Tailored Carbon Nanocomposites for Efficient CO2 Capture
Abstract
1. Introduction
2. Results and Discussion
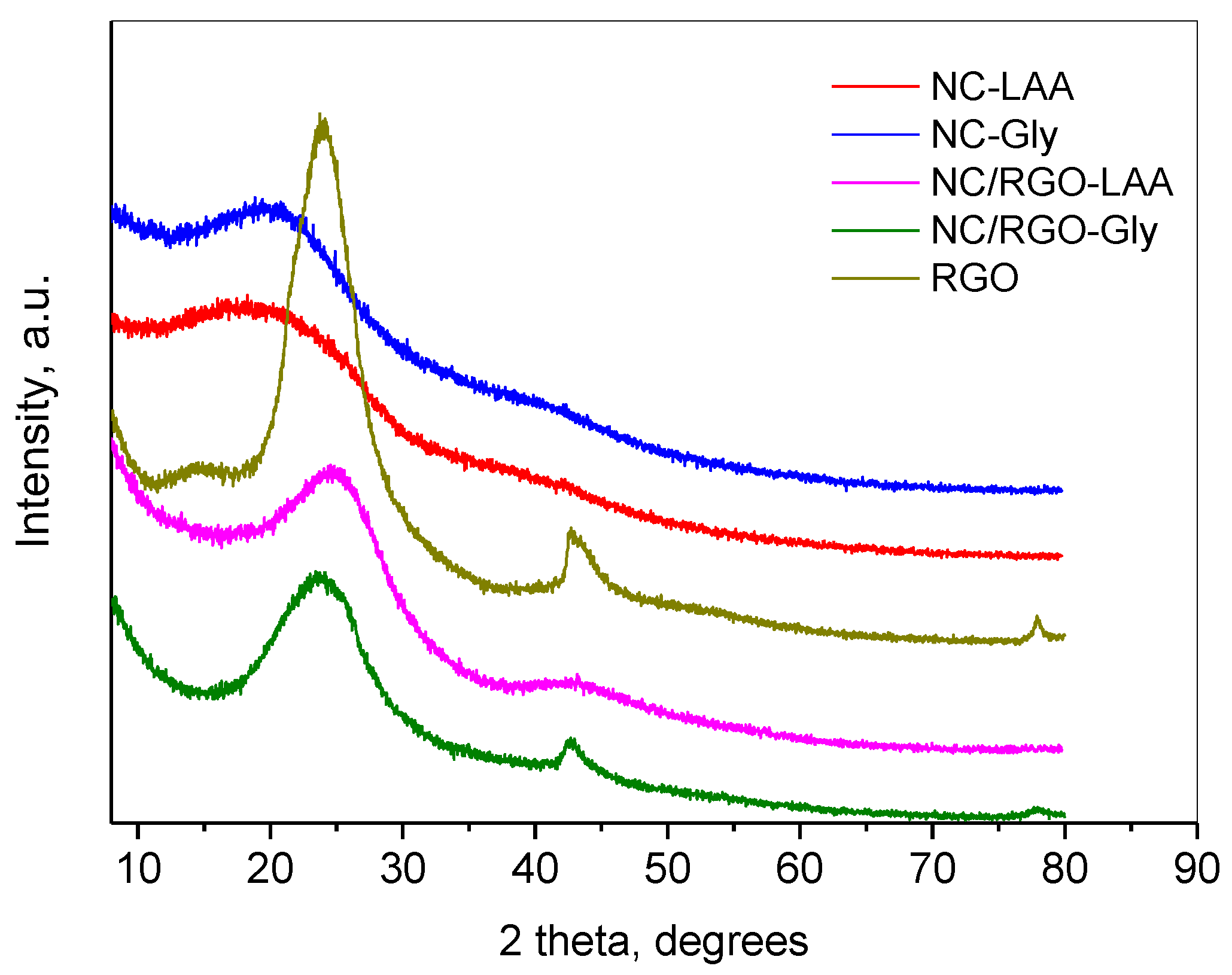
2.1. XRD
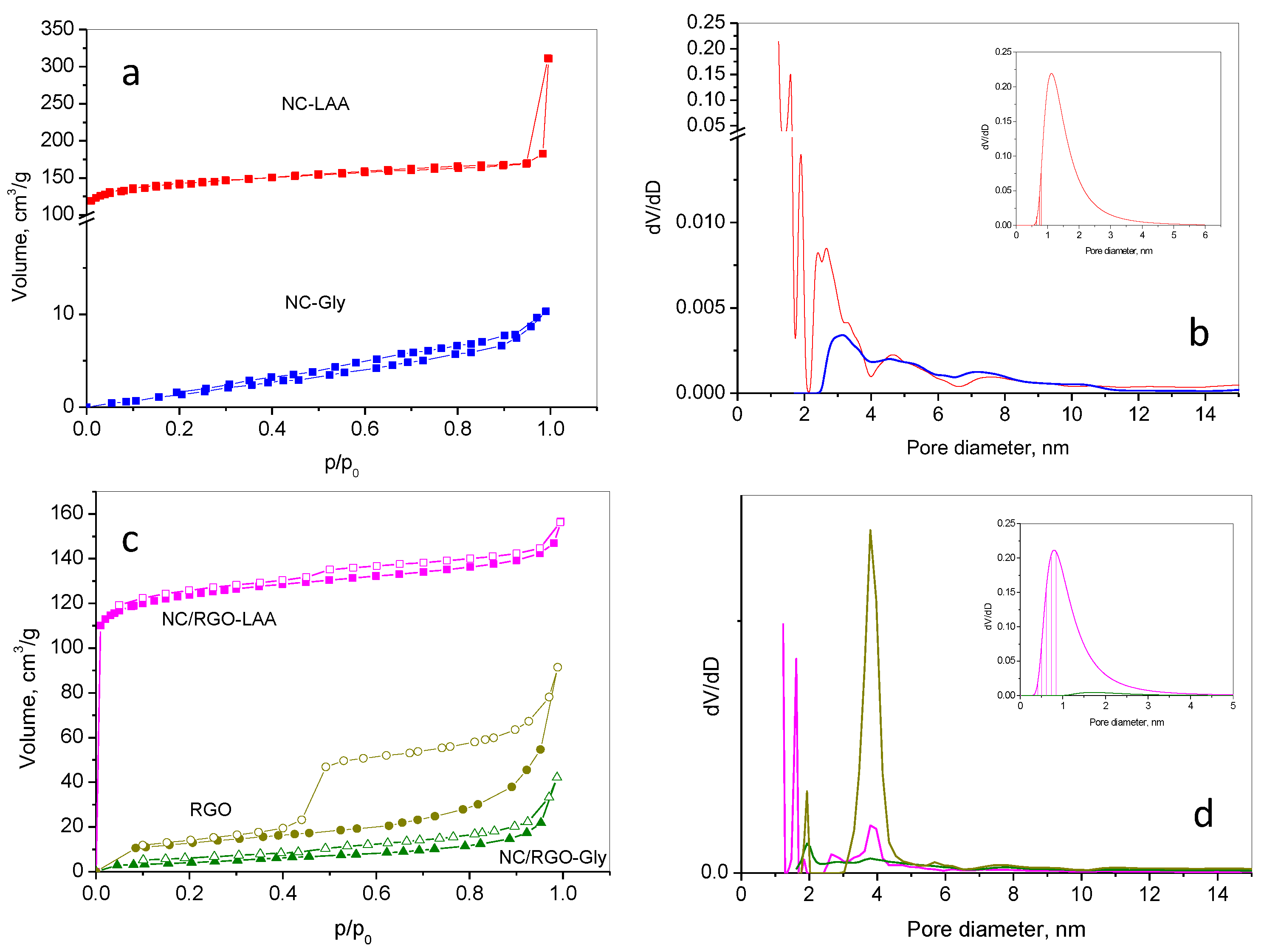
2.2. Nitrogen Adsorption
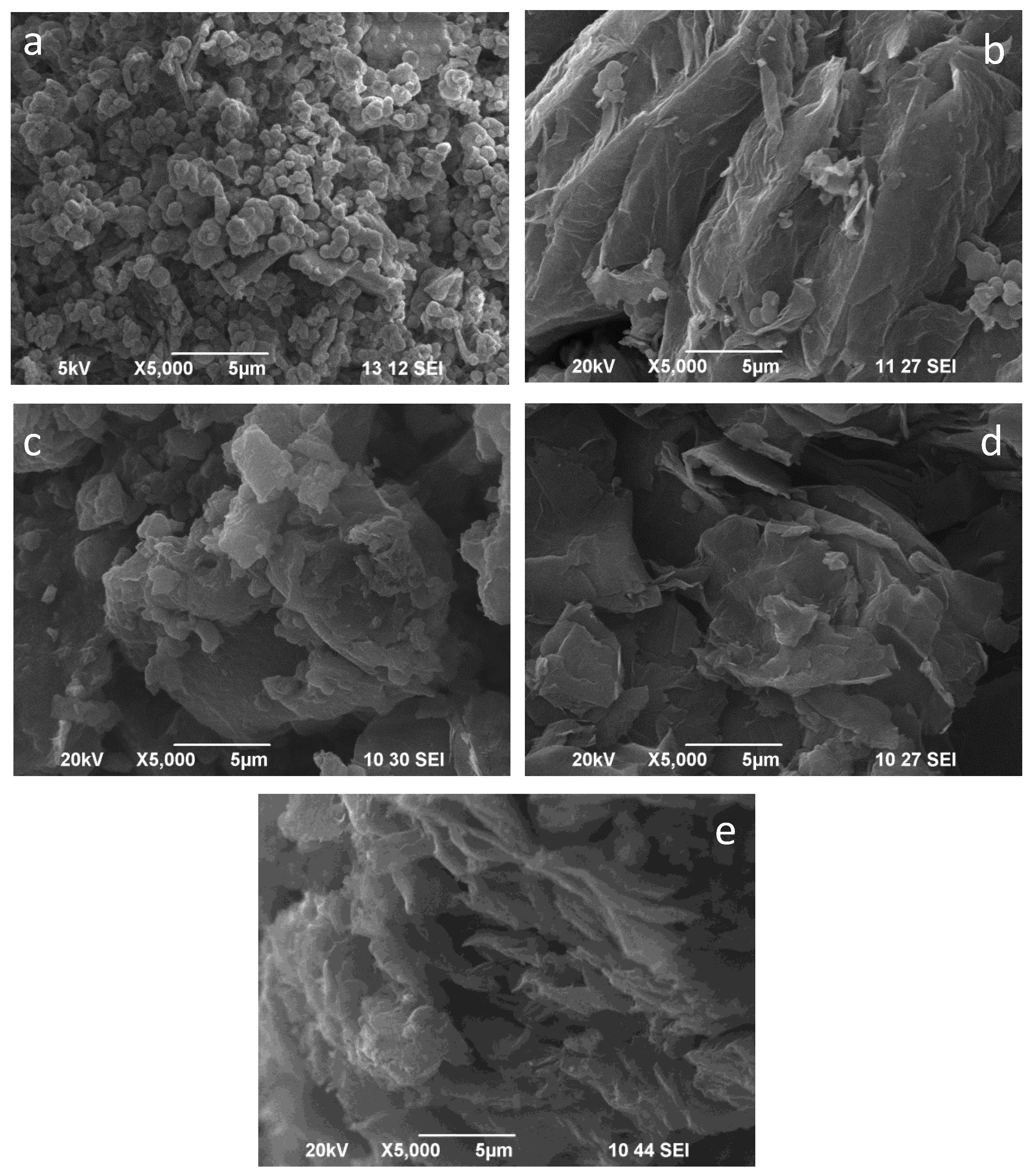
2.3. SEM
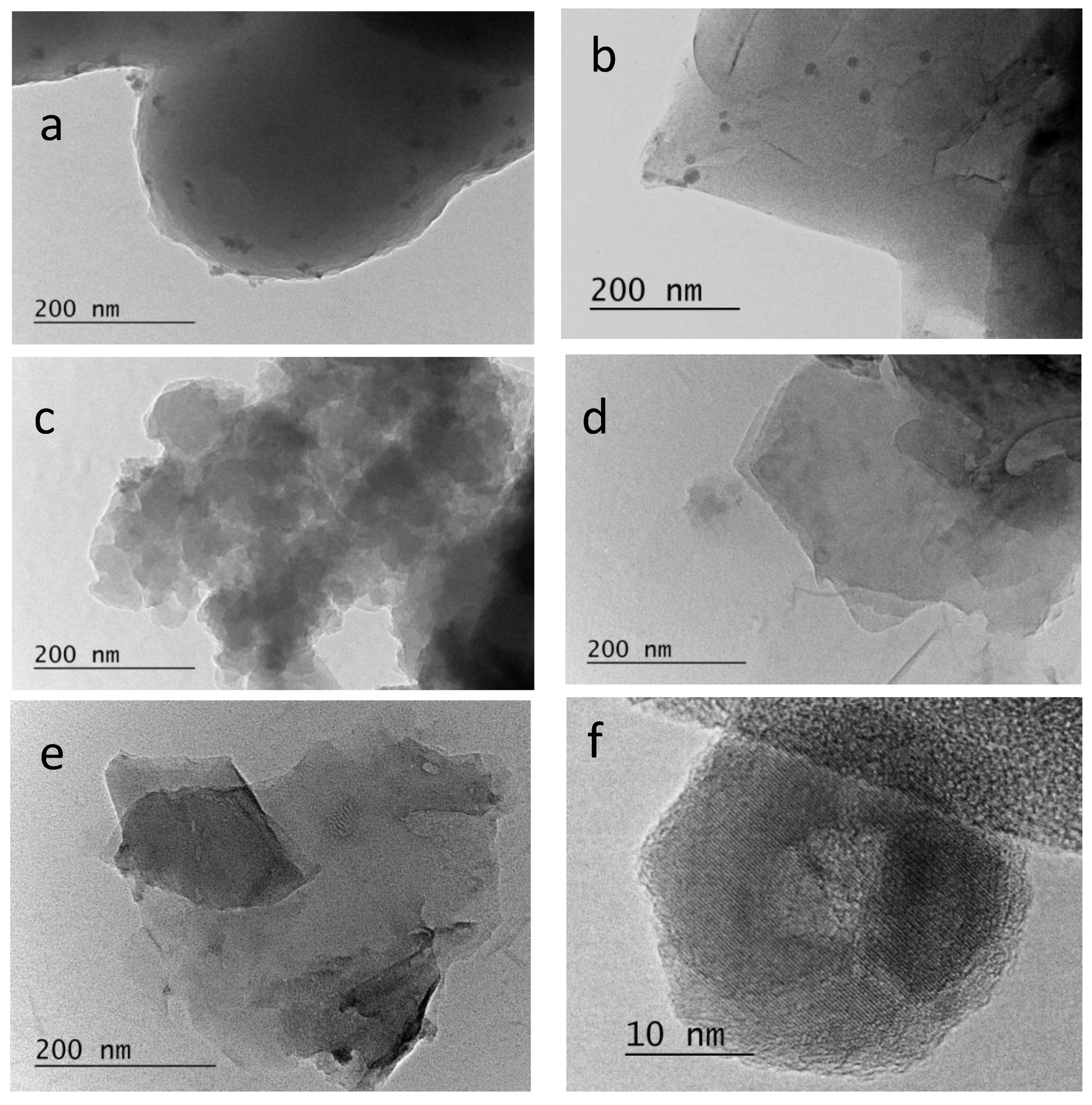
2.4. TEM
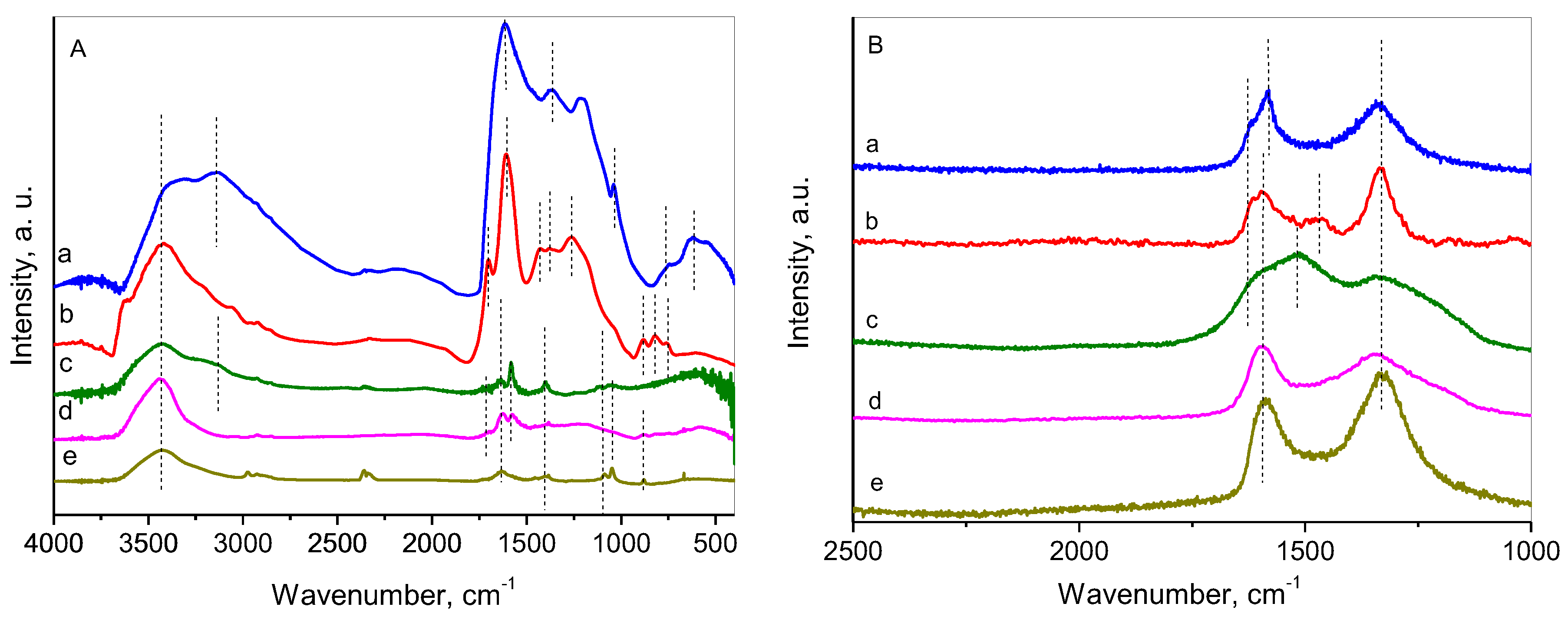
2.5. FTIR and Raman
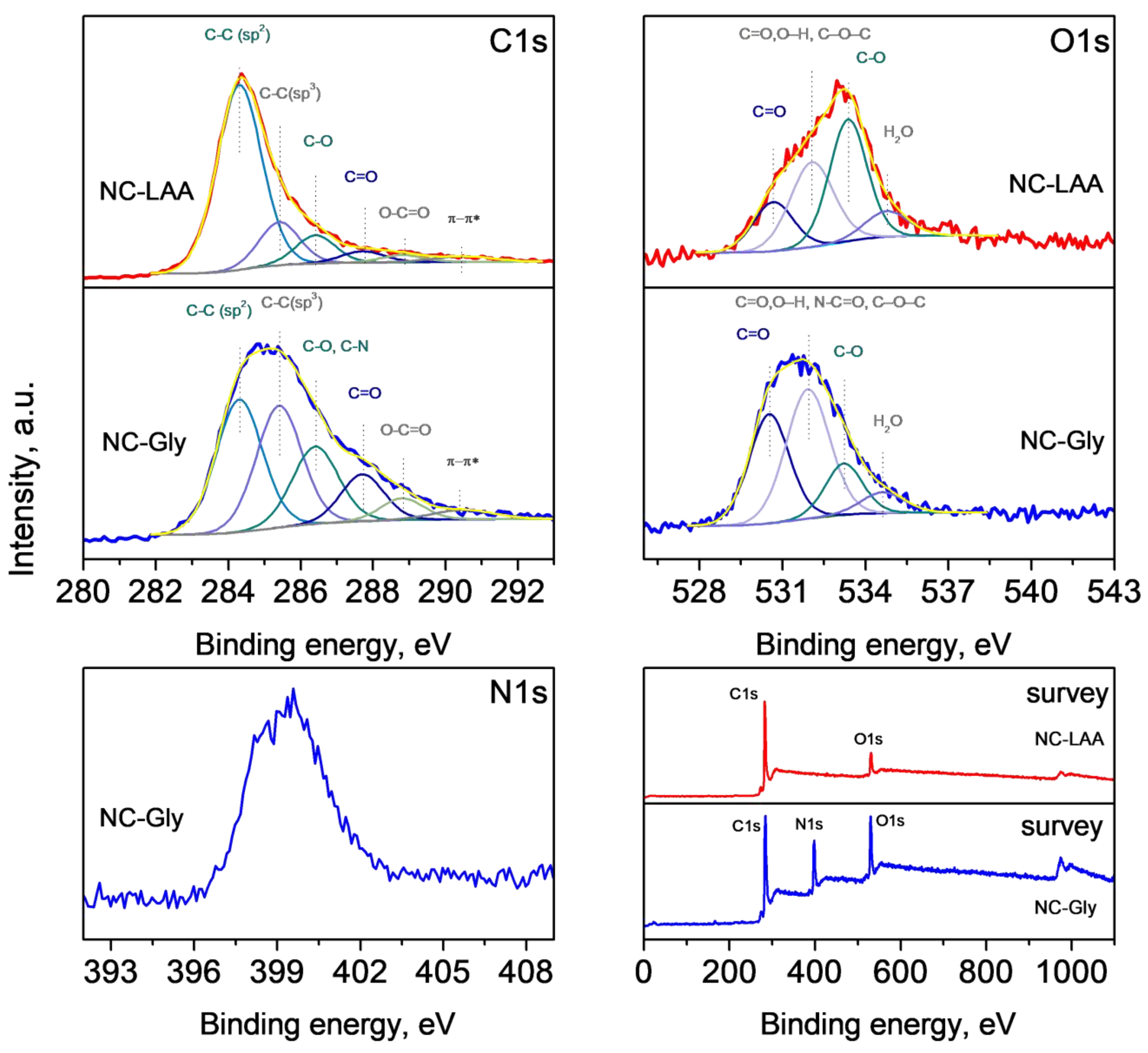
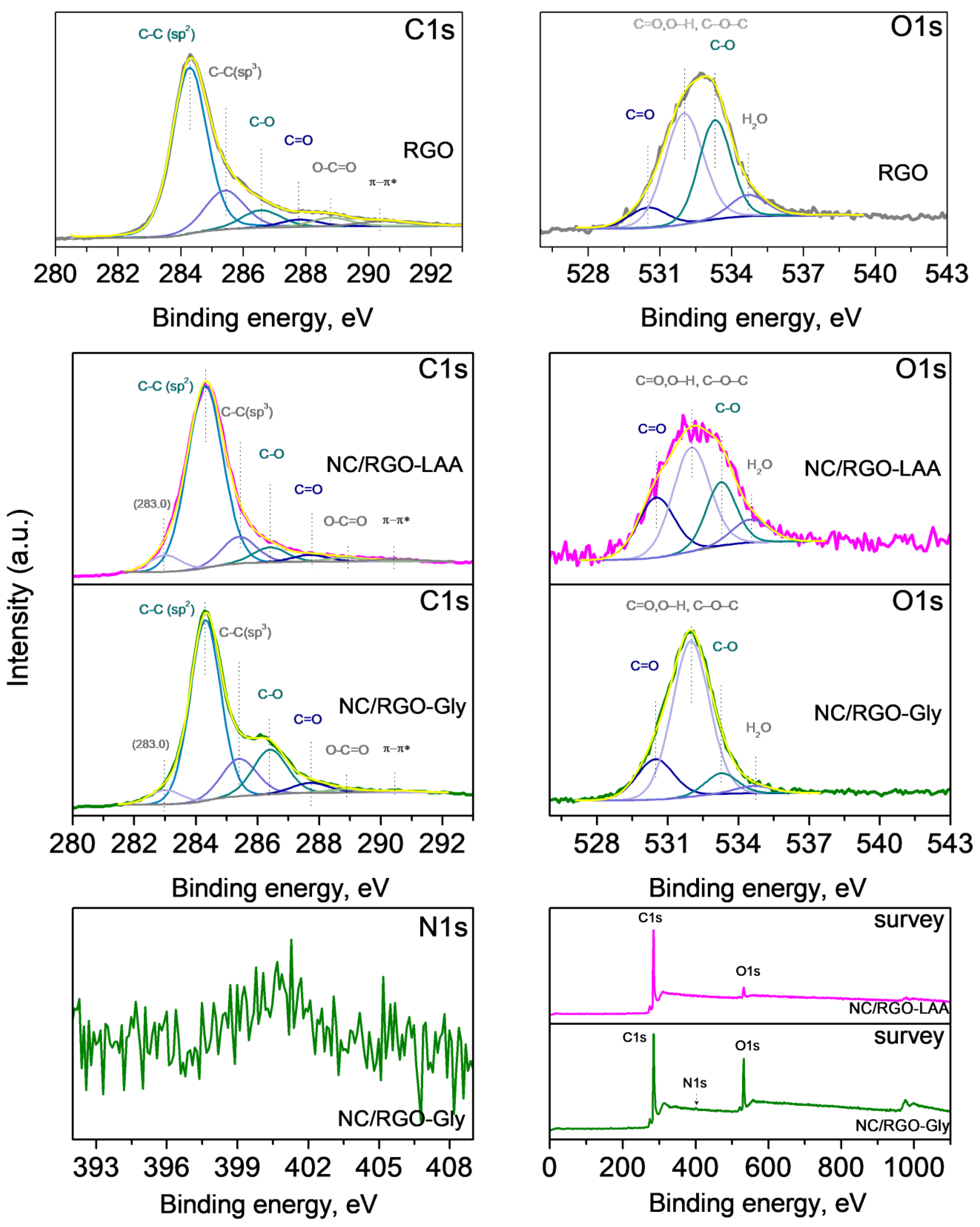
2.6. XPS
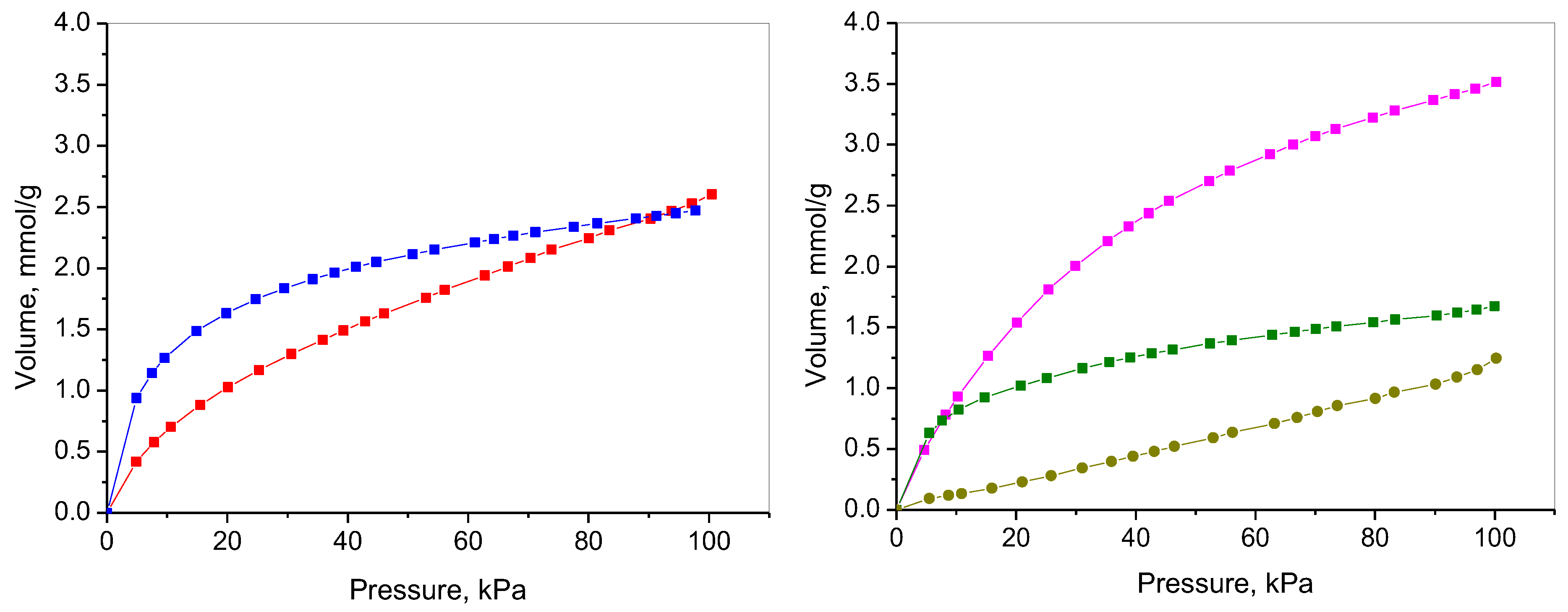
2.7. CO2 Adsorption
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Composites
3.3. Synthesis of Nanocarbons
3.4. Synthesis of RGO
3.5. Characterization
3.6. CO2 Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, M.; Frame, D.; Huntingford, C.; Jones, C.D.; Lowe, J.A.; Meinshausen, M.; Meinshausen, N. Warming caused by cumulative carbon emissions towards the trillionth tonne. Nature 2009, 458, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.D.; Gillett, N.P.; Stott, P.A. The proportionality of global warming to cumulative carbon emissions. Nature 2009, 459, 829–832. [Google Scholar] [CrossRef]
- Hack, J.; Maeda, N.; Meier, D. Review on CO2 capture using amine-functionalized materials. ACS Omega 2022, 7, 39520–39530. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y.; Serna-Guerero, R. Flue gas treatment via CO2 adsorption. Chem. Eng. J. 2011, 171, 760–774. [Google Scholar] [CrossRef]
- Maina, J.W.; Pozo-Gonzalo, C.; Kong, L.; Schütz, J.; Hill, M.; Dumée, L.F. Metal organic framework based catalysts for CO2 conversion. Mater. Horiz. 2017, 4, 345–361. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Karadas, F.; Park, J.; Deniz, E.; Stucky, G.D.; Jung, Y.; Atilhan, M.; Yavuz, C.T. Amidoximes: Promising candidates for CO2 capture. Energy Environ. Sci. 2011, 4, 4528–4531. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, C.; Zhang, Z.; Usadi, A.; Calabro, D.; Baugh, L.; Zhao, D. Evaluation of Schiff-Base Covalent Organic Frameworks for CO2 Capture: Structure–Performance Relationships, Stability, and Performance under Wet Conditions. ACS Sustain. Chem. Eng. 2022, 10, 332–341. [Google Scholar] [CrossRef]
- Zentou, H.; Hoque, B.; Abdalla, M.A.; Saber, A.F.; Abdelaziz, O.Y.; Aliyu, M.; Alkhedhair, A.M.; Alabduly, A.J.; Abdelnaby, M.M. Recent advances and challenges in solid sorbents for CO2 capture. Carbon Capture Sci. Technol. 2025, 15, 100386. [Google Scholar] [CrossRef]
- Patel, H.A.; Karadas, F.; Canlier, A.; Park, J.; Deniz, E.; Jung, Y.; Atilhan, M.; Yavuz, C.T. High capacity carbon dioxide adsorption by inexpensive covalent organic polymers. J. Mater. Chem. 2012, 22, 8431–8437. [Google Scholar] [CrossRef]
- Xu, S.; Luo, Y.; Tan, B. Recent Development of Hypercrosslinked Microporous Organic Polymers. Macromol. Rapid Commun. 2013, 34, 471–484. [Google Scholar] [CrossRef]
- Karadas, F.; Yavuz, C.T.; Zulfiqar, S.; Aparicio, S.; Stucky, G.D.; Atilhan, M. CO2 Adsorption Studies on Hydroxy Metal Carbonates M(CO3)x(OH)y (M = Zn, Zn−Mg, Mg, Mg−Cu, Cu, Ni, and Pb) at High Pressures up to 175 bar. Langmuir 2011, 27, 10642–10647. [Google Scholar] [CrossRef] [PubMed]
- Andonova, S.; Akbari, S.S.; Karadaş, F.; Spassova, I.; Paneva, D.; Hadjiivanov, K. Structure and properties of KNi–hexacyanoferrate Prussian Blue Analogues for efficient CO2 capture: Host–guest interaction chemistry and dynamics of CO2 adsorption. J. CO2 Util. 2021, 50, 101593. [Google Scholar] [CrossRef]
- Franchi, R.S.; Harlick, P.J.E.; Sayari, A. Applications of pore expanded mesoporous silica. 2. Development of a high-capacity, water-tolerant adsorbent for CO2. Ind. Eng. Chem. Res. 2005, 44, 8007–8013. [Google Scholar] [CrossRef]
- Bae, T.-H.; Hudson, M.R.; Mason, J.A.; Queen, W.L.; Dutton, J.J.; Sumida, K.; Micklash, K.J.; Kaye, S.S.; Brown, C.M.; Long, J.R. Evaluation of cation-exchanged zeolite adsorbents for post-combustion carbon dioxide capture. Energy Environ. Sci. 2013, 6, 128–138. [Google Scholar] [CrossRef]
- Khandaker, T.; Hossain, M.S.; Dhar, P.K.; Rahman, M.S.; Hossain, M.A.; Ahmed, M.B. Efficacies of Carbon-Based Adsorbents for Carbon Dioxide Capture. Processes 2020, 8, 654. [Google Scholar] [CrossRef]
- Mehra, P.; Paul, A. CO2 Adsorption on Nitrogen-Rich Porous Carbon Materials Synthesized via a Soft-Templating Approach. ACS Omega 2022, 7, 34538–34546. [Google Scholar] [CrossRef]
- Li, M.; Huang, K.; Schott, J.A.; Wu, Z.; Dai, S. Effect of Metal Oxides Modification on CO2 Adsorption Performance over Mesoporous Carbon. Microporous Mesoporous Mater. 2017, 249, 34–41. [Google Scholar] [CrossRef]
- Jin, X.; Li, Z.-Q.; Ge, J.; Zhu, L.; Liu, C.; Li, Q.; Liu, J.; Yin, C.; Su, G. Rapid Synthesis of MOF CaBTC Using an Ultrasonic Irradiation Method and Its Derivative Materials for CO2 Capture. New J. Chem. 2025. accepted manuscript. [Google Scholar] [CrossRef]
- Kirren, P.; Barka, L.; Rahmani, S.; Bondon, N.; Donzel, N.; Trens, P.; Bessière, A.; Raehm, L.; Charnay, C.; Durand, J.-O. Periodic Mesoporous Organosilica Nanoparticles for CO2 Adsorption at Standard Temperature and Pressure. Molecules 2022, 27, 4245. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Chen, R.; Wang, C.; Li, H.; Wang, S. Heteroatom-Doped Nanoporous Carbon Derived from MOF-5 for CO2 Capture. Appl. Surf. Sci. 2018, 435, 494–502. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Gao, Q.; Wei, W. High-Performance CO2 Adsorption over Functionalized Porous Carbon Nanomaterials. ACS Appl. Nano Mater. 2025, 8, 7190–7199. [Google Scholar] [CrossRef]
- Gao, H.; Li, Q.; Ren, S. Progress on CO2 Capture by Porous Organic Polymers. Curr. Opin. Green Sustain. Chem. 2019, 16, 55–63. [Google Scholar] [CrossRef]
- Dani, A.; Crocellà, V.; Magistris, C.; Santoro, V.; Yuana, J.; Bordiga, S. CO2 Adsorption on Porous Polymers Containing Polar Groups: Structure–Property Relationship. J. Mater. Chem. A 2017, 5, 372–383. [Google Scholar] [CrossRef]
- Velikova, N.; Spassova, I. Amine Functionalized Mesoporous Hybrid Materials: Influence of KCl and Xylene on the Textural Characteristics and CO2 Sorption. J. Sol-Gel Sci. Technol. 2019, 91, 374–384. [Google Scholar] [CrossRef]
- Velikova, N.; Spassova, I. Bifunctional Mesoporous Hybrid Sol–Gel Prepared Silicas for CO2 Adsorption. J. Sol-Gel Sci. Technol. 2021, 100, 326–340. [Google Scholar] [CrossRef]
- Manyà, J.J.; González, B.; Azuara, M.; Arner, G. Ultra-Microporous Adsorbents Prepared from Vine Shoots-Derived Biochar with High CO2 Uptake and CO2/N2 Selectivity. Chem. Eng. J. 2018, 345, 631–639. [Google Scholar] [CrossRef]
- Cheung, O.; Bacsik, Z.; Fil, N.; Krokidas, P.; Wardecki, D.; Hedin, N. Highly Efficient Carbon Dioxide Adsorption by Porous Carbon Materials Derived from Polymer Blends. ACS Omega 2020, 5, 25371–25380. [Google Scholar] [CrossRef]
- Li, S.; Jia, S.; Nagasaka, T.; Bai, H.; Yang, L. CO2 Adsorption Properties of Amine-Modified Zeolites Synthesized Using Different Types of Solid Waste. Sustainability 2023, 15, 10144. [Google Scholar] [CrossRef]
- Sun, M.; Gu, Q.; Hanif, A.; Wang, T.; Shang, J. Transition Metal Cation-Exchanged SSZ-13 Zeolites for CO2 Capture and Separation from N2. Chem. Eng. J. 2019, 370, 1450–1458. [Google Scholar] [CrossRef]
- Singh, G.; Kim, Y.; Lakhi, K.S.; Joseph, S.; Srivastava, P.; Naidu, R.; Vinu, A. Heteroatom Functionalized Activated Porous Biocarbons and Their Excellent Performance for CO2 Capture at High Pressure. J. Mater. Chem. A 2017, 5, 21196–21204. [Google Scholar] [CrossRef]
- Yang, B.; Hu, H.; Yu, Q.; Zhang, X.; Li, Z.; Lei, L. Pretreated multiwalled carbon nanotube adsorbents with amine-grafting for removal of carbon dioxide in confined spaces. RSC Adv. 2014, 4, 56224–56234. [Google Scholar] [CrossRef]
- Bhanja, P.; Das, S.K.; Patra, A.K.; Bhaumik, A. Functionalized graphene oxide as an efficient adsorbent for CO2 capture and support for heterogeneous catalysis. RSC Adv. 2016, 6, 72055–72068. [Google Scholar] [CrossRef]
- Wen, C.; Qiu, Z.; Zhao, G.; Wei, X.; Zhu, Z.; Wang, Y.; Cui, P.; Zhong, L. Preparation and Characterization of UiO-66-(OH)2/MWCNTs Composites for CO2/N2 Adsorption Separation. Fuel 2024, 373, 132292. [Google Scholar] [CrossRef]
- Gautam, S.; Sahoo, S. Experimental Investigation on Different Activated Carbons as Adsorbents for CO2 Capture. Therm. Sci. Eng. Prog. 2022, 33, 101339. [Google Scholar] [CrossRef]
- Xie, L.; Li, Q.; Demir, M.; Yu, Q.; Hu, X.; Jiang, Z.; Wang, L. Lotus Seed Pot-Derived Nitrogen Enriched Porous Carbon for CO2 Capture Application. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130226. [Google Scholar] [CrossRef]
- Chew, T.L.; Ahmad, A.L.; Bhatia, S. Ordered mesoporous silica (OMS) as an adsorbent and membrane for separation of carbon dioxide (CO2). Adv. Colloid Interface Sci. 2010, 153, 43–57. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; Colin McCarthy, M.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Dos Santos, M.C.; Maynart, M.C.; Aveiro, L.R.; da Paz, E.C.; dos Santos Pinheiro, V. Carbon-Based Materials: Recent Advances, Challenges, and Perspectives. In Reference Module in Materials Science and Materials Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 1–12. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Stein, A.; Wang, Z.; Fierke, M.A. Functionalization of porous carbon materials with designed pore architecture. Adv. Mater. 2009, 21, 265–293. [Google Scholar] [CrossRef]
- Kundu, S.; Khandaker, T.; Anik, M.A.M.; Hasan, M.K.; Dhar, P.K.; Dutta, S.K.; Latif, M.A.; Hossain, M.S. A comprehensive review of enhanced CO2 capture using activated carbon derived from biomass feedstock. RSC Adv. 2024, 14, 29693. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ma, F.; Wu, G.; Ma, D.; Geng, W.; Wan, J. Facile self-templating large scale preparation of biomass-derived 3D hierarchical porous carbon for advanced supercapacitors. J. Mater. Chem. A 2015, 3, 18154–18162. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Pham, V.P.; Jang, H.S.; Whang, D.; Choi, J.Y. Direct growth of graphene on rigid and flexible substrates: Progress, applications, and challenges. Chem. Soc. Rev. 2017, 46, 6276–6300. [Google Scholar] [CrossRef]
- Cigala, R.M.; De Luca, G.; Ielo, I.; Crea, F. Biopolymeric Nanocomposites for CO2 Capture. Polymers 2024, 16, 1063. [Google Scholar] [CrossRef]
- Barker-Rothschild, D.; Chen, J.; Wan, Z.; Renneckar, S.; Burgert, I.; Ding, Y.; Lu, Y.; Rojas, O.J. Lignin-based porous carbon adsorbents for CO2 capture. Chem. Soc. Rev. 2025, 54, 623. [Google Scholar] [CrossRef]
- Verma, S.K.; Tripathi, P.; Bhatnagar, A. Carbon Nanotubes for CO2 Capture and Conversion. In Nanomaterials for Carbon Dioxide Capture and Conversion Technologies; Mazari, S.A., Mubarak, N.M., Tripathi, M., Eds.; Micro and Nano Technologies Series; Elsevier: Amsterdam, The Netherlands, 2023; pp. 245–260. [Google Scholar] [CrossRef]
- Broud, M.T.; Samandari, M.; Yu, L.; Harper, D.P.; Keffer, D.J. Selective Carbon Dioxide Binding on Carbon Quantum Dots. J. Phys. Chem. C 2023, 127, 13639–13650. [Google Scholar] [CrossRef]
- Jung, S.; Park, Y.; Kwon, E.E. Strategic use of biochar for CO2 capture and sequestration. J. CO2 Util. 2019, 32, 128–139. [Google Scholar] [CrossRef]
- Ekhlasi, L.; Younesi, H.; Rashidi, A.; Bahramifar, N. Populus wood biomass-derived graphene for high CO2 capture at atmospheric pressure and estimated cost of production. Process Saf. Environ. Prot. 2018, 113, 97–108. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, K.; Yang, Z.; Bauer, R.A.; Hong, N.; Verweij, H. Nanometer-thick supported graphene oxide membrane for CO2 capture. ACS Appl. Nano Mater. 2020, 3, 6654–6663. [Google Scholar] [CrossRef]
- Ai, N.; Lou, S.; Lou, F.; Xu, C.; Wang, Q.; Zeng, G. Facile synthesis of macroalgae-derived graphene adsorbents for efficient CO2 capture. Process Saf. Environ. Prot. 2021, 148, 1048–1059. [Google Scholar] [CrossRef]
- Politakos, N.; Barbarin, I.; Cantador, L.S.; Cecilia, J.A.; Mehravar, E.; Tomovska, R. Graphene-Based Monolithic Nanostructures for CO2 Capture. Ind. Eng. Chem. Res. 2020, 59, 8612–8621. [Google Scholar] [CrossRef]
- Isah, M.; Lawal, R.; Onaizi, S.A. CO2 Capture and Conversion Using Graphene-Based Materials: A Review on Recent Progresses and Future Outlooks. Green Chem. Eng. 2024, 4. in press. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhang, S.; Zhang, J.; Li, K. Ab Initio Investigation of the Adsorption of CO2 Molecules on Defect Sites of Graphene Surfaces: Role of Local Vacancy Structures. Materials 2023, 16, 981. [Google Scholar] [CrossRef]
- Chandra, V.; Yu, S.U.; Kim, S.H.; Yoon, Y.S.; Kim, D.Y.; Kwon, A.H.; Meyyappan, M.; Kim, K.S. Highly selective CO2 capture on N-doped carbon produced by chemical activation of polypyrrole functionalized graphene sheets. Chem. Commun. 2012, 48, 735–737. [Google Scholar] [CrossRef]
- Gadipelli, S.; Lu, Y.; Skipper, N.T.; Yildirim, T.; Guo, Z. Design of hyperporous graphene networks and their application in solid-amine based carbon capture systems. J. Mater. Chem. A 2017, 5, 17833–17840. [Google Scholar] [CrossRef]
- Kumar, R.; Raut, D.; Ramamurty, U.; Rao, C.N. Remarkable improvement in the mechanical properties and CO2 uptake of MOFs brought about by covalent linking to graphene. Angew. Chem. Int. Ed. 2016, 55, 7857–7861. [Google Scholar] [CrossRef]
- Stankovic, B.; Barbarin, I.; Sanz, O.; Tomovska, R.; Ruipérez, F. Experimental and theoretical study of the effect of different functionalities of graphene oxide/polymer composites on selective CO2 capture. Sci. Rep. 2022, 12, 15992. [Google Scholar] [CrossRef]
- Sari Yilmaz, M. Preparation and CO2 capture performances of KIT-6@reduced graphene oxide composites. J. Porous. Mater. 2023, 30, 1555–1564. [Google Scholar] [CrossRef]
- Bhowmik, K.; Chakravarty, A.; Bysakh, S.; De, G. γ-alumina nanorod/reduced graphene oxide as support for poly(ethylenimine) to capture carbon dioxide from flue gas. Energy Technol. 2016, 4, 1409–1419. [Google Scholar] [CrossRef]
- Che Othman, F.E.; Yusof, N.; González-Benito, J.; Fan, X.; Ismail, A.F. Electrospun Composites Made of Reduced Graphene Oxide and Polyacrylonitrile-Based Activated Carbon Nanofibers (rGO/ACNF) for Enhanced CO2 Adsorption. Polymers 2020, 12, 2117. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.D.; Trivedi, D.H.; Jadhav, N.L.; Pinjari, D.V. Sustainable and Green Synthesis of Carbon Nanomaterials: A Review. J. Environ. Chem. Eng. 2021, 9, 106118. [Google Scholar] [CrossRef]
- Ashourirad, B.; Sekizkardes, A.K.; Altarawneh, S.; El-Kaderi, H.M. Exceptional Gas Adsorption Properties by Nitrogen-Doped Porous Carbons Derived from Benzimidazole-Linked Polymers. Chem. Mater. 2015, 27, 1349–1358. [Google Scholar] [CrossRef]
- Chen, J. Acid/base-treated activated carbons: Characterization of functional groups and metal adsorptive properties. Langmuir 2004, 20, 2233–2242. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Sun, H.; Suvorova, A.; Saunders, M.; Tadé, M.; Wang, S. Heteroatom (N or N-S)-doping induced layered and honeycomb microstructures of porous carbons for CO2 capture and energy applications. Adv. Funct. Mater. 2016, 26, 8651–8661. [Google Scholar] [CrossRef]
- Creamer, A.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef]
- Sahoo, P.C.; Singh, R.; Sivagurunathan, P.; Singh, D.; Kumar, M.; Gupta, R.P.; Srivastava, U. Carbon Dot-Blended Mixed Amine for Efficient CO2 Capture under Highly Oxidative Flue Gas Conditions. Int. J. Greenh. Gas Control 2025, 142, 104340. [Google Scholar] [CrossRef]
- Rajan, A.S.; Sampath, S.; Shukla, A.K. An In Situ Carbon-Grafted Alkaline Iron Electrode for Iron-Based Accumulators. Energy Environ. Sci. 2014, 7, 1110–1116. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yi, C.; Pan, Y.; Fang, Y. Chapter 5—Surface Engineering of Carbon Nanodots (C-Dots) for Biomedical Applications. In Micro and Nano Technologies, Novel Nanomaterials for Biomedical, Environmental and Energy Applications; Wang, X., Chen, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–188. [Google Scholar] [CrossRef]
- Farias, M.D.P.; Albuquerque, P.B.S.; Soares, P.A.G.; de Sá, D.M.A.T.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Xyloglucan from Hymenaea courbaril var. courbaril seeds as encapsulating agent of L-ascorbic acid. Int. J. Biol. Macromol. 2018, 107, 1559–1566. [Google Scholar] [CrossRef]
- Kumar, R.A.; Vizhi, R.E.; Sivakumar, N.; Vijayan, N.; Rajan Babu, D. Crystal growth, optical and thermal studies of nonlinear optical γ-glycine single crystal grown from lithium nitrate. Optik 2012, 123, 409–413. [Google Scholar] [CrossRef]
- Rammal, A.; Perrin, E.; Chabbert, B.; Bertrand, I.; Habrant, A.; Lecart, B.; Vrabie, V. Evaluation of lignocellulosic biomass degradation by combining mid- and near-infrared spectra by the outer product and selecting discriminant wavenumbers using a genetic algorithm. Appl. Spectrosc. 2015, 69, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Mehta, S.; Chen, Y.; Ma, L.; Renner, P.; Parkinson, D.; Liang, H. Correction to "Design and synthesis of lignin-based flexible supercapacitors". ACS Sustain. Chem. Eng. 2020, 8, 9597–9598. [Google Scholar] [CrossRef]
- Demirel, G.; Temiz, A.; Jebrane, M.; Terzıev, N.; Gezer, E. Micro-distribution, water absorption, and dimensional stability of wood treated with epoxidized plant oils. Bioresources 2018, 13, 5124–5138. [Google Scholar] [CrossRef]
- Gaidukevič, J.; Pauliukaitė, R.; Niaura, G.; Matulaitienė, I.; Opuchovič, O.; Radzevič, A.; Barkauskas, J. Synthesis of reduced graphene oxide with adjustable microstructure using regioselective reduction in the melt of boric acid: Relationship between structural properties and electrochemical performance. Nanomaterials 2018, 8, 889. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Tian, Y.; Yang, Z.; Xiao, Q.; Guo, X.; Sun, K. Insight into the capacitive properties of reduced graphene oxide. ACS Appl. Mater. Interfaces 2014, 6, 2248–2254. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Vyas, R.; Kumari, M.; Kaushal, A.; Gupta, R.; Sharma, S.K.; Sachdev, K. A New Sustainable Green Protocol for Production of Reduced Graphene Oxide and Its Gas Sensing Properties. J. Sci. Adv. Mater. Devices 2019, 4, 473–482. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman Spectroscopy of Carbon Materials and Their Composites: Graphene, Nanotubes and Fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; da Silva, M.M.; de Souza Santos, L.V.; Jacob, R.S.; de Vasconcelos, C.K.B.; Viana, M.M. Graphene Oxide in the Remediation of Norfloxacin from Aqueous Matrix: Simultaneous Adsorption and Degradation Process. Environ. Sci. Pollut. Res. 2020, 27, 34513–34528. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Avramova, I.; Dimov, D.A.; Stankova, N.; Petrov, M.; Karaivanova, D.; Avdeev, G.; Russev, S.; Karashanova, D.; Georgieva, B.; Valcheva, E.; et al. Novel Approach for Synthesis of Graphene-like Phases by Pulsed Laser Ablation in a Flow-Mode Suspension. Materials 2022, 15, 7870. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Yang, C.-C.; Tsai, M.-H.; Yang, Z.-R.; Yang, Y.-W.; Tseng, Y.-C.; Wang, C.-H. An Effective Charge Neutralization Enabled by Graphene Overlayer in Ambient Pressure XPS Measurements of Insulators. Adv. Mater. Interfaces 2023, 10, 2201926. [Google Scholar] [CrossRef]
- Matsuoka, M.; Isotani, S.; Mansano, R.D.; Sucasaire, W.; Pinto, R.A.C.; Mittani, J.C.R.; Ogata, K.; Kuratani, N. X-Ray Photoelectron Spectroscopy and Raman Spectroscopy Studies on Thin Carbon Nitride Films Deposited by Reactive RF Magnetron Sputtering. World J. Nano Sci. Eng. (WJNSE) 2012, 2, 92–102. [Google Scholar] [CrossRef][Green Version]
- Radim, P.L.; Otyepka, M.M. Spectroscopic Fingerprints of Graphitic, Pyrrolic, Pyridinic, and Chemisorbed Nitrogen in N-Doped Graphene. J. Phys. Chem. C 2019, 123, 10695–10702. [Google Scholar] [CrossRef]
- Dawson, R.; Stöckel, E.; Holst, J.; Adams, D.; Cooper, A. Microporous organic polymers for carbon dioxide capture. Energy Environ. Sci. 2011, 4, 4239. [Google Scholar] [CrossRef]
- Hong, S.; Jang, E.; Dysart, A.; Pol, V.; Lee, K. CO2 capture in the sustainable wheat-derived activated microporous carbon compartments. Sci. Rep. 2016, 6, 34590. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Furmaniak, S.; Gauden, P.; Terzyk, A. Carbon dioxide adsorption-induced deformation of microporous carbons. J. Phys. Chem. C 2010, 114, 5126–5133. [Google Scholar] [CrossRef]
- Ge, C.; Song, J.; Qin, Z.; Wang, J.; Fan, W. Polyurethane foam-based ultramicroporous carbons for CO2 capture. ACS Appl. Mater. Interfaces 2016, 8, 18849–18859. [Google Scholar] [CrossRef]
- Jiménez, V.; Ramírez-Lucas, A.; Díaz, J.; Sánchez, P.; Romero, A. CO2 capture in different carbon materials. Environ. Sci. Technol. 2012, 46, 7407–7414. [Google Scholar] [CrossRef]
- Sevilla, M.; Parra, J.; Fuertes, A. Assessment of the role of micropore size and N-doping in CO2 capture by porous carbons. ACS Appl. Mater. Interfaces 2013, 5, 6360–6368. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.; Vijayan, S.; Prabhakaran, K. Waste-fish-derived nitrogen self-doped microporous carbon as effective sorbent for CO2 capture. ChemistrySelect 2018, 3, 9555–9563. [Google Scholar] [CrossRef]
- Sudeep, P.; Narayanan, T.; Ganesan, A.; Shaijumon, M.; Yang, H.; Özden, Ş.; Ajayan, P. Covalently interconnected three-dimensional graphene oxide solids. ACS Nano 2013, 7, 7034–7040. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Impact of Surface Functional Groups and Their Introduction Methods on the Mechanisms of CO2 Adsorption on Porous Carbonaceous Adsorbents. Cisco Certif. Support Tech. (CCST) 2022, 3, 100045. [Google Scholar] [CrossRef]
- Nakao, S.; Yogo, K.; Goto, K.; Kai, T.; Yamada, H. Introduction. In Advanced CO2 Capture Technologies; Springer Briefs in Energy; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Qian, D.; Cheng, L.; Wang, E.; Li, W.; Lu, A. A method for creating microporous carbon materials with excellent CO2-adsorption capacity and selectivity. ChemSusChem 2013, 7, 291–298. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1964, 11, 121–127. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhanja, P.; Das, S.; Sen, T.; Bhaumik, A. Triazine containing N-rich microporous organic polymers for CO2 capture and unprecedented CO2/N2 selectivity. J. Solid State Chem. 2017, 247, 113–119. [Google Scholar] [CrossRef]
- Ziaee, A.; Chovan, D.; Lusi, M.; Perry, J.; Zaworotko, M.; Tofail, S. Theoretical optimization of pore size and chemistry in SIFSIX-3-M hybrid ultramicroporous materials. Cryst. Growth Des. 2016, 16, 3890–3897. [Google Scholar] [CrossRef]
- Keskın, S.; Sholl, D. Efficient methods for screening of metal organic framework membranes for gas separations using atomically detailed models. Langmuir 2009, 25, 11786–11795. [Google Scholar] [CrossRef]
- Graham, C.; Imrie, D.A.; Raab, R.E. Measurement of the Electric Quadrupole Moments of CO2, CO, N2, Cl2, and BF3. Mol. Phys. 1998, 93, 49–56. [Google Scholar] [CrossRef]
- KIDA. Carbon Dioxide (CO2)—Kinetic Database for Astrochemistry. Available online: https://kida.astrochem-tools.org/species/56/CO2.html (accessed on 14 May 2025).
- KIDA. Nitrogen (N2)—Kinetic Database for Astrochemistry. Available online: https://kida.astrochem-tools.org/species/32/N2 (accessed on 14 May 2025).
- Marcano, C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
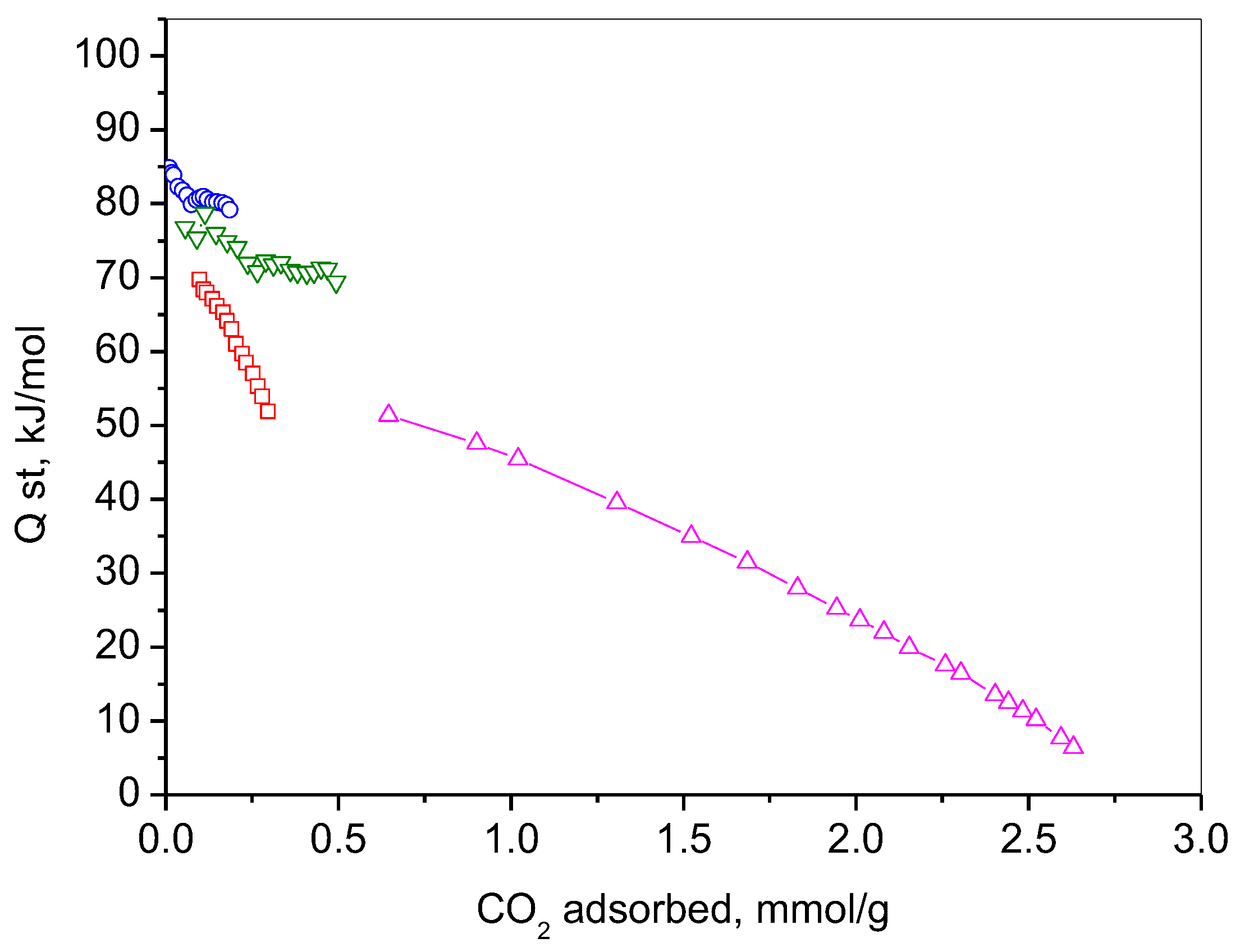
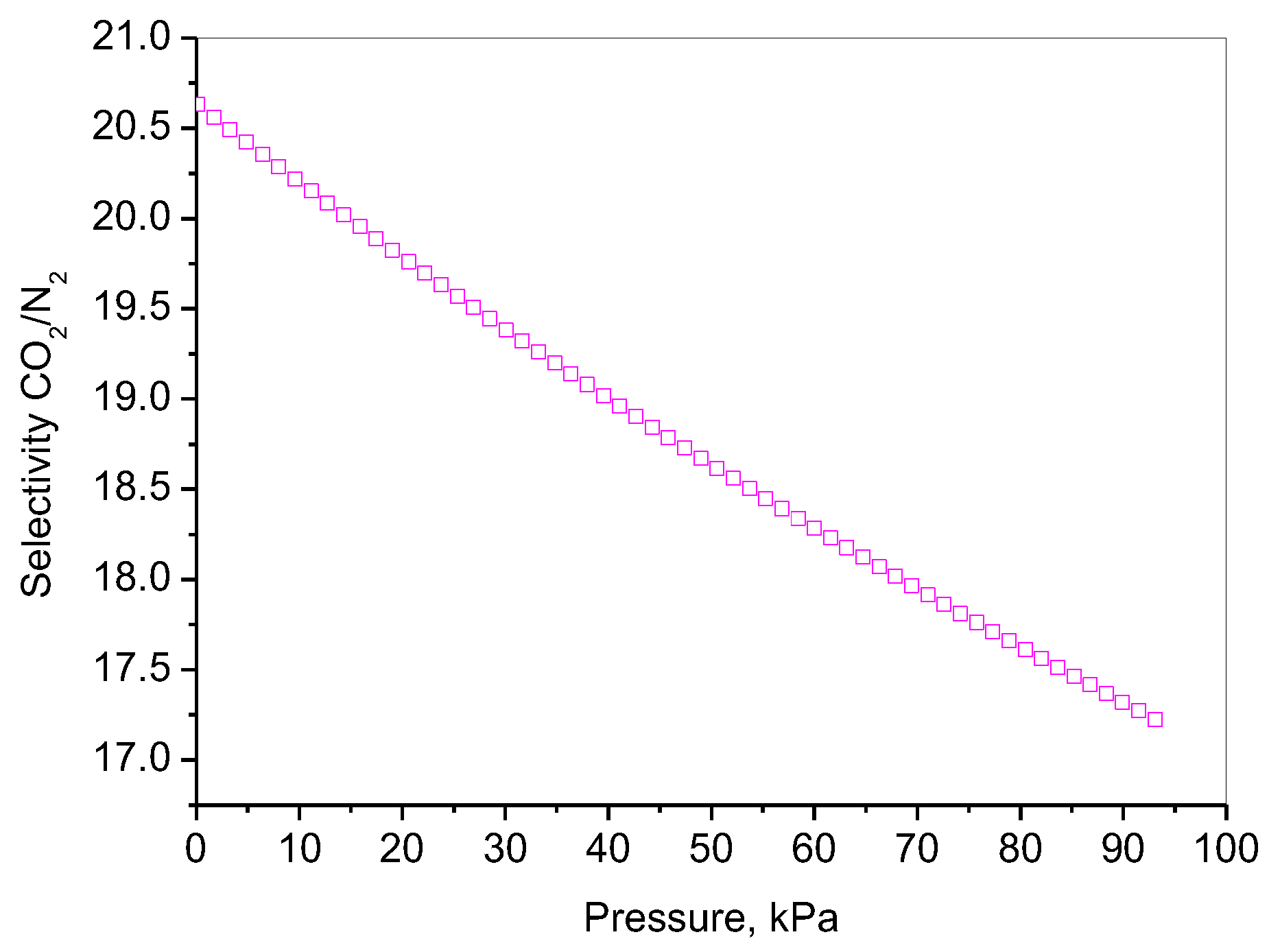
| Adsorbent | Adsorption Capacity | Adsorption Mechanism | Reference |
|---|---|---|---|
| Potassium nickel hexacyanoferrate Prussian Blue analogs (K-NiFe-PBAs) | 3.0 mmol·g−¹ | Physical | [12] |
| N and B-doped graphene aerogels | 2.9 mmol·g−¹ | Not pointed | [15] |
| Chitosan aerogels with graphene oxide nanosheets | 4.14 mmol·g−¹ | Not pointed | [15] |
| Biordered ultramicroporous graphitic carbon | 7.81 mmol·g−¹ | Physical | [16] |
| Reduced graphene | 2.36 mmol·g−¹ | Physical | [16] |
| Activated carbon | 4.66 mmol·g−¹ | Physical | [17] |
| CaBTC-derived MOF (CaO/CN-5) | 2.30 mmol·g−¹ | Chemical | [18] |
| Periodic mesoporous organosilica (PMO) nanoparticles | 2.26 mmol·g−¹ | Physical | [19] |
| Metal–organic framework | 3.7 mmol·g−¹ | Physical | [20] |
| MIP-206-OH-Gly MOF | 2.15 mmol·g−¹ | Physical | [21] |
| Covalent Organic Frameworks (COFs) | 3.2 mmol·g−¹ | Not pointed | [22] |
| Click-based porous cationic polymer | 2 mmol·g−¹ | Mixed | [23] |
| Amine-functionalized mesoporous silica | 0.7 mmol·g−¹ | Chemical | [24] |
| Bifunctionalized mesoporous silica materials | 1.22 mmol·g−¹ | Physical | [25] |
| Biochar derived from vine shoots | 4.07 mmol·g⁻¹ | Physical | [26] |
| Zeolite Na-ZK-4 (2.3) | 4.86 mmol·g⁻¹ | Physical | [27] |
| Amine-modified zeolite NaA | 80 cm3·g⁻¹ | Chemical | [28] |
| Ni(II)/SSZ-13 | 4.49 mmol·g−¹ | Mixed | [29] |
| N-doped activated biocarbon | 3.6 mmol·g−¹ | Physical | [30] |
| Multi-walled CNTs | 0.64 mmol·g−¹ | Not pointed | [31] |
| Graphene oxide with 2,6-diformyl-4-methyl phenol | 8.10 mmol·g−¹ | Physical | [32] |
| Composite of UiO-66-(OH)2 and MWCNTs | 5.75 mmol·g−¹ | Physical | [33] |
| AC CARB 6X12 55 | 4.53 mmol·g−¹ | Physical | [34] |
| Activated carbon Norit RB 4 | 3.02 mmol·g−¹ | Physical | [34] |
| Lotus seed pot-derived N-doped porous carbon | 6.2 mmol·g−¹ | Mixed | [35] |
| Sample | S, m2/g | V, cm3/g | Dav, nm | Smi, m2/g | Sext, m2/g | Vmi, cm3/g |
|---|---|---|---|---|---|---|
| NC-LAA | 493 | 0.27 | 2.2 | 367 | 126 | 0.15 |
| NC/RGO-LAA | 487 | 0.24 | 2.0 | 430 | 56 | 0.17 |
| NC-Gly | 15 | 0.02 | 14 | - | - | - |
| NC/RGO-Gly | 88 | 0.12 | 5.3 | 63 | 25 | 0.03 |
| RGO | 45 | 0.15 | 9 | 4 | 41 | 0.002 |
| Sample | C, at% | O, at% | N, at% |
|---|---|---|---|
| NC-LAA | 90.2 | 9.8 | - |
| NC-Gly | 64.8 | 14.8 | 22.4 |
| NC/RGO-LAA | 87.3 | 12.7 | - |
| NC/RGO-Gly | 81.7 | 16.2 | 2.1 |
| RGO | 93.4 | 6.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kichukova, D.; Lazarova, T.; Atanasova, G.; Kovacheva, D.; Spassova, I. Tailored Carbon Nanocomposites for Efficient CO2 Capture. Molecules 2025, 30, 2408. https://doi.org/10.3390/molecules30112408
Kichukova D, Lazarova T, Atanasova G, Kovacheva D, Spassova I. Tailored Carbon Nanocomposites for Efficient CO2 Capture. Molecules. 2025; 30(11):2408. https://doi.org/10.3390/molecules30112408
Chicago/Turabian StyleKichukova, Diana, Tsvetomila Lazarova, Genoveva Atanasova, Daniela Kovacheva, and Ivanka Spassova. 2025. "Tailored Carbon Nanocomposites for Efficient CO2 Capture" Molecules 30, no. 11: 2408. https://doi.org/10.3390/molecules30112408
APA StyleKichukova, D., Lazarova, T., Atanasova, G., Kovacheva, D., & Spassova, I. (2025). Tailored Carbon Nanocomposites for Efficient CO2 Capture. Molecules, 30(11), 2408. https://doi.org/10.3390/molecules30112408









