Development of Xanthyletin-Loaded Nanoparticles for the Control of Leucoagaricus gongylophorus
Abstract
1. Introduction
2. Results
2.1. Biopolymeric Nanoparticle Characterization
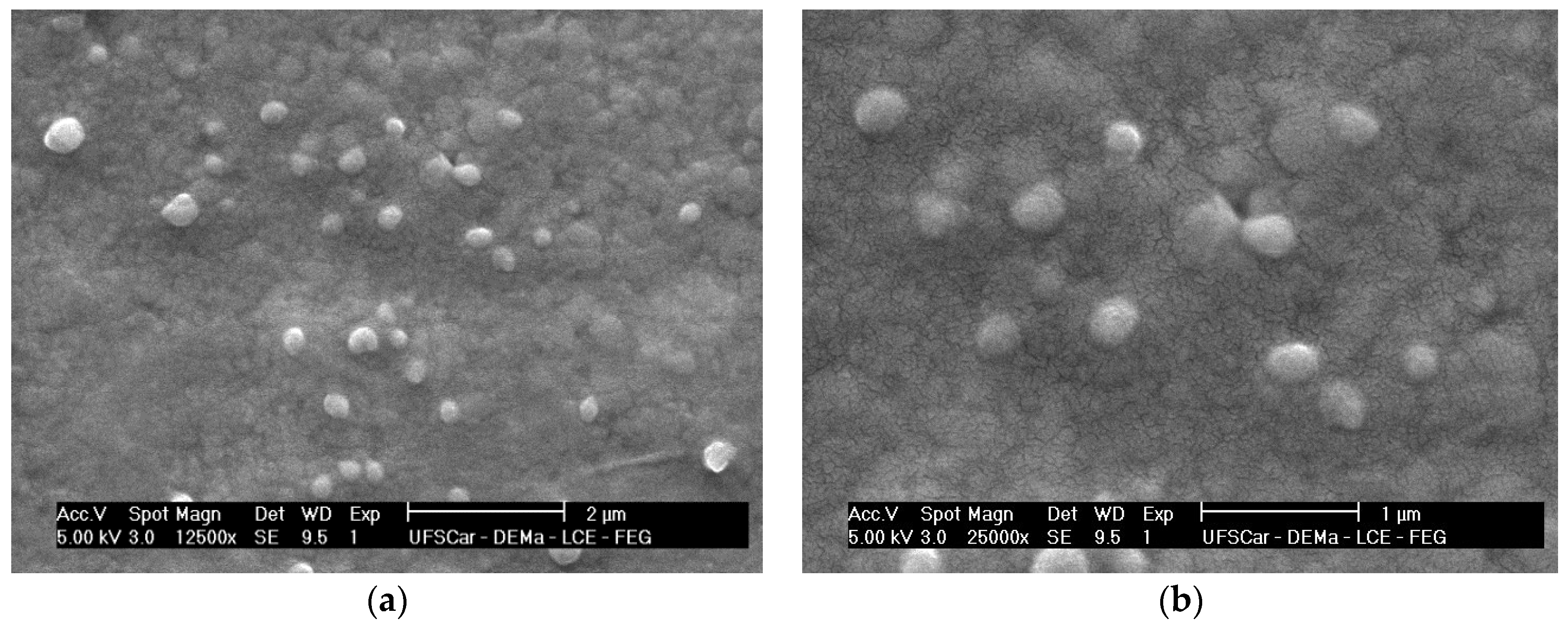
2.2. Morphological Analysis
2.3. Stability Studies
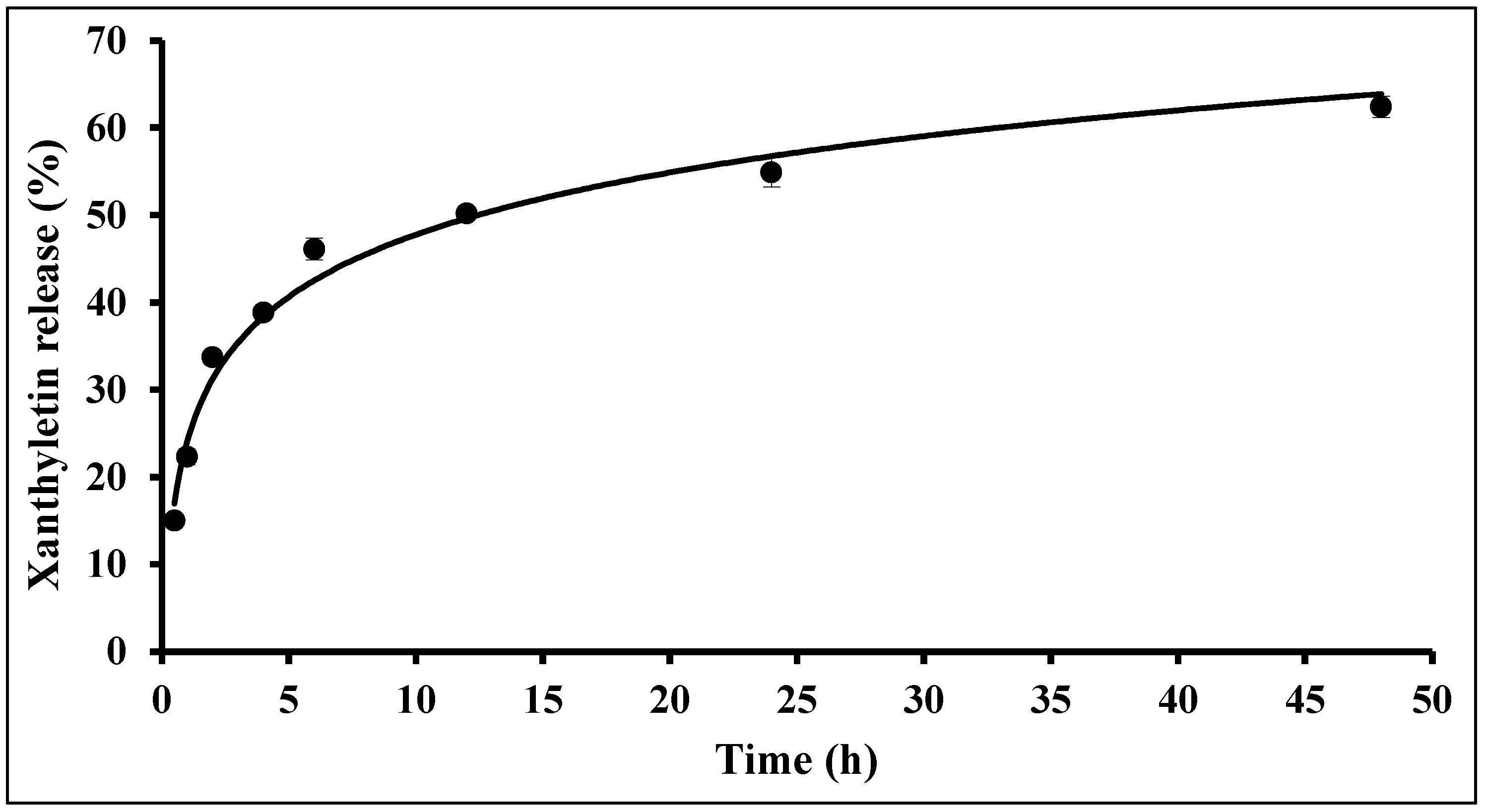
2.4. In Vitro Release Studies
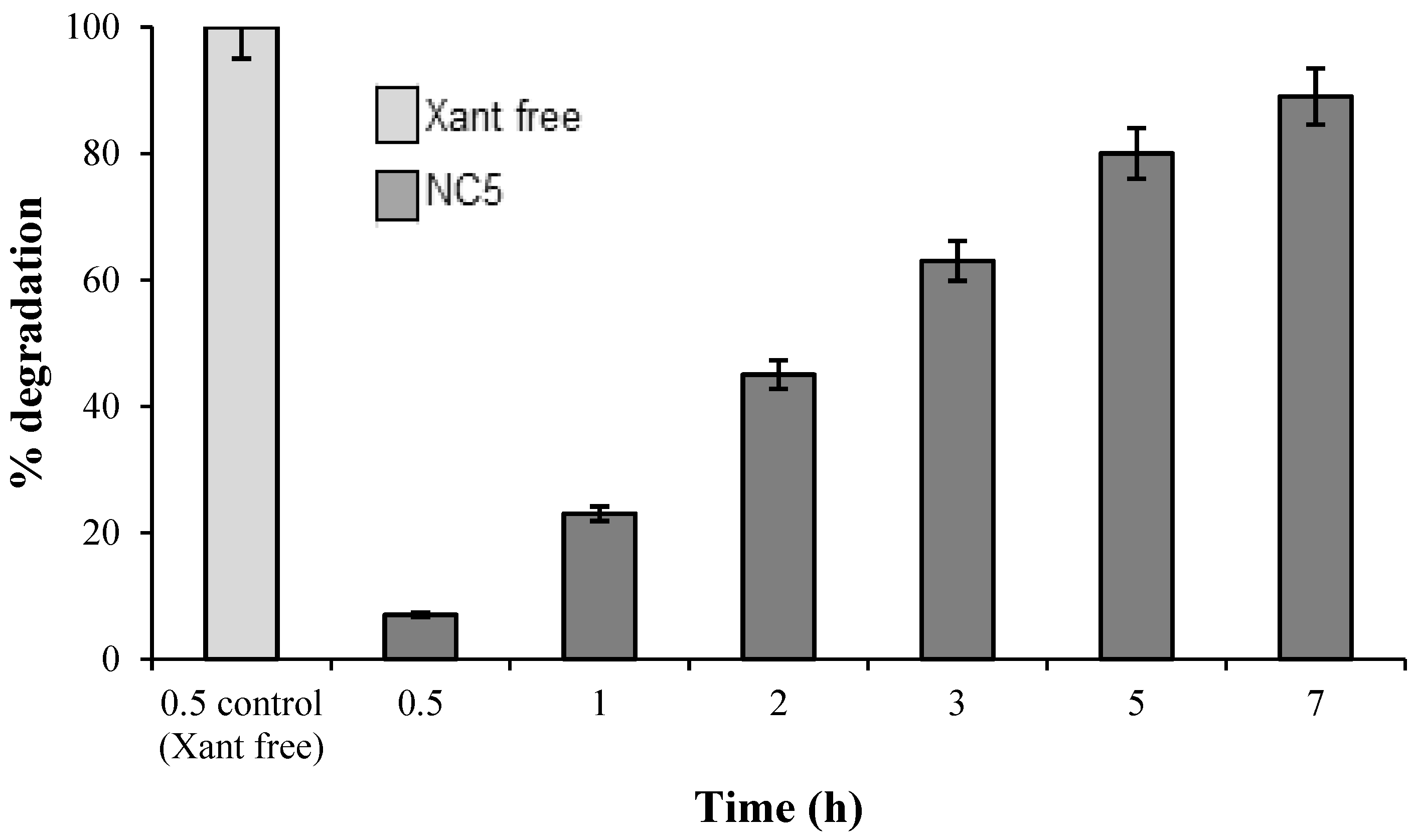
2.5. UV Light-Accelerated Degradation
2.6. Fungicidal Bioassay
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Purification and Identification of Xanthyletin
4.3. Reagents and Standards
4.4. Nanoparticle Preparation
4.5. Biopolymeric Nanoparticle Characterization
4.5.1. Xanthyletin Quantification
4.5.2. Determination of Total Xanthyletin Content
4.5.3. Determination of the Amount of Encapsulated Xanthyletin
4.6. Particle Size and Zeta Potential Determination
4.7. Morphological Analysis
4.8. Stability Studies
4.9. Nanoparticle In Vitro Release Studies
4.10. Fungicidal Bioassay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ejaz, S.; Akram, W.; Lim, C.W.; Lee, J.J.; Hussain, I. Endocrine disrupting pesticides: A leading cause of cancer among rural people in Pakistan. Exp. Oncol. 2004, 26, 98–105. [Google Scholar]
- Gupta, S.; Gupta, R.; Sharma, S. Impact of chemical- and bio-pesticides on bacterial diversity in rhizosphere of Vigna radiata. Ecotoxicology 2013, 22, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C. Insecticide discovery: An evaluation and analysis. Pestic. Biochem. Physiol. 2013, 107, 8–17. [Google Scholar] [CrossRef]
- Odukkathil, G.; Vasudevan, N. Toxicity and bioremediation of pesticides in agricultural soil. Rev. Environ. Sci. Biotechnol. 2013, 12, 421–444. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, R.; Thakur, K.; Mahajan, D.; Brar, B.; Sharma, D.; Kumar, S.; Sharma, A.K. Impact of Pesticides Application on Aquatic Ecosystem and Biodiversity: A Review. Biol. Bull. 2023, 50, 1362–1375. [Google Scholar] [CrossRef]
- Qamar, W.; Shahid, M.U.; Irfan, M.; Abbas, R.Z.; Faraz, A.; Hussain, R.; Alvi, M.A. Thiamethoxam Toxicity: A Review in One-Health Perspective. Kafkas Univ. Vet. Fak. Derg. 2023, 29, 557–570. [Google Scholar] [CrossRef]
- Pinto, A.C.; Silva, D.H.S.; Bolzani, V.S.; Lopes, N.P.; Epifanio, R.A. Produtos naturais: Atualidade, desafios e perspectivas. Quim. Nova 2002, 25, 45–61. [Google Scholar] [CrossRef]
- Castâno-Quintana, K.; Montoya-Lerma, J.; Giraldo-Echeverri, C. Toxicity of foliage extracts of Tithonia diversifolia (Asteraceae) on Atta cephalotes (Hymenoptera: Myrmicinae) workers. Ind. Crops Prod. 2013, 44, 391–395. [Google Scholar] [CrossRef]
- Sparks, T.C.; Bryant, R.J. Innovation in insecticide discovery: Approaches to the discovery of new classes of insecticides. Pest Manag. Sci. 2022, 78, 3226–3247. [Google Scholar] [CrossRef]
- Abdelgaleil, S.M.A.; Gad, H.A.; Ramadan, G.R.M.; El-Bakry, A.M.; ElSabrout, A.M. Monoterpenes for Management of Field Crop Insect Pests. J. Agric. Sci. Technol. 2023, 25, 769–784. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, D.; Chen, J.; Zhang, Z. Plants in the Genus Tephrosia: Valuable Resources for Botanical Insecticides. Insects 2020, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Kilani-Morakchi, S.; Morakchi-Goudjil, H.; Sifi, K. Azadirachtin-Based Insecticide: Overview, Risk Assessments, and Future Directions. Front. Agron. 2021, 3, 676208. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef]
- Godoy, M.F.P.; Victor, S.R.; Bellini, A.M.; Guerreiro, G.; Rocha, W.C.; Bueno, O.C.; Hebling, M.J.A.; Bacci, M.C.; da Silva, M.F.G.F.; Vieira, P.C.; et al. Inhibition of the symbiotic fungus of leaf-cutting ants by coumarins. J. Braz. Chem. Soc. 2005, 16, 669–672. [Google Scholar] [CrossRef]
- Xiong, Y.; Huang, G.; Yao, Z.; Zhao, C.; Zhu, X.; Wu, Q.; Zhou, X.; Li, J. Screening Effective Antifungal Substances from the Bark and Leaves of Zanthoxylum avicennae by the Bioactivity-Guided Isolation Method. Molecules 2019, 24, 4207. [Google Scholar] [CrossRef] [PubMed]
- Boulogne, I.; Ozier-Lafontaine, H.; Germosen-Robineau, L.; Desfontaines, L.; Loranger-Merciris, G. Acromyrmex octospinosus (Hymenoptera: Formicidae) management: Effects of TRAMILs fungicidal plant extracts. J. Econ. Entomol. 2012, 105, 1224–1233. [Google Scholar] [CrossRef]
- Motta, A.C.Q.; Avelino, D.S.; Marinho, C.G.S.; Fadini, M.A.M.; Melo, J.O.F. Leucoagaricus gongylophorus provides protection for Atta sexdens against plant extracts. Cienc. Florest. 2022, 32, 86–101. [Google Scholar] [CrossRef]
- Araújo, S.; Seibert, J.; Ruani, A.; de la Cruz, R.A.; Cruz, A.; Pereira, A.; Doraí Zandonai, D.; Forim, M.; Silva, M.F.; Bueno, O.; et al. The Symbiotic Fungus Leucoagaricus gongylophorus (Möller) Singer (Agaricales, Agaricaceae) as a Target Organism to Control Leaf-Cutting Ants. Insects 2022, 13, 359. [Google Scholar] [CrossRef]
- Zanuncio, J.C.; Lopes, E.T.; Leite, H.G.; Zanetti, R.; Sediyama, C.S.; Fialho, M.C.Q. Sampling methods for monitoring the number and area of colonies of leaf-cutting ants (Hymenoptera: Formicidae) in Eucalyptus plantations in Brazil. Sociobiology 2004, 44, 337–344. [Google Scholar]
- Nickele, M.A.; Reis Filho, W.; Oliveira, E.B.; Iede, E.T.; Caldato, N.; Strapasson, P. Leaf-cutting ant attack in initial pine plantations and growth of defoliated plants. Pesqui. Agropecuária Bras. 2012, 47, 892–899. [Google Scholar] [CrossRef]
- Buteler, M.; Alma, A.M.; Herrera, M.L.; Gorosito, N.B.; Fernández, P.C. Novel organic repell ent for leaf-cutting ants: Tea tree oil and its potential use as a management tool. Int. J. Pest Manag. 2019, 67, 1–9. [Google Scholar] [CrossRef]
- Justi Junior, J.; Imenes, S.L.; Bergmann, E.L.; Campos-Farinha, A.E.C.; Zorzenon, F.J. Formigas cortadeiras. Bol. Técn. Inst. Biol. 1996, 4, 1–31. [Google Scholar]
- Liu, Y.; Tong, Z.; Prud’homme, R.K. Stabilized polymeric nanoparticles for controlled and efficient release of bifenthrin. Pest Manag. Sci. 2008, 64, 808–812. [Google Scholar] [CrossRef]
- Paula, H.C.B.; Sombra, F.M.; Abreu, F.O.M.S.; De Paula, R.C.M. Lippia sidoides essential oil encapsulation by angico gum/chitosan nanoparticles. J. Braz. Chem. Soc. 2010, 21, 2359–2366. [Google Scholar] [CrossRef]
- Yang, F.L.; Li, X.G.; Zhu, F.; Lei, C.L. Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Agric. Food Chem. 2009, 57, 10156–10162. [Google Scholar] [CrossRef]
- Beyki, M.; Zhaveh, S.; Khalili, S.T.; Rahmani-Cherati, T.; Abollahi, A.; Bayat, M.; Tabatabaei, M.; Mohsenifar, A. Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crops Prod. 2014, 54, 310–319. [Google Scholar] [CrossRef]
- Forim, M.R.; Costa, E.S.; da Silva, M.F.G.F.; Fernandes, J.B.; Mondego, J.M.; Boiça, A.L., Jr. Development of a new method to prepare nano-/microparticles loaded with extracts of Azadirachta indica, their characterization and use in controlling Plutella xylostella. J. Agric. Food Chem. 2013, 61, 9131–9139. [Google Scholar] [CrossRef]
- Buteler, M.; Garcia, G.L.; Stadler, T. Potential of nanostructured alumina for leaf-cutting ants Acromyrmex lobicornis (Hymenoptera: Formicidae) management. Austral Entomol. 2018, 57, 292–296. [Google Scholar] [CrossRef]
- Decool, G.; Kfoury, M.; Paitel, L.; Sardo, A.; Fourmentin, S. Cyclodextrins as molecular carriers for biopesticides: A review. Environ. Chem. Lett. 2023, 22, 321–353. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Pohlmann, A.R.; Freitas, L.D.; Guterres, S.S. Caracterização e estudo de estabilidade de suspensões de nanocápsulas e de nanoesferas poliméricas contendo diclofenaco. Acta Farm. Bonaer. 2002, 21, 99–106. [Google Scholar]
- Schaffazick, S.R.; Guterres, S.S.U.; Freitas, L.D.; Pohlmann, A.R. Physicochemical characterization and stability of the polymeric nanoparticle systems for drug administration. Quim. Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Nascimento, T.G.; Silva, P.F.; Azevedo, L.F.; Rocha, L.G.; Moraes Porto, I.C.C.M.; Moura, T.F.A.L.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B.; Fonseca, E.J.S.; et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanoscale Res. Lett. 2016, 11, 301. [Google Scholar] [CrossRef]
- Peres, M.C.; Costa, G.C.S.; Reis, L.E.L.; Silva, L.D.; Peixoto, M.F.; Alves, C.C.F.; Forim, M.R.; Quintela, E.D.; Araújo, W.L.; Cazal, C.M. In natura and nanoencapsulated essential oils from Xylopia aromatica reduce oviposition of Bemisia tabaci in Phaseolus vulgaris. J. Pest Sci. 2020, 93, 807–821. [Google Scholar] [CrossRef]
- Bala, I.; Hariharan, S.; Kumar, M. PLGA nanoparticles in drug delivery: The state of the art. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 387–422. [Google Scholar] [CrossRef] [PubMed]
- Bodmeier, R.D.; Mainceint, O. Polymer dispersions as drug carriers. In Pharmaceutical Dosage Forms: Disperse Systems; Lieberman, H.A., Rieger, M.M., Banker, G.S., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 87–103. [Google Scholar]
- Magenheim, B.; Benita, S. Nanoparticle characterization: A comprehensive physicochemical approach. Pharma Sci. 1991, 1, 221–241. [Google Scholar]
- Guterres, S.S.; Fessi, H.; Barratt, G.; Devissaguet, J.P.; Puisieux, F. Poly-(DL-lactide) nanocapsules containing diclofenac, 1. Formulation and stability study. Int. J. Pharm. 1995, 113, 57–63. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanism of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Li, Z.Z.; Xu, S.A.; Wen, L.X.; Liu, F.; Liu, A.Q.; Wang, Q.; Sun, H.Y.; Yu, W.; Chen, J.F. Controlled release of avermectin from porous hollow silica nanoparticles: Influence of shell thickness on loading efficiency, UV-shielding property and release. J. Control. Release 2006, 111, 81–88. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Galindo-Rodriguez, S.A.; Puel, F.; Briancon, S.; Allemann, E.; Doelker, E.; Fessi, H. Comparative scale-up of three methods for producing ibuprofen-loaded nanoparticles. Eur. J. Pharm. Sci. 2005, 25, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Polymeric materials for drug delivery systems. J. Control. Release 2011, 267, 112–126. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer-Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Choi, J.-H.; Woo, J.-J.; Kim, I. Sustainable Polycaprolactone Polyol-Based Thermoplastic Poly(ester ester) Elastomers Showing Superior Mechanical Properties and Biodegradability. Polymers 2023, 15, 3209. [Google Scholar] [CrossRef]
- Rosa, D.S.; Penteado, D.F.; Calil, M.R. Thermal properties and biodegradability of PCL and PHB submitted in fungi pool. Rev. Cienc. Tecnol. 2000, 15, 75–80. [Google Scholar]
- Christofoli, M.; Costa, E.C.C.; Bicalho, K.U.; Domingues, V.C.; Peixoto, M.F. Insecticidal effect of nanoencapsulated essential oils from Zanthoxylum rhoifolium (Rutaceae) in Bemisia tabaci populations. Ind. Crop Prod. 2015, 70, 301–308. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Vinha, G.L.; Alcántara-de la Cruz, R.; Della Lucia, T.M.C.; Wilcken, C.F.; da Silva, E.D.; Lemes, P.G.; Zanuncio, J.C. Leaf-cutting ants in commercial forest plantations of Brazil: Biological aspects and control methods. South. J. Sci. 2020, 82, 95–103. [Google Scholar] [CrossRef]
- Torres, F.B.M.; Guida, Y.; Weber, R.; Torres, J.P.M. Brazilian overview of per- and polyfluoroalkyl substances listed as persistent organic pollutants in the Stockholm Convention. Chemosphere 2022, 291, 132674. [Google Scholar] [CrossRef]
- Yammine, J.; Chihib, N.-E.; Gharsallaoui, A.; Dumas, E.; Ismail, A.; Karam, L. Essential oils and their active components applied as: Free, encapsulated and in hurdle technology to fight microbial contaminations. Heliyon 2022, 8, e12472. [Google Scholar] [CrossRef]
- Miastkowska, M.; Michalczyk, A.; Figacz, K.; Sikora, E. Nanoformulations as a modern form of biofungicide. J. Environ. Health Sci. Eng. 2020, 18, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Della Lucia, T.M.; Gandra, L.C.; Guedes, R.N. Managing leaf-cutting ants: Peculiarities, trends and challenges. Pest Manag. Sci. 2014, 70, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Cazal, C.M.; Domingues, V.C.; Batalhao, J.R.; Bueno, O.C.; Rodrigues Filho, E.; da Silva, M.F.G.F.; Vieira, P.C.; Fernandes, J.B. Isolation of xanthyletin, an inhibitor of ants’ symbiotic fungus, by high-speed counter-current chromatography. J. Chromatogr. A 2009, 1216, 4307–4312. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P. Procédé de Préparation de Systémes Colloidaux Dispersibles d’une Substance Sous Forme de Nanoparticules. EP0274961A1, 20 July 1988. [Google Scholar]
- Cazal, C.M.; Forim, M.R.; da Silva, M.F.G.F.; Vieira, P.C.; Fernandes, J.B. Development and validation of a RP-HPLC method to determine the xanthyletin content in biodegradable polymeric nanoparticles. Quim. Nova 2014, 37, 1145–1150. [Google Scholar] [CrossRef]
- Levy, M.Y.; Benita, S. Drug release from submicronized o/w emulsion: A new in vitro kinetic evaluation model. Int. J. Pharm. 1990, 66, 29–37. [Google Scholar] [CrossRef]
- Bicalho, K.U.; Terezan, A.P.; Martins, D.C.; Freitas, T.G.; Fernandes, J.B.; da Silva, M.F.G.F.; Vieira, P.C.; Pagnocca, F.C.; Bueno, O.C. Evaluation of the toxicity of Virola sebifera crude extracts, fractions and isolated compounds on the nest of leaf-cutting ants. Psyche 2011, 2012, 785424. [Google Scholar] [CrossRef]



| Formulations | pH | PD (nm) | ZP (mV) | (Rec %) | (EE %) |
|---|---|---|---|---|---|
| NS1 | 5.91 ± 0.31 | 202 ± 1.58 | −25.1 ± 0.09 | 97.2 ± 1.22 | 91.9 ± 0.83 |
| NS2 | 5.92 ± 0.22 | 203 ± 0.98 | −27.0 ± 0.11 | 99.2 ± 0.93 | 79.3 ± 0.72 |
| NC3 | 5.87 ± 0.14 | 296 ± 1.22 | −31.3 ± 0.07 | 98.4 ± 1.01 | 94.5 ± 0.48 |
| NC4 | 5.94 ± 0.18 | 293 ± 0.99 | −26.3 ± 0.13 | 98.9 ± 0.87 | 97.6 ± 0.79 |
| NC5 | 5.33 ± 0.15 | 304 ± 1.17 | −29.3 ± 0.07 | 96.5 ± 0.94 | 98.0 ± 0.97 |
| Formulations | Xanthyletin (mg) | Isodecyl Oleate (mg) |
|---|---|---|
| NS1 | 10 | 0 |
| NS2 | 30 | 0 |
| NC1 | 10 | 1000 |
| NC2 | 30 | 1000 |
| NC3 | 30 | 800 |
| NC4 | 30 | 700 |
| NC5 | 30 | 600 |
| NC6 | 30 | 500 |
| NC7 | 33 | 600 |
| Fixed variables | ||
| Acetone | 30.0 mL | |
| Span® 60 | 50.0 mg | |
| Aqueous phase volume | 60.0 mL | |
| Tween® 80 | 50.0 mg | |
| PCL | 150.0 mg | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazal, C.d.M.; Forim, M.R.; Terezan, A.P.; Matos, A.P.; Cunha, G.O.S.; da Silva, M.F.d.G.F.; Vieira, P.C.; Pagnocca, F.C.; Fernandes, J.B. Development of Xanthyletin-Loaded Nanoparticles for the Control of Leucoagaricus gongylophorus. Molecules 2025, 30, 2469. https://doi.org/10.3390/molecules30112469
Cazal CdM, Forim MR, Terezan AP, Matos AP, Cunha GOS, da Silva MFdGF, Vieira PC, Pagnocca FC, Fernandes JB. Development of Xanthyletin-Loaded Nanoparticles for the Control of Leucoagaricus gongylophorus. Molecules. 2025; 30(11):2469. https://doi.org/10.3390/molecules30112469
Chicago/Turabian StyleCazal, Cristiane de Melo, Moacir Rossi Forim, Ana Paula Terezan, Andreia Pereira Matos, Gracielle Oliveira Sabbag Cunha, Maria Fátima das Graças Fernandes da Silva, Paulo Cezar Vieira, Fernando Carlos Pagnocca, and João Batista Fernandes. 2025. "Development of Xanthyletin-Loaded Nanoparticles for the Control of Leucoagaricus gongylophorus" Molecules 30, no. 11: 2469. https://doi.org/10.3390/molecules30112469
APA StyleCazal, C. d. M., Forim, M. R., Terezan, A. P., Matos, A. P., Cunha, G. O. S., da Silva, M. F. d. G. F., Vieira, P. C., Pagnocca, F. C., & Fernandes, J. B. (2025). Development of Xanthyletin-Loaded Nanoparticles for the Control of Leucoagaricus gongylophorus. Molecules, 30(11), 2469. https://doi.org/10.3390/molecules30112469







