Removal of Hexavalent Chromium from Wastewater Originating from Spent Bricks by Modified Biochars Derived from Honeybee Biomass
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Studies
2.2. Cr(VI) Adsorption Tests
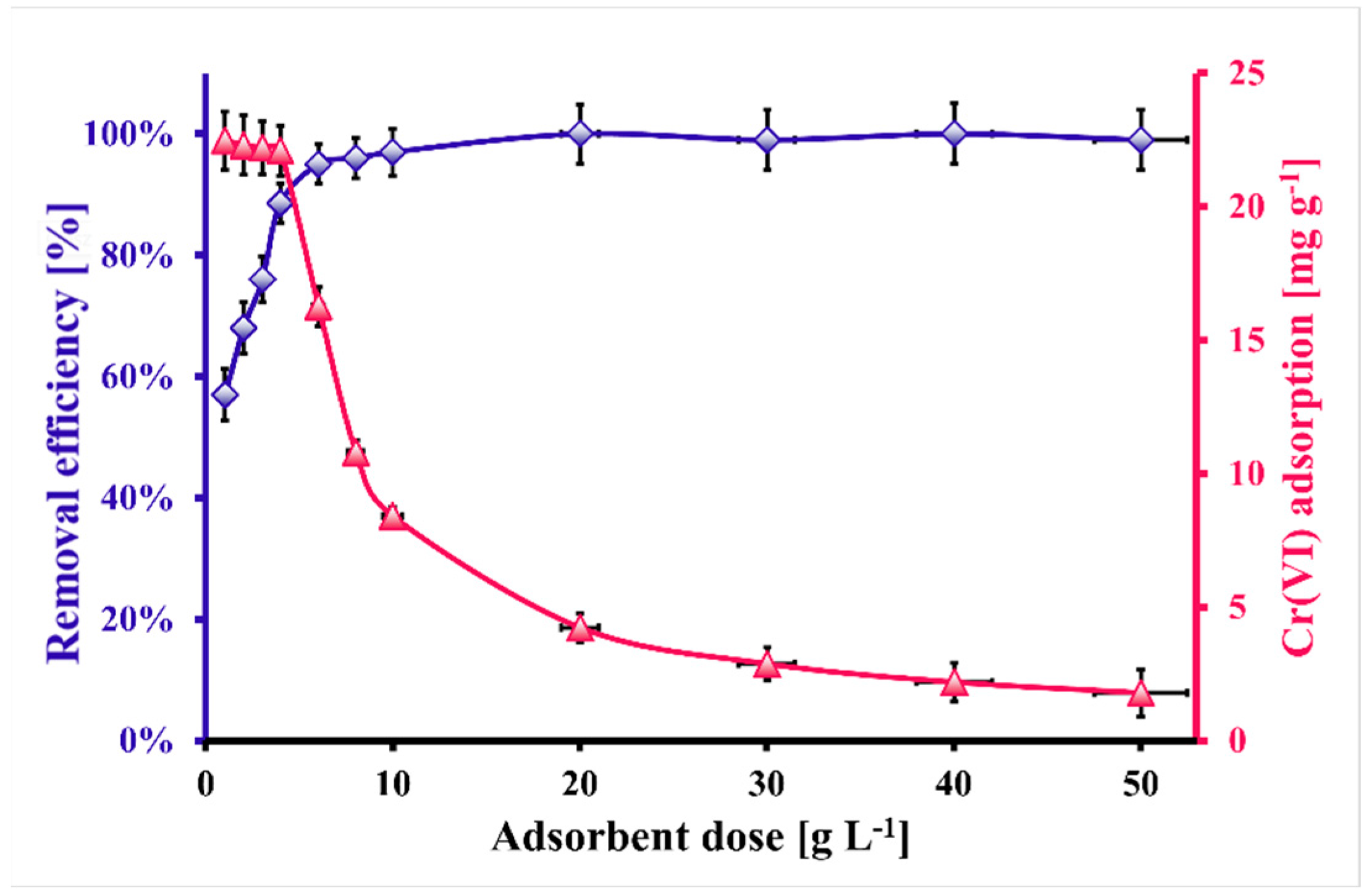
2.3. Cr(VI) Removal from the Brick-Originated Wastewater
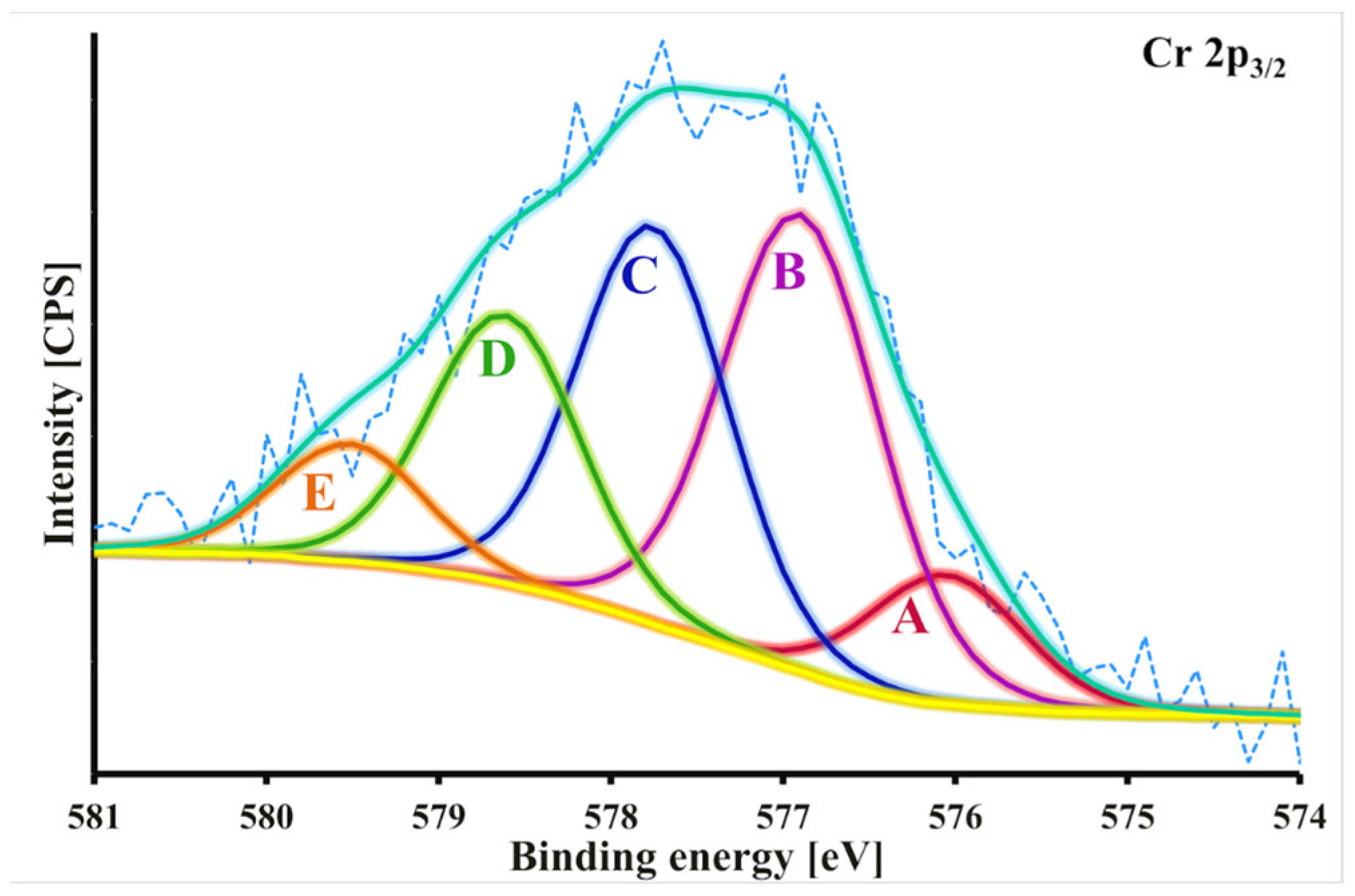
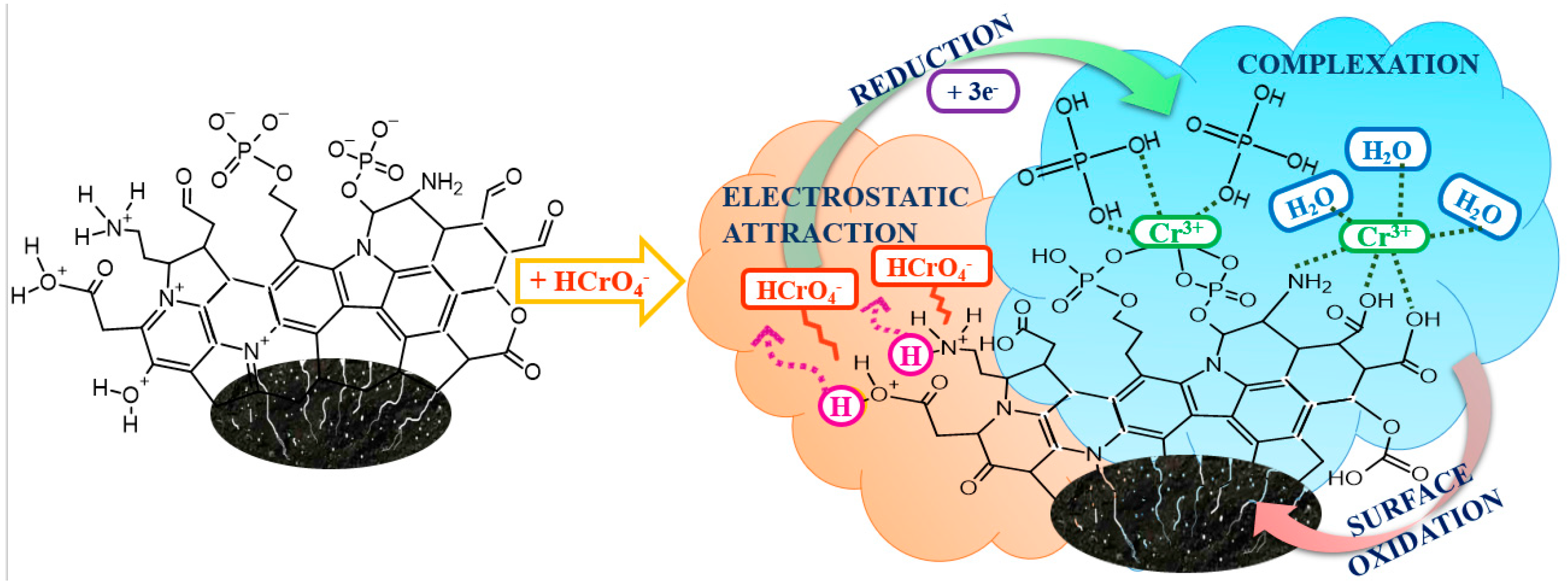
2.4. Cr(VI) Adsorption Mechanism
3. Materials and Methods
3.1. Raw Material and Reagents
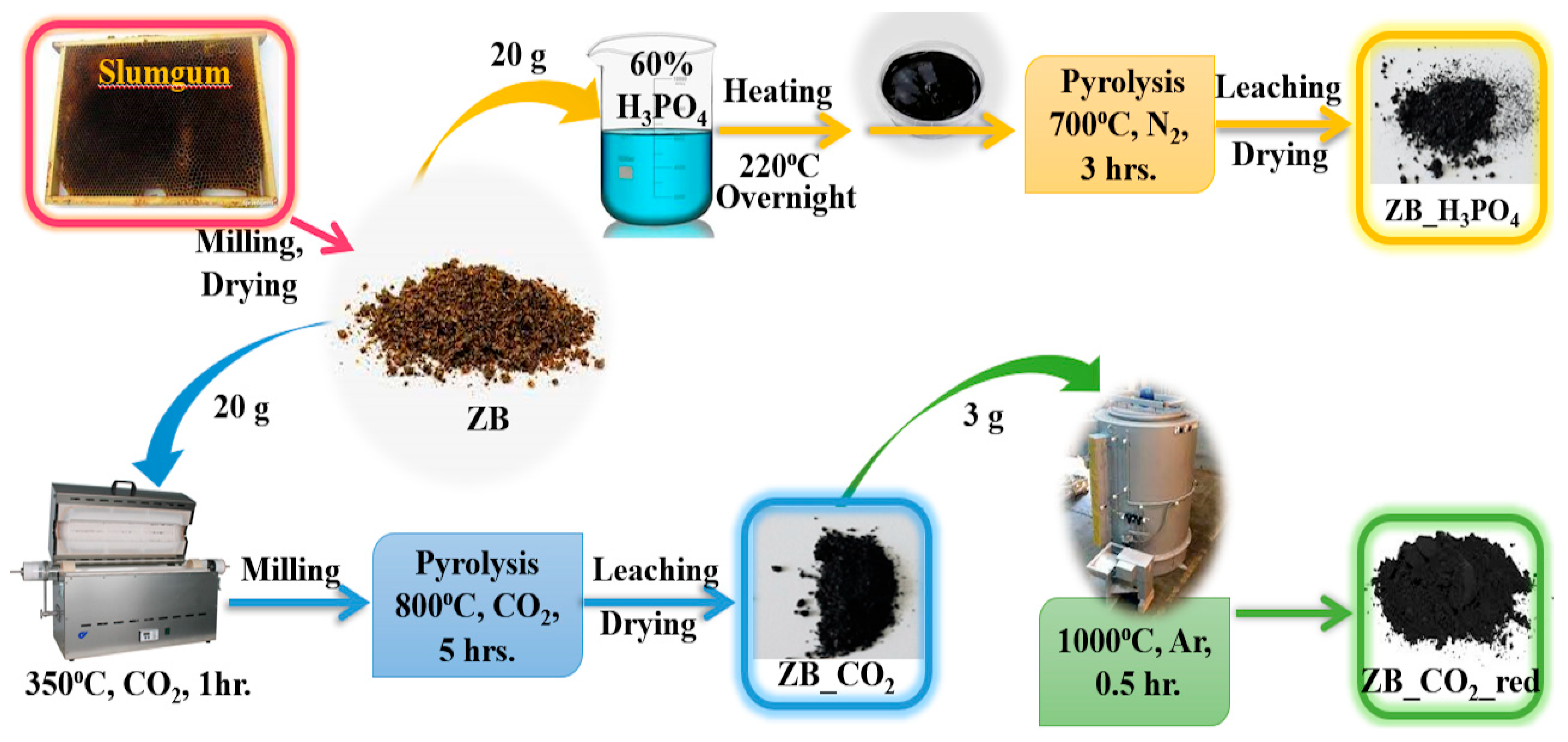
3.2. Preparation of Biochar Materials
3.3. Physicochemical Characteristics
3.4. Cr(VI) Batch Adsorption Studies
3.5. Removal of Cr(VI) from Brick-Originated Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Pesticide residues in honey bees, pollen and beeswax: Assessing beehive exposure. Environ. Pollut. 2018, 241, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Morales-Corts, M.R.; Gómez-Sánchez, M.Á.; Pérez-Sánchez, R. Evaluation of green/pruning wastes compost and vermicompost, slumgum compost and their mixes as growing media for horticultural production. Sci. Hortic. 2014, 172, 155–160. [Google Scholar] [CrossRef]
- Morales-Corts, R.; Gomez-Sanchez, M.A.; Perez-Sanchez, R.; Prieto-Calvo, C. Characterization of beekeeping wastes for using in seedling production. Span. J. Agric. Res. 2010, 8, 493–500. [Google Scholar] [CrossRef]
- Bilbao, D.C.; Machin, E.B.; Pedroso, D.T.; Machin, A.B. Theoretical assessment of energy recovery from beekeeping waste (slumgum). Sustain. Energy Technol. Assess. 2022, 49, 101700. [Google Scholar] [CrossRef]
- Jamaludin, N.; Rashid, S.A.; Tan, T. Natural biomass as carbon sources for the synthesis of photoluminescent carbon dots. In Synthesis, Technology and Applications of Carbon Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–134. [Google Scholar] [CrossRef]
- Qu, Q.; Guo, X.; Shao, Z.; Wang, X.; Zhu, M.; Qiu, L. Adsorption performance and mechanism of Fe-loaded biochar derived from waste zanthoxylum branch for removing Cr(VI) from aqueous solution. Biomass Convers. Biorefin. 2022, 103, 10201–10215. [Google Scholar] [CrossRef]
- Li, A.; Xie, H.; Qiu, Y.; Liu, L.; Lu, T.; Wang, W.; Qiu, G. Resource utilization of rice husk biomass: Preparation of MgO flake-modified biochar for simultaneous removal of heavy metals from aqueous solution and polluted soil. Environ. Pollut. 2022, 310, 119869. [Google Scholar] [CrossRef]
- Zhang, A.; Li, X.; Xing, J.; Xu, G. Adsorption of potentially toxic elements in water by modified biochar: A review. J. Environ. Chem. Eng. 2020, 8, 104196. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest trends in heavy metal removal from wastewater by biochar based sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Shooto, N.D.; Thabede, P.M. Binary adsorption of chromium and cadmium metal ions by hemp (Cannabis sativa) based adsorbents. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100683. [Google Scholar] [CrossRef]
- Wei, D.; Li, B.; Huang, H.; Luo, L.; Zhang, J.; Yang, Y.; Guo, J.; Tang, L.; Zeng, G.; Zhou, Y. Biochar-based functional materials in the purification of agricultural wastewater: Fabrication, application and future research needs. Chemosphere 2018, 197, 165–180. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, L.; Qin, Y.; Xu, M.; Jia, X.; Chen, Z. Removal of aqueous Cr(VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour. Technol. 2018, 256, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bonilla Mancilla, H.D.; Pino Moreyra, J.D.; Bullon Rosas, J.J.; Bernal Marcelo, A.R.; Tovar, C.T.; Jindal, M.K.; Kumar, D.; Sillanpää, M.; Ghernaout, D. Pinus radiata forest residue: A bio-adsorbent of choice for Cr (VI) removal from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2025, 23, 101042. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, W.; Yin, Y.; Chen, Z.; Qiu, R.; Simonnot, M.-O.; Wang, X. Adsorption-reduction removal of Cr(VI) by tobacco petiole pyrolytic biochar: Batch experiment, kinetic and mechanism studies. Bioresour. Technol. 2018, 268, 149–157. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Naiya, T.; Mandal, S.; Das, S. Adsorption, kinetics and equilibrium studies on removal of Cr(VI) from aqueous solutions using different low-cost adsorbents. Chem. Eng. J. 2008, 137, 529–541. [Google Scholar] [CrossRef]
- Han, J.; Jiao, F.; Liu, W.; Qin, W.; Xu, T.; Xue, K.; Zhang, T. Innovative methodology for comprehensive utilization of spent MgO-Cr2O3 bricks: Copper flotation. ACS Sustain. Chem. Eng. 2016, 4, 5503–5510. [Google Scholar] [CrossRef]
- Jiao, F.; Li, W.; Xue, K.; Yang, C.; Qin, W. Recovery of chromium and magnesium from spent magnesia-chrome refractories by acid leaching combined with alkali precipitation and evaporation. Seperation Purif. Technol. 2019, 227, 115705. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Selvasembian, R.; Ashiq, A.; Gunarathne, V.; Ekanayake, A.; Perera, V.O.; Wijesekera, H.; Mia, S.; Ahmad, M.; Vithanage, M.; et al. A systematic review on adsorptive removal of hexavalent chromium from aqueous solutions: Recent advances. Sci. Total Environ. 2022, 809, 152055. [Google Scholar] [CrossRef]
- Sivakumar, D. Biosorption of hexavalent chromium in a tannery industry wastewater using fungi species. Glob. J. Environ. Sci. Manag. 2016, 2, 105–124. [Google Scholar] [CrossRef]
- Xue, K.; Li, W.; Jiao, F.; Qin, W.; Yang, C. Comprehensive recovery of valuable metals from spent magnesia–chrome refractories by ferric chloride–hydrochloride leaching. J. Sustain. Metall. 2021, 7, 898–907. [Google Scholar] [CrossRef]
- Xue, K.; Han, J.; Jiao, F.; Liu, W.; Qin, W.; Cai, L.; Xu, T. Comprehensive utilization of spent magnesia-chrome refractories with gravity separation followed by flotation. Miner. Eng. 2018, 127, 125–133. [Google Scholar] [CrossRef]
- Dobrzyński, D. Chromium pollution of underground waters in surroudings of the cement plant “Pokój” in Rejowiec Fabryczny. Prz. Geol. 1990, 38, 496–501. [Google Scholar]
- Kim, Y.; Oh, J.-I.; Vithanage, M.; Park, Y.-K.; Lee, J.; Kwon, E.E. Modification of biochar properties using CO2. Chem. Eng. J. 2019, 372, 383–389. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Alvear-Daza, J.J.; Canneva, A.; Donadelli, J.A.; Manrique-Holguin, M.; Rengifo-Herrera, J.A.; Pizzio, L.R. Removal of diclofenac and ibuprofen on mesoporous activated carbon from agro-industrial wastes prepared by optimized synthesis employing a central composite design. Biomass Conv. Bioref. 2022, 277, 13197–13219. [Google Scholar] [CrossRef]
- Jawad, A.H.; Malek, N.N.A.; Khadiran, T.; ALOthman, Z.A.; Yaseen, Z.M. Mesoporous high-surface-area activated carbon from biomass waste via microwave-assisted-H3PO4 activation for methylene blue dye adsorption: An optimized process. Diam. Relat. Mater. 2022, 128, 109288. [Google Scholar] [CrossRef]
- Ngueabouo, A.M.S.; Tagne, R.F.T.; Tchuifon, D.R.T.; Fotsop, C.G.; Tamo, A.K.; Anagho, S.G. Strategy for optimizing the synthesis and characterization of activated carbons obtained by chemical activation of coffee husk. Mater. Adv. 2022, 3, 8361–8374. [Google Scholar] [CrossRef]
- Olchowski, R.; Podkościelna, B.; Zawisza, B.; Morlo, K.; Dobrowolski, R. U(VI) removal from water by novel P-modified activated carbon derived from polymeric microspheres. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100788. [Google Scholar] [CrossRef]
- Özçifçi, Z.; Özşanlı, M.A.; Akçay, H.T.; Yumak, T. Sucrose-based activated carbon foams as an electrode active material for supercapacitors. J. Porous Mater. 2023, 30, 797–810. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Gawdzik, B.; Tascón, J.M.D. Phosphorus-containing carbons: Preparation, properties and utilization. Carbon 2020, 157, 796–846. [Google Scholar] [CrossRef]
- Goskula, S.; Siliveri, S.; Gujjula, S.R.; Chirra, S.; Narayanan, V. Synthesis of sustainable acid biochar catalysts derived from waste biomass for esterification of glycerol. ChemistrySelect 2023, 8, e202203662. [Google Scholar] [CrossRef]
- Kim, J.A.; Vijayaraghavan, K.; Reddy, D.H.K.; Yun, Y.-S. A phosphorus-enriched biochar fertilizer from bio-fermentation waste: A potential alternative source for phosphorus fertilizers. J. Clean. Prod. 2018, 196, 163–171. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Chen, H.; Wang, W.; Yang, Y. Comparative study of biochar modified with different functional groups for efficient removal of Pb(II) and Ni(II). Int. J. Environ. Res. Public Health 2022, 19, 11163. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Mierzwa-Hersztek, M.; Nawrocka, A.; Pińkowska, H.; Bajda, T.; Stanek-Tarkowska, J.; Szostek, M. FT-IR analysis and the content of phenolic compounds in exogenous organic matter produced from plant biomass. J. Elem. 2019, 24, 879–896. [Google Scholar] [CrossRef]
- Wei, L.; Huang, Y.; Huang, L.; Li, Y.; Huang, Q.; Xu, G.; Müller, K.; Wang, H.; Ok, Y.S.; Liu, Z. The ratio of H/C is a useful parameter to predict adsorption of the herbicide metolachlor to biochars. Environ. Res. 2020, 184, 109324. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.; Qi, R.; Wang, M.; Zhang, K.; Jiang, E.; Ren, Y.; Hu, Z. Study on the infiltration mechanism of molten urea and biochar for a novel fertilizer preparation. Ind. Crops Prod. 2020, 153, 112558. [Google Scholar] [CrossRef]
- Koinuma, M.; Tateishi, H.; Hatakeyama, K.; Miyamoto, S.; Ogata, C.; Funatsu, A.; Taniguchi, T.; Matsumoto, Y. Analysis of reduced graphene oxides by X-ray photoelectron spectroscopy and electrochemical capacitance. Chem. Lett. 2013, 42, 924–926. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Kirilenko, D.A.; Ulin, N.V.; Baidakova, M.V.; Shnitov, V.V.; Pavlov, S.I.; Chumakov, R.G.; Stolyarova, D.Y.; Besedina, N.A.; et al. From graphene oxide towards aminated graphene: Facile synthesis, its structure and electronic properties. Sci. Rep. 2020, 10, 6902. [Google Scholar] [CrossRef]
- Larciprete, R.; Gardonio, S.; Petaccia, L.; Lizzit, S. Atomic oxygen functionalization of double walled C nanotubes. Carbon 2009, 47, 2579–2589. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yoo, J.J.; Kim, Y.I.; Yoon, J.K.; Yoon, H.N.; Kim, J.-H.; Park, S.B. Oxygen functional groups and electrochemical capacitive behavior of incompletely reduced graphene oxides as a thin-film electrode of supercapacitor. Electrochim. Acta 2014, 116, 118–128. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Dideikin, A.T.; Kirilenko, D.A.; Baidakova, M.V.; Shnitov, V.V.; Roth, F.; Konyakhin, S.V.; Besedina, N.A.; Pavlov, S.I.; Kuricyn, R.A.; et al. Facile reduction of graphene oxide suspensions and films using glass wafers. Sci. Rep. 2018, 8, 14154. [Google Scholar] [CrossRef] [PubMed]
- Wagstaffe, M.; Thomas, A.G.; Jackman, M.J.; Torres-Molina, M.; Syres, K.L.; Handrup, K. An experimental investigation of the adsorption of a phosphonic acid on the anatase TiO2 (101) Surface. J. Phys. Chem. C 2016, 120, 1693–1700. [Google Scholar] [CrossRef]
- Arrigo, R.; Hävecker, M.; Wrabetz, S.; Blume, R.; Lerch, M.; McGregor, J.; Parrott, E.P.J.; Zeitler, J.A.; Gladden, L.F.; Knop-Gericke, A.; et al. Tuning the acid/base properties of nanocarbons by functionalization via amination. J. Am. Chem. Soc. 2010, 132, 9616–9630. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Peng, B.; Haller, G.L.; Ember, E.E.; Lercher, J.A. Nitrogen modified carbon nano-materials as stable catalysts for phosgene synthesis. ACS Catal. 2016, 6, 5843–5855. [Google Scholar] [CrossRef]
- Raicopol, M.; Andronescu, C.; Atasiei, R.; Hanganu, A.; Pilan, L. Post-polymerization electrochemical functionalization of a conducting polymer: Diazonium salt electroreduction at polypyrrole electrodes. J. Electrochem. Soc. 2014, 161, G103–G113. [Google Scholar] [CrossRef]
- Demri, B.; Muster, D. XPS study of some calcium compounds. J. Mater. Process. Technol. 1995, 55, 311–314. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Stefaniak, E. Study of chromium(VI) adsorption from aqueous solution onto activated carbon. Adsorp. Sci. Technol. 2000, 18, 97–106. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhang, Y.; Li, M.; Zhao, Z. Efficient removal of Cr(VI) by modified sodium alginate via synergistic adsorption and photocatalytic reduction. Appl. Surf. Sci. 2022, 579, 152259. [Google Scholar] [CrossRef]
- Wu, S.; Han, C.; Xin, L.; Li, M.; Long, H.; Gao, X. Synthesis of triethylenetetramine modified sodium alginate/CuS nanocrystal composite for enhanced Cr(VI) removal: Performance and mechanism. Int. J. Biol. Macromol. 2023, 238, 124283. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Jeyakumar, P.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.; Bolan, N.; Wang, H. A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1027–1078. [Google Scholar] [CrossRef]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A critical assessment of chromium in the environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Archundia, C.; Bonato, P.S.; Lugo Rivera, J.F.; Mascioli, L.C.; Collins, K.E.; Collins, C.H. Reduction of low concentration Cr(VI) in acid solutions. Sci. Total Environ. 1993, 130–131, 231–236. [Google Scholar] [CrossRef]
- Dizge, N.; Aydiner, C.; Demirbas, E.; Kobya, M.; Kara, S. Adsorption of reactive dyes from aqueous solutions by fly ash: Kinetic and equilibrium studies. J. Hazard. Mater. 2008, 150, 737–746. [Google Scholar] [CrossRef]
- Stefaniak, E.; Dobrowolski, R.; Staszczuk, P. On the adsorption of chromium(VI) Ions on dolomite and ‘dolomitic sorbents’. Adsorp. Sci. Technol. 2000, 18, 107–115. [Google Scholar] [CrossRef]
- Mahajan, T.; Paikaray, S.; Mahajan, P. Applicability of the equilibrium adsorption isotherms and the statistical tools on to them: A case study for the adsorption of fluoride onto Mg-Fe-CO3 LDH. J. Phys. Conf. Ser. 2023, 2603, 012056. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Prelot, B. Adsorption processes for the removal of contaminants from wastewater. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Budimir, F.; Ptacek, C.J.; Amos, R.T.; Blowes, D.W. Chromium isotope fractionation during the removal of hexavalent chromium by oak-based biochar. Chemosphere 2024, 369, 143880. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, F.; Yang, C.; Lu, L.; Zhao, Y.; Tu, B.; Li, L. Adsorption and reduction of Cr(VI) by C-P-O groups in biochar: Performance and mechanisms. Solid State Sci. 2025, 160, 107815. [Google Scholar] [CrossRef]
- Chęć, M.; Olszewski, K.; Dziechciarz, P.; Skowronek, P.; Pietrow, M.; Borsuk, G.; Bednarczyk, M.; Jasina, G.; Jasina, J.; Gagoś, M. Effect of stearin and paraffin adulteration of beeswax on brood survival. Apidologie 2021, 52, 432–446. [Google Scholar] [CrossRef]
- Dos Santos Silva, L.; De Oliveira Carvalho, J.; De Sousa Bezerra, R.D.; Da Silva, M.; Ferreira, F.; Osajima, J.; Da Silva Filho, E. Potential of cellulose functionalized with carboxylic acid as biosorbent for the removal of cationic dyes in aqueous solution. Molecules 2018, 23, 743. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; An, Q.; Jin, L.; Luo, N.; Li, Z.; Jiang, J. Unraveling sorption of Cr(VI) from aqueous solution by FeCl3 and ZnCl2-modified corn stalks biochar: Implicit mechanism and application. Bioresour. Technol. 2020, 297, 122466. [Google Scholar] [CrossRef]
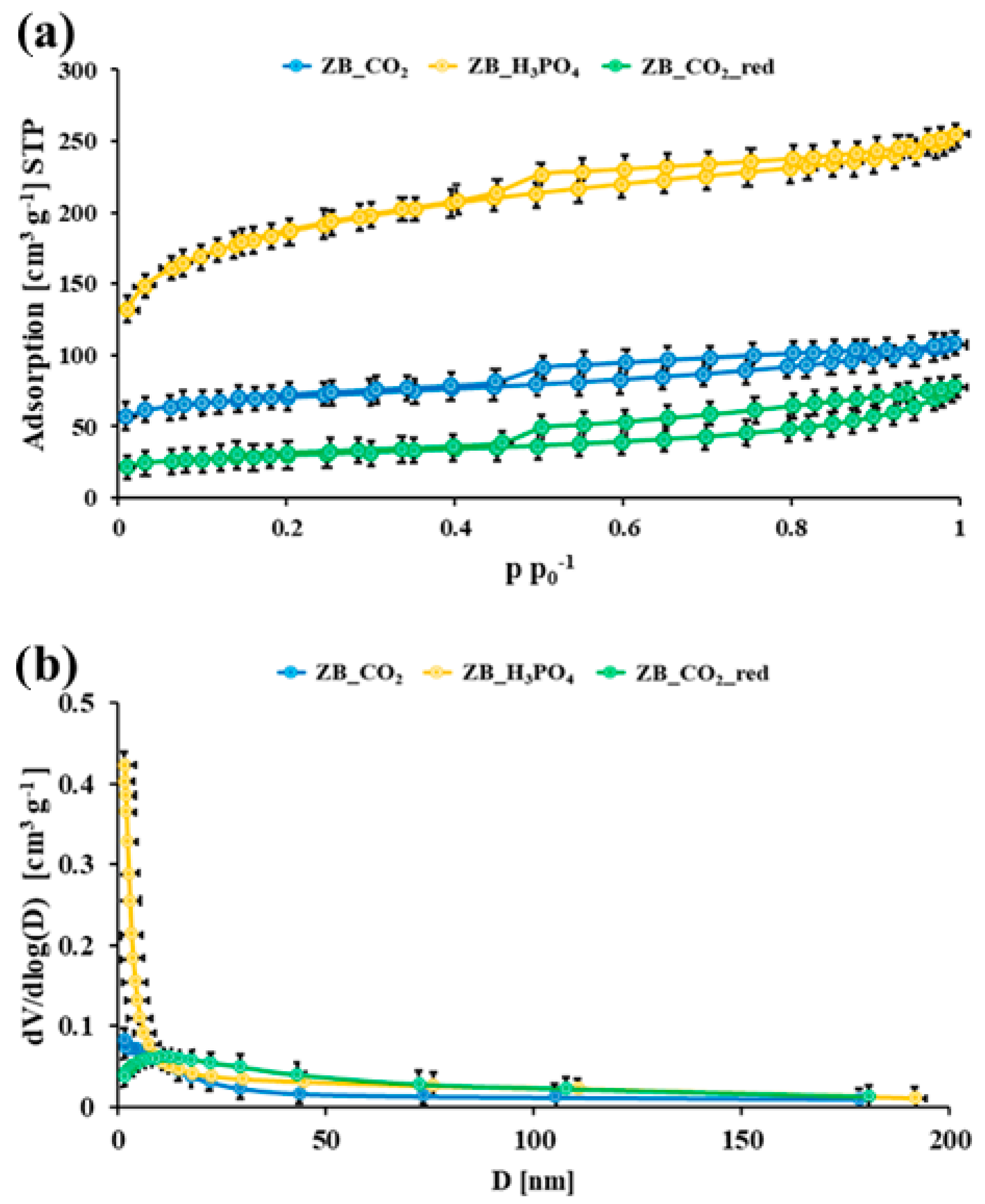
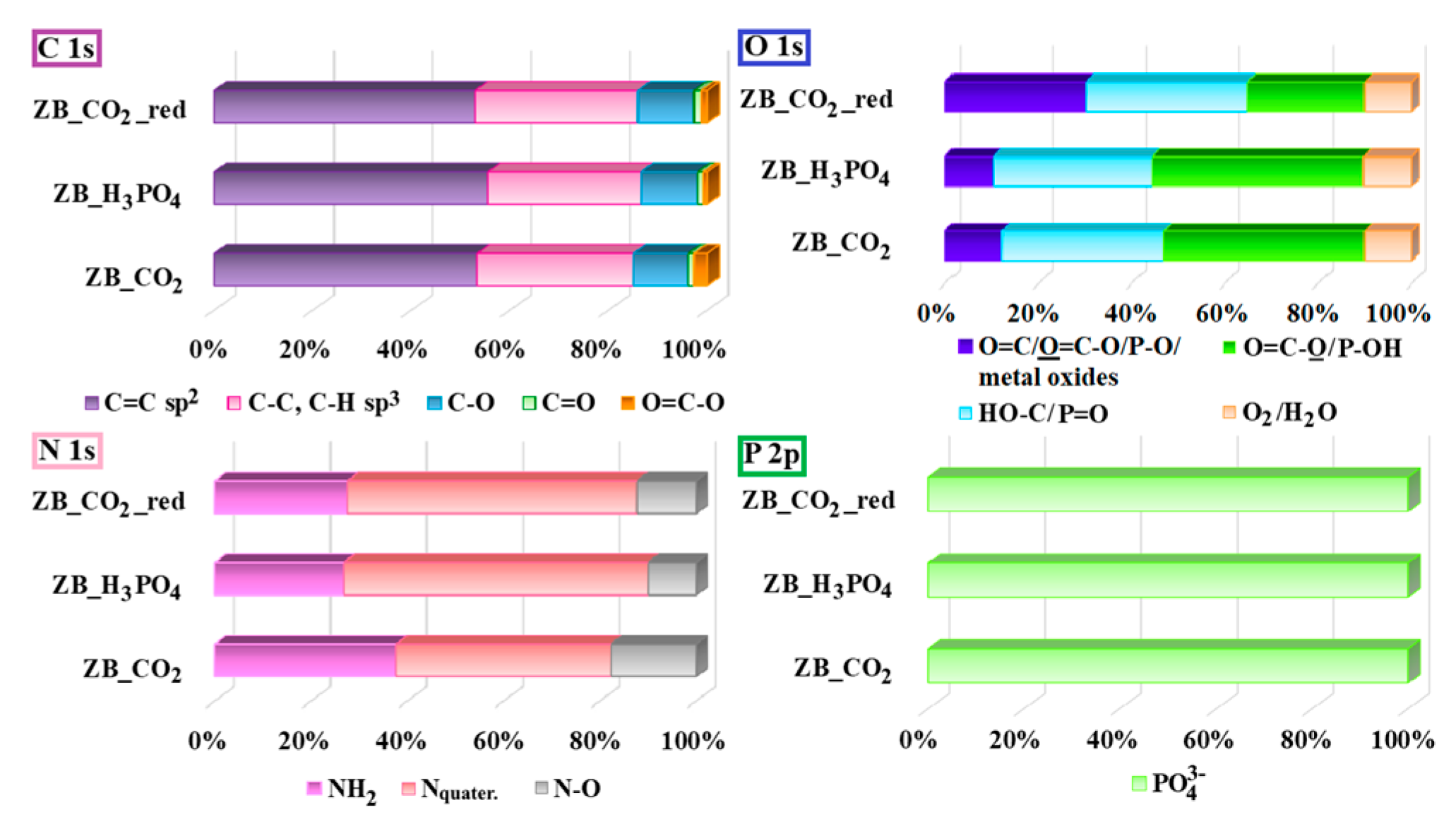
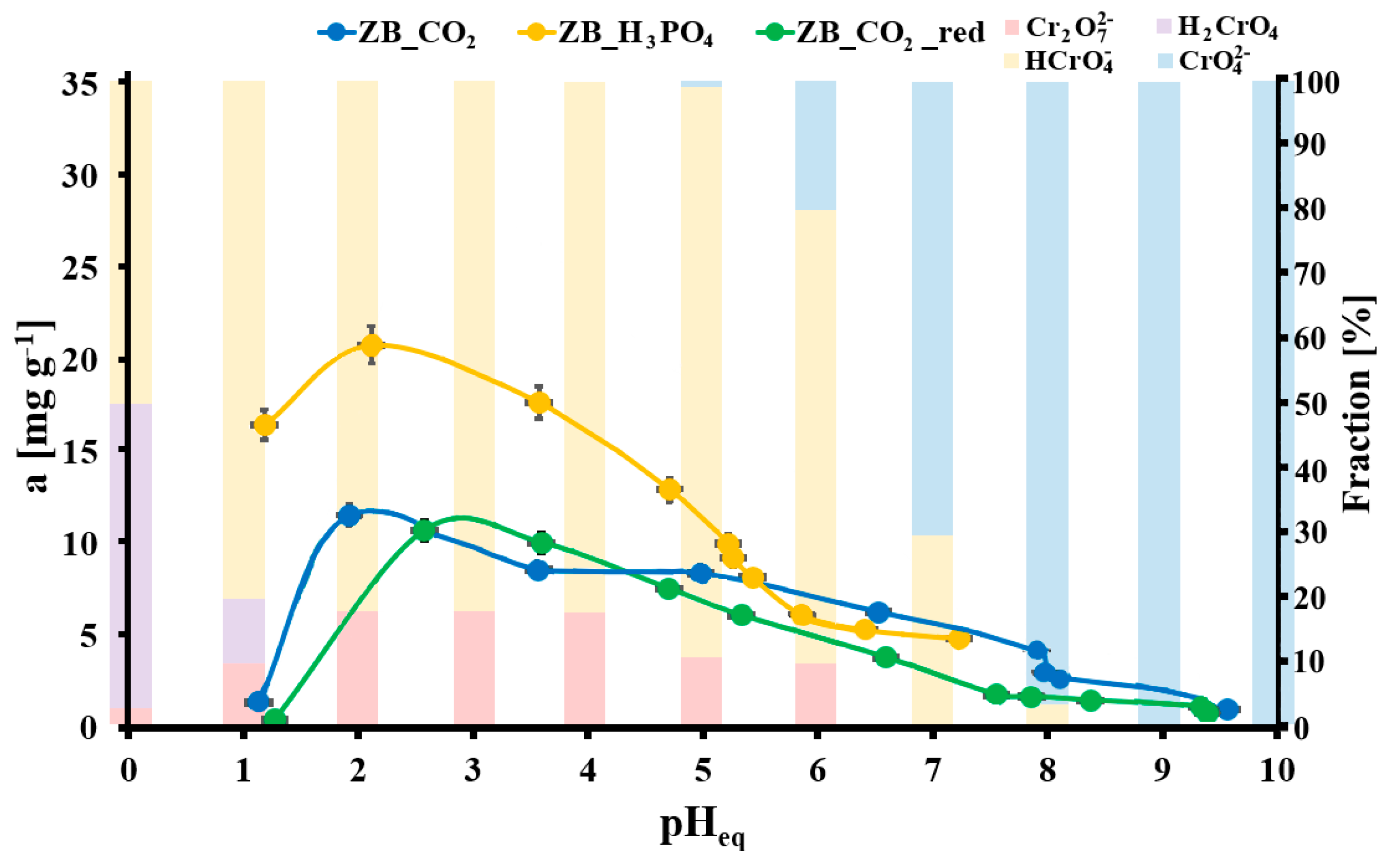
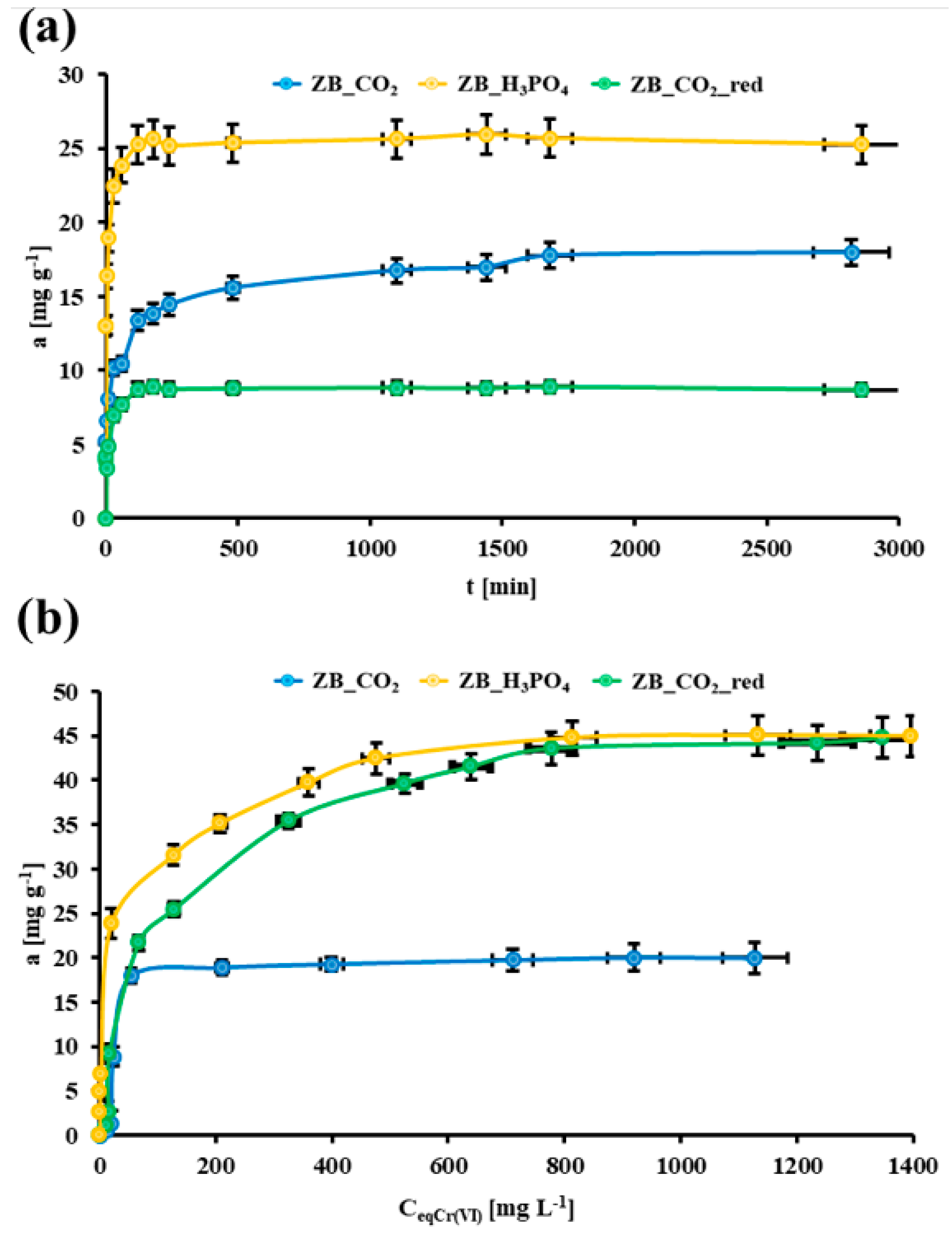
| Material Symbol | SBET [m2 g−1] | VTot. [cm3 g−1] | dBJH,des. [nm] |
|---|---|---|---|
| ZB | 0 * | 0 * | 11.9 $ ± 0.4 # |
| ZB_CO2 | 254 $ ± 8 # | 0.17 $ ± 0.01 # | 4.4 $ ± 0.1 # |
| ZB_CO2_red | 105 $ ± 4 # | 0.12 $ ± 0.01 # | 5.7 $ ± 0.2 # |
| ZB_H3PO4 | 663 $ ± 10 # | 0.39 $ ± 0.02 # | 3.5 $ ± 0.1 # |
| Material | CHN | XPS | ||||||
|---|---|---|---|---|---|---|---|---|
| C [wt. %] | H [wt. %] | N [wt. %] | C [wt. %] | N [wt. %] | O [wt. %] | P [wt. %] | K [wt. %] | |
| ZB | 59.2 $ ± 2.4 # | 10.7 $ ± 0.4 # | 5.2 $ ± 0.3 # | nd | nd | nd | nd | nd |
| ZB_CO2_red | 68.3 $ ± 1.7 # | 1.6 $ ± 0.1 # | 3.9 $ ± 0.2 # | 71.4 $ ± 1.8 # | 2.6 $ ± 0.1 # | 17.2 $ ± 0.6 # | 1.8 $ ± 0.1 # | 5.1 $ ± 0.2 # |
| ZB_CO2 | 84.7 $ ± 1.2 # | 2.9 $ ± 0.3 # | 3.8 $ ± 0.1 # | 88.8 $ ± 1.3 # | 2.1 $ ± 0.2 # | 7.6 $ ± 0.4 # | 1.9 $ ± 0.1 # | 4.9 $ ± 0.2 # |
| ZB_H3PO4 | 52.1 $ ± 2.3 # | 3.4 $ ± 0.3 # | 3.9 $ ± 0.1 # | 65.9 $ ± 3.1 # | 3.9 $ ± 0.1 # | 20.6 $ ± 0.9 # | 8.1 $ ± 0.3 # | 0 * |
| Material | PFO | PSO | Elovich | IPD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qeq.calc. [mg g−1] | k1 [min−1] | R2 | qeq.calc. [mg g−1] | k2 [g mg−1 min−1] | R2 | qeq.exp. [mg g−1] | α [mg g−1 min−1] | β [g mg−1] | R2 | kid [mg g−1 min−1/2] | C [mg g−1] | R2 | |
| ZB_CO2 | 8.96 $ ± 0.45 # | 0.002 $& | 0.890 | 13.05 $ ± 0.73 # | 0.046 $& | 0.705 | 17.9 $ ± 0.9 # | 21.1 $ ± 1.1 # | 0.57 $ ± 0.03 # | 0.984 | 0.27 $ ± 0.01 # | 7.26 $ ± 0.36 # | 0.710 |
| ZB_CO2_red | 1.40 $ ± 0.07 # | 0.002 $& | 0.408 | 7.50 $ ± 0.03 # | 0.108 $& | 0.535 | 8.90 $ ± 0.45 # | 115 $ ± 5 # | 1.32 $ ± 0.07 # | 0.840 | 0.11 $& | 4.94 $ ± 0.25 # | 0.454 |
| ZB_H3PO4 | 3.21 $ ± 0.16 # | 0.001 $& | 0.295 | 24.0 $ ± 0.9 # | 0.039 $& | 0.820 | 25.9 $ ± 1.3 # | 8399 $ ± 420 # | 0.59 $ ± 0.02 # | 0.848 | 0.26 $ ± 0.01 # | 16.4 $ ± 0.8 # | 0.372 |
| Material | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qm,exp. [mg g−1] | qm,teoret. [mg g−1] | kL [L mg−1] | R2 | nF [a. u.] | kF [mg1−nF LnF g−1] | R2 | |
| ZB_CO2 | 20.0 $ ± 1.0 # | 80.0 $ ± 4.0 # | 0.001 & | 0.562 | 1.37 $ ± 0.02 # | 0.58 $ ± 0.08 # | 0.616 |
| ZB_CO2_red | 44.7 $ ± 1.2 # | 103.5 $ ± 2.4 # | 0.002 & | 0.833 | 1.48 $ ± 0.03 # | 0.29 $ ± 0.04 # | 0.840 |
| ZB_H3PO4 | 45.0 $ ± 2.3 # | 33.0 $ ± 1.7 # | 0.217 $ ± 0.010 # | 0.909 | 2.84 $ ± 0.02 # | 5.25 $ ± 0.32 # | 0.965 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olchowski, R.; Morlo, K.; Dobrzyńska, J.; Dobrowolski, R. Removal of Hexavalent Chromium from Wastewater Originating from Spent Bricks by Modified Biochars Derived from Honeybee Biomass. Molecules 2025, 30, 2421. https://doi.org/10.3390/molecules30112421
Olchowski R, Morlo K, Dobrzyńska J, Dobrowolski R. Removal of Hexavalent Chromium from Wastewater Originating from Spent Bricks by Modified Biochars Derived from Honeybee Biomass. Molecules. 2025; 30(11):2421. https://doi.org/10.3390/molecules30112421
Chicago/Turabian StyleOlchowski, Rafał, Kinga Morlo, Joanna Dobrzyńska, and Ryszard Dobrowolski. 2025. "Removal of Hexavalent Chromium from Wastewater Originating from Spent Bricks by Modified Biochars Derived from Honeybee Biomass" Molecules 30, no. 11: 2421. https://doi.org/10.3390/molecules30112421
APA StyleOlchowski, R., Morlo, K., Dobrzyńska, J., & Dobrowolski, R. (2025). Removal of Hexavalent Chromium from Wastewater Originating from Spent Bricks by Modified Biochars Derived from Honeybee Biomass. Molecules, 30(11), 2421. https://doi.org/10.3390/molecules30112421







