Egyptian Blue into Carboxymetylcellulose: New Dual-Emissive Solid-State Luminescent Films
Abstract
1. Introduction
2. Results and Discussion
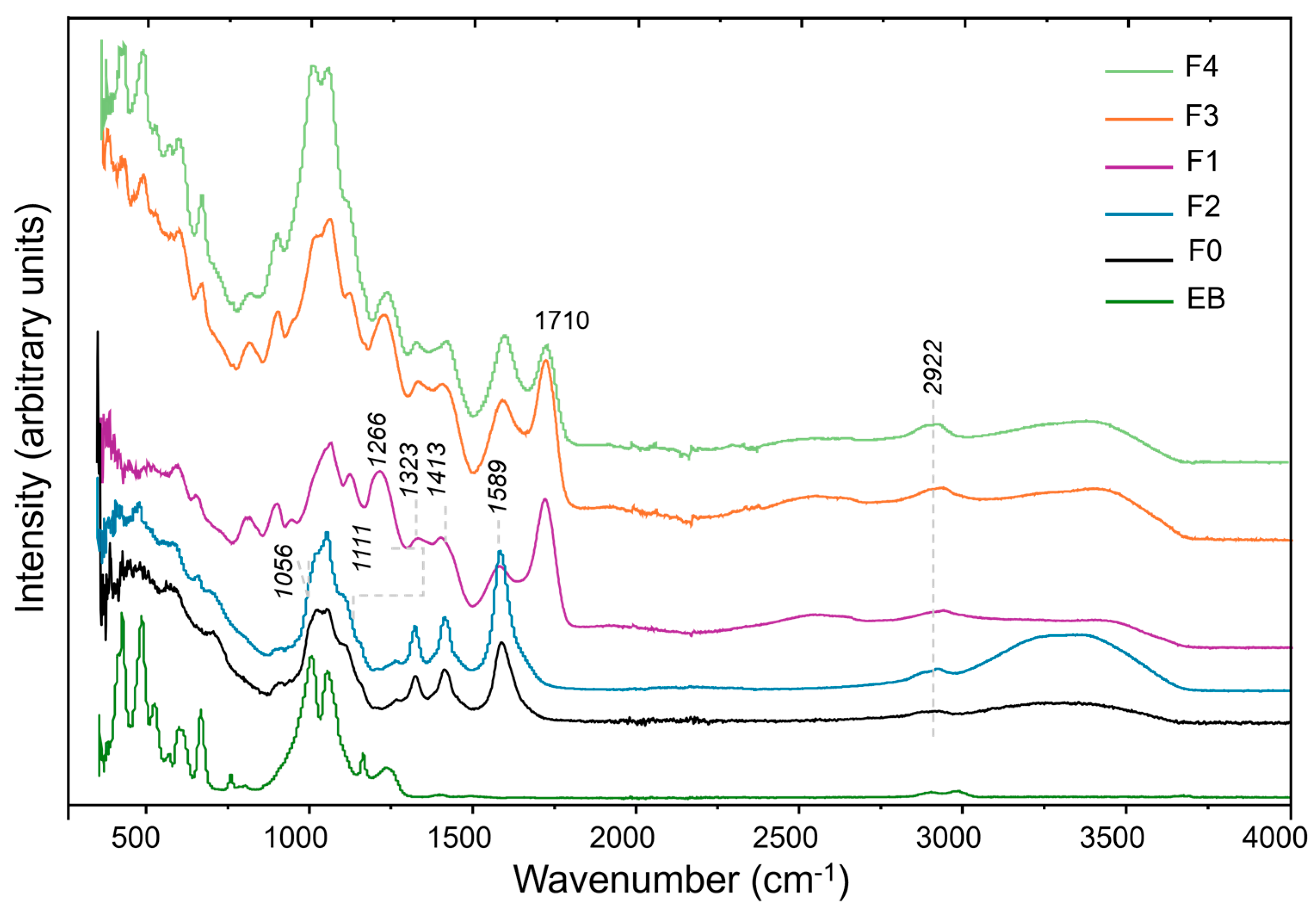
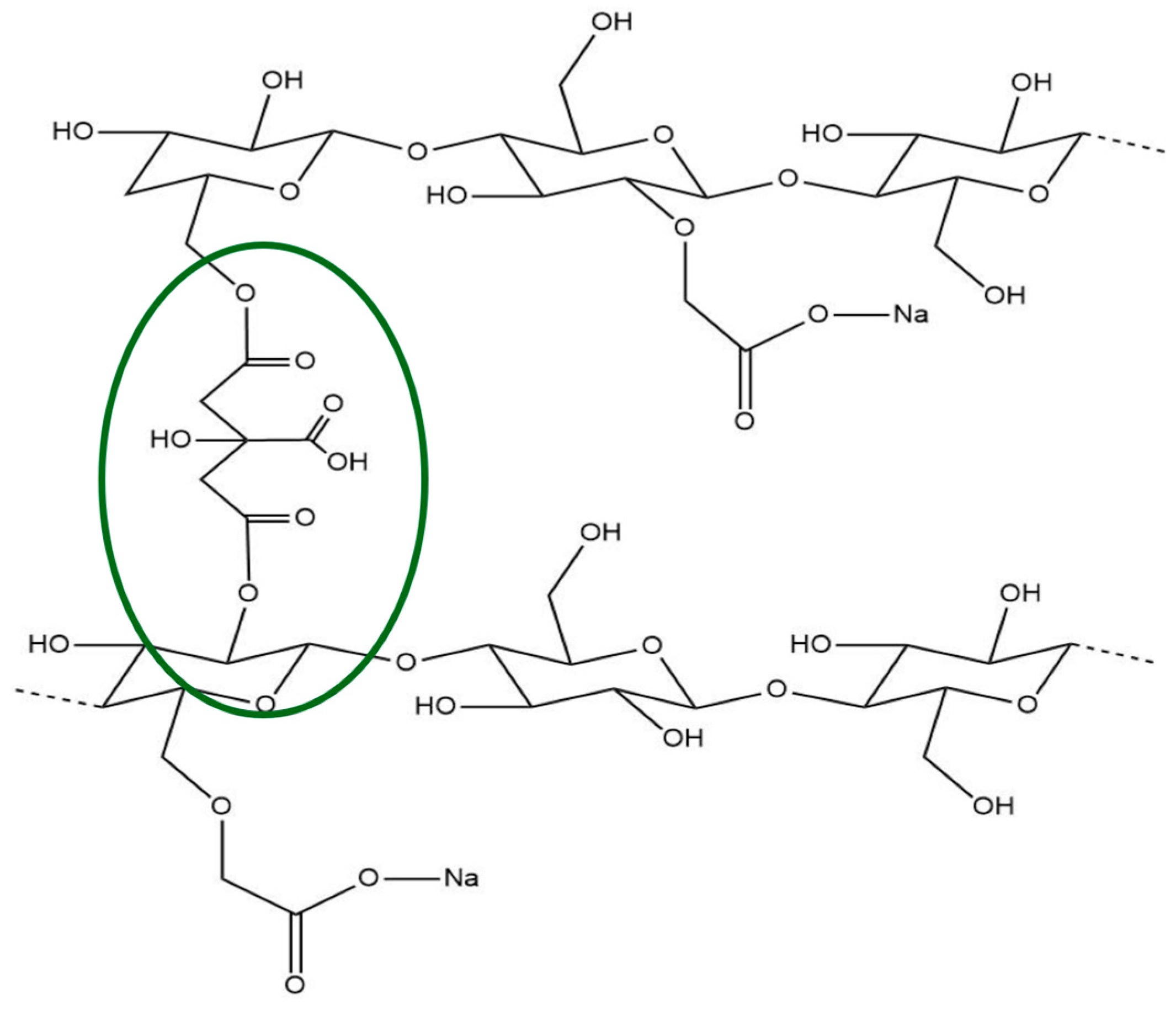
2.1. ATR Spectroscopy
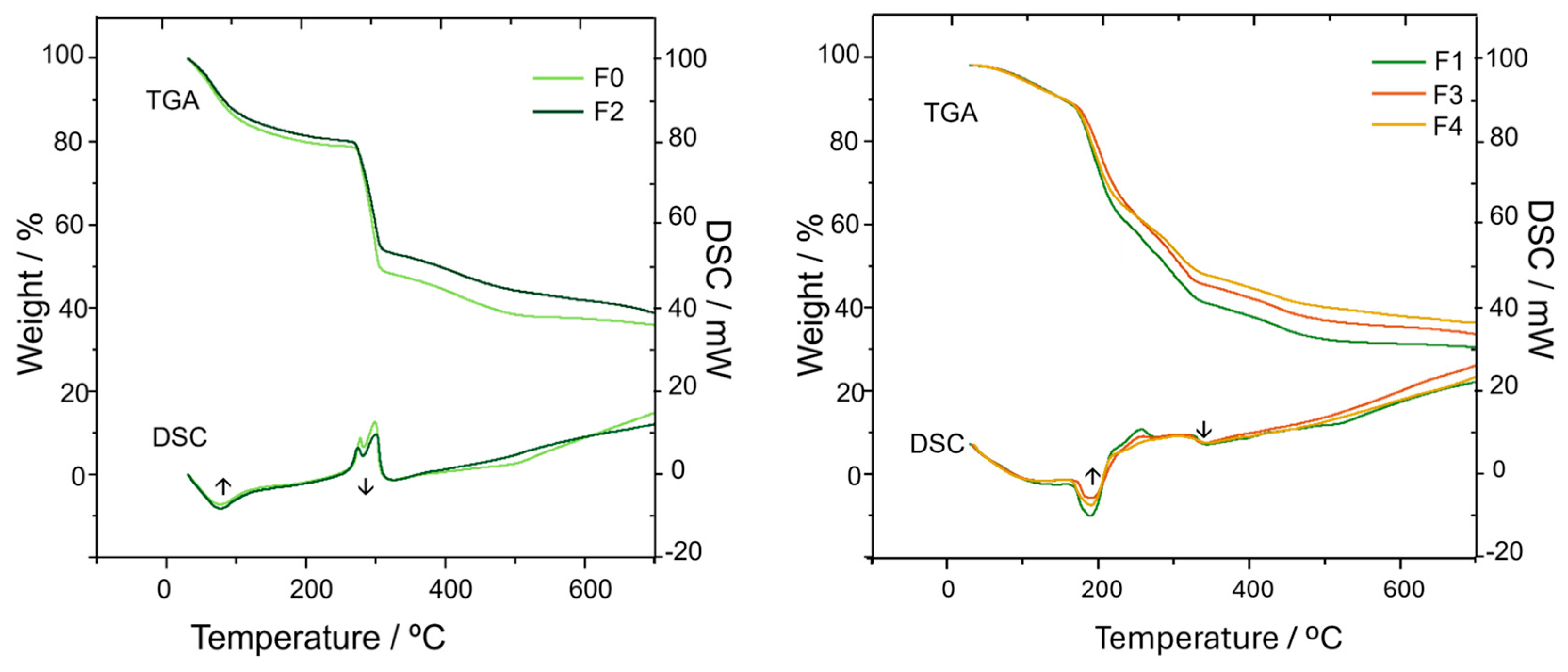
2.2. Thermal Analysis
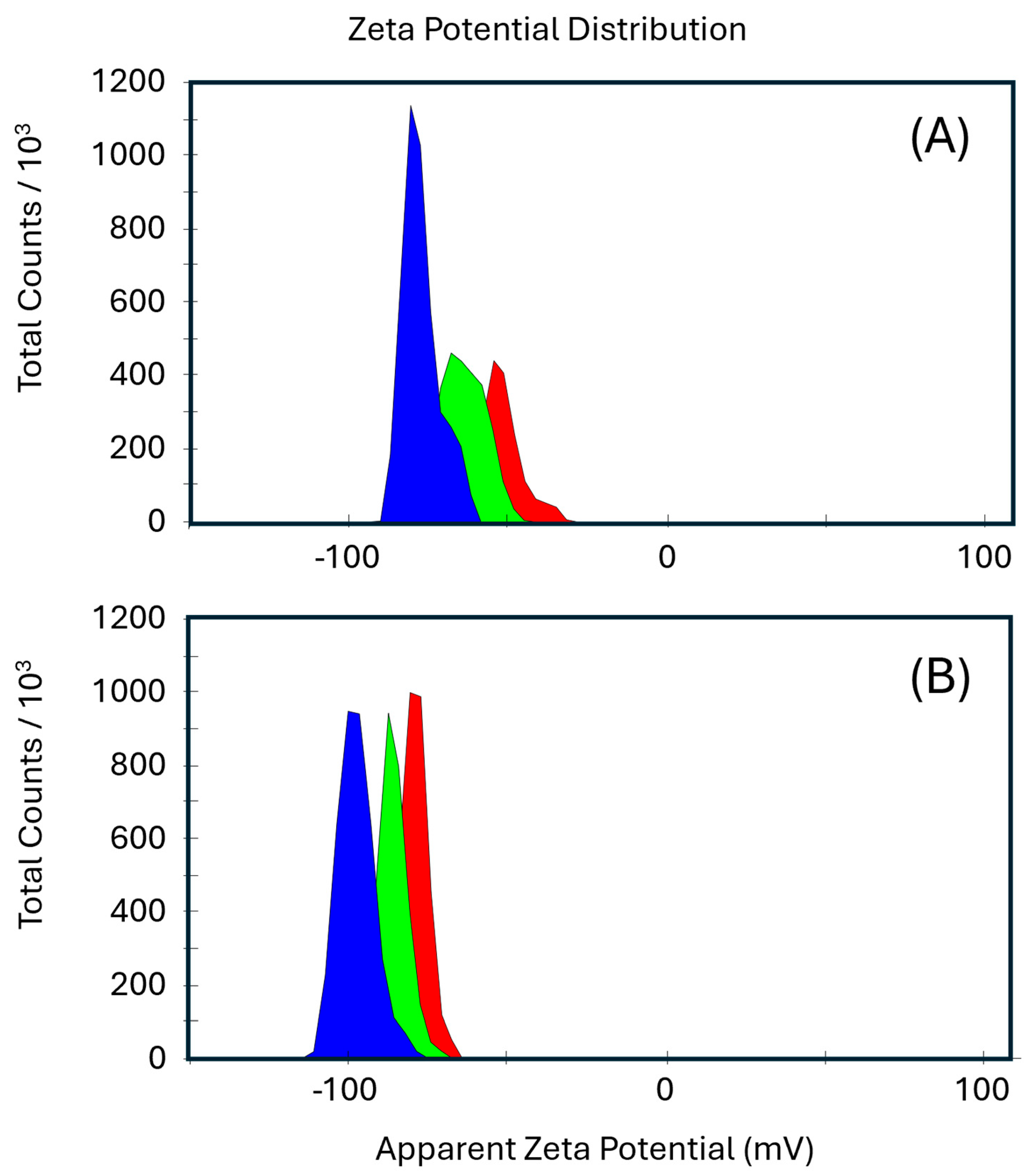
2.3. Zeta Potential
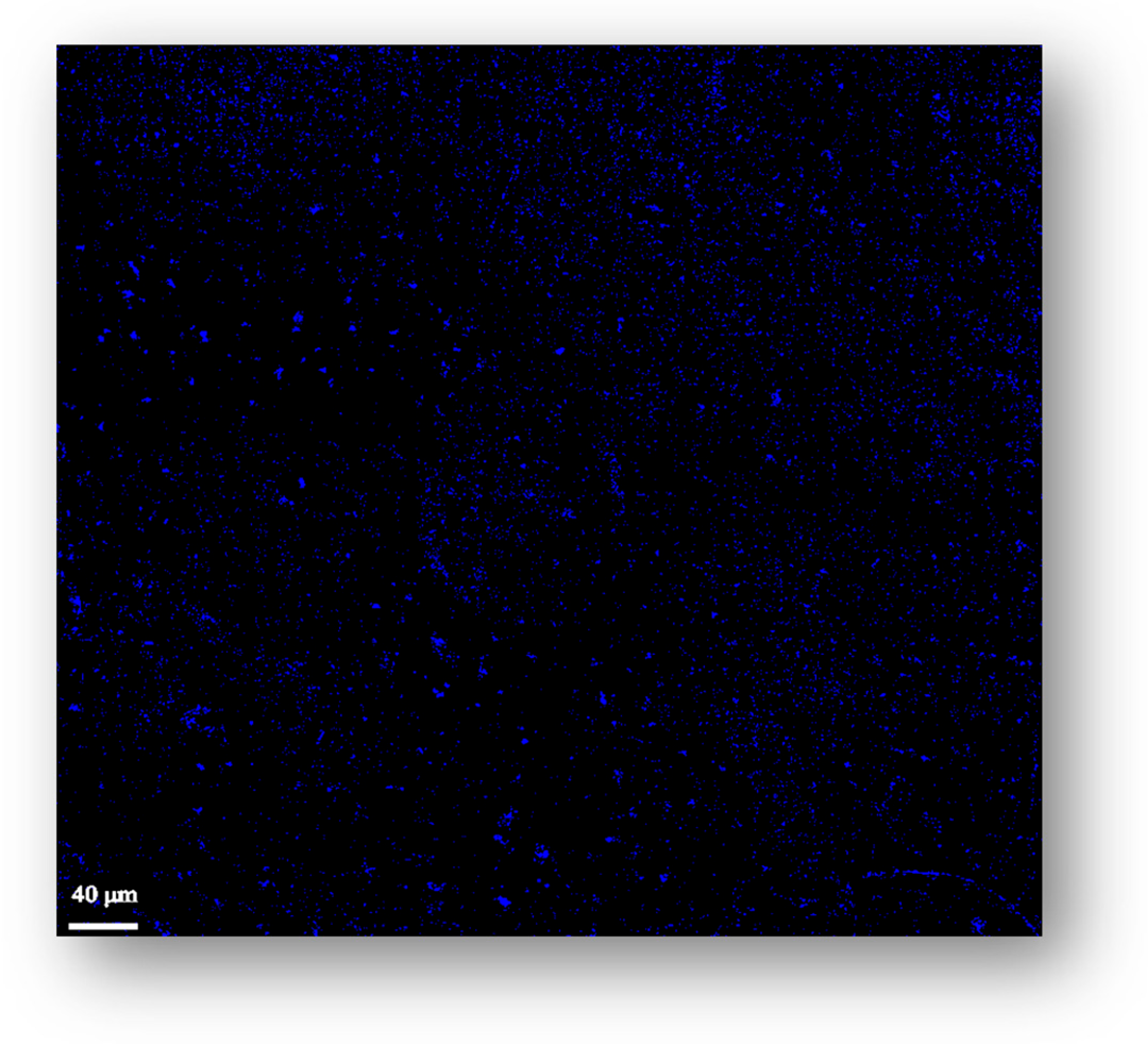
2.4. Microscopy Imaging
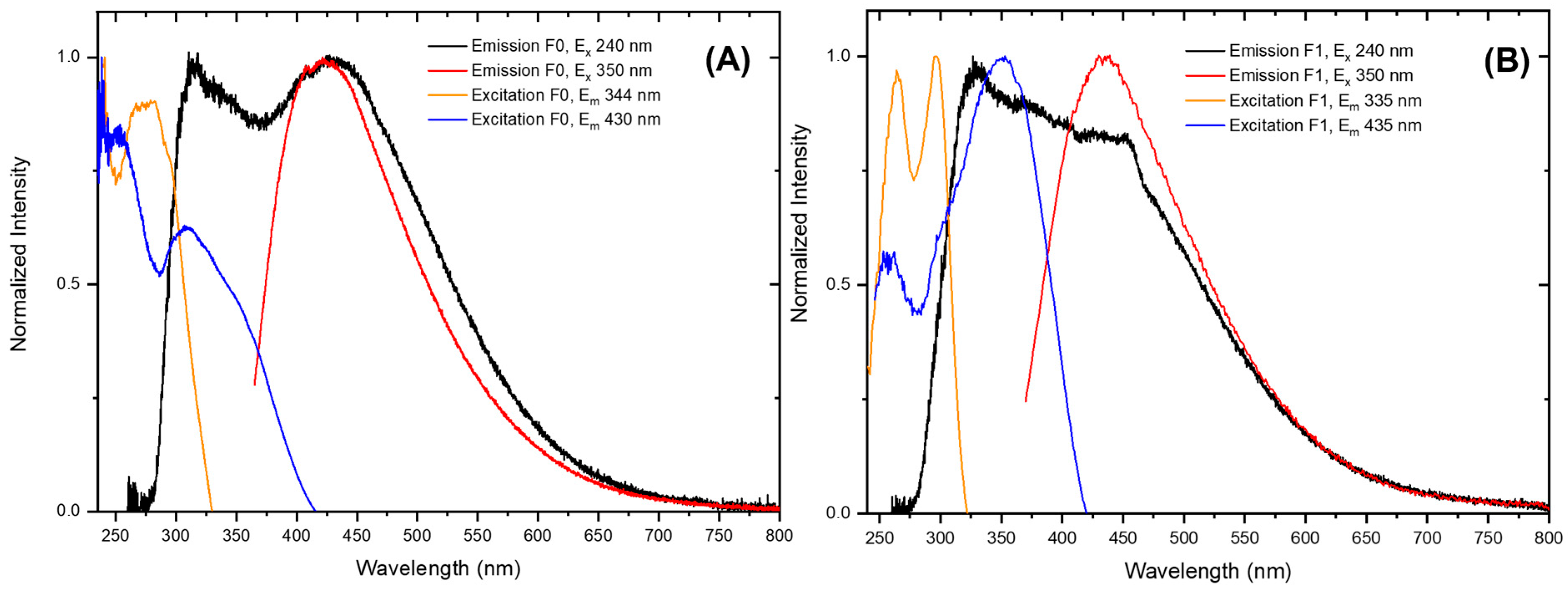
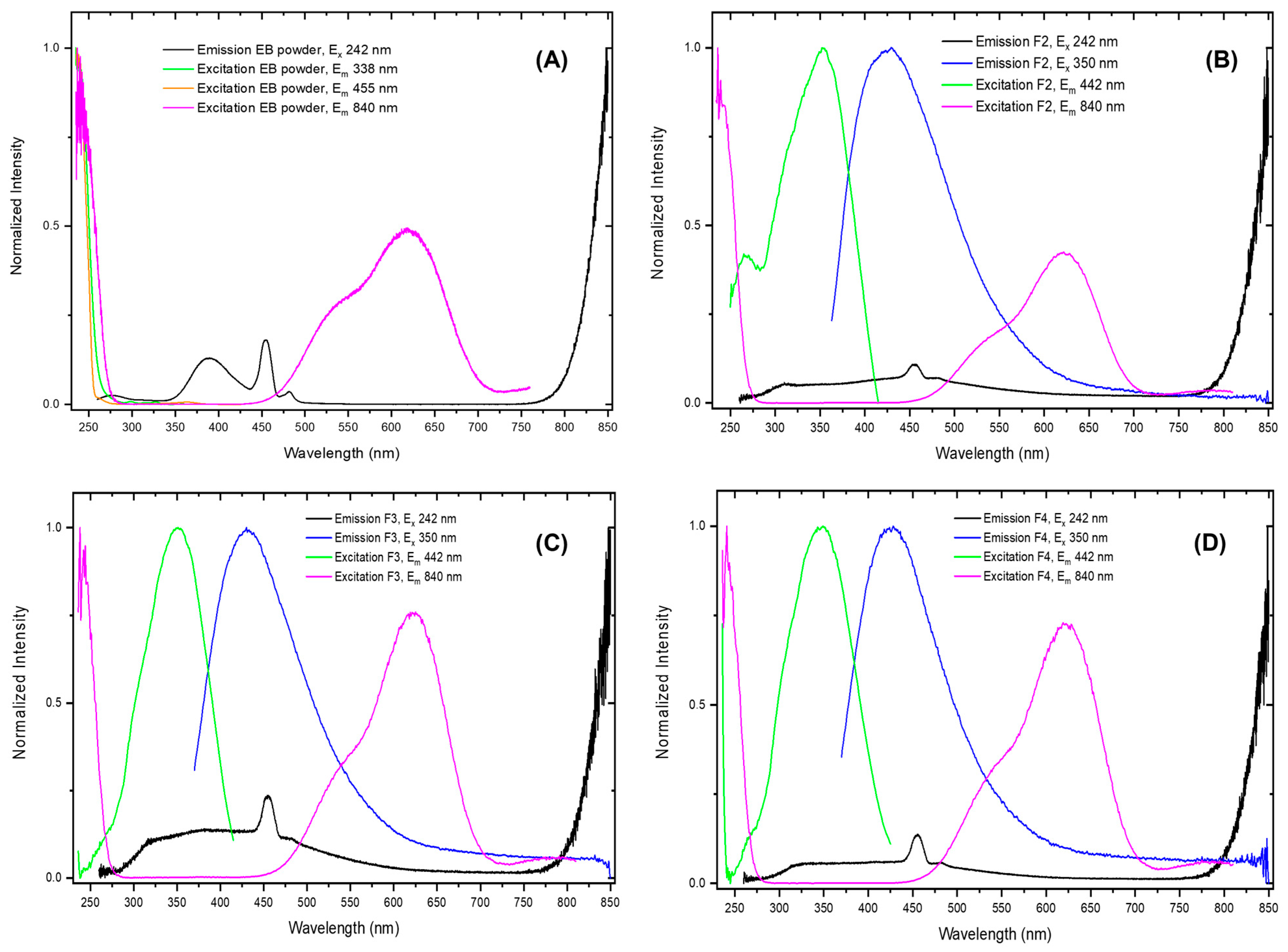
2.5. UV–Vis and PL Spectroscopy
3. Materials and Methods
3.1. Reagents
3.2. Preparation of Films
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| ATR | Attenuated total reflection |
| CA | Citric acid |
| CARS | Coherent anti-Stokes Raman scattering |
| CMC-CA | Citric acid-crosslinked carboxymethylcellulose |
| CMC | Carboxymethylcellulose |
| CTE | Clustering-triggered emission |
| DSC | Differential scanning calorimetry |
| NIR | Near-infrared |
| PL | Photoluminescence |
| TGA | Thermogravimetric analysis |
| ZP | Zeta potential |
References
- Liu, Y.; Zu, B.; Dou, X. Cellulose-based fluorescent materials for chemical sensing applications. Coord. Chem. Rev. 2025, 532, 216505. [Google Scholar] [CrossRef]
- Bresolí-Obach, R.; Castro-Osma, J.A.; Nonell, S.; Lara-Sánchez, A.; Martín, C. Polymers showing cluster triggered emission as potential materials in biophotonic applications. J. Photochem. Photobio. C Photochem. Rev. 2024, 58, 100653. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, B.Z. Through-Space Interactions in Clusteroluminescence. J. Am. Chem. Soc. 2021, 1, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhu, D.; Su, Z.; Bryce, M.R. Recent Advances in Oligomers/Polymers with Unconventional Chromophores. Mater. Chem. Front. 2021, 5, 60–75. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; McGonigal, P.R.; Ye, R.; Liu, S.; Lam, J.W.Y.; Kwok, R.T.K.; Yuan, W.Z.; Xie, J.; Rogach, A.L.; et al. Clusterization-triggered emission: Uncommon luminescence from common materials. Mater. Today 2020, 32, 275–292. [Google Scholar] [CrossRef]
- Tang, S.; Yang, T.; Zhao, Z.; Zhu, T.; Zhang, Q.; Hou, W.; Yuan, W.Z. Nonconventional Luminophores: Characteristics, Advancements and Perspectives. Chem. Soc. Rev. 2021, 50, 12616–12655. [Google Scholar] [CrossRef]
- Chen, C.-X.; Cai, H.-W.; Sun, X.-L.; Wan, W.-M.; Huang, B.-Q. Multicolor, Non Traditional Intrinsically Luminescent Polymers: Recent Advances. ChemPlusChem 2025, 90, e202400633. [Google Scholar] [CrossRef]
- Dou, X.; Zhou, Q.; Chen, X.; Tan, Y.; He, X.; Lu, P.; Sui, K.; Tang, B.Z.; Zhang, Y.; Yuan, W.Z. Clustering-Triggered Emission and Persistent Room Temperature Phosphorescence of Sodium Alginate. Biomacromolecules 2018, 19, 2014–2022. [Google Scholar] [CrossRef]
- Du, L.; He, G.; Gong, Y.; Yuan, W.Z.; Wang, S.; Yu, C.; Liu, Y.; Wei, C. Efficient persistent room temperature phosphorescence achieved through Zn2+ doped sodium carboxymethyl cellulose composites. Compos. Commun. 2018, 8, 106–110. [Google Scholar] [CrossRef]
- Du, L.-L.; Jiang, B.-L.; Chen, X.-H.; Wang, Y.-Z.; Zou, L.-M.; Liu, Y.-L.; Gong, Y.-Y.; Wei, C.; Yuan, W.-Z. Clustering-triggered Emission of Cellulose and Its Derivatives. Chin. J. Polym. Sci. 2019, 37, 409–415. [Google Scholar] [CrossRef]
- Meng, L.; Li, X.; An, X.; Chen, Z.; Xiao, H. Clustering-Triggered Emission of Carboxymethylated Nanocellulose. Front. Chem. 2019, 7, 447. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, H.; Liu, M.; Chen, J.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. Facile preparation of luminescent cellulose nanocrystals with aggregation-induced emission feature through Ce(IV) redox polymerization. Carbohydr. Polym. 2019, 223, 115102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Z.; Yuan, W.Z. Intrinsic Luminescence from Nonaromatic Biomolecules. ChemPlusChem 2020, 85, 1065–1080. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, M.; Li, C.; Lu, S.; Lei, B.; Jiang, J.; Yin, Y.; Zhang, Y.; Shen, Y. Tunable Photoluminescence Properties of Cotton Fiber with Gradually Changing Crystallinity. Front. Chem. 2022, 10, 805252. [Google Scholar] [CrossRef]
- Ge, M.; Liu, S.; Li, J.; Li, M.; Li, S.; James, T.D.; Chen, Z. Luminescent materials derived from biomass resources. Coord. Chem. Rev. 2023, 477, 214951. [Google Scholar] [CrossRef]
- Gao, Q.; Lü, B.; Peng, F. Natural polysaccharide-based room-temperature phosphorescence materials: Designs, properties, and applications. Prog. Mater. Sci. 2025, 148, 101372. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, J.; Li, S.; James, T.D.; Chen, Z. Photoluminescent materials from woody biomass resources. Trends Chem. 2024, 6, 753–767. [Google Scholar] [CrossRef]
- Poisson, J.; Zhang, K. Unique Optical Properties of Cellulosic Materials. Acc. Mater. Res. 2024, 5, 920–932. [Google Scholar] [CrossRef]
- Guo, J.; Wu, M.; Zhang, L.; Peng, J.; Li, X.; Chen, C.; Ma, H. Multicolor room temperature phosphorescence cellulose with source-boosting effect for information encryption. Mater. Today Chem. 2024, 40, 102211. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Edo, M.G.; Saborío, M.G.; Estrany, F.; Alemán, C. Pastes and hydrogels from carboxymethyl cellulose sodium salt as supporting electrolyte of solid electrochemical supercapacitors. Carbohydr. Polym. 2018, 200, 456–467. [Google Scholar] [CrossRef]
- Akhlaq, M.; Mushtaq, U.; Naz, S.; Uroos, M. Carboxymethyl Cellulose-Based Materials as an Alternative Source for Sustainable Electrochemical Devices: A Review. RSC Adv. 2023, 13, 5723–5743. [Google Scholar] [CrossRef] [PubMed]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable carboxymethyl cellulose based material for sustainable packaging application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Hasan, S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.M.; Martins, I.; Bruno, S.M.; Vaz, P.D.; Ribeiro-Claro, P.J.A.; Rudić, S.; Nolasco, M.M. Shedding Light on Cuprorivaite, the Egyptian Blue Pigment: Joining Neutrons and Photons for a Computational Spectroscopy Study. Cryst. Growth Des. 2023, 23, 4961–4969. [Google Scholar] [CrossRef]
- Sgamellotti, A.; Anselmi, C. An evergreen blue. Spectroscopic properties of Egyptian blue from pyramids to Raphael, and beyond. Inorg. Chim. Acta 2022, 530, 120699. [Google Scholar] [CrossRef]
- Binet, L.; Lizion, J.; Bertaina, S.; Gourier, D. Magnetic and New Optical Properties in the UV–visible Range of the Egyptian Blue Pigment Cuprorivaite CaCuSi4O10. J. Phys. Chem. C 2021, 125, 25189–25196. [Google Scholar] [CrossRef]
- Accorsi, G.; Verri, G.; Bolognesi, M.; Armaroli, N.; Clementi, C.; Miliani, C.; Romani, A. The Exceptional Near-Infrared Luminescence Properties of Cuprorivaite (Egyptian Blue). Chem. Commun. 2009, 23, 3392–3394. [Google Scholar] [CrossRef]
- Pozza, G.; Ajò, D.; Chiari, G.; De Zuane, F.; Favaro, M. Photoluminescence of the inorganic pigments Egyptian blue, Han blue and Han purple. J. Cult. Herit. 2020, 1, 393–398. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Materials for Fluorescence-Based Optical Chemical Sensors. J. Mater. Chem. 2005, 15, 2657–2669. [Google Scholar] [CrossRef]
- Borisov, S.M.; Würth, C.; Resch-Genger, U.; Klimant, I. New Life of Ancient Pigments: Application in High-Performance Optical Sensing Materials. Anal. Chem. 2013, 85, 9371–9377. [Google Scholar] [CrossRef]
- Shahbazi, S.; Goodpaster, J.V.; Smith, G.D.; Becker, T.; Lewis, S.W. Preparation, characterization, and application of a lipophilic coated exfoliated Egyptian blue for near infrared luminescent latent fingermark detection. Forensic Chem. 2020, 18, 100208. [Google Scholar] [CrossRef]
- Armetta, F.; Lo Bianco, A.; Boiko, V.; Hreniak, D.; Saladino, M.L. Multimodal Anti-Counterfeiting Inks: Modern Use of an Ancient Pigment in Synergy with a Persistent Phosphor. J. Mater. Chem. C 2025, 13, 1188–1197. [Google Scholar] [CrossRef]
- Tyler, J.L.; Sacci, R.L.; Ning, J.; Mullins, D.R.; Liang, K.; Nanda, J.; Sun, J.; Naguib, M. Egyptian Blue: From Pigment to Battery Electrodes. RSC Adv. 2021, 11, 19885–19889. [Google Scholar] [CrossRef]
- Selvaggio, G.; Chizhik, A.; Nißler, R.; Kuhlemann, L.; Meyer, D.; Vuong, L.; Preiß, H.; Herrmann, N.; Mann, F.A.; Lv, Z.; et al. Exfoliated near Infrared Fluorescent Silicate Nanosheets for (Bio)Photonics. Nat. Commun. 2020, 11, 1495. [Google Scholar] [CrossRef]
- Li, Y.-J.; Ye, S.; Wang, C.H.; Wang, X.-M.; Zhang, Q.Y. Temperature-Dependent near-Infrared Emission of Highly Concentrated Cu2+ in CaCuSi4O10 Phosphor. J. Mater. Chem. C 2014, 2, 10395–103402. [Google Scholar] [CrossRef]
- Sobik, P.; Jeremiasz, O.; Nowak, P.; Sala, A.; Pawłowski, B.; Kulesza-Matlak, G.; Sypień, A.; Drabczyk, K. Towards Efficient Luminescent Solar Energy Concentrator Using Cuprorivaite Infrared Phosphor (CaCuSi4O10)—Effect of Dispersing Method on Photoluminescence Intensity. Materials 2021, 14, 3952. [Google Scholar] [CrossRef]
- Nongnual, T.; Butprom, N.; Boonsang, S.; Kaewpirom, S. Citric acid crosslinked carboxymethyl cellulose edible films: A case study on preserving freshness in bananas. Int. J. Biol. Macromol. 2024, 267, 131135. [Google Scholar] [CrossRef]
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; Vulpoi, A.; David, L.; Astilean, S.; Botiz, I. Enhancing Photoluminescence Quenching in Donor–Acceptor PCE11:PPCBMB Films through the Optimization of Film Microstructure. Nanomaterials 2019, 9, 1757. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Luner, P.; Caluwe, P. Mechanism of Crosslinking of Papers with Polyfunctional Carboxylic Acid. J. Appl. Polym. Sci. 1995, 58, 1523–1534. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Coma, V.; Sebti, I.; Pardon, P.; Pichavant, F.H.; Deschamps, A. Film properties from crosslinking of cellulosic derivatives with a polyfunctional carboxylic acid. Carbohydr. Polym. 2003, 51, 265–271. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Citric Acid as a Benign Alternative to Metal Catalysts for the Production of Cellulose-Grafted-Polycaprolactone Copolymers. Polym. Chem. 2012, 3, 679–684. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel Superabsorbent Cellulose-Based Hydrogels Crosslinked with Citric Acid. J. Appl. Polym. Sc. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Seki, Y.; Altinisik, A.; Demircioğlu, B.; Tetik, C. Carboxymethylcellulose (CMC) Hydroxyethylcellulose (HEC) Based Hydrogels: Synthesis and Characterization. Cellulose 2014, 21, 1689–1698. [Google Scholar] [CrossRef]
- Magri, V.R.; Duarte, A.; Perotti, G.F.; Constantino, V.R.L. Investigation of Thermal Behavior of Layered Double Hydroxides Intercalated with Carboxymethylcellulose Aiming Bio-Carbon Based Nanocomposites. ChemEngineering 2019, 3, 55. [Google Scholar] [CrossRef]
- Kumar, B.; Deeba, F.; Priyadarshi, R.; Singh, S.; Bano, S.; Kumar, A.; Negi, Y.S. Development of Novel Cross-Linked Carboxymethyl Cellulose/Poly(Potassium 1-Hydroxy Acrylate): Synthesis, Characterization and Properties. Polym. Bull. 2020, 77, 4555–4570. [Google Scholar] [CrossRef]
- Akar, E.; Altınışık, A.; Seki, Y. Preparation of pH- and ionic-strength responsive biodegradable fumaric acid crosslinked carboxymethyl cellulose. Carbohydr. Polym. 2012, 90, 1634–1641. [Google Scholar] [CrossRef]
- El-Sayed, S.; Mahmoud, K.H.; Fatah, A.A.; Hassen, A. DSC, TGA and dielectric properties of carboxymethyl cellulose/polyvinyl alcohol blends. Phys. B Condens. Matter 2011, 406, 4068–4076. [Google Scholar] [CrossRef]
- El Ghazzaqui Barbosa, A.; Constantino, A.B.T.; Bastos, L.P.H.; Garcia-Rojas, E.E. Encapsulation of sacha inchi oil in complex coacervates formed by carboxymethylcellulose and lactoferrin for controlled release of β-carotene. Food Hydrocoll. Health 2022, 2, 100047. [Google Scholar] [CrossRef]
- Benyounes, K.; Abdelbaki, B. Rheological and Electrokinetic Properties of Carboxymethylcellulose-Water Dispersions in the Presence of Salts. Int. J. Phys. Sci. 2012, 7, 1790–1798. [Google Scholar] [CrossRef]
- Alqahtani, N.K.; Alnemr, T.M.; Shulaybi, F.A.; Mohamed, H.A.; Gouda, M. Carboxymethyl-Cellulose-Containing Ag Nanoparticles as an Electrochemical Working Electrode for Fast Hydroxymethyl-Furfural Sensing in Date Molasses. Polymers 2023, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Bichara, L.C.; Lanús, H.E.; Ferrer, E.G.; Gramajo, M.B.; Brandán, S.A. Vibrational Study and Force Field of the Citric Acid Dimer Based on the SQM Methodology. Adv. Phys. Chem. 2011, 1, 347072. [Google Scholar] [CrossRef]
- Badry, R.; Ezzat, H.A.; El-Khodary, S.; Morsy, M.; Elhaes, H.; Nada, N.; Ibrahim, M. Spectroscopic and thermal analyses for the effect of acetic acid on the plasticized sodium carboxymethyl cellulose. J. Mol. Struct. 2021, 1224, 129013. [Google Scholar] [CrossRef]
- Chowdhury, F.I.; Dick, C.; Meng, L.; Mahpeykar, S.M.; Ahvazi, B.; Wang, X. Cellulose nanocrystals as host matrix and waveguide materials for recyclable luminescent solar concentrators. RSC Adv. 2017, 7, 32436–32441. [Google Scholar] [CrossRef]
- Griffini, G. Host Matrix Materials for Luminescent Solar Concentrators: Recent Achievements and Forthcoming Challenges. Front. Mater. 2019, 6, 29. [Google Scholar] [CrossRef]
- Trukhin, A.N. Excitons in SiO2: A review. J. Non-Cryst. Solids 1992, 149, 32–45. [Google Scholar] [CrossRef]
- Itoh, C.; Tanimura, K.; Itoh, N. Optical Studies of Self-Trapped Excitons in SiO2. J. Phys. C Solid State Phys. 1988, 21, 4693. [Google Scholar] [CrossRef]
- Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D.; Marinotto, D. The intriguing effect of thiolates as co-ligands in platinum(II) complexes bearing a cyclometalated 1,3-di(2-pyridyl)benzene. Inorg. Chim. Acta 2022, 532, 120744. [Google Scholar] [CrossRef]
- Roberto, D.; Colombo, A.; Dragonetti, C.; Fagnani, F.; Cocchi, M.; Marinotto, D. A Novel Class of Cyclometalated Platinum(II) Complexes for Solution-Processable OLEDs. Molecules 2022, 27, 5171. [Google Scholar] [CrossRef]
- Fagnani, F.; Colombo, A.; Malandrino, G.; Dragonetti, C.; Pellegrino, A.L. Luminescent 1,10-Phenanthroline β-Diketonate Europium Complexes with Large Second-Order Nonlinear Optical Properties. Molecules 2022, 27, 6990. [Google Scholar] [CrossRef]
- Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D.; Marinotto, D. New members of a class of cyclometalated 1,3-di-(2-pyridyl)benzene platinum(II) complexes bearing a tetrazole-thiolate ancillary ligand. Inorg. Chim. Acta 2023, 550, 121446. [Google Scholar] [CrossRef]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishic, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef]



| Sample | τav λex = 242 nm λem = 390 nm | τav λex = 242 nm λem = 455 nm | τav λex = 621 nm λem = 840 nm | Φ (%) λex = 620 nm |
|---|---|---|---|---|
| EB powder | 109.54 ± 0.38 µs | 128.84 ± 0.49 µs | 124.81 ± 1.64 µs | 11.0 |
| F2 | 55.90 ± 1.68 µs | 115.62 ± 4.36 µs | 123.29 ± 0.59 µs | 10.9 |
| F3 | 99.50 ± 2.25 µs | 124.05 ± 3.70 µs | 122.97 ± 0.69 µs | 12.8 |
| F4 | 149.05 ± 4.13 µs | 150.19 ± 6.10 µs | 122.10 ± 0.71 µs | 11.3 |
| CMC 6.5-EB-CA | Egyptian Blue/%(w/w) | Citric Acid/% (w/w) |
|---|---|---|
| F0 | - | - |
| F1 | - | 10 |
| F2 | 1.5 | - |
| F3 | 1.5 | 10 |
| F4 | 3.0 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coimbra, M.; Fagnani, F.; Peres, G.; Ribeiro-Claro, P.; Otero, J.C.; Marinotto, D.; Roberto, D.; Nolasco, M. Egyptian Blue into Carboxymetylcellulose: New Dual-Emissive Solid-State Luminescent Films. Molecules 2025, 30, 2359. https://doi.org/10.3390/molecules30112359
Coimbra M, Fagnani F, Peres G, Ribeiro-Claro P, Otero JC, Marinotto D, Roberto D, Nolasco M. Egyptian Blue into Carboxymetylcellulose: New Dual-Emissive Solid-State Luminescent Films. Molecules. 2025; 30(11):2359. https://doi.org/10.3390/molecules30112359
Chicago/Turabian StyleCoimbra, Mariana, Francesco Fagnani, Gisele Peres, Paulo Ribeiro-Claro, Juan Carlos Otero, Daniele Marinotto, Dominique Roberto, and Mariela Nolasco. 2025. "Egyptian Blue into Carboxymetylcellulose: New Dual-Emissive Solid-State Luminescent Films" Molecules 30, no. 11: 2359. https://doi.org/10.3390/molecules30112359
APA StyleCoimbra, M., Fagnani, F., Peres, G., Ribeiro-Claro, P., Otero, J. C., Marinotto, D., Roberto, D., & Nolasco, M. (2025). Egyptian Blue into Carboxymetylcellulose: New Dual-Emissive Solid-State Luminescent Films. Molecules, 30(11), 2359. https://doi.org/10.3390/molecules30112359










