Facile One-Pot Fischer–Suzuki–Knoevenagel Microwave-Assisted Synthesis of Fluorescent 5-Aryl-2-Styryl-3H-Indoles
Abstract
1. Introduction
2. Results and Discussion
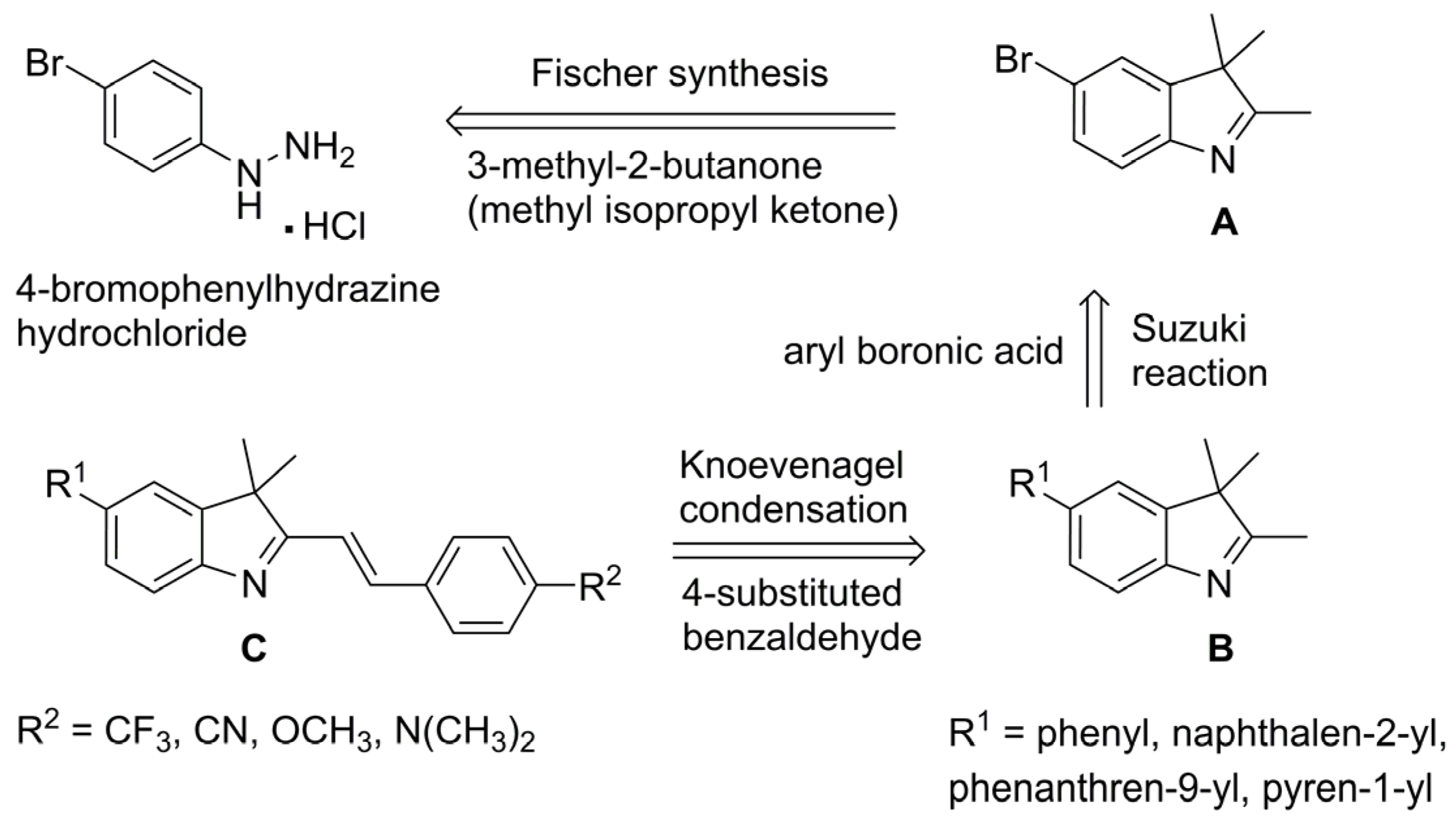
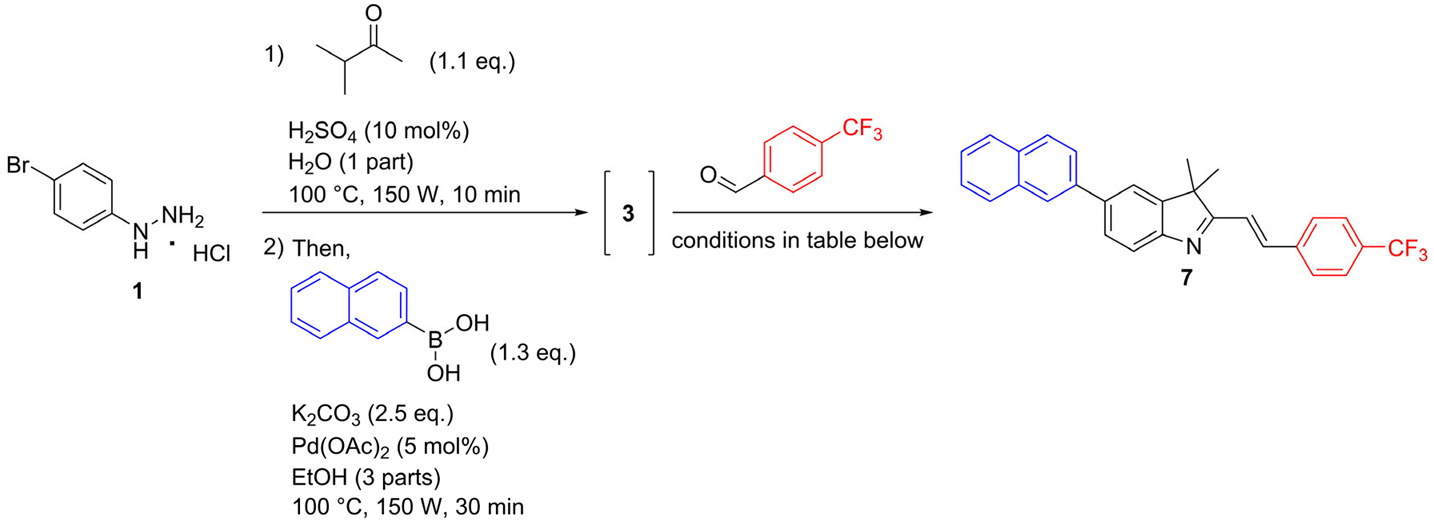
2.1. Chemistry
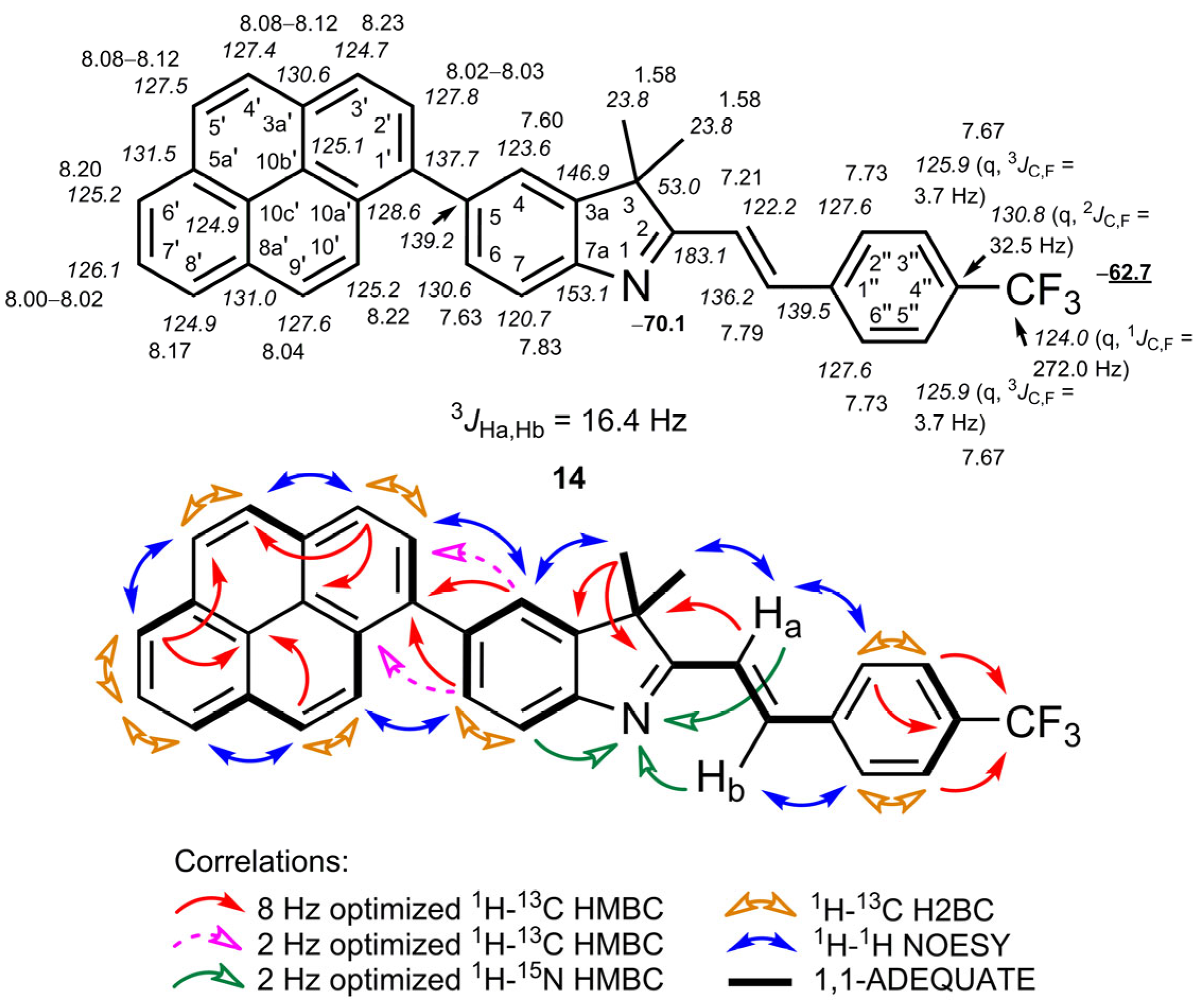
2.2. NMR Spectroscopic Investigations
2.3. Optical Properties
3. Materials and Methods
3.1. General
3.2. Synthetic Procedures
3.2.1. Synthesis of 5-Bromo-2,3,3-trimethyl-3H-indole (2)
3.2.2. General Procedures for the Synthesis of 5-aryl-2,3,3-trimethyl-3H-indoles (3–6)
2,3,3-Trimethyl-5-(naphthalen-2-yl)-3H-indole (3)
2,3,3-Trimethyl-5-(phenanthren-9-yl)-3H-indole (4)
2,3,3-Trimethyl-5-(pyren-1-yl)-3H-indole (5)
2,3,3-Trimethyl-5-phenyl-3H-indole (6)
3.2.3. General Procedure for the Synthesis of 5-aryl-3,3-dimethyl-2-styryl-3H-indoles via One-Pot Fischer–Suzuki–Knoevenagel Approach (7–17)
3,3-Dimethyl-5-(naphthalen-2-yl)-2-{(E)-2-[4-(trifluoromethyl)phenyl]ethenyl}-3H-indole (7)
4-{(E)-2-[3,3-Dimethyl-5-(naphthalen-2-yl)-3H-indol-2-yl]ethenyl}benzonitrile (8)
2-[(E)-2-(4-Methoxyphenyl)ethenyl]-3,3-dimethyl-5-(naphthalen-2-yl)-3H-indole (9)
4-{(E)-2-[3,3-Dimethyl-5-(naphthalen-2-yl)-3H-indol-2-yl]ethenyl}-N,N-dimethylaniline (10)
3,3-Dimethyl-5-(phenanthren-9-yl)-2-{(E)-2-[4-(trifluoromethyl)phenyl]ethenyl}-3H-indole (11)
4-{(E)-2-[3,3-Dimethyl-5-(phenanthren-9-yl)-3H-indol-2-yl]ethenyl}benzonitrile (12)
2-[(E)-2-(4-Methoxyphenyl)ethenyl]-3,3-dimethyl-5-(phenanthren-9-yl)-3H-indole (13)
3,3-Dimethyl-5-(pyren-1-yl)-2-{(E)-2-[4-(trifluoromethyl)phenyl]ethenyl}-3H-indole (14)
4-{(E)-2-[3,3-Dimethyl-5-(pyren-1-yl)-3H-indol-2-yl]ethenyl}benzonitrile (15)
2-[(E)-2-(4-Methoxyphenyl)ethenyl]-3,3-dimethyl-5-(pyren-1-yl)-3H-indole (16)
4-{(E)-2-[3,3-Dimethyl-5-phenyl-3H-indol-2-yl]ethenyl}benzonitrile (17)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhurta, D.; Bharate, S.B. Styryl Group, a Friend or Foe in Medicinal Chemistry. ChemMedChem 2022, 17, e202100706. [Google Scholar] [CrossRef] [PubMed]
- Deligeorgiev, T.; Vasilev, A.; Kaloyanova, S.; Vaquero, J.J. Styryl Dyes—Synthesis and Applications during the Last 15 Years. Color. Technol. 2010, 126, 55–80. [Google Scholar] [CrossRef]
- Salem, M.; Ayyad, R.; Sakr, H. Design and Synthesis of Some New Oxadiazole Derivatives as Anticancer Agents. IJOC 2022, 12, 64–74. [Google Scholar] [CrossRef]
- Antonioletti, R.; Righi, G.; Ricelli, A.; Rossetti, I.; Viglianti, A.; Frezza, C.; Marino-Merlo, F.; Macchi, B. Synthesis and Biological Evaluation of Styrylheterocycles Analogs of Resveratrol as Apoptosis-Inducing Agents. Curr. Org. Chem. 2017, 21, 939–948. [Google Scholar] [CrossRef]
- Hranjec, M.; Kralj, M.; Piantanida, I.; Sedić, M.; Šuman, L.; Pavelić, K.; Karminski-Zamola, G. Novel Cyano- and Amidino-Substituted Derivatives of Styryl-2-Benzimidazoles and Benzimidazo[1,2- a ]Quinolines. Synthesis, Photochemical Synthesis, DNA Binding, and Antitumor Evaluation, Part 3. J. Med. Chem. 2007, 50, 5696–5711. [Google Scholar] [CrossRef]
- Atanasov, G.; Rusew, R.I.; Gelev, V.M.; Chanev, C.D.; Nikolova, R.; Shivachev, B.L.; Petrov, O.I.; Apostolova, M.D. New Heterocyclic Combretastatin A-4 Analogs: Synthesis and Biological Activity of Styryl-2(3H)-Benzothiazolones. Pharmaceuticals 2021, 14, 1331. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.W.; Wang, J.; Okuda, K.S.; Su, Q.; Zhang, Y.; Abas, F.; Chia, S.L.; Yusoff, K. Discovery of a Novel Dual Functional Phenylpyrazole-Styryl Hybrid That Induces Apoptotic and Autophagic Cell Death in Bladder Cancer Cells. Eur. J. Med. Chem. 2023, 254, 115335. [Google Scholar] [CrossRef]
- Flynn, D.L.; Belliotti, T.R.; Boctor, A.M.; Connor, D.T.; Kostlan, C.R.; Nies, D.E.; Ortwine, D.F.; Schrier, D.J.; Sircar, J.C. Styrylpyrazoles, Styrylisoxazoles, and Styrylisothiazoles. Novel 5-Lipoxygenase and Cyclooxygenase Inhibitors. J. Med. Chem. 1991, 34, 518–525. [Google Scholar] [CrossRef]
- Nauduri, D.; Reddy, G.B.S. Antibacterials and Antimycotics: Part 1: Synthesis and Activity of 2-Pyrazoline Derivatives. Chem. Pharm. Bull. 1998, 46, 1254–1260. [Google Scholar] [CrossRef]
- Mishra, S.; Karmodiya, K.; Surolia, N.; Surolia, A. Synthesis and Exploration of Novel Curcumin Analogues as Anti-Malarial Agents. Bioorg. Med. Chem. 2008, 16, 2894–2902. [Google Scholar] [CrossRef]
- Sherin, D.R.; Rajasekharan, K.N. Mechanochemical Synthesis and Antioxidant Activity of Curcumin-Templated Azoles. Arch. Pharm. 2015, 348, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Silva, J.; Silva, V.L.M.; Silva, A.M.S.; Corvo, M.L.; Freitas, M.; Fernandes, E. Pyrazoles Have a Multifaceted Anti-Inflammatory Effect Targeting Prostaglandin E2, Cyclooxygenases and Leukocytes’ Oxidative Burst. Int. J. Biochem. Cell Biol. 2024, 172, 106599. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.M.O.; Ouro, P.M.S.; Silva, A.M.S.; Silva, V.L.M. Styrylpyrazoles: Properties, Synthesis and Transformations. Molecules 2020, 25, 5886. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.M.; Silva, A.M.S. An Overview of 2-Styrylchromones: Natural Occurrence, Synthesis, Reactivity and Biological Properties. Eur. J. Org. Chem. 2017, 2017, 3115–3133. [Google Scholar] [CrossRef]
- Lucas, M.; Freitas, M.; Silva, A.M.S.; Fernandes, E.; Ribeiro, D. Styrylchromones: Biological Activities and Structure-Activity Relationship. ChemMedChem 2025, 20, e202400782. [Google Scholar] [CrossRef]
- Buettelmann, B.; Alanine, A.; Bourson, A.; Gill, R.; Heitz, M.-P.; Mutel, V.; Pinard, E.; Trube, G.; Wyler, R. 2-Styryl-Pyridines and 2-(3,4-Dihydro-Naphthalen-2-Yl)Pyridines as Potent NR1/2B Subtype Selective NMDA Receptor Antagonists. Chimia 2004, 58, 630. [Google Scholar] [CrossRef]
- Gouleni, N.; Di Rienzo, A.; Yılmaz, A.; Selvitopi, H.; Arslan, M.E.; Mardinoglu, A.; Turkez, H.; Di Stefano, A.; Vassiliou, S.; Cacciatore, I. Novel Styryl-Thiazole Hybrids as Potential Anti-Alzheimer’s Agents. RSC Med. Chem. 2023, 14, 2315–2326. [Google Scholar] [CrossRef]
- Kamal, A.; Rahim, A.; Riyaz, S.; Poornachandra, Y.; Balakrishna, M.; Kumar, C.G.; Hussaini, S.M.A.; Sridhar, B.; Machiraju, P.K. Regioselective Synthesis, Antimicrobial Evaluation and Theoretical Studies of 2-Styryl Quinolines. Org. Biomol. Chem. 2015, 13, 1347–1357. [Google Scholar] [CrossRef]
- Jagani, C.L.; Sojitra, N.A.; Vanparia, S.F.; Patel, T.S.; Dixit, R.B.; Dixit, B.C. Microwave Promoted Synthesis and Antimicrobial Activity of 3-Thiazole Substituted 2-Styryl-4(3H)-Quinazolinone Derivatives. J. Saudi Chem. Soc. 2012, 16, 363–369. [Google Scholar] [CrossRef]
- Antonioletti, R.; Viglianti, A.; Cristofoli, S.; Ricelli, A. Role of Some Neosynthesized Styryl Heterocycles in Ochratoxin A Control in Aspergillus Carbonarius. Curr. Org. Chem. 2016, 20, 2029–2035. [Google Scholar] [CrossRef][Green Version]
- De Fatima, A.; Modolo, L.; Conegero, L.; Pilli, R.; Ferreira, C.; Kohn, L.; De Carvalho, J. Styryl Lactones and Their Derivatives: Biological Activities, Mechanisms of Action and Potential Leads for Drug Design. Curr. Med. Chem. 2006, 13, 3371–3384. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, M.A.; Bermejo, A.; Zafra-Polo, M.C.; Cortes, D. Styryl-Lactones from Goniothalamus Species— A Review. Phytochem. Anal. 1999, 10, 161–170. [Google Scholar] [CrossRef]
- Rasol, N.E.; Ahmad, F.B.; Mai, C.-W.; Bihud, N.V.; Abdullah, F.; Awang, K.; Ismail, N.H. Styryl Lactones from Roots and Barks Goniothalamus Lanceolatus. Nat. Prod. Commun. 2018, 13, 1934578X1801301203. [Google Scholar] [CrossRef]
- De Fátima, Â.; Kohn, L.K.; Antônio, M.A.; De Carvalho, J.E.; Pilli, R.A. (R)-Goniothalamin: Total Syntheses and Cytotoxic Activity against Cancer Cell Lines. Bioorg. Med. Chem. 2005, 13, 2927–2933. [Google Scholar] [CrossRef]
- Varvuolytė, G.; Malina, L.; Bieliauskas, A.; Hošíková, B.; Simerská, H.; Kolářová, H.; Kleizienė, N.; Kryštof, V.; Šačkus, A.; Žukauskaitė, A. Synthesis and Photodynamic Properties of Pyrazole-Indole Hybrids in the Human Skin Melanoma Cell Line G361. Dyes Pigm. 2020, 183, 108666. [Google Scholar] [CrossRef]
- Varvuolytė, G.; Řezníčková, E.; Bieliauskas, A.; Kleizienė, N.; Vojáčková, V.; Opichalová, A.; Žukauskaitė, A.; Kryštof, V.; Šačkus, A. Synthesis and Photodynamic Activity of New 5-[(E)-2-(3-Alkoxy-1-phenyl-1H-pyrazol-4-yl)ethenyl]-2-phenyl-3H-indoles. Arch. Pharm. 2024, 357, e2400282. [Google Scholar] [CrossRef] [PubMed]
- Ustimova, M.A.; Fedorov, Y.V.; Chmelyuk, N.S.; Abakumov, M.A.; Fedorova, O.A. Fluorescence Turn-on Probes for Intracellular DNA/RNA Distribution Based on Asymmetric Bis(Styryl) Dyes. Spectrochim. Acta Mol. Biomol. Spectrosc. 2022, 279, 121446. [Google Scholar] [CrossRef]
- Supabowornsathit, K.; Faikhruea, K.; Ditmangklo, B.; Jaroenchuensiri, T.; Wongsuwan, S.; Junpra-ob, S.; Choopara, I.; Palaga, T.; Aonbangkhen, C.; Somboonna, N.; et al. Dicationic Styryl Dyes for Colorimetric and Fluorescent Detection of Nucleic Acids. Sci. Rep. 2022, 12, 14250. [Google Scholar] [CrossRef]
- Pfeuffer, B.; Geng, P.; Wagenknecht, H. Two-Factor Fluorogenic Cyanine-Styryl Dyes with Yellow and Red Fluorescence for Bioorthogonal Labelling of DNA. ChemBioChem 2024, 25, e202300739. [Google Scholar] [CrossRef]
- Cesaretti, A.; Calzoni, E.; Montegiove, N.; Bianconi, T.; Alebardi, M.; La Serra, M.A.; Consiglio, G.; Fortuna, C.G.; Elisei, F.; Spalletti, A. Lighting-Up the Far-Red Fluorescence of RNA-Selective Dyes by Switching from Ortho to Para Position. Int. J. Mol. Sci. 2023, 24, 4812. [Google Scholar] [CrossRef]
- Pithan, P.M.; Decker, D.; Druzhinin, S.I.; Ihmels, H.; Schönherr, H.; Voß, Y. 8-Styryl-Substituted Coralyne Derivatives as DNA Binding Fluorescent Probes. RSC Adv. 2017, 7, 10660–10667. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Fu, Y.-J.; Liu, Q.-L.; Lu, D.-T.; Dong, C.; Shuang, S.-M. Novel Far-Visible and near-Infrared pH Probes Based on Styrylcyanine for Imaging Intracellular pH in Live Cells. Chem. Commun. 2012, 48, 11202. [Google Scholar] [CrossRef]
- Varvuolytė, G.; Řezníčková, E.; Krikštolė, S.; Tamulienė, R.; Bieliauskas, A.; Malina, L.; Vojáčková, V.; Duben, Z.; Kolářová, H.; Kleizienė, N.; et al. Synthesis and Photo-Induced Anticancer Activity of New 2-Phenylethenyl-1H-Benzo[e]Indole Dyes. Eur. J. Med. Chem. 2024, 277, 116777. [Google Scholar] [CrossRef]
- Orrego-Hernández, J.; Cobo, J.; Portilla, J. Synthesis, Photophysical Properties, and Metal-Ion Recognition Studies of Fluoroionophores Based on 1-(2-Pyridyl)-4-Styrylpyrazoles. ACS Omega 2019, 4, 16689–16700. [Google Scholar] [CrossRef]
- Kido, J.; Endo, J. Orange Color Electroluminescence from Bis(2-Styryl-8-Quinolinolato)Zinc(II). Chem. Lett. 1997, 26, 633–634. [Google Scholar] [CrossRef]
- Al-lehaib, L.A.; Ali, E.M.M.; Al-Footy, K.O.; El-Shishtawy, R.M. Novel Styryl-Heterocyclic Hybrids: Synthesis, Characterization and Anticancer Activity. Results Chem. 2024, 7, 101374. [Google Scholar] [CrossRef]
- Vasilev, A.; Deligeorgiev, T.; Gadjev, N.; Kaloyanova, S.; Vaquero, J.J.; Alvarez-Builla, J.; Baeza, A.G. Novel Environmentally Benign Procedures for the Synthesis of Styryl Dyes. Dyes Pigm. 2008, 77, 550–555. [Google Scholar] [CrossRef][Green Version]
- Dabiri, M.; Baghbanzadeh, M.; Delbari, A.S. Novel and Efficient One-Pot Tandem Synthesis of 2-Styryl-Substituted 4(3 H )-Quinazolinones. J. Comb. Chem. 2008, 10, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Šačkus, A.; Žukauskaitė, Ž.; Kleizienė, N.; Bieliauskas, A.; Amankavičienė, V.; Holzer, W.; Martynaitis, V. One-Pot Synthesis of Polycyclic Heterocyclic Compounds by Condensation of 1-Carbamoylmethyl-2,3,3-Trimethyl-3H-Indolium Salts with Pyridine-2, 3, and 4- and Quinoline-4-Carboxaldehydes. Tetrahedron 2018, 74, 3679–3690. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Kazlauskas, K.; Miasojedovas, A.; Juršėnas, S.; Jankauskas, V.; Holzer, W.; Getautis, V.; Šačkus, A. Pyrazolyl-Substituted Polyconjugated Molecules for Optoelectronic Applications. Dyes Pigm. 2010, 85, 79–85. [Google Scholar] [CrossRef]
- Kazlauskas, K.; Kreiza, G.; Arbačiauskienė, E.; Bieliauskas, A.; Getautis, V.; Šačkus, A.; Juršėnas, S. Morphology and Emission Tuning in Fluorescent Nanoparticles Based on Phenylenediacetonitrile. J. Phys. Chem. C 2014, 118, 25261–25271. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Martynaitis, V.; Krikštolaitytė, S.; Holzer, W.; Šačkus, A. Synthesis of 3-Substituted 1-Phenyl-1H-Pyrazole-4-Carbaldehydes and the Corresponding Ethanones by Pd-Catalysed Cross-Coupling Reactions. Arkivoc 2011, 2011, 1–21. [Google Scholar] [CrossRef]
- Nandi, S.; Jamatia, R.; Sarkar, R.; Sarkar, F.K.; Alam, S.; Pal, A.K. One-Pot Multicomponent Reaction: A Highly Versatile Strategy for the Construction of Valuable Nitrogen-Containing Heterocycles. ChemistrySelect 2022, 7, e202201901. [Google Scholar] [CrossRef]
- Kornet, M.M.; Müller, T.J.J. Recent Advances in Sequentially Pd-Catalyzed One-Pot Syntheses of Heterocycles. Molecules 2024, 29, 5265. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef]
- Levi, L.; Müller, T.J.J. Multicomponent syntheses of functional chromophores. Chem. Soc. Rev. 2016, 45, 2825–2846. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000; ISBN 978-0-19-850698-0. [Google Scholar]
- Tietze, L.F. Domino Reactions in Organic Synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef]
- Brandner, L.; Müller, T.J.J. Multicomponent synthesis of chromophores—The one-pot approach to functional π-systems. Front. Chem. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, A.; Qadri, M.M.; Ansari, I.; Gautam, A.; Chakraborti, A.K. In(OTf)3-Catalyzed Synthesis of 2-Styryl Quinolines: Scope and Limitations of Metal Lewis Acids for Tandem Friedländer Annulation–Knoevenagel Condensation. RSC Adv. 2015, 5, 2920–2927. [Google Scholar] [CrossRef]
- Meyer, T.; Krug, R.; Müller, T.J.J. Three-Component Suzuki–Knoevenagel Synthesis of Merocyanine Libraries and Correlation Analyses of Their Oxidation Potentials and Optical Band Gaps. Molecules 2021, 26, 5149. [Google Scholar] [CrossRef]
- Sajjadifar, S.; Vahedi, H.; Massoudi, A.; Louie, O. New 3H-Indole Synthesis by Fischer’s Method. Part I. Molecules 2010, 15, 2491–2498. [Google Scholar] [CrossRef]
- Seen, S.B.; Gong, Y.; Ashton, M. The Application of the Fischer Indole Synthesis in Medicinal Chemistry. In Advances in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; Volume 139, pp. 1–85. ISBN 978-0-443-19314-9. [Google Scholar]
- Yan, X.; Chen, X.; Shan, Z.; Bi, L. Innovative Cyanine-Based Fluorescent Dye for Targeted Mitochondrial Imaging and Its Utility in Whole-Brain Visualization. ACS Omega 2024, 9, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; Hennig, L. Ring Transformations of Heterocyclic Compounds. XXII. Pyrido[1,2-a] Indolium Salts from 2-methyl-3H-indoles by Pyrylium Mediated Three Carbon Annelation. J. Heterocycl. Chem 2002, 39, 263–269. [Google Scholar] [CrossRef]
- Liu, H.; Yin, J.; Xing, E.; Du, Y.; Su, Y.; Feng, Y.; Meng, S. Halogenated Cyanine Dyes for Synergistic Photodynamic and Photothermal Therapy. Dyes Pigm. 2021, 190, 109327. [Google Scholar] [CrossRef]
- Fong, D.; Andrews, G.M.; Adronov, A. Preparation of Stimulus-responsive, Polyfluorene-wrapped Carbon Nanotubes via Palladium Cross Coupling. J. Polym. Sci. A Polym. Chem. J. 2018, 56, 2723–2729. [Google Scholar] [CrossRef]
- Gaur, P.; Kumar, A.; Dey, G.; Kumar, R.; Bhattacharyya, S.; Ghosh, S. Selenium Incorporated Cationic Organochalcogen: Live Cell Compatible and Highly Photostable Molecular Stain for Imaging and Localization of Intracellular DNA. ACS Appl. Mater. Interfaces 2016, 8, 10690–10699. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-C.; Yu, J.; Ye, F.; Rong, Y.; Gallina, M.E.; Fujimoto, B.S.; Zhang, Y.; Chan, Y.-H.; Sun, W.; Zhou, X.-H.; et al. Squaraine-Based Polymer Dots with Narrow, Bright Near-Infrared Fluorescence for Biological Applications. J. Am. Chem. Soc. 2015, 137, 173–178. [Google Scholar] [CrossRef]
- Chandra Saha, P.; Das, R.S.; Chatterjee, T.; Bhattacharyya, M.; Guha, S. Supramolecular β-Sheet Forming Peptide Conjugated with Near-Infrared Chromophore for Selective Targeting, Imaging, and Dysfunction of Mitochondria. Bioconjugate Chem. 2020, 31, 1301–1306. [Google Scholar] [CrossRef]
- Owens, E.A.; Bruschi, N.; Tawney, J.G.; Henary, M. A Microwave-Assisted and Environmentally Benign Approach to the Synthesis of near-Infrared Fluorescent Pentamethine Cyanine Dyes. Dyes Pigm. 2015, 113, 27–37. [Google Scholar] [CrossRef]
- Ning, J.-J.; Wang, J.-F.; Ren, Z.-G.; Young, D.J.; Lang, J.-P. Versatile Palladium(II)-Catalyzed Suzuki–Miyaura Coupling in Ethanol with a Novel, Stabilizing Ligand. Tetrahedron 2015, 71, 4000–4006. [Google Scholar] [CrossRef]
- Kolagkis, P.X.; Serviou, S.K.; Stini, N.A.; Demertzidou, V.P.; Poursaitidis, E.T.; Galathri, E.M.; Mountanea, O.G.; Skolia, E.; Kokotos, C.G. Deciphering the Knoevenagel Condensation: Towards a Catalyst-Free and Water-Mediated Process. Org. Biomol. Chem. 2024, 22, 8293–8299. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Whiffing, C.A.; Perry, A. Three Complementary One-Pot Four-Component Reaction Sequences for Rapid, General and Direct Spiropyran Synthesis. Eur. J. Org. Chem. 2023, 26, e202201245. [Google Scholar] [CrossRef]
- Dissanayake, D.S.; McCandless, G.T.; Stefan, M.C.; Biewer, M.C. Systematic Variation of Thiophene Substituents in Photochromic Spiropyrans. Photochem. Photobiol. Sci. 2017, 16, 1057–1062. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, T.; Wang, R.; Gao, J.; Sun, J.; Ou-Yang, Z.; Liu, Y.; Gu, X.; Zhao, C. A Water-Soluble Fluorescent Probe for Real-Time Visualization of γ-Glutamyl Transpeptidase Activity in Living Cells. Bioorg. Med. Chem. Lett. 2022, 68, 128762. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Zhan, K.; Zhong, K.-L.; Chen, X.-L.; Xia, X.-H. A Novel Indole Derivative with Superior Photophysical Performance for Fluorescent Probe, pH-Sensing, and Logic Gates. Int. J. Mol. Sci. 2023, 24, 1711. [Google Scholar] [CrossRef]
- Fors, B.P.; Krattiger, P.; Strieter, E.; Buchwald, S.L. Water-Mediated Catalyst Preactivation: An Efficient Protocol for C−N Cross-Coupling Reactions. Org. Lett. 2008, 10, 3505–3508. [Google Scholar] [CrossRef]
- Adrio, L.A.; Nguyen, B.N.; Guilera, G.; Livingston, A.G.; Hii, K.K. (Mimi) Speciation of Pd(OAc) 2 in Ligandless Suzuki–Miyaura Reactions. Catal. Sci. Technol. 2012, 2, 316–323. [Google Scholar] [CrossRef]
- Žukauskaitė, Ž.; Buinauskaitė, V.; Solovjova, J.; Malinauskaitė, L.; Kveselytė, A.; Bieliauskas, A.; Ragaitė, G.; Šačkus, A. Microwave-Assisted Synthesis of New Fluorescent Indoline-Based Building Blocks by Ligand Free Suzuki-Miyaura Cross-Coupling Reaction in Aqueous Media. Tetrahedron 2016, 72, 2955–2963. [Google Scholar] [CrossRef]
- Zheng, J.; Deng, L.; Chen, M.; Xiao, X.; Xiao, S.; Guo, C.; Xiao, G.; Bai, L.; Ye, W.; Zhang, D.; et al. Elaboration of Thorough Simplified Vinca Alkaloids as Antimitotic Agents Based on Pharmacophore Similarity. Eur. J. Med. Chem. 2013, 65, 158–167. [Google Scholar] [CrossRef]
- Hu, W.; Zeng, L.; Zhai, S.; Li, C.; Feng, W.; Feng, Y.; Liu, Z. Developing a Ratiometric Two-Photon Probe with Baseline Resolved Emissions by through Band Energy Transfer Strategy: Tracking Mitochondrial SO2 during Neuroinflammation. Biomaterials 2020, 241, 119910. [Google Scholar] [CrossRef]
- Deniz, E.; Ray, S.; Tomasulo, M.; Impellizzeri, S.; Sortino, S.; Raymo, F.M. Photoswitchable Fluorescent Dyads Incorporating BODIPY and [1,3]Oxazine Components. J. Phys. Chem. A 2010, 114, 11567–11575. [Google Scholar] [CrossRef] [PubMed]




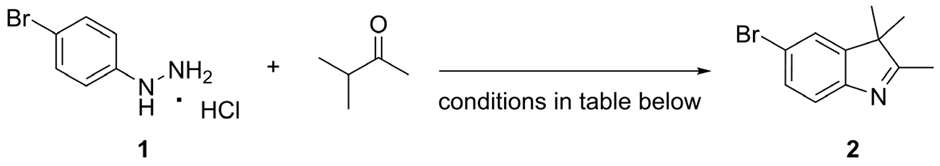 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst (mol%) | Solvent | Ketone (eq.) | Time (min) | Yield (%) b |
| 1 c | H2SO4 (10) | EtOH | 1.5 | 18 hours d | 55 |
| 2 | H2SO4 (10) | EtOH | 1.5 | 10 | 58 |
| 3 | - | AcOH | 1.2 | 10 | 54 |
| 4 e | H2SO4 (10) | H2O | 1.1 | 10 | 67 |
| 5 | H2SO4 (10) | H2O | 1.5 | 15 | 67 |
| 6 f | H2SO4 (10) | EtOH:H2O | 1.5 | 10 | 55 |
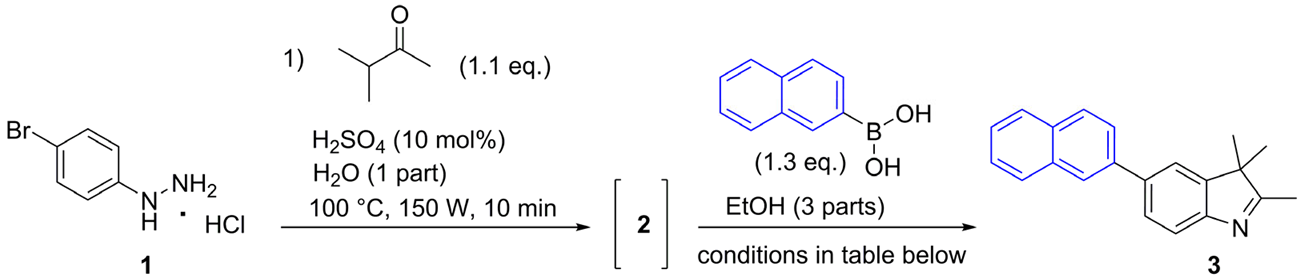 | ||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst (mol%) | Base (eq.) | Ligand (eq.) | Temperature (°C) | Time (min) | Overall Yield (%) b |
| 1 | Pd(OAc)2 (10) | Cs2CO3 (3) | PPh3 (0.4) | 80 | 40 | 45 |
| 2 c | Pd(OAc)2 (10) | Cs2CO3 (3) | PPh3 (0.4) | 80 | 40 | 35 |
| 3 c | Pd(OAc)2 (10) | Cs2CO3 (3) | PPh3 (0.4) | 80 | 2 h | 48 |
| 4 | Pd(OAc)2 (5) | Cs2CO3 (3) | PPh3 (0.4) | 100 | 30 | 48 |
| 5 | Pd(OAc)2 (5) | Cs2CO3 (2.5) | - | 100 | 30 | 46 |
| 6 d | Pd(OAc)2 (5) | K2CO3 (2.5) | - | 100 | 30 | 48 |
| 7 e | Pd(OAc)2 (5) | K2CO3 (2) | - | 100 | 30 | 29 |
 | ||||
|---|---|---|---|---|
| Entry | Additive (eq.) | Aldehyde (eq.) | Time (min) | Overall Yield (%) b |
| 1 | - | 1.5 | 60 | Traces |
| 2 | Piperidine (0.5) | 1.5 | 60 | Traces |
| 3 | L-Proline (0.5) | 1.5 | 60 | Traces |
| 4 | AcOH (10) | 1.3 | 60 | Traces |
| 5 c | AcOH d | 1.3 | 20 | 29 |
| Entry | Compound | λabs (nm) a | ε × 103 (M−1cm−1) b | λem (nm) | Stokes Shift (cm−1) | λex c (nm) | Φf (%) |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 304 | 24.5 | 351 367 | 4405 5647 | 320 | <0.1 |
| 2 | 4 | 300 | 17.3 | 360 376 | 5556 6738 | 300 | 9.5 |
| 3 | 5 | 344 | 32.8 | 389 403 | 3363 4256 | 350 | 46.6 |
| 4 | 6 | 283 | 19.0 | 367 | 8088 | 320 | 3.2 |
| 5 | 7 | 368 | 35.6 | 490 | 6766 | 380 | 49.4 |
| 6 | 8 | 376 | 20.2 | 507 | 6872 | 380 | 71.5 |
| 7 | 9 | 370 | 44.7 | 464 491 | 5475 6660 | 380 | 0.2 |
| 8 | 10 | 412 | 49.5 | 538 | 5684 | 380 | <0.1 |
| 9 | 11 | 355 | 26.2 | 485 | 7550 | 350 | 42.2 |
| 10 | 12 | 368 | 29.8 | 508 | 7489 | 350 | 67.9 |
| 11 | 13 | 361 | 31.3 | 448 | 5379 | 350 | 1.3 |
| 12 | 14 | 366 | 40.2 | 538 | 8735 | 380 | 70.1 |
| 13 | 15 | 371 | 38.6 | 552 | 8838 | 380 | 71.0 |
| 14 | 16 | 372 | 43.8 | 492 | 6557 | 380 | 5.4 |
| 15 | 17 | 369 | 33.4 | 491 | 6734 | 380 | 53.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkus, M.R.; Kleizienė, N.; Bieliauskas, A.; Šačkus, A. Facile One-Pot Fischer–Suzuki–Knoevenagel Microwave-Assisted Synthesis of Fluorescent 5-Aryl-2-Styryl-3H-Indoles. Molecules 2025, 30, 2503. https://doi.org/10.3390/molecules30122503
Bartkus MR, Kleizienė N, Bieliauskas A, Šačkus A. Facile One-Pot Fischer–Suzuki–Knoevenagel Microwave-Assisted Synthesis of Fluorescent 5-Aryl-2-Styryl-3H-Indoles. Molecules. 2025; 30(12):2503. https://doi.org/10.3390/molecules30122503
Chicago/Turabian StyleBartkus, Martynas Rojus, Neringa Kleizienė, Aurimas Bieliauskas, and Algirdas Šačkus. 2025. "Facile One-Pot Fischer–Suzuki–Knoevenagel Microwave-Assisted Synthesis of Fluorescent 5-Aryl-2-Styryl-3H-Indoles" Molecules 30, no. 12: 2503. https://doi.org/10.3390/molecules30122503
APA StyleBartkus, M. R., Kleizienė, N., Bieliauskas, A., & Šačkus, A. (2025). Facile One-Pot Fischer–Suzuki–Knoevenagel Microwave-Assisted Synthesis of Fluorescent 5-Aryl-2-Styryl-3H-Indoles. Molecules, 30(12), 2503. https://doi.org/10.3390/molecules30122503







