A Systematic Study of Lysine Succinylation in the Pathogenic Bacterium Vibrio harveyi in Aquatic Animals
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Ksuc Peptides and Proteins in V. harveyi
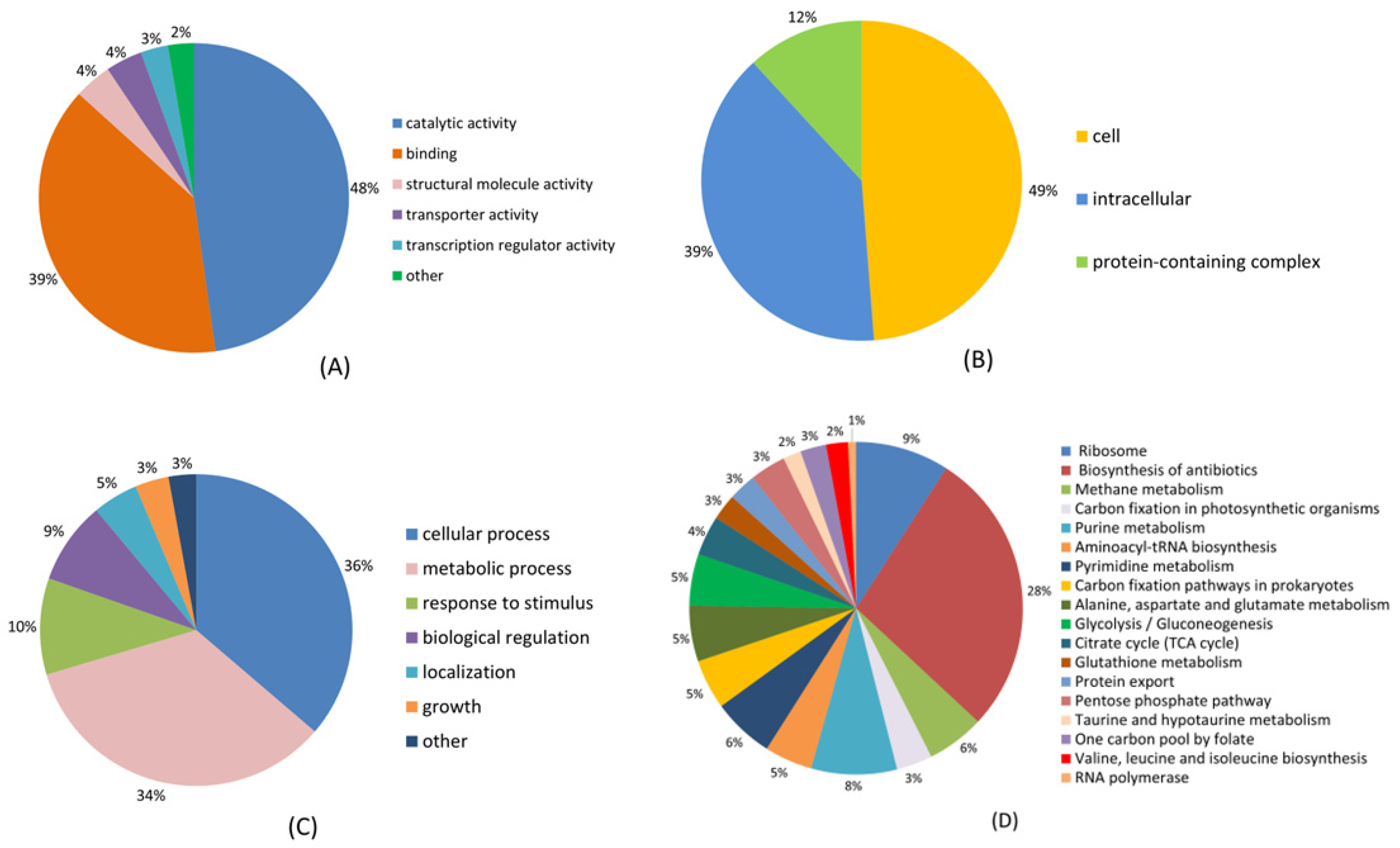
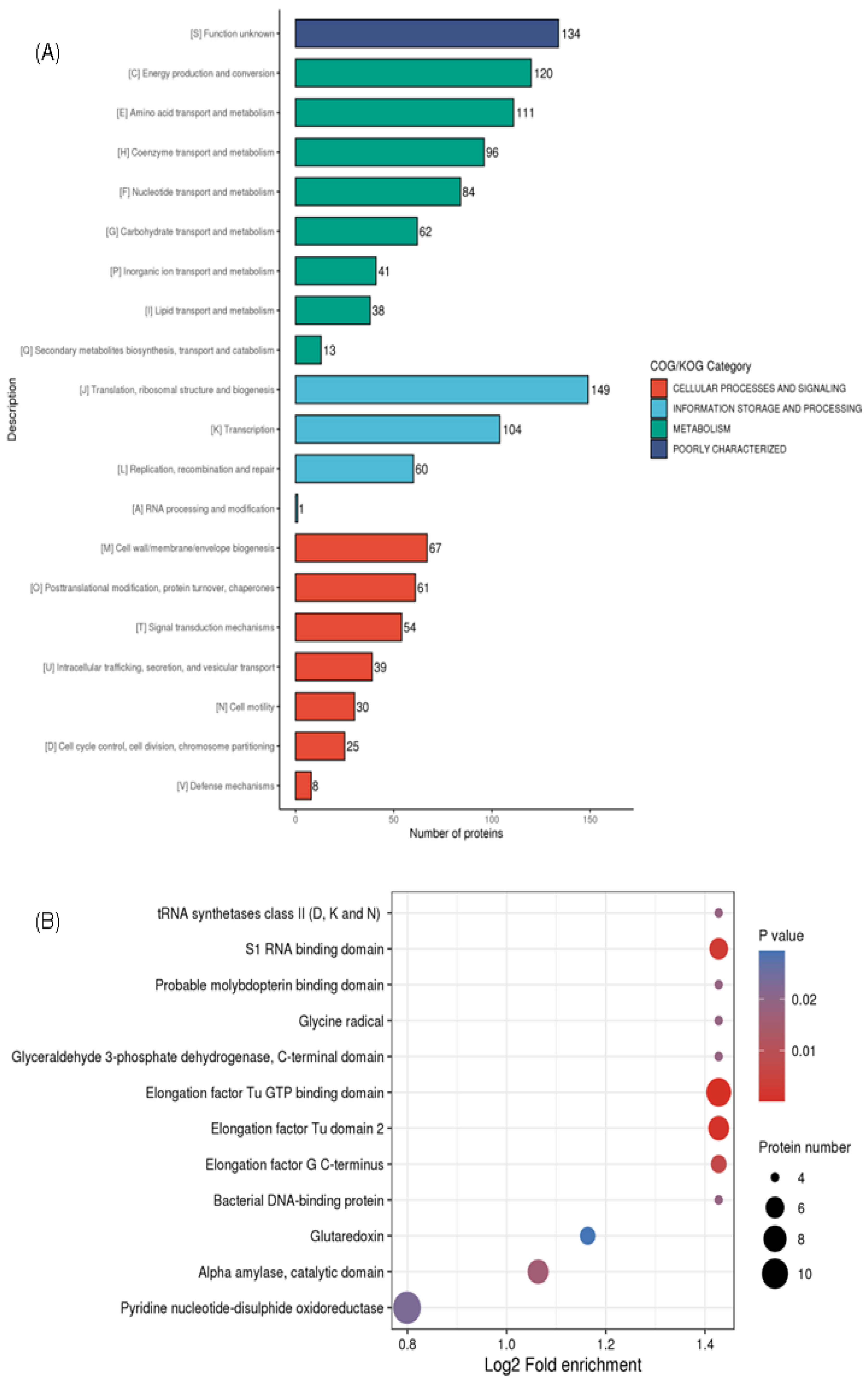
2.2. Functional Description of V. harveyi’s Lysine Succinylome
2.3. Motif of Ksuc Peptides in V. harveyi
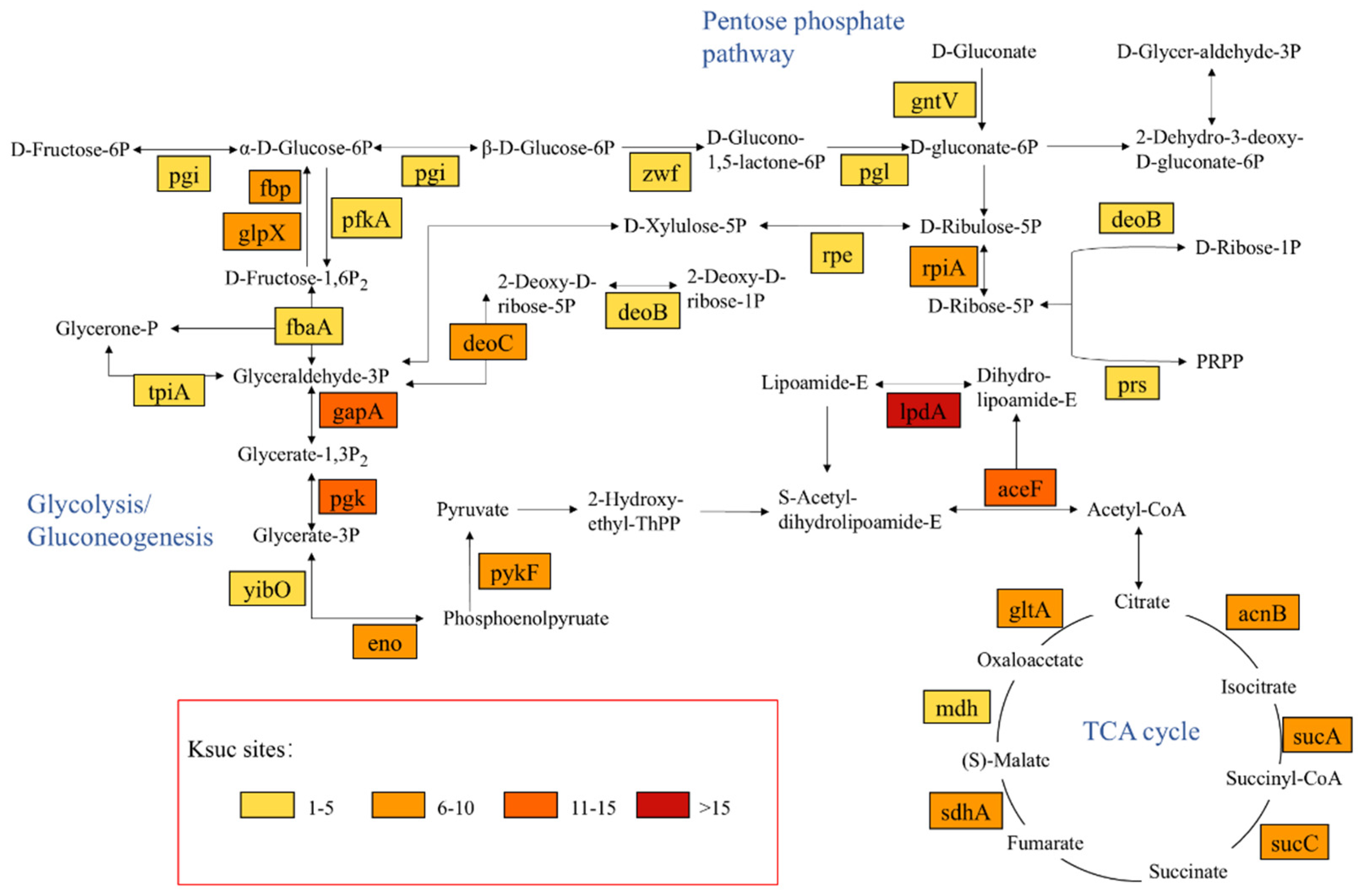
2.4. Succinylation-Modified Proteases Are Widely Involved in Various Metabolic Pathways
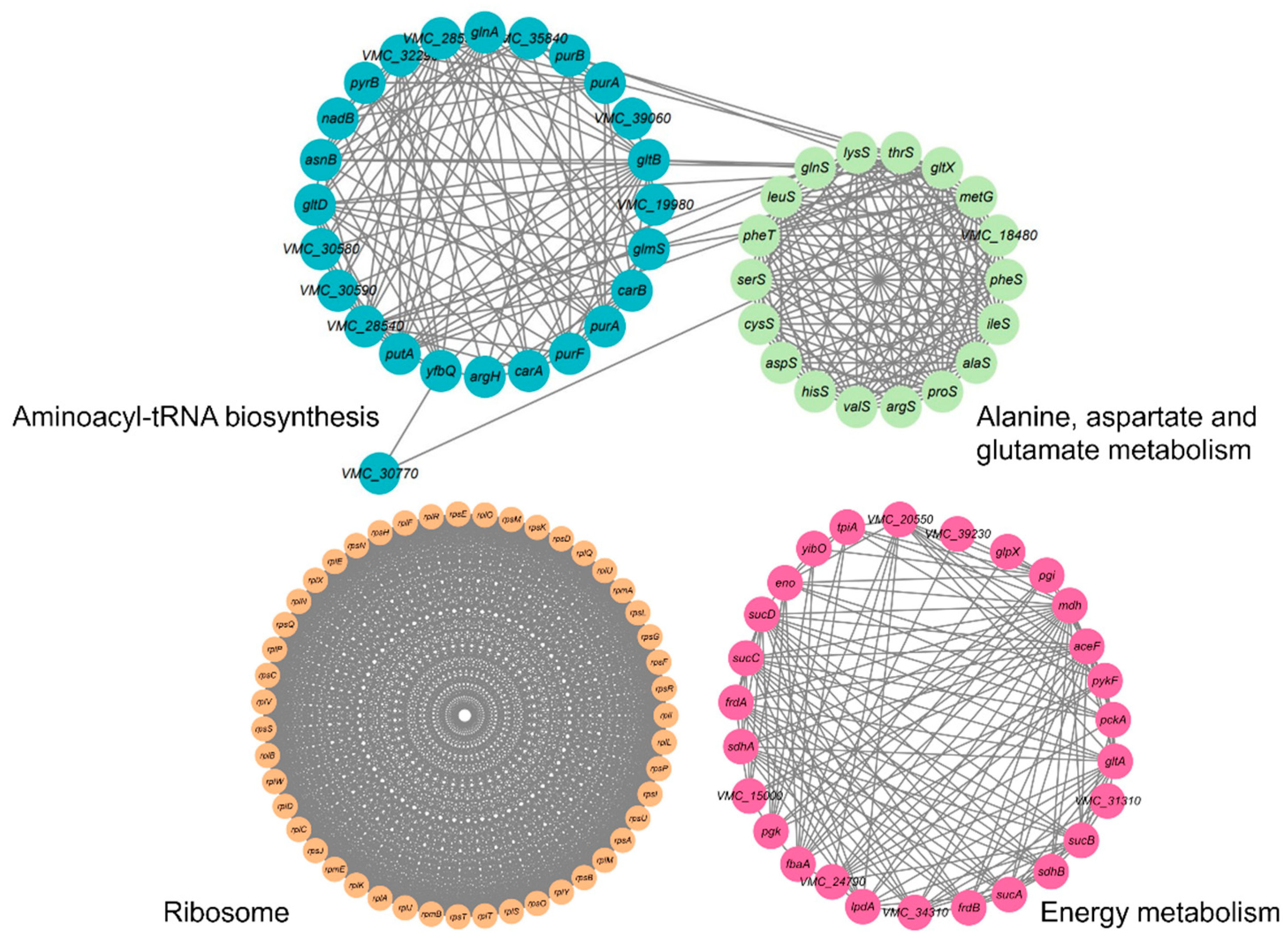
2.5. Analysis of V. harveyi’s Succinylated Protein PPI Network
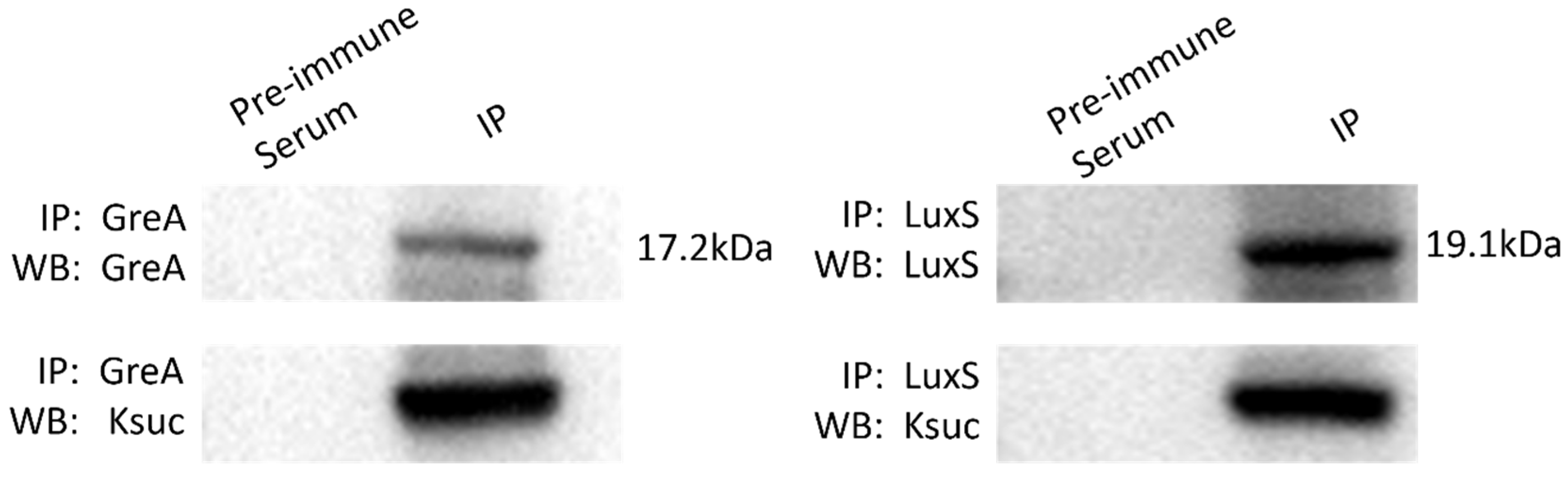
2.6. Western Blot and Immunoprecipitation Are Used to Validate the GreA and LuxS Ksuc Proteins
3. Materials and Methods
3.1. Bacterial Strains and Protein Extraction
3.2. Enrichment of Ksuc Peptides
3.3. LC-MS/MS Analysis
3.4. Data Processing
3.5. Immunoprecipitation and Western Blot
3.6. Bioinformatics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hermann, J.; Schurgers, L.; Jankowski, V. Identification and Characterization of Post-Translational Modifications: Clinical Implications. Mol. Asp. Med. 2022, 86, 101066. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ye, T.; Li, C.; Praveen, P.; Hu, Z.; Li, W.; Shang, C. Embracing the Era of Antimicrobial Peptides with Marine Organisms. Nat. Prod. Rep. 2024, 41, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Longarini, E.J.; Matic, I. The Fast-Growing Business of Serine ADP-Ribosylation. DNA Repair 2022, 118, 103382. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, J.; Zhang, W.; Ni, J.; Huang, R.; Wang, Z.; Cheng, S.; Wang, Y.; Tian, Z.; Zhou, Q.; et al. Methylation of PhoP by CheR Regulates Salmonella Virulence. mBio 2021, 12, e0209921. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, X.; Li, F.; Li, C.; Marquez-Lago, T.; Leier, A.; Akutsu, T.; Webb, G.I.; Xu, D.; Smith, A.I.; et al. Large-Scale Comparative Assessment of Computational Predictors for Lysine Post-Translational Modification Sites. Brief. Bioinform. 2019, 20, 2267–2290. [Google Scholar] [CrossRef]
- Azevedo, C.; Saiardi, A. Why Always Lysine? The Ongoing Tale of One of the Most Modified Amino Acids. Adv. Biol. Regul. 2016, 60, 144–150. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, J.; Lin, S.; Deng, W.; Zhang, Y.; Xue, Y. PLMD: An Updated Data Resource of Protein Lysine Modifications. J. Genet. Genom. 2017, 44, 243–250. [Google Scholar] [CrossRef]
- Zhao, G.; Zhen, J.; Liu, X.; Guo, J.; Li, D.; Xie, J.; Xie, L. Protein Post-Translational Modification by Lysine Succinylation: Biochemistry, Biological Implications, and Therapeutic Opportunities. Genes Dis. 2023, 10, 1242–1262. [Google Scholar] [CrossRef]
- Kutuzova, G.D.; Ugarova, N.N.; Berezin, I.V. General Characteristics of the Changes in the Thermal Stability of Proteins and Enzymes After the Chemical Modification of Their Functional Groups. Russ. Chem. Rev. 1984, 53, 1078. [Google Scholar] [CrossRef]
- Zhou, C.; Dai, J.; Lu, H.; Chen, Z.; Guo, M.; He, Y.; Gao, K.; Ge, T.; Jin, J.; Wang, L.; et al. Succinylome Analysis Reveals the Involvement of Lysine Succinylation in the Extreme Resistance of Deinococcus Radiodurans. Proteomics 2019, 19, 1900158. [Google Scholar] [CrossRef]
- Sun, L.; Yao, Z.; Guo, Z.; Zhang, L.; Wang, Y.; Mao, R.; Lin, Y.; Fu, Y.; Lin, X. Comprehensive Analysis of the Lysine Acetylome in Aeromonas Hydrophila Reveals Cross-Talk between Lysine Acetylation and Succinylation in LuxS. Emerg. Microbes Infect. 2019, 8, 1229–1239. [Google Scholar] [CrossRef]
- Zeng, J.; Wu, L.; Chen, Q.; Wang, L.; Qiu, W.; Zheng, X.; Yin, X.; Liu, J.; Ren, Y.; Li, Y. Comprehensive Profiling of Protein Lysine Acetylation and Its Overlap with Lysine Succinylation in the Porphyromonas Gingivalis Fimbriated Strain ATCC 33277. Mol. Oral Microbiol. 2020, 35, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tan, M.; Xie, Z.; Dai, L.; Chen, Y.; Zhao, Y. Identification of Lysine Succinylation as a New Post-Translational Modification. Nat. Chem. Biol. 2011, 7, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Pang, H.; Chen, Y.; Zheng, H.; Li, W.; Ramanathan, S.; Hoare, R.; Monaghan, S.J.; Lin, X.; Jian, J. First Succinylome Profiling of Vibrio Alginolyticus Reveals Key Role of Lysine Succinylation in Cellular Metabolism and Virulence. Front. Cell. Infect. Microbiol. 2021, 10, 626574. [Google Scholar] [CrossRef]
- Sreedhar, A.; Wiese, E.K.; Hitosugi, T. Enzymatic and Metabolic Regulation of Lysine Succinylation. Genes Dis. 2020, 7, 166–171. [Google Scholar] [CrossRef]
- Chen, X.; Lei, W.; Meng, H.; Jiang, Y.; Zhang, S.; Chen, H.; Du, M.; Xue, X. Succinylation Modification Provides New Insights for the Treatment of Immunocompromised Individuals with Drug-Resistant Aspergillus Fumigatus Infection. Front. Immunol. 2023, 14, 1161642. [Google Scholar] [CrossRef] [PubMed]
- Stastna, M. Post-Translational Modifications of Proteins in Cardiovascular Diseases Examined by Proteomic Approaches. FEBS J. 2025, 292, 28–46. [Google Scholar] [CrossRef]
- Xia, J.; Liu, J.; Xu, F.; Zhou, H. Proteomic Profiling of Lysine Acetylation and Succinylation in Staphylococcus Aureus. Clin. Transl. Med. 2022, 12, e1058. [Google Scholar] [CrossRef]
- Adejor, J.; Tumukunde, E.; Li, G.; Lin, H.; Xie, R.; Wang, S. Impact of Lysine Succinylation on the Biology of Fungi. Curr. Issues Mol. Biol. 2024, 46, 1020–1046. [Google Scholar] [CrossRef]
- Dong, Y.; Li, P.; Li, P.; Chen, C. First Comprehensive Analysis of Lysine Succinylation in Paper Mulberry (Broussonetia Papyrifera). BMC Genom. 2021, 22, 255. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Defoirdt, T.; Ina-Salwany, M.Y.; Yusoff, F.M.; Shariff, M.; Ismail, S.I.; Natrah, I. Vibrio Parahaemolyticus and Vibrio Harveyi Causing Acute Hepatopancreatic Necrosis Disease (AHPND) in Penaeus Vannamei (Boone, 1931) Isolated from Malaysian Shrimp Ponds. Aquaculture 2019, 511, 734227. [Google Scholar] [CrossRef]
- Luna, G.M.; Bongiorni, L.; Gili, C.; Biavasco, F.; Danovaro, R. Vibrio Harveyi as a Causative Agent of the White Syndrome in Tropical Stony Corals. Environ. Microbiol. Rep. 2010, 2, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bu, L.; Jin, S.; Wang, X.; Zhao, Q.; Zhou, S.; Xu, Y. Outbreak of Vibriosis Caused by Vibrio Harveyi and Vibrio Alginolyticus in Farmed Seahorse Hippocampus Kuda in China. Aquaculture 2020, 523, 735168. [Google Scholar] [CrossRef]
- Triga, A.; Smyrli, M.; Katharios, P. Pathogenic and Opportunistic Vibrio Spp. Associated with Vibriosis Incidences in the Greek Aquaculture: The Role of Vibrio Harveyi as the Principal Cause of Vibriosis. Microorganisms 2023, 11, 1197. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xu, H.; Su, Y.; Liu, S.; Xu, L.; Guo, Z.; Wu, J.; Cheng, C.; Feng, J. Horizontal Gene Transfer Contributes to Virulence and Antibiotic Resistance of Vibrio Harveyi 345 Based on Complete Genome Sequence Analysis. BMC Genom. 2019, 20, 761. [Google Scholar] [CrossRef]
- Anetzberger, C.; Reiger, M.; Fekete, A.; Schell, U.; Stambrau, N.; Plener, L.; Kopka, J.; Schmitt-Kopplin, P.; Hilbi, H.; Jung, K. Autoinducers Act as Biological Timers in Vibrio Harveyi. PLoS ONE 2012, 7, e48310. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, J.; Todd, J.D.; Zhang, X.-H.; Zhang, Y. Quorum Sensing Regulates the Production of Methanethiol in Vibrio Harveyi. Microorganisms 2024, 12, 35. [Google Scholar] [CrossRef]
- Lin, H.-N.; Huang, X.-H.; Miao, X.-J.; Hu, W.-L.; Lou, Y.-L.; Xie, D.-L. Quorum Sensing: An Emerging Role for Vibrio Infection and Host Defense. Infect. Microbes Dis. 2024, 6, 47. [Google Scholar] [CrossRef]
- Eickhoff, M.J.; Fei, C.; Cong, J.-P.; Bassler, B.L. LuxT Is a Global Regulator of Low-Cell-Density Behaviors, Including Type III Secretion, Siderophore Production, and Aerolysin Production, in Vibrio Harveyi. mBio 2022, 13, e03621. [Google Scholar] [CrossRef]
- Lorenz, N.; Shin, J.Y.; Jung, K. Activity, Abundance, and Localization of Quorum Sensing Receptors in Vibrio Harveyi. Front. Microbiol. 2017, 8, 634. [Google Scholar] [CrossRef]
- Morot, A.; Lambert, C.; Bidault, A.; Dufour, A.; Rodrigues, S.; Delavat, F.; Paillard, C. Vibrio Harveyi Uses Both Type III Secretion System and Quorum Sensing for the Colonization of the European Abalone. Fish Shellfish Immunol. 2025, 157, 110103. [Google Scholar] [CrossRef] [PubMed]
- McRose, D.L.; Baars, O.; Seyedsayamdost, M.R.; Morel, F.M. MQuorum Sensing and Iron Regulate a Two-for-One Siderophore Gene Cluster in Vibrio Harveyi. Proc. Natl. Acad. Sci. USA 2018, 115, 7581–7586. [Google Scholar] [CrossRef]
- Xie, L.; Liu, W.; Li, Q.; Chen, S.; Xu, M.; Huang, Q.; Zeng, J.; Zhou, M.; Xie, J. First Succinyl-Proteome Profiling of Extensively Drug-Resistant Mycobacterium Tuberculosis Revealed Involvement of Succinylation in Cellular Physiology. J. Proteome Res. 2015, 14, 107–119. [Google Scholar] [CrossRef]
- Colak, G.; Xie, Z.; Zhu, A.Y.; Dai, L.; Lu, Z.; Zhang, Y.; Wan, X.; Chen, Y.; Cha, Y.H.; Lin, H.; et al. Identification of Lysine Succinylation Substrates and the Succinylation Regulatory Enzyme CobB in Escherichia Coli*. Mol. Cell. Proteom. 2013, 12, 3509–3520. [Google Scholar] [CrossRef]
- Wu, L.; Gong, T.; Zhou, X.; Zeng, J.; Huang, R.; Wu, Y.; Li, Y. Global Analysis of Lysine Succinylome in the Periodontal Pathogen Porphyromonas Gingivalis. Mol. Oral Microbiol. 2019, 34, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, R.; Li, C.; Li, W.; Ye, Z. Global Analysis of Protein Lysine Succinylation Profiles and Their Overlap with Lysine Acetylation in the Marine Bacterium Vibrio Parahemolyticus. J. Proteome Res. 2015, 14, 4309–4318. [Google Scholar] [CrossRef]
- Ye, T.; Wang, D.; Sun, Y.; Xie, S.; Liu, T.; Tian, N.; Tan, M.; Xu, J.-Y. Characterization of Acidic Lysine Acylations in Mycobacteria. Front. Microbiol. 2024, 15, 1503184. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Zhang, L.; Cai, Q.; Lin, Y.; Lin, L.; Lin, X. Proteomics Analysis Reveals the Effect of Aeromonas Hydrophila Sirtuin CobB on Biological Functions. J. Proteom. 2020, 225, 103848. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Fingleton, C.; Zeden, M.S.; Bueno, E.; Gallagher, L.A.; Shinde, D.; Ahn, J.; Olson, H.M.; Fillmore, T.L.; Adkins, J.N.; et al. Accumulation of Succinyl Coenzyme A Perturbs the Methicillin-Resistant Staphylococcus Aureus (MRSA) Succinylome and Is Associated with Increased Susceptibility to Beta-Lactam Antibiotics. mBio 2021, 12, e0053021. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, L.; Song, H.; Liao, J.; Lin, L.; Jiang, W.; Wu, X.; Wang, G. Acetylome and Succinylome Profiling of Edwardsiella Tarda Reveals Key Roles of Both Lysine Acylations in Bacterial Antibiotic Resistance. Antibiotics 2022, 11, 841. [Google Scholar] [CrossRef]
- Won, K.M.; Park, S.I. Pathogenicity of Vibrio Harveyi to Cultured Marine Fishes in Korea. Aquaculture 2008, 285, 8–13. [Google Scholar] [CrossRef]
- Fu, S.; Ni, P.; Yang, Q.; Hu, H.; Wang, Q.; Ye, S.; Liu, Y. Delineating the Key Virulence Factors and Intraspecies Divergence of Vibrio Harveyi via Whole-Genome Sequencing. Can. J. Microbiol. 2021, 67, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.; Ni, B.; Laganenka, L.; Sourjik, V. Multiple Functions of Flagellar Motility and Chemotaxis in Bacterial Physiology. FEMS Microbiol. Rev. 2021, 45, fuab038. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.D.; Bagdasarian, M.; Hol, W.G.J.; Sandkvist, M. In Vivo Cross-Linking of EpsG to EpsL Suggests a Role for EpsL as an ATPase-Pseudopilin Coupling Protein in the Type II Secretion System of Vibrio Cholerae. Mol. Microbiol. 2011, 79, 786–798. [Google Scholar] [CrossRef][Green Version]
- Yao, Z.; Guo, Z.; Wang, Y.; Li, W.; Fu, Y.; Lin, Y.; Lin, W.; Lin, X. Integrated Succinylome and Metabolome Profiling Reveals Crucial Role of S-Ribosylhomocysteine Lyase in Quorum Sensing and Metabolism of Aeromonas Hydrophila*[S]. Mol. Cell. Proteom. 2019, 18, 200–215. [Google Scholar] [CrossRef]
- Huang, Y.; Jian, J.; Lu, Y.; Cai, S.; Wang, B.; Tang, J.; Pang, H.; Ding, Y.; Wu, Z. Draft Genome Sequence of the Fish Pathogen Vibrio Harveyi Strain ZJ0603. J. Bacteriol. 2012, 194, 6644–6645. [Google Scholar] [CrossRef]
- Medina, A.; Mancera, J.M.; Martínez-Manzanares, E.; Moriñigo, M.A.; Arijo, S. Identification of Vibrio Harveyi Proteins Involved in the Specific Immune Response of Senegalese Sole (Solea Senegalensis, Kaup). Fish Shellfish Immunol. 2015, 47, 377–380. [Google Scholar] [CrossRef]
- Nurhafizah, W.W.I.; Lee, K.L.; Laith A, A.R.; Nadirah, M.; Danish-Daniel, M.; Zainathan, S.C.; Najiah, M. Virulence Properties and Pathogenicity of Multidrug-Resistant Vibrio Harveyi Associated with Luminescent Vibriosis in Pacific White Shrimp, Penaeus Vannamei. J. Invertebr. Pathol. 2021, 186, 107594. [Google Scholar] [CrossRef]
- Umezawa, K.; Tsumoto, H.; Kawakami, K.; Miura, Y. A Chemical Probe for Proteomic Analysis and Visualization of Intracellular Localization of Lysine-Succinylated Proteins. Analyst 2023, 148, 95–104. [Google Scholar] [CrossRef]
- He, D.; Wang, Q.; Li, M.; Damaris, R.N.; Yi, X.; Cheng, Z.; Yang, P. Global Proteome Analyses of Lysine Acetylation and Succinylation Reveal the Widespread Involvement of Both Modification in Metabolism in the Embryo of Germinating Rice Seed. J. Proteome Res. 2016, 15, 879–890. [Google Scholar] [CrossRef]
- Huang, H.-J.; Ye, Z.-X.; Lu, G.; Zhang, C.-X.; Chen, J.-P.; Li, J.-M. Identification of Salivary Proteins in the Whitefly Bemisia Tabaci by Transcriptomic and LC–MS/MS Analyses. Insect Sci. 2021, 28, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.-S.; Sacharz, J.; Leeming, M.G.; Nie, S.; Varshney, S.; Scott, N.E.; Williamson, N.A. Getting More out of FLAG-Tag Co-Immunoprecipitation Mass Spectrometry Experiments Using FAIMS. J. Proteom. 2022, 254, 104473. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Su, J.; Moradian-Oldak, J. Co-Immunoprecipitation Reveals Interactions Between Amelogenin and Ameloblastin via Their Self-Assembly Domains. Front. Physiol. 2020, 11, 622086. [Google Scholar] [CrossRef] [PubMed]
- Middleton-Davis, F.; Davis, A.; Middleton, K. Method for Detecting Acetylated PD-L1 in Cell Lysates by Immunoprecipitation and Western Blot Analysis. PLoS ONE 2022, 17, e0268887. [Google Scholar] [CrossRef]
- Cheng, A.; Grant, C.E.; Noble, W.S.; Bailey, T.L. MoMo: Discovery of Statistically Significant Post-Translational Modification Motifs. Bioinformatics 2019, 35, 2774–2782. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]


| Protein | Gene Name | Ksuc Site | VF Class |
|---|---|---|---|
| D0WSF4 | cheX | K13 | Bacterial chemotaxis |
| D0WUX7 | VMC_09770 | K273, K46, K44, K229 | Bacterial chemotaxis |
| D0WUX8 | cheV | K39, K48 | Bacterial chemotaxis |
| D0WVM2 | cheW | K10 | Bacterial chemotaxis |
| D0WVP7 | fliG | K298 | Bacterial chemotaxis |
| D0WSX5 | epsG | K96 | Type II secretion system |
| D0WU68 | yajC | K48, K56, K69 | Quorum sensing |
| D0WXB6 | luxS | K23, K29 | Quorum sensing |
| D0X2T3 | luxO | K77, K193, K270, K277, K293 | Quorum sensing |
| D0WYV3 | secB | K43 | Quorum sensing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhou, P.; Zhang, W.; Zhang, Y.; Guo, H.; Wei, Y.; Wen, X.; Jian, J.; Wang, N.; Pang, H. A Systematic Study of Lysine Succinylation in the Pathogenic Bacterium Vibrio harveyi in Aquatic Animals. Molecules 2025, 30, 2418. https://doi.org/10.3390/molecules30112418
Yang S, Zhou P, Zhang W, Zhang Y, Guo H, Wei Y, Wen X, Jian J, Wang N, Pang H. A Systematic Study of Lysine Succinylation in the Pathogenic Bacterium Vibrio harveyi in Aquatic Animals. Molecules. 2025; 30(11):2418. https://doi.org/10.3390/molecules30112418
Chicago/Turabian StyleYang, Shuai, Peng Zhou, Weijie Zhang, Yujia Zhang, Haiwei Guo, Yingzhu Wei, Xiaoxin Wen, Jichang Jian, Na Wang, and Huanying Pang. 2025. "A Systematic Study of Lysine Succinylation in the Pathogenic Bacterium Vibrio harveyi in Aquatic Animals" Molecules 30, no. 11: 2418. https://doi.org/10.3390/molecules30112418
APA StyleYang, S., Zhou, P., Zhang, W., Zhang, Y., Guo, H., Wei, Y., Wen, X., Jian, J., Wang, N., & Pang, H. (2025). A Systematic Study of Lysine Succinylation in the Pathogenic Bacterium Vibrio harveyi in Aquatic Animals. Molecules, 30(11), 2418. https://doi.org/10.3390/molecules30112418






