon 2002, 40, 1287–1294. [Google Scholar] [CrossRef]
- De Smedt, J.; Soroush, S.; Heynderickx, P.M.; Arauzo, P.J.; Ronsse, F. The feasibility of activated carbon derived from waste seaweed via molten salt activation in a eutectic mixture of ZnCl2-NaCl-KCl for adsorption of anionic dyes. Biomass Conv. Bioref. 2025, 15, 12891–12903. [Google Scholar] [CrossRef]
- Kunarbekova, M.; Busquets, R.; Sailaukhanuly, Y.; Mikhalovsky, S.V.; Toshtay, K.; Kudaibergenov, K.; Azat, S. Carbon adsorbents for the uptake of radioactive iodine from contaminated water effluents: A systematic review. Chem. Eng. J. 2024, 67, 106174. [Google Scholar] [CrossRef]
- Roberts, D.G.; Harris, D.J. Char gasification in mixtures of CO2 and H2O: Competition and inhibition. Fuel 2007, 86, 2672–2678. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.T.; Fan, M.; Daugaard, D.; Brown, R.C. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- Acevedo, B.; Barriocanal, C.; Lupul, I.; Gryglewicz, G. Properties and performance of mesoporous activated carbons from scrap tyres, bituminous wastes and coal. Fuel 2015, 151, 83–90. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, A.; Zhao, Y. Preparation and characterisation of activated carbon from waste tea by physical activation using steam. J. Air Waste Manag. Assoc. 2018, 68, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Ordabaeva, A.T.; Muldakhmetov, Z.M.; Gazaliev, A.M.; Kim, S.V.; Shaikenova, Z.S.; Meiramov, M.G. Production of activated carbon from sifted coke and determination of its physicochemical characteristics. Molecules 2023, 28, 5661. [Google Scholar] [CrossRef]
- Minkova, V.; Marinov, S.P.; Zanzi, R.; Bjornbom, E.; Budinova, T.; Stefanova, M.; Lakov, L. Thermochemical treatment of biomass in a flow of steam or in a mixture of steam and carbon dioxide. Fuel Process. Technol. 2000, 62, 45–52. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Berger, J.; Siemieniewska, T.; Tomków, K. Development of porosity in brown-coal chars on activation with carbon dioxide. Fuel 1976, 55, 9–15. [Google Scholar] [CrossRef]
- Lan, X.; Jiang, X.; Song, Y.; Jing, X.; Xing, X. The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation. Green Process. Synth. 2019, 8, 837–845. [Google Scholar] [CrossRef]
- Maya, J.C.; Macías, R.; Gómez, C.A.; Chejne, F. On the evolution of pore microstructure during coal char activation with steam/CO2 mixtures. Carbon 2020, 157, 482–496. [Google Scholar] [CrossRef]
- Kan, Y.; Yue, Q.; Kong, J.; Gao, B.; Li, Q. The application of activated carbon produced from waste printed circuit boards (PCBs) by H3PO4 and steam activation for the removal of malachite green. Chem. Eng. J. 2015, 260, 541–549. [Google Scholar] [CrossRef]
- Guedidi, H.; Reinert, L.; Lévêque, J.-M.; Soneda, Y.; Bellakhal, N.; Duclaux, L. The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen. Carbon 2013, 54, 432–443. [Google Scholar] [CrossRef]
- Jaria, G.; Lourenço, M.A.O.; Silva, C.P.; Ferreira, P.; Otero, M.; Calisto, V.; Esteves, V.I. Effect of the surface functionalization of a waste-derived activated carbon on pharmaceuticals’ adsorption from water. J. Mol. Liq. 2019, 294, 112098. [Google Scholar] [CrossRef]
- Pakuła, M.; Walczyk, M.; Biniak, S.; Świątkowski, A. Electrochemical and FTIR studies of the mutual influence of lead(II) or iron(III) and phenol on their adsorption from aqueous acid solution by modified activated carbons. Chemosphere 2007, 69, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Sulaymon, A.H.; Abbood, D.W.; Ali, A.H. A comparative adsorption/biosorption for the removal of phenol and lead onto granular activated carbon and dried anaerobic sludge. Desalination Water Treat. 2013, 51, 2055–2067. [Google Scholar] [CrossRef]
- Gökçe, Y.; Aktaş, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- Tran, H.N.; Huang, F.-C.; Lee, C.-K.; Chao, H.-P. Activated carbon derived from spherical hydrochar functionalized with triethylenetetramine: Synthesis, characterizations, and adsorption application. Green Process. Synth. 2017, 6, 511–524. [Google Scholar] [CrossRef]
- Alade, A.O.; Amuda, O.S.; Ogunleye, O.O.; Okoya, A.A. Evaluation of interaction of carbonization temperatures and concentrations on the adsorption capacities and removal efficiencies of activated carbons using response surface methodology (RSM). J. Bioremed. Biodegrad. 2012, 3, 134. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 478–544. [Google Scholar]
- Habeeb, O.A.; Kanthasamy, R.; Ali, G.A.M.; Yunus, R.M. Application of response surface methodology for optimization of palm kernel shell activated carbon preparation factors for removal of H2S from industrial wastewater. J. Teknol. 2017, 79, 1–10. [Google Scholar] [CrossRef]
- GOST R 55661—2013; SOLID MINERAL FUEL Determination of Ash (ISO 1171:2010 Solid Mineral Fuels—Determination of Ash (MOD)). Prepared by the Federal State Unitary Enterprise “All-Russian Scientific Research Center for Standardization, Information and Certification of Raw Materials, Materials and Substances” (FSUE “VNITSMV”) on the Basis of Its Own Authentic Translation into Russian of the Standard Specified in Paragraph 4. Federal Agency for Technical Regulation and Metrology: Moscow, Russia, 2013. Available online: http://www.omegametal.ru/Data2/1/4293775/4293775966.pdf (accessed on 17 April 2025).
- GOST R 32558—2013; Coal—Determination of Bulk Density (ISO 23499:2008, MOD). Developed by the Federal State Unitary Enterprise All-Russian Scientific Research Center for Standardization, Information and Certification of Raw Materials, Materials and Substances (FSUE VNITSMV) on the Basis of Its Own Authentic Translation into Russian of the International Standard Specified in Paragraph 5. TC 1792, Interstate Council for Standardization, Metrology and Certification (ISC): Moscow, Russia, 2013. Available online: https://ohranatruda.ru/upload/iblock/8e0/4293774832.pdf (accessed on 17 April 2025).
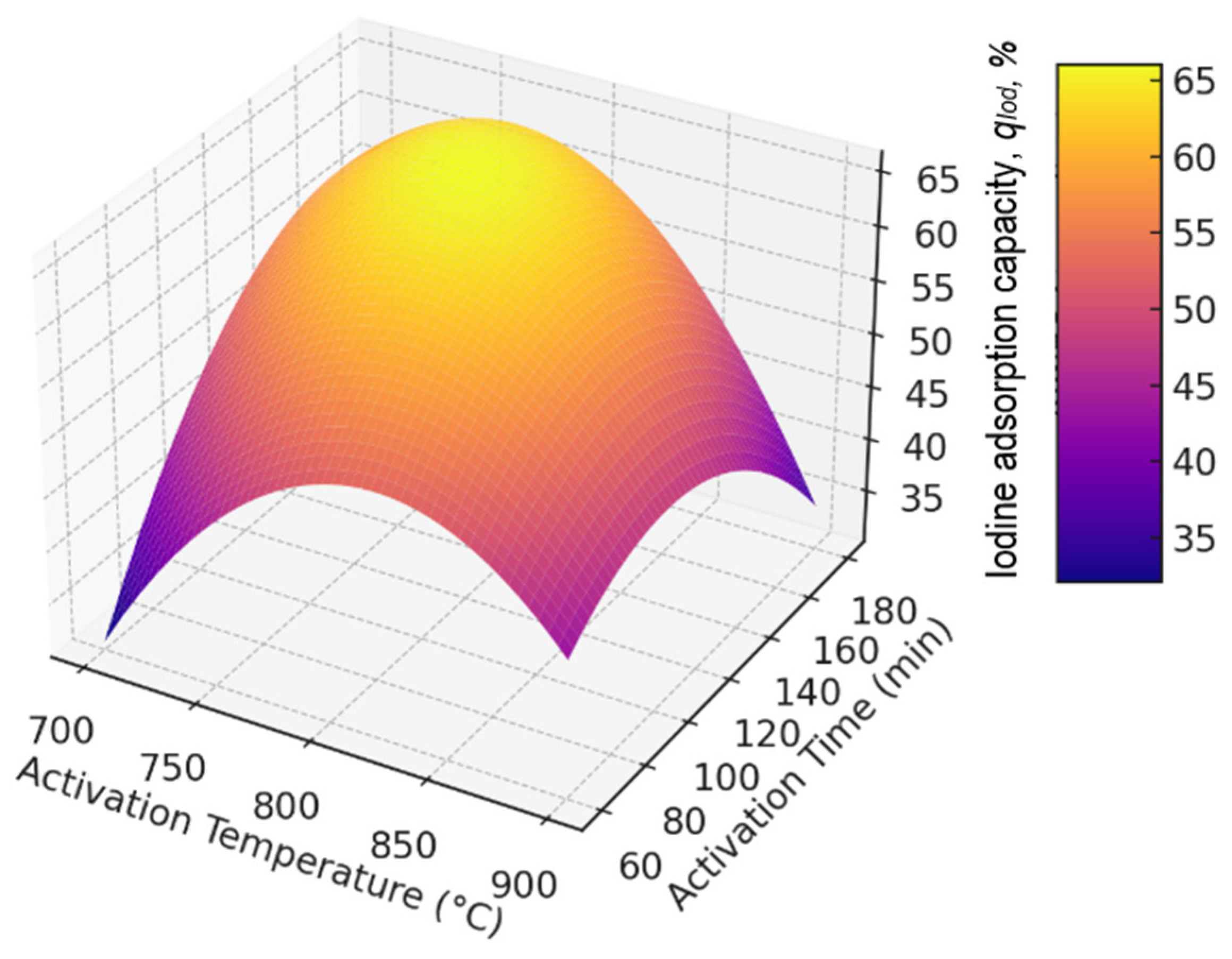
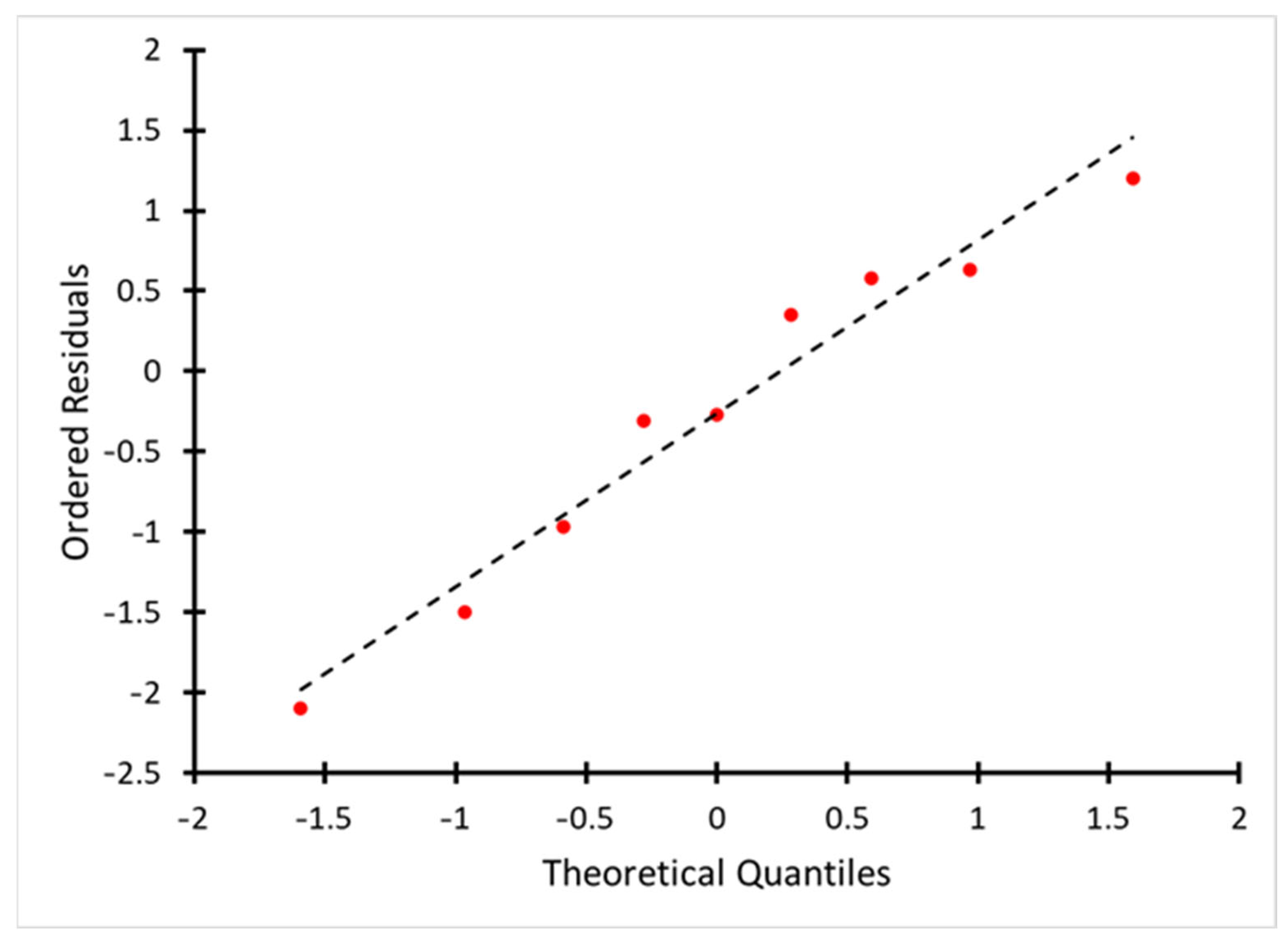
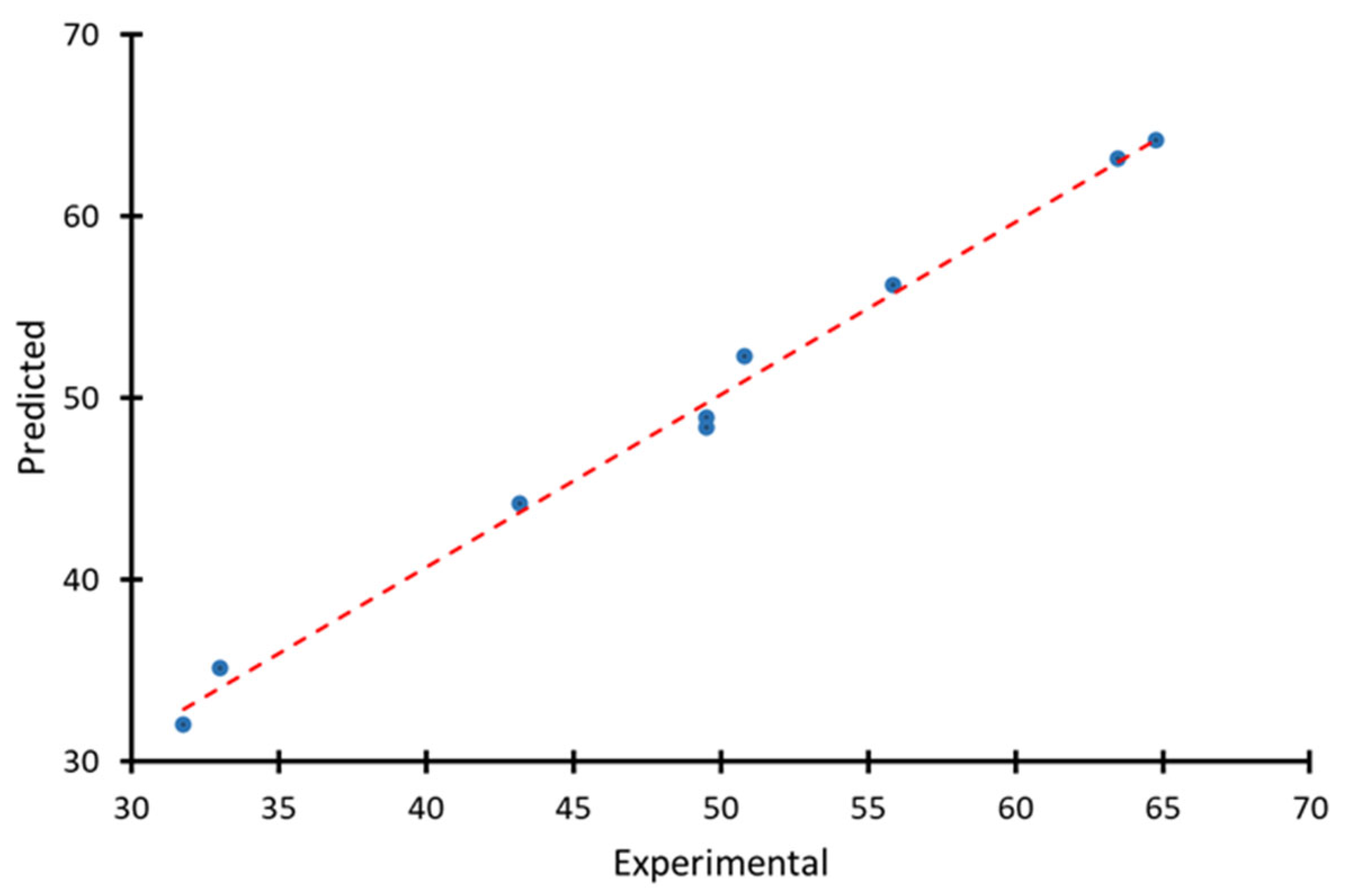

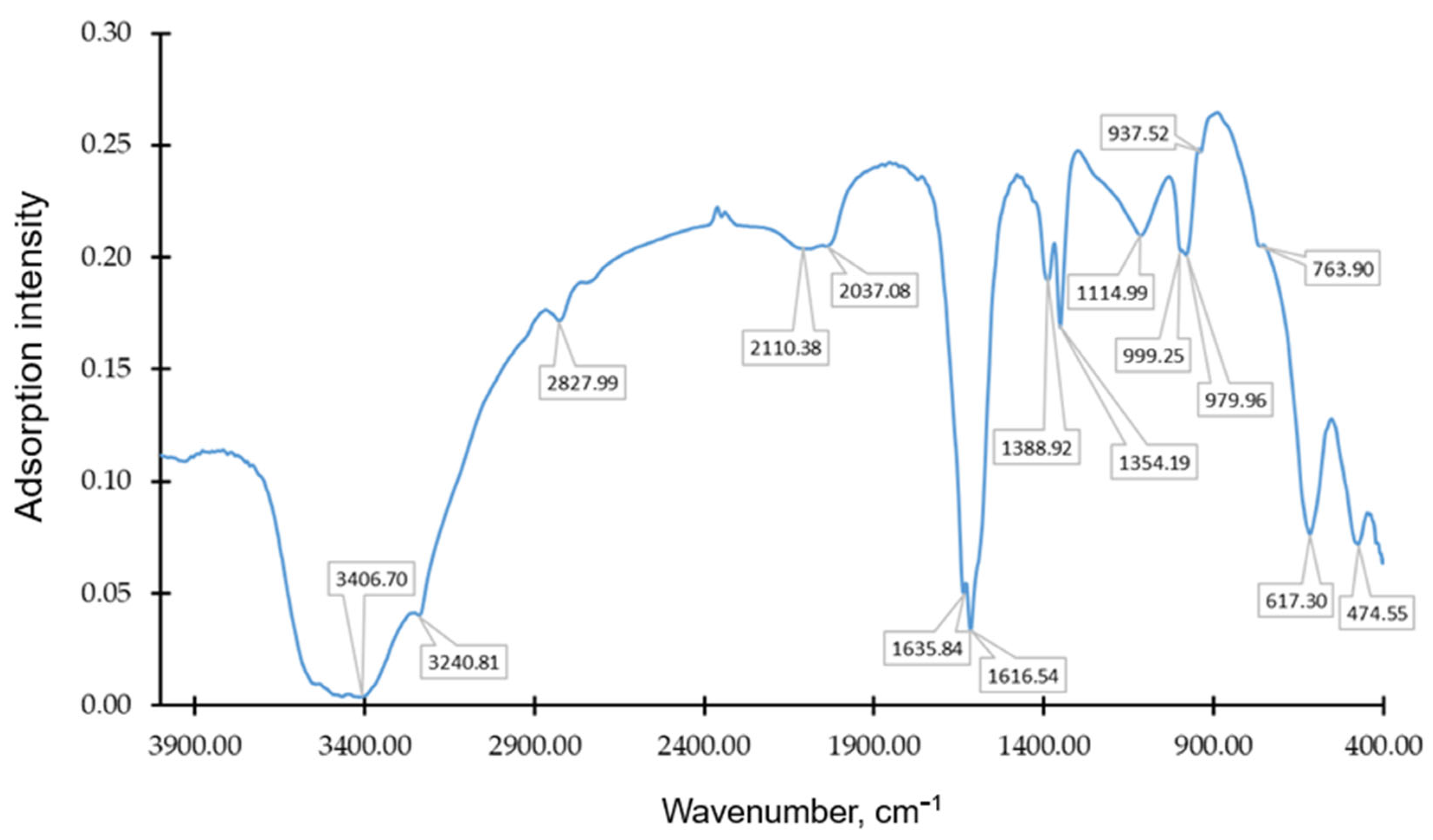


| Factor 1 | Factor 2 | Response | Burn-Off, % | Ash, % | Bulk Density, g/dm3 |
|---|---|---|---|---|---|
| Temperature, T °C | Activation Time, τ, min | Iodine Capacity qIod experiment, % | |||
| 700 | 180 | 55.88 | 29.51 | 9.36 | 537 |
| 700 | 120 | 50.8 | 23.93 | 6.41 | 538 |
| 700 | 60 | 33.02 | 17.56 | 7.02 | 595 |
| 800 | 180 | 63.5 | 64.88 | 15.17 | 376 |
| 800 | 120 | 64.77 | 46.36 | 10.83 | 476 |
| 800 | 60 | 49.53 | 28.97 | 7.20 | 539 |
| 900 | 180 | 31.75 | 83.09 | 26.27 | 411 |
| 900 | 120 | 49.53 | 77.62 | 22.58 | 427 |
| 900 | 60 | 43.18 | 46.42 | 9.71 | 510 |
| Coefficient | Estimate (βᵢ) | Standard Error SE (βᵢ) | t-Value | p-Value |
| β0 | 65.19 | 2.52 | 25.85 | 0.0001 |
| β1 | −2.54 | 1.39 | −1.83 | 0.165 |
| β2 | 4.23 | 1.39 | 3.04 | 0.056 |
| β3 | −15.24 | 2.38 | −6.4 | 0.0077 |
| β4 | −8.89 | 2.38 | −3.73 | 0.0336 |
| β5 | −8.57 | 1.69 | −5.08 | 0.0133 |
| Temperature (T), °C | Activation Time (τ), min | Experimental qIod experiment, % | Predicted qIod, % | Residual (Error), % |
|---|---|---|---|---|
| 700 | 180 | 55.88 | 56.19 | −0.31 |
| 700 | 120 | 50.8 | 52.3 | −1.5 |
| 700 | 60 | 33.02 | 35.12 | −2.1 |
| 800 | 180 | 63.5 | 63.15 | 0.35 |
| 800 | 120 | 64.77 | 64.19 | 0.58 |
| 800 | 60 | 49.53 | 48.9 | 0.63 |
| 900 | 180 | 31.75 | 32.02 | −0.27 |
| 900 | 120 | 49.53 | 48.33 | 1.2 |
| 900 | 60 | 43.18 | 44.15 | −0.97 |
| Source of Variation | Sum of Squares (SS) | Degrees of Freedom (df) | Mean Square (MS) | F-Statistic | p-Value |
|---|---|---|---|---|---|
| Model | 1062.76 | 5 | 212.55 | 18.75 | 0.018 |
| Residual (Error) | 34.01 | 3 | 11.34 | — | — |
| Total | 1096.77 | 8 | — | — | — |
| Coke Fines | Obtained Sorbent | ||||||
|---|---|---|---|---|---|---|---|
| Di, nm | dDi, nm | dVi, cm3 | dVi/Vsum, % | Di, nm | dDi, nm | dVi, cm3 | dVi/Vsum, % |
| 3.496 | 0.76746 | 0.00097 | 4.4951 | 3.496 | 0.767 | 0.027 | 14.65 |
| 4.43 | 1.1007 | 0.0019 | 8.7793 | 4.43 | 1.101 | 0.018 | 9.892 |
| 5.863 | 1.7661 | 0.00095 | 4.3632 | 5.863 | 1.766 | 0.013 | 7.128 |
| 8.441 | 3.389 | 0.00169 | 7.788 | 8.441 | 3.389 | 0 | 0 |
| 14.998 | 9.725 | 0.00047 | 2.1774 | 14.998 | 9.725 | 0 | 0 |
| 29.351 | 18.982 | 0 | 0 | 29.351 | 18.982 | 0 | 0 |
| 43.558 | 9.4333 | 0 | 0 | 43.558 | 9.433 | 0 | 0 |
| 56.122 | 15.694 | 0.01392 | 64.23 | 56.122 | 15.694 | 0.116 | 63.18 |
| 79.642 | 31.345 | 0.00177 | 8.1675 | 79.642 | 31.345 | 0.009 | 5.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordabaeva, A.T.; Muldakhmetov, Z.M.; Meiramov, M.G.; Kim, S.V. Activation of Coke Fines Using CO2 and Steam: Optimization and Characterization of Carbon Sorbents. Molecules 2025, 30, 2528. https://doi.org/10.3390/molecules30122528
Ordabaeva AT, Muldakhmetov ZM, Meiramov MG, Kim SV. Activation of Coke Fines Using CO2 and Steam: Optimization and Characterization of Carbon Sorbents. Molecules. 2025; 30(12):2528. https://doi.org/10.3390/molecules30122528
Chicago/Turabian StyleOrdabaeva, Aigul T., Zainulla M. Muldakhmetov, Mazhit G. Meiramov, and Sergey V. Kim. 2025. "Activation of Coke Fines Using CO2 and Steam: Optimization and Characterization of Carbon Sorbents" Molecules 30, no. 12: 2528. https://doi.org/10.3390/molecules30122528
APA StyleOrdabaeva, A. T., Muldakhmetov, Z. M., Meiramov, M. G., & Kim, S. V. (2025). Activation of Coke Fines Using CO2 and Steam: Optimization and Characterization of Carbon Sorbents. Molecules, 30(12), 2528. https://doi.org/10.3390/molecules30122528






