Green and Efficient Synthetic Protocol for 1,3,5-Triazine Derivatives with Anticancer Potential Against Colorectal Cancer
Abstract
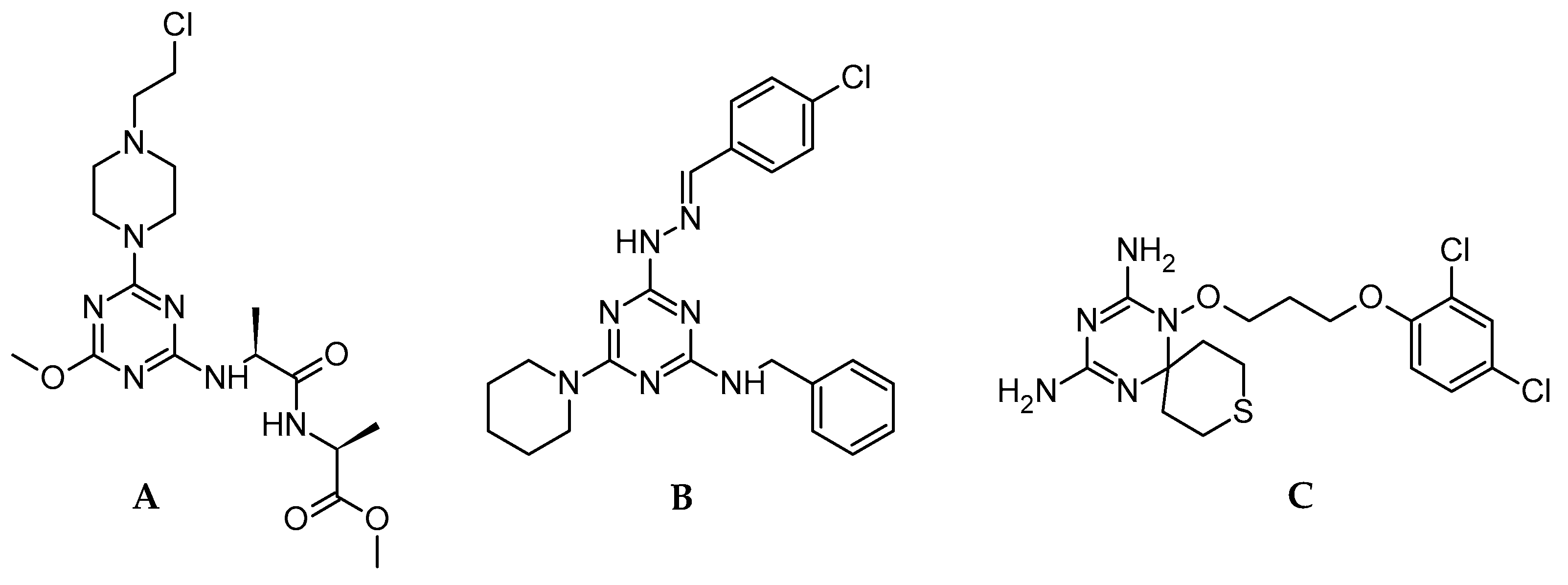
1. Introduction
2. Results and Discussion
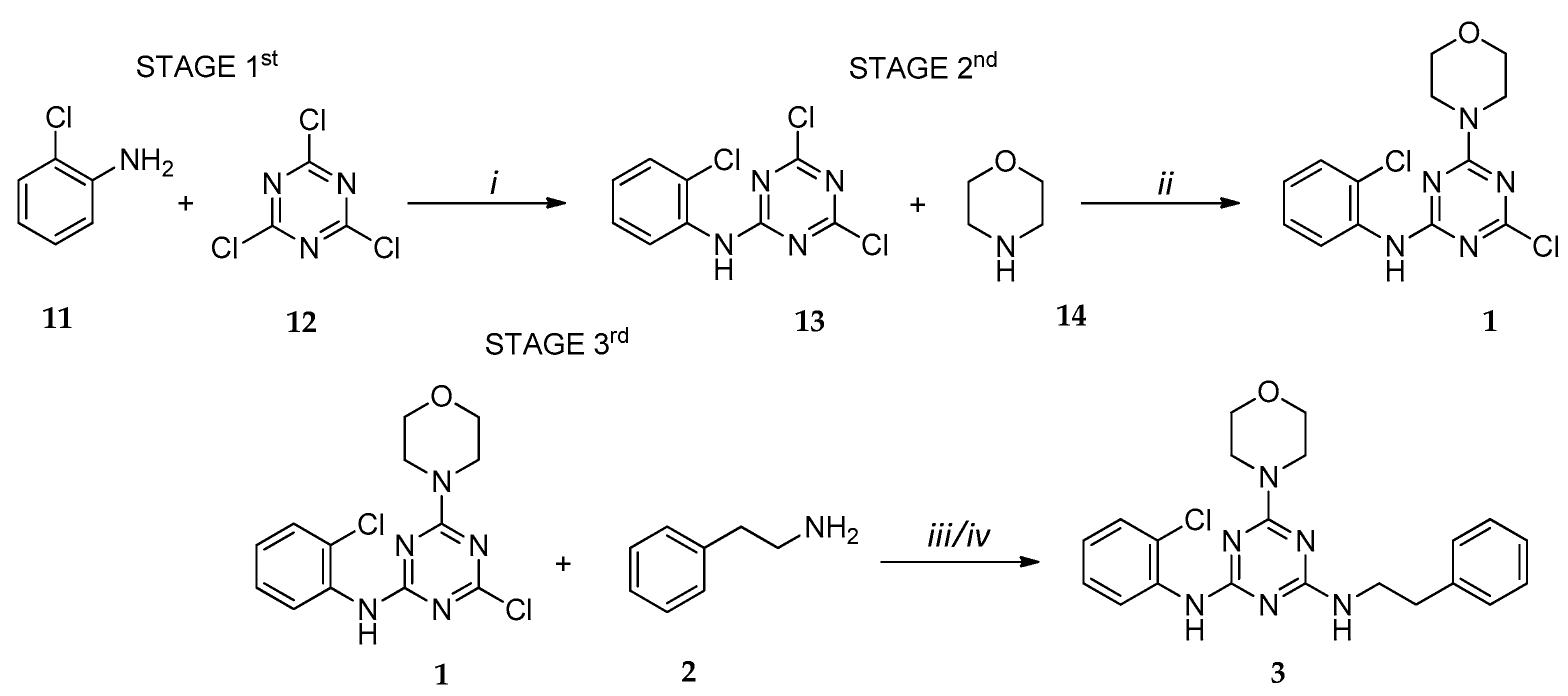
2.1. Chemistry
2.2. In Vitro Results and ADME Evaluation
3. Materials and Methods
3.1. Chemistry
3.2. General Procedure for the Synthesis of Compound 3 (Reaction Number 1–14) and Final Compounds 4–11 (Microwave Synthesis)
3.2.1. N2-(2-chlorophenyl)-6-morpholino-N4-phenethyl-1,3,5-triazine-2,4-diamine hydrochloride (3) Was Isolated as Follows
3.2.2. N2-(2-chlorophenyl)-6-morpholino-N4-phenyl-1,3,5-triazine-2,4-diamine hydrochloride (4) Was Isolated as Follows
3.2.3. N2-benzyl-N4-(2-chlorophenyl)-6-morpholino-1,3,5-triazine-2,4-diamine hydrochloride (5) Was Isolated as Follows
3.2.4. N2-(2-chlorophenyl)-6-morpholino-N4-(3-phenylpropyl)-1,3,5-triazine-2,4-diamine hydrochloride (6) Was Isolated as Follows
3.2.5. N2-(2-chlorophenyl)-N4-(2-(4-(4-fluorophenyl)piperazin-1-yl)ethyl)-6-morpholino-1,3,5-triazine-2,4-diamine hydrochloride (7) Was Isolated as Follows
3.2.6. N-(2-chlorophenyl)-4-morpholino-6-(4-phenylpiperazin-1-yl)-1,3,5-triazin-2-amine hydrochloride (8) Was Isolated as Follows
3.2.7. 4-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)-N-(2-chlorophenyl)-6-morpholino-1,3,5-triazin-2-amine hydrochloride (9) Was Isolated as Follows
3.2.8. N2-(2-chlorophenyl)-6-morpholino-N4-(2-(phenylamino)ethyl)-1,3,5-triazine-2,4-diamine hydrochloride (10) Was Isolated as Follows
3.2.9. N2-(2-chlorophenyl)-6-morpholino-N4-(2-phenoxyethyl)-1,3,5-triazine-2,4-diamine hydrochloride (11) Was Isolated as Follows
3.2.10. Procedure for the Synthesis of Compound 13
3.2.11. Procedure for the Synthesis of Compound 1
3.2.12. General Procedure for the Synthesis of Final Compounds 3, 5, 6, 8–11 (Sonochemistry)
3.3. In Vitro Screening and ADME Evaluation
3.3.1. In Silico Tools
3.3.2. Lipophilicity and Phospholipids Affinity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abedizadeh, R.; Majidi, F.; Khorasani, H.R.; Abedi, H.; Sabour, D. Colorectal cancer: A comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments. Cancer Metastasis Rev. 2024, 43, 729–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, X.; Chen, G.; Du, J. Drug Resistance in Colorectal Cancer: From Mechanism to Clinic. Cancers 2022, 14, 2928. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.; Manogaran, P. Mechanisms and Strategies to Overcome Drug Resistance in Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 1988. [Google Scholar] [CrossRef]
- Norcic, G. Liquid Biopsy in Colorectal Cancer-Current Status and Potential Clinical Applications. Micromachines 2018, 9, 300. [Google Scholar] [CrossRef]
- Han, C.J.; Ning, X.; Burd, C.E.; Spakowicz, D.J.; Tounkara, F.; Kalady, M.F.; Noonan, A.M.; McCabe, S.; Von Ah, D. Chemotoxicity and Associated Risk Factors in Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2597. [Google Scholar] [CrossRef]
- Mneimneh, A.T.; Darwiche, N.; Mehanna, M.M. Investigating the therapeutic promise of drug-repurposed-loaded nanocarriers: A pioneering strategy in advancing colorectal cancer treatment. Int. J. Pharm. 2024, 664, 124473. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, N.; Georgieva, M. Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials. Pharmaceutics 2022, 14, 1213. [Google Scholar] [CrossRef]
- Wang, N.; Chen, L.; Huang, W.; Gao, Z.; Jin, M. Current Advances of Nanomaterial-Based Oral Drug Delivery for Colorectal Cancer Treatment. Nanomaterials 2024, 14, 557. [Google Scholar] [CrossRef]
- Singh, U.P.; Pathak, M.; Dubey, V.; Bhat, H.R.; Gahtori, P.; Singh, R.K. Design, Synthesis, Antibacterial Activity, and Molecular Docking Studies of Novel Hybrid 1,3-Thiazine-1,3,5-Triazine Derivatives as Potential Bacterial Translation Inhibitor. Chem. Biol. Drug Des. 2012, 80, 572–583. [Google Scholar] [CrossRef]
- Singh, U.P.; Bhat, H.R.; Gahtori, P. Antifungal Activity, SAR and Physicochemical Correlation of Some Thiazole-1,3,5-Triazine Derivatives. J. Mycol. Med. 2012, 22, 134–141. [Google Scholar] [CrossRef]
- Gravestock, D.; Rousseau, A.L.; Lourens, A.C.U.; Moleele, S.S.; Van Zyl, R.L.; Steenkamp, P.A. Expeditious Synthesis and Biological Evaluation of Novel 2,N6-Disubstituted 1,2-Dihydro-1,3,5-Triazine-4,6-Diamines as Potential Antimalarials. Eur. J. Med. Chem. 2011, 46, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Kolesińska, B.; Frączyk, J.; Kamiński, Z.J.; Tankiewicz-Kwedlo, A.; Hermanowicz, J.; Czarnomysy, R.; Maliszewski, D.; Drozdowska, D. Synthesis and Cellular Effects of Novel 1,3,5-Triazine Derivatives in DLD and Ht-29 Human Colon Cancer Cell Lines. Invest. New Drugs 2020, 38, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, G.; Shao, R.; Huang, R. Synthesis and Antivirus Activity of 1,3,5-Triazine Derivatives. Heteroatom Chem. 2003, 14, 542–545. [Google Scholar] [CrossRef]
- Patil, V.; Noonikara-Poyil, A.; Joshi, S.D.; Patil, S.A.; Patil, S.A.; Lewis, A.M.; Bugarin, A. Synthesis, Molecular Docking Studies, and In Vitro Evaluation of 1,3,5-Triazine Derivatives as Promising Antimicrobial Agents. J. Mol. Struct. 2020, 1220, 128687. [Google Scholar] [CrossRef]
- Marín-Ocampo, L.; Veloza, L.A.; Abonia, R.; Sepúlveda-Arias, J.C. Anti-Inflammatory Activity of Triazine Derivatives: A Systematic Review. Eur. J. Med. Chem. 2019, 162, 435–447. [Google Scholar] [CrossRef]
- Gajula, M.R.; Reddy, Y.V.R. Synthesis, Characterization and In Vitro Biological Evaluation of Some New 1,3,5-Triazine-Bis-Azomethine Hybrid Molecules as Potential Antitubercular Agents. Eur. J. Chem. 2014, 5, 374–379. [Google Scholar] [CrossRef][Green Version]
- Padalkar, V.S.; Patil, V.S.; Sekar, N. Synthesis and Photo-Physical Properties of Fluorescent 1,3,5-Triazine Styryl Derivatives. Chem. Cent. J. 2011, 5, 77. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; Xiong, L.; Zhongyi, H. Tribological Performance of N-Containing Heterocyclic Triazine Derivative as Lubricant Additive in Ethylene Glycol. Surf. Interface Anal. 2021, 53, 1027–1034. [Google Scholar] [CrossRef]
- Giacomelli, G.; Porcheddu, A.; De Luca, L. 1,3,5-Triazine: A Versatile Heterocycle in Current Applications of Organic Chemistry. Curr. Org. Chem. 2005, 36, 1497–1519. [Google Scholar] [CrossRef]
- Al Rasheed, H.H.; Malebari, A.M.; Dahlous, K.A.; Fayne, D.; El-Faham, A. Synthesis, Anti-Proliferative Activity, and Molecular Docking Study of New Series of 1,3,5-Triazine Schiff Base Derivatives. Molecules 2020, 25, 4065. [Google Scholar] [CrossRef]
- Maliszewski, D.; Drozdowska, D. Recent Advances in the Biological Activity of s-Triazine Core Compounds. Pharmaceuticals 2022, 15, 221. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Triazine-Based Reagents: Organic Synthesis and Medicinal Chemistry Applications. Front. Chem. 2018, 6, 516. [Google Scholar] [CrossRef]
- Mattson, R.J.; Denhart, D.J.; Catt, J.D.; Dee, M.F.; Deskus, J.A.; Ditta, J.L.; Epperson, J.; King, H.D.; Gao, A.; Poss, M.A.; et al. Synthesis and Biological Evaluation of Novel Triazine Derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 4245–4248. [Google Scholar] [CrossRef] [PubMed]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and Heterogeneous Catalysis: A Review on Selected Catalytic Processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef]
- Herrera, A.; Riaño, A.; Moreno, R.; Caso, B.; Pardo, Z.D.; Fernández, I.; Sáez, E.; Molero, D.; Sánchez-Vázquez, A.; Martínez-Alvarez, R. One-Pot Synthesis of 1,3,5-Triazine Derivatives via Controlled Cross-Cyclotrimerization of Nitriles: A Mechanism Approach. J. Org. Chem. 2014, 79, 7012–7024. [Google Scholar] [CrossRef]
- Díaz-Ortiz, Á.; Elguero, J.; de la Hoz, A.; Jiménez, A.; Moreno, A.; Moreno, S.; Sánchez-Migallón, A. Microwave-Assisted Synthesis and Dynamic Behaviour of N₂,N₄,N₆-Tris(1H-pyrazolyl)-1,3,5-triazine-2,4,6-triamines. QSAR Comb. Sci. 2005, 24, 649–659. Available online: https://api.semanticscholar.org/CorpusID:98351437 (accessed on 1 April 2025). [CrossRef]
- Lim, H.Y.; Tan, Y.S.; Yeo, C.I.; Dolzhenko, A.V. A New One-Pot Microwave-Assisted Synthesis and Structural Analysis of 6,N₂,N₄-Trisubstituted 1,3,5-Triazine-2,4-Diamines. J. Mol. Struct. 2025, 1325, 140965. [Google Scholar] [CrossRef]
- Shahari, M.S.B.; Junaid, A.; Tiekink, E.R.T.; Dolzhenko, A.V. A New One-Pot Three-Component Synthesis of 4-Aryl-6-Cycloamino-1,3,5-Triazin-2-Amines under Microwave Irradiation. Synthesis 2021, 53, 2457–2468. [Google Scholar] [CrossRef]
- Kułaga, D.; Drabczyk, A.K.; Zaręba, P.; Jaśkowska, J.; Chrzan, J.; Greber, K.E.; Ciura, K.; Plażuk, D.; Wielgus, E. Green Synthesis of 1,3,5-Triazine Derivatives Using a Sonochemical Protocol. Ultrason. Sonochem. 2024, 108, 106951. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Ebenso, E.E. Green Ultrasound Assisted Synthesis of N2,N4,N6-tris((Pyridin-2-ylamino)methyl)-1,3,5-triazine-2,4,6-triamine as Effective Corrosion Inhibitor for Mild Steel in 1 M Hydrochloric Acid Medium. Int. J. Electrochem. Sci. 2013, 8, 10864–10877. [Google Scholar] [CrossRef]
- Al-Rasheed, H.H.; Alshaikh, M.; Khaled, J.M.; Alharbi, N.S.; El-Faham, A. Ultrasonic Irradiation: Synthesis, Characterization, and Preliminary Antimicrobial Activity of Novel Series of 4,6-Disubstituted-1,3,5-Triazine Containing Hydrazone Derivatives. J. Chem. 2016, 2016, 3464758. [Google Scholar] [CrossRef]
- Makosza, M. Phase-transfer catalysis. A general green methodology in organic synthesis. Pure Appl. Chem. 2000, 72, 1399–1403. [Google Scholar] [CrossRef]
- Díaz-Ortiz, A.; Hoz, A.; Moreno, A.; Sánchez-Migallón, A.; Valiente, G. Synthesis of 1,3,5-triazines in solvent-free conditions catalysed by silica-supported lewis acids. Green Chem. 2002, 4, 339–343. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Bálint, E. Microwave Irradiation and Phase Transfer Catalysis in C-, O- and N-Alkylation Reactions. Curr. Org. Synth. 2013, 10, 541–558. [Google Scholar] [CrossRef]
- Özgeriş, F.B.; Özgeriş, B. Synthesis, Characterization, and Biological Evaluations of Substituted Phenethylamine-Based Urea as Anticancer and Antioxidant Agents. Monatsh. Chem. 2021, 152, 1241–1250. [Google Scholar] [CrossRef]
- Rani, P.; Pal, D.; Hegde, R.R.; Hashim, S.R. Anticancer, Anti-Inflammatory, and Analgesic Activities of Synthesized 2-(Substituted Phenoxy) Acetamide Derivatives. Biomed. Res. Int. 2014, 2014, 386473. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.S.; Abou El Ella, D.A.; El-Gazzar, M.G.; El-Sayed, M.A. Synthesis and Characterization of New Series of 1,3-5-Triazine Hydrazone Derivatives with Promising Antiproliferative Activity. Molecules 2020, 25, 2755. [Google Scholar] [CrossRef]
- Ranjbari, S.; Behzadi, M.; Sepehri, S.; Dadkhah Aseman, M.; Jarrahpour, A.; Mohkam, M.; Ghasemi, Y.; Reza Akbarizadeh, A.; Kianpour, S.; Atioğlu, Z.; et al. Investigations of Antiproliferative and Antioxidant Activity of β-Lactam Morpholino-1,3,5-Triazine Hybrids. Bioorg. Med. Chem. 2020, 28, 115408. [Google Scholar] [CrossRef]
- Jaśkowska, J.; Drabczyk, A.K.; Śliwa, P.; Jodłowski, P.; Pindelska, E.; Kułaga, D.; Zaręba, P.; Majka, Z.; Siwek, A.; Wolak, M.; et al. Ultrasound Assisted One-Pot Synthesis and Preliminary in Vitro Studies of Salicylamide Arylpiperazines as Dual 5-HT1A/5-HT7 Ligands. J. Mol. Struct. 2023, 1275, 134585. [Google Scholar] [CrossRef]
- Zaręba, P.; Drabczyk, A.K.; Wnorowski, A.; Pindelska, E.; Latacz, G.; Jaśkowska, J. Eco-Friendly Methods of Synthesis and Preliminary Biological Evaluation of Sulfonamide Derivatives of Cyclic Arylguanidines. Ultrason. Sonochem. 2022, 90, 106165. [Google Scholar] [CrossRef]
- Drabczyk, A.K.; Kułaga, D.; Zaręba, P.; Tylińska, W.; Bachowski, W.; Archała, A.; Wnorowski, A.; Tzani, A.; Detsi, A.; Jaśkowska, J. Eco-Friendly Synthesis of New Olanzapine Derivatives and Evaluation of Their Anticancer Potential. RSC Adv. 2023, 13, 20467–20476. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Valkó, K.L.; Zhang, T. Biomimetic Properties and Estimated In Vivo Distribution of Chloroquine and Hydroxy-Chloroquine Enantiomers. ADMET DMPK 2021, 9, 151–165. [Google Scholar] [CrossRef]
- Valkó, K.L. Lipophilicity and Biomimetic Properties Measured by HPLC to Support Drug Discovery. J. Pharm. Biomed. Anal. 2016, 130, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Bunnally, S.; Young, R.J. The Role and Impact of High Throughput Biomimetic Measurements in Drug Discovery. ADMET DMPK 2018, 6, 74–84. [Google Scholar] [CrossRef]
- Bayliss, M.K.; Butler, J.; Feldman, P.L.; Green, D.V.S.; Leeson, P.D.; Palovich, M.R.; Taylor, A.J. Quality Guidelines for Oral Drug Candidates: Dose, Solubility and Lipophilicity. Drug Discov. Today 2016, 21, 1719–1727. [Google Scholar] [CrossRef]
- Baumer, A.; Bittermann, K.; Klüver, N.; Escher, B.I. Baseline Toxicity and Ion-Trapping Models to Describe the pH-Dependence of Bacterial Toxicity of Pharmaceuticals. Environ. Sci. Process. Impacts 2017, 19, 901–916. [Google Scholar] [CrossRef]
- Escher, B.I.; Eggen, R.I.L.; Schreiber, U.; Schreiber, Z.; Vye, E.; Wisner, B.; Schwarzenbach, R.P. Baseline Toxicity (Narcosis) of Organic Chemicals Determined by In Vitro Membrane Potential Measurements in Energy-Transducing Membranes. Environ. Sci. Technol. 2002, 36, 1971–1979. [Google Scholar] [CrossRef]
- Jiang, Z.; Reilly, J. Chromatography Approaches for Early Screening of the Phospholipidosis-Inducing Potential of Pharmaceuticals. J. Pharm. Biomed. Anal. 2012, 61, 184–190. [Google Scholar] [CrossRef]
- Ulenberg, S.; Ciura, K.; Georgiev, P.; Pastewska, M.; Ślifirski, G.; Król, M.; Herold, F.; Bączek, T. Use of Biomimetic Chromatography and In Vitro Assay to Develop Predictive GA-MLR Model for Use in Drug-Property Prediction among Anti-Depressant Drug Candidates. Microchem. J. 2022, 175, 107183. [Google Scholar] [CrossRef]
- Ciura, K.; Kovačević, S.; Pastewska, M.; Kapica, H.; Kornela, M.; Sawicki, W. Prediction of the Chromatographic Hydrophobicity Index with Immobilized Artificial Membrane Chromatography Using Simple Molecular Descriptors and Artificial Neural Networks. J. Chromatogr. A 2021, 1660, 462666. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, C.; Tsopelas, F.; Valkó, K.L. Prediction of hERG Inhibition of Drug Discovery Compounds Using Biomimetic HPLC Measurements. ADMET DMPK 2021, 9, 191–207. [Google Scholar] [CrossRef] [PubMed]



| Reaction No. | Base | PTC | Solvent | Yield [%] |
|---|---|---|---|---|
| 1 | Na2CO3 | TBAB | DMF | 87 |
| 2 | K2CO3 | 64 | ||
| 3 | KOH | 48 | ||
| 4 | NaOH | 23 | ||
| 5 | DIPEA | 23 | ||
| 6 | TEA | 56 | ||
| 7 | NH3∙H2O | 2 | ||
| 8 | Na2CO3 | TEBA | DMF | 56 |
| 9 | TEAC | 80 | ||
| 10 | CTBA | 85 | ||
| 11 | KI | 44 | ||
| 12 | - | 53 | ||
| 13 | TBAB | H2O | 10 | |
| 14 | - | 8 |
| Compound No. | Amines | Yield [%] | ||
|---|---|---|---|---|
| Microwave Synthesis | Sonochemistry | |||
| 3 | 2 |  | 87 | 90 |
| 4 | 2a |  | 80 | - |
| 5 | 2b | 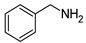 | 70 | 89 |
| 6 | 2c | 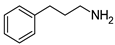 | 52 | 85 |
| 7 | 2d | 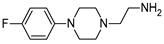 | 82 | - |
| 8 | 2e | 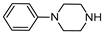 | 96 | 87 |
| 9 | 2f | 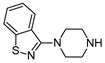 | 59 | 94 |
| 10 | 2g | 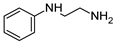 | 77 | 41 |
| 11 | 2h | 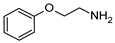 | 54 | 69 |
| Compound No. | SW480 | SW620 |
|---|---|---|
| 3 | >100 | >100 |
| 4 | >100 | 36.09 ± 46.48 |
| 5 | 43.4 ± 12.22 | 32.83 ± 23.37 |
| 6 | >100 | >100 |
| 7 | >100 | >100 * |
| 8 | 20.57 ± 24.87 | >100 * |
| 9 | >100 | 22.1 ± 17.29 |
| 10 | >100 | >100 * |
| 11 | >100 * | 5.85 ± 14.34 |
| 5-fluorouracil | 15.45 ± 7.825 | 21.74 ± 2.754 |
| Compound No. | RP-HPLC | IAM-HPLC | Perpecpta | SwissADME | ADMET-AI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| chrom logD | Ar | PFI | CHI IAM | clogD | logP | iLOGP | XLOGP3 | WLOGP | MLOGP | Silicos-IT Log P | Consensus Log P | logP | |
| 3 | 3.95 | 4 | 7.95 | 55.97 | 2.84 | 1.11 | 4.29 | 5.36 | 2.90 | 2.33 | 3.12 | 3.60 | 4.04 |
| 4 | 4.10 | 3 | 7.10 | 46.34 | 3.61 | 1.80 | 4.11 | 4.54 | 3.02 | 1.95 | 2.63 | 3.25 | 3.60 |
| 5 | 3.95 | 3 | 6.95 | 46.45 | 3.27 | 1.26 | 3.89 | 4.56 | 2.87 | 1.95 | 2.31 | 3.12 | 3.63 |
| 6 | 4.71 | 3 | 7.71 | 51.63 | 2.74 | 0.81 | 4.08 | 4.61 | 2.29 | 2.37 | 2.08 | 3.09 | 3.43 |
| 7 | 4.44 | 3 | 7.44 | 49.59 | 4.06 | 2.51 | 4.28 | 5.19 | 3.58 | 2.68 | 3.48 | 3.84 | 4.15 |
| 8 | 4.15 | 3 | 7.15 | 47.79 | 3.45 | 1.63 | 3.73 | 3.04 | 3.47 | 2.29 | 2.34 | 2.97 | 3.85 |
| 9 | 4.23 | 3 | 7.23 | 48.02 | 3.70 | 2.07 | 4.13 | 4.83 | 3.19 | 2.47 | 3.10 | 3.54 | 3.76 |
| 10 | 3.94 | 3 | 6.94 | 44.55 | 2.97 | 1.86 | 4.75 | 4.61 | 2.57 | 2.38 | 2.48 | 3.36 | 3.48 |
| 11 | 4.01 | 3 | 7.01 | 45.92 | 3.51 | 1.66 | 4.01 | 4.37 | 2.99 | 2.25 | 2.71 | 3.27 | 3.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrzan, J.; Drabczyk, A.K.; Siemińska, I.; Baj-Krzyworzeka, M.; Greber, K.E.; Jaśkowska, J.; Kułaga, D.; Ciura, K. Green and Efficient Synthetic Protocol for 1,3,5-Triazine Derivatives with Anticancer Potential Against Colorectal Cancer. Molecules 2025, 30, 2437. https://doi.org/10.3390/molecules30112437
Chrzan J, Drabczyk AK, Siemińska I, Baj-Krzyworzeka M, Greber KE, Jaśkowska J, Kułaga D, Ciura K. Green and Efficient Synthetic Protocol for 1,3,5-Triazine Derivatives with Anticancer Potential Against Colorectal Cancer. Molecules. 2025; 30(11):2437. https://doi.org/10.3390/molecules30112437
Chicago/Turabian StyleChrzan, Julia, Anna Karolina Drabczyk, Izabela Siemińska, Monika Baj-Krzyworzeka, Katarzyna Ewa Greber, Jolanta Jaśkowska, Damian Kułaga, and Krzesimir Ciura. 2025. "Green and Efficient Synthetic Protocol for 1,3,5-Triazine Derivatives with Anticancer Potential Against Colorectal Cancer" Molecules 30, no. 11: 2437. https://doi.org/10.3390/molecules30112437
APA StyleChrzan, J., Drabczyk, A. K., Siemińska, I., Baj-Krzyworzeka, M., Greber, K. E., Jaśkowska, J., Kułaga, D., & Ciura, K. (2025). Green and Efficient Synthetic Protocol for 1,3,5-Triazine Derivatives with Anticancer Potential Against Colorectal Cancer. Molecules, 30(11), 2437. https://doi.org/10.3390/molecules30112437








