ZIF-8 as Potential Pesticide Adsorbent Medium for Wastewater Treatment: The Case Study of Model Linuron Extraction Conditions Optimization via Design of Experiment
Abstract
1. Introduction
2. Results and Discussion
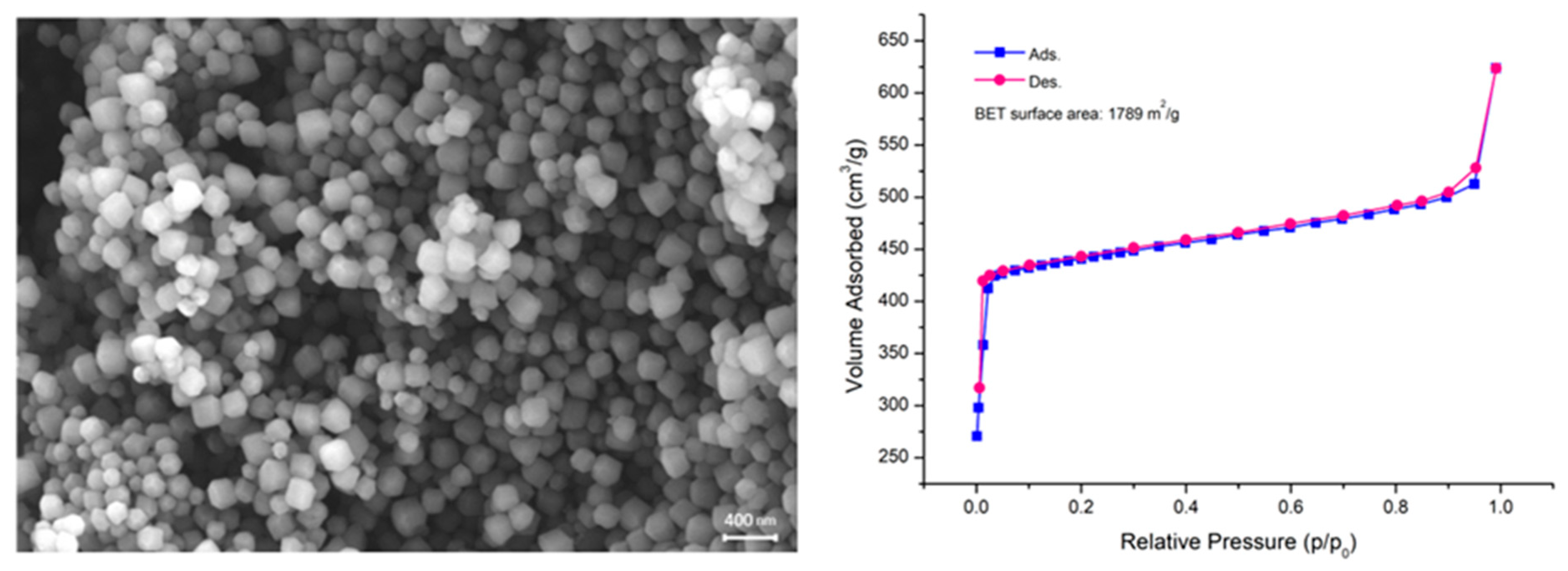
2.1. Characterization of the Adsorbent Material
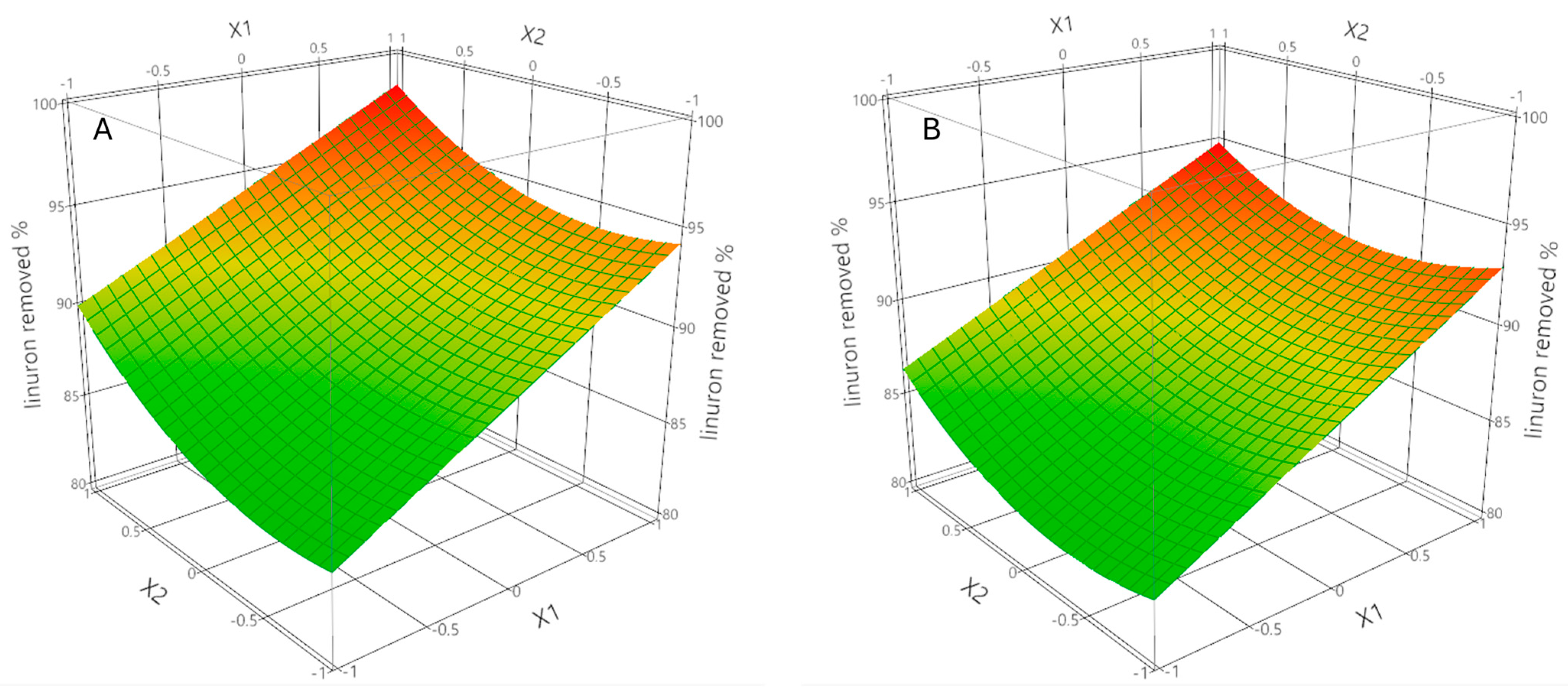
2.2. Response Surface Methodology and Model Fitting
3. Materials and Methods
3.1. Materials
3.2. ZIF-8 Synthesis
3.3. Adsorption Experiments
3.4. Experimental Design
3.5. Characterization Techniques
3.5.1. XRPD
3.5.2. Attenuated Total Reflectance Mid Infrared Spectroscopy (ATR-MIR)
3.5.3. Surface Area Analysis
3.5.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO; WHO. Report 2023—Pesticide Residues in Food; Joint FAO/WHO Meeting on Pesticide Residues: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Madani, N.A.; Carpenter, D.O. Effects of Glyphosate and Glyphosate-Based Herbicides like RoundupTM on the Mammalian Nervous System: A Review. Environ. Res. 2022, 214, 113933. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.M.A.; Videira, R.A.; Fernandes, M.A.S.; Santos, M.S.; Moreno, A.J.M.; Vicente, J.A.F.; Jurado, A.S. Toxicity of the Herbicide Linuron as Assessed by Bacterial and Mitochondrial Model Systems. Toxicol. In Vitro 2014, 28, 932–939. [Google Scholar] [CrossRef]
- Ding, H.; Zheng, W.; Han, H.; Hu, X.; Hu, B.; Wang, F.; Su, L.; Li, H.; Li, Y. Reproductive Toxicity of Linuron Following Gestational Exposure in Rats and Underlying Mechanisms. Toxicol. Lett. 2017, 266, 49–55. [Google Scholar] [CrossRef]
- Swarcewicz, M.; Gregorczyk, A.; Sobczak, J. Comparison of Linuron Degradation in the Presence of Pesticide Mixtures in Soil under Laboratory Conditions. Environ. Monit. Assess. 2013, 185, 8109–8114. [Google Scholar] [CrossRef]
- Zahoor, M. Removal of Paraquat and Linuron from Water by Continuous Flow Adsorption/Ultrafiltration Membrane Processes. J. Chem. Soc. Pak. 2013, 35, 577. [Google Scholar]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, M.; Lin, Y.S. Stability of ZIF-8 in Water under Ambient Conditions. Microporous Mesoporous Mater. 2019, 279, 201–210. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid Synthesis of Zeolitic Imidazolate Framework-8 (ZIF-8) Nanocrystals in an Aqueous System. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Dai, H.; Yuan, X.; Jiang, L.; Wang, H.; Zhang, J.; Zhang, J.; Xiong, T. Recent Advances on ZIF-8 Composites for Adsorption and Photocatalytic Wastewater Pollutant Removal: Fabrication, Applications and Perspective. Coord. Chem. Rev. 2021, 441, 213985. [Google Scholar] [CrossRef]
- Sann, E.E.; Pan, Y.; Gao, Z.; Zhan, S.; Xia, F. Highly Hydrophobic ZIF-8 Particles and Application for Oil-Water Separation. Sep. Purif. Technol. 2018, 206, 186–191. [Google Scholar] [CrossRef]
- Kida, K.; Okita, M.; Fujita, K.; Tanaka, S.; Miyake, Y. Formation of High Crystalline ZIF-8 in an Aqueous Solution. CrystEngComm 2013, 15, 1794–1801. [Google Scholar] [CrossRef]
- Vatanpour, V.; Yuksekdag, A.; Ağtaş, M.; Mehrabi, M.; Salehi, E.; Castro-Muñoz, R.; Koyuncu, I. Zeolitic Imidazolate Framework (ZIF-8) Modified Cellulose Acetate NF Membranes for Potential Water Treatment Application. Carbohydr. Polym. 2023, 299, 120230. [Google Scholar] [CrossRef]
- Yu, R.; Wu, Z. High Adsorption for Ofloxacin and Reusability by the Use of ZIF-8 for Wastewater Treatment. Microporous Mesoporous Mater. 2020, 308, 110494. [Google Scholar] [CrossRef]
- Lai, Z. Development of ZIF-8 Membranes: Opportunities and Challenges for Commercial Applications. Curr. Opin. Chem. Eng. 2018, 20, 78–85. [Google Scholar] [CrossRef]
- Foschi, M.; Capasso, P.; Maggi, M.A.; Ruggieri, F.; Fioravanti, G. Experimental Design and Response Surface Methodology Applied to Graphene Oxide Reduction for Adsorption of Triazine Herbicides. ACS Omega 2021, 6, 16943–16954. [Google Scholar] [CrossRef]
- Lee, Y.R.; Jang, M.S.; Cho, H.Y.; Kwon, H.J.; Kim, S.; Ahn, W.S. ZIF-8: A Comparison of Synthesis Methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- di Nicola, N.; Paolucci, V.; Daniele, V.; Taglieri, G.; Crucianelli, M.; Guidoni, L.; Lazzarini, A. ZIF-8 as Potential Vector for Enhanced Target Delivery of Sulfathiazole for the Treatment of Bovine Ruminal Acidosis. Eur. J. Inorg. Chem. 2024, 27, e202400504. [Google Scholar] [CrossRef]
- Balliana, E.; Marchand, M.; Di Matteo, V.; Ballarin, B.; Cassani, M.C.; Panzavolta, S.; Zendri, E. Application of Zinc-Based Metal-Organic Framework ZIF-8 on Paper: A Pilot Study on Visual Appearance and Effectiveness. Polymers 2025, 17, 1369. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of Metal-Organic Framework Hybrid Nanocomposites Based on GO and CNT with High Adsorption Capacity for Dye Removal. Chem. Eng. J. 2017, 326, 1145–1158. [Google Scholar] [CrossRef]
- Krokidas, P.; Castier, M.; Moncho, S.; Brothers, E.; Economou, I.G. Molecular Simulation Studies of the Diffusion of Methane, Ethane, Propane, and Propylene in ZIF-8. J. Phys. Chem. C 2015, 119, 27028–27037. [Google Scholar] [CrossRef]
- Schmidt, B.E.; Cnudde, P.; Van Speybroeck, V.; Vanduyfhuys, L. In-Depth Thermodynamic and Kinetic Analysis of Ethane Diffusion in ZIF-8. J. Phys. Chem. C 2024, 128, 18509–18523. [Google Scholar] [CrossRef]
- Verploegh, R.J.; Nair, S.; Sholl, D.S. Temperature and Loading-Dependent Diffusion of Light Hydrocarbons in ZIF-8 as Predicted Through Fully Flexible Molecular Simulations. J. Am. Chem. Soc. 2015, 137, 15760–15771. [Google Scholar] [CrossRef]
- Zhong, Y.; Mu, X.; Cheang, U.K. High-Performance and Selective Adsorption of ZIF-8/MIL-100 Hybrids towards Organic Pollutants. Nanoscale Adv. 2022, 4, 1431–1444. [Google Scholar] [CrossRef]
- Feng, D.; Xia, Y. Comparisons of Glyphosate Adsorption Properties of Different Functional Cr-Based Metal–Organic Frameworks. J. Sep. Sci. 2018, 41, 732–739. [Google Scholar] [CrossRef]
- Tanaka, S.; Tanaka, Y. A Simple Step toward Enhancing Hydrothermal Stability of ZIF-8. ACS Omega 2019, 4, 19905–19912. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhang, J.; Zhang, X.; Liu, J.; Du, X.; Lu, X. Yolk-Shell MOF-on-MOF Hybrid Solid-Phase Microextraction Coatings for Efficient Enrichment and Detection of Pesticides: Structural Regulation Cause Performance Differences. Talanta 2024, 278, 126474. [Google Scholar] [CrossRef]
- Rouahna, N.; Salem, D.B.; Bouchareb, I.; Nouioua, A.; Ouakouak, A.; Fadel, A.; Hamdi, N.; Boopathy, R. Reduction of Crystal Violet Dye from Water by Pomegranate Peel–Derived Efficient Biochar: Influencing Factors and Adsorption Behaviour. Water Air Soil. Pollut. 2023, 234, 324. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, D.; Mei, G.; Hong, X.; Wu, J.; Zheng, J.; Pang, J.; Yan, Z. Preparation of Konjac Glucomannan-Based Zeolitic Imidazolate Framework-8 Composite Aerogels with High Adsorptive Capacity of Ciprofloxacin from Water. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 187–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Liu, F.; Yuan, Y.; Wu, H.; Li, A. Effects of Ionic Strength on Removal of Toxic Pollutants from Aqueous Media with Multifarious Adsorbents: A Review. Sci. Total Environ. 2019, 646, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Hu, X.; Sun, Z. Magnetic Zeolite Imidazole Framework Material-8 as an Effective and Recyclable Adsorbent for Removal of Ceftazidime from Aqueous Solution. J. Hazard. Mater. 2020, 384, 121406. [Google Scholar] [CrossRef] [PubMed]
- Aranda-García, E.; Chávez-Camarillo, G.M.; Cristiani-Urbina, E. Effect of Ionic Strength and Coexisting Ions on the Biosorption of Divalent Nickel by the Acorn Shell of the Oak Quercus Crassipes Humb. & Bonpl. Processes 2020, 8, 1229. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of Solution PH, Ionic Strength, and Temperature on Adsorption Behavior of Reactive Dyes on Activated Carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- El-Nahhal, Y.Z.; Lagaly, G. Salt Effects on the Adsorption of a Pesticide on Modified Bentonites. Colloid. Polym. Sci. 2005, 283, 968–974. [Google Scholar] [CrossRef]


| Run | Temperature [°C] | Linuron Concentration [μg/mL] | Ionic Strength [mg/mL] | X1 | X2 | X3 | Linuron Removed (%) |
|---|---|---|---|---|---|---|---|
| 1 | 40 | 2 | 0 | +1 | −1 | −1 | 91.4 |
| 2 | 40 | 8 | 50 | +1 | +1 | −1 | 97.5 |
| 3 | 40 | 8 | 50 | +1 | +1 | +1 | 93.4 |
| 4 | 40 | 2 | 0 | +1 | −1 | +1 | 92.9 |
| 5 | 40 | 5 | 25 | +1 | 0 | 0 | 92.1 |
| 6 | 30 | 5 | 25 | 0 | 0 | 0 | 91.5 |
| 7 * | 30 | 5 | 25 | 0 | 0 | 0 | 89.6 |
| 8 * | 30 | 5 | 25 | 0 | 0 | 0 | 89.1 |
| 9 | 30 | 2 | 0 | 0 | −1 | 0 | 87.7 |
| 10 | 30 | 8 | 50 | 0 | 1 | 0 | 94.6 |
| 11 | 20 | 8 | 50 | −1 | 1 | −1 | 86.9 |
| 12 | 20 | 5 | 25 | −1 | 0 | 0 | 83.2 |
| 13 | 30 | 5 | 25 | 0 | 0 | −1 | 85.9 |
| 14 | 30 | 5 | 25 | 0 | 0 | 1 | 88.9 |
| 15 * | 30 | 5 | 25 | 0 | 0 | −1 | 85.9 |
| 16 * | 30 | 5 | 25 | 0 | 0 | 1 | 86.9 |
| 17 | 20 | 2 | 0 | −1 | −1 | −1 | 81.3 |
| 18 | 20 | 2 | 0 | −1 | −1 | 1 | 82.7 |
| 19 * | 20 | 8 | 50 | −1 | 1 | −1 | 88.7 |
| 20 | 20 | 8 | 50 | −1 | 1 | 1 | 85.8 |
| 21 * | 20 | 5 | 25 | −1 | 0 | 0 | 86.6 |
| 22 * | 20 | 5 | 25 | −1 | 0 | 0 | 86.6 |
| Parameters | Value ± SD | R2 | Adj-R2 | Q2 | |
|---|---|---|---|---|---|
| intercept | 89.5 ± 0.5 | ||||
| * X1 | 4.4 ± 0.4 | ||||
| * X2 | 2.5 ± 0.4 | 0.909 | 0.873 | 0.755 | |
| X3 | 0.4 ± 0.4 | ||||
| * X2·X3 | –1.1 ± 0.5 | ||||
| * X22 | 1.9 ± 0.7 | ||||
| Variation source | Sum of squares | Degrees of freedom | Mean square | F-value | p-value |
| lack of fit | 16.4 | 8 | 2.05 | 0.986 | 0.5140 |
| pure error | 14.5 | 7 | 2.1 | ||
| model | 309.9 | 6 | 51.66 | 25.7 | <0.001 |
| residual | 30.9 | 15 | 2.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Nicola, N.; Di Pelino, M.; Foschi, M.; Passalacqua, R.; Lazzarini, A.; Ruggieri, F. ZIF-8 as Potential Pesticide Adsorbent Medium for Wastewater Treatment: The Case Study of Model Linuron Extraction Conditions Optimization via Design of Experiment. Molecules 2025, 30, 2480. https://doi.org/10.3390/molecules30122480
di Nicola N, Di Pelino M, Foschi M, Passalacqua R, Lazzarini A, Ruggieri F. ZIF-8 as Potential Pesticide Adsorbent Medium for Wastewater Treatment: The Case Study of Model Linuron Extraction Conditions Optimization via Design of Experiment. Molecules. 2025; 30(12):2480. https://doi.org/10.3390/molecules30122480
Chicago/Turabian Styledi Nicola, Nicola, Mariacristina Di Pelino, Martina Foschi, Rosalba Passalacqua, Andrea Lazzarini, and Fabrizio Ruggieri. 2025. "ZIF-8 as Potential Pesticide Adsorbent Medium for Wastewater Treatment: The Case Study of Model Linuron Extraction Conditions Optimization via Design of Experiment" Molecules 30, no. 12: 2480. https://doi.org/10.3390/molecules30122480
APA Styledi Nicola, N., Di Pelino, M., Foschi, M., Passalacqua, R., Lazzarini, A., & Ruggieri, F. (2025). ZIF-8 as Potential Pesticide Adsorbent Medium for Wastewater Treatment: The Case Study of Model Linuron Extraction Conditions Optimization via Design of Experiment. Molecules, 30(12), 2480. https://doi.org/10.3390/molecules30122480









