Photocatalysis and Photodynamic Therapy in Diabetic Foot Ulcers (DFUs) Care: A Novel Approach to Infection Control and Tissue Regeneration
Abstract
1. Introduction
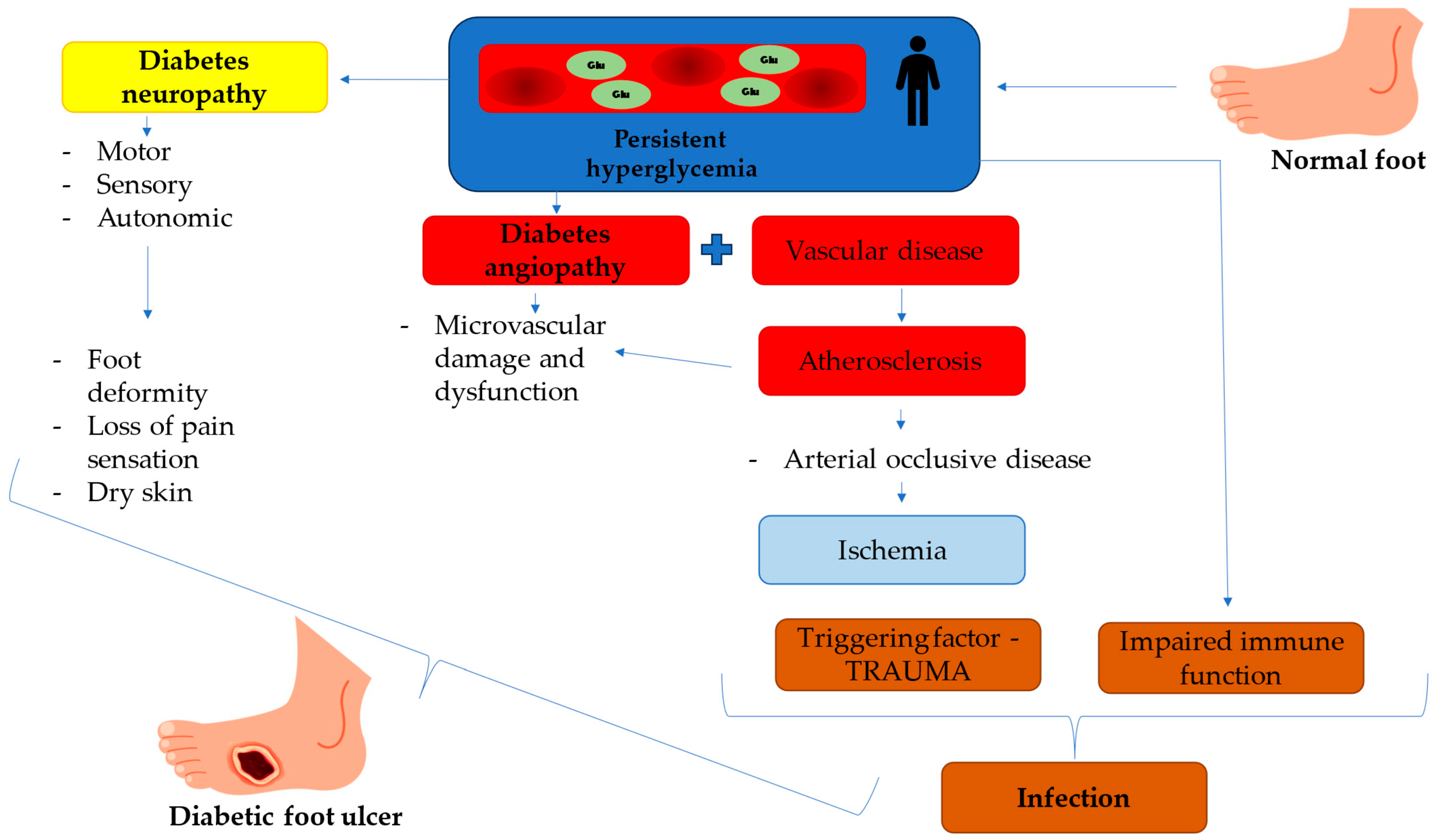
1.1. Diabetic Foot Ulcers (DFUs)—Devastating Complication of Diabetes Mellitus
1.2. Pathophysiology of Diabetic Foot Ulcers
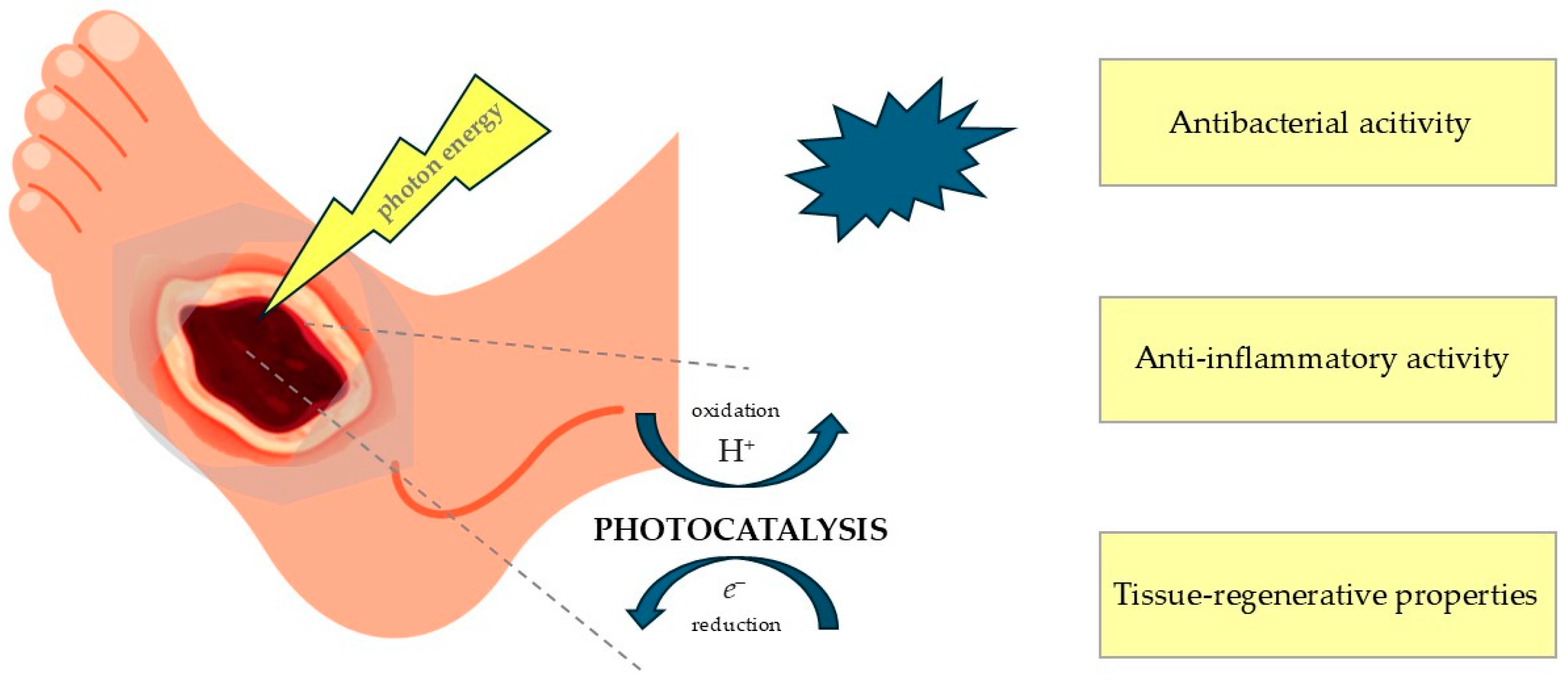
1.3. Photocatalysis and Photodynamic Therapy as Potential Clinical Approaches for the Management and Treatment of Diabetic Foot Ulcers
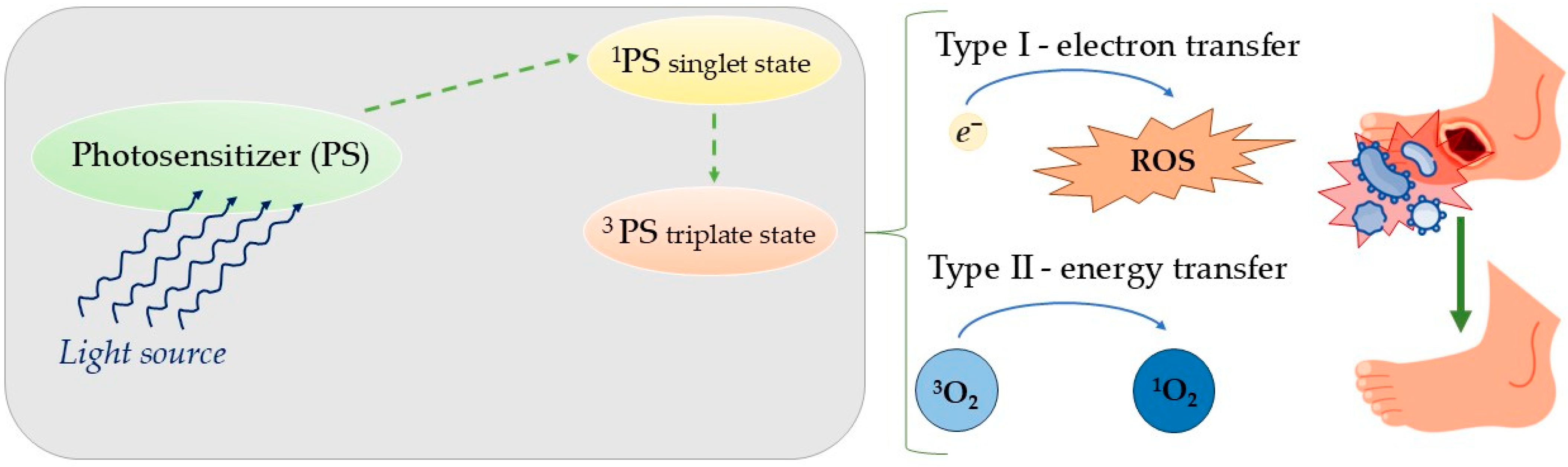
1.4. Photocatalysis
1.5. Classification of Photocatalysts
1.5.1. Inorganic Photocatalysts—Metal Oxides
1.5.2. Carbon Dots
1.5.3. Silver-Doped Photocatalysts (Ag-TiO2, Ag-ZnO)
1.6. The Effect of Photocatalysis on Gram-Positive Versus Gram-Negative Bacteria
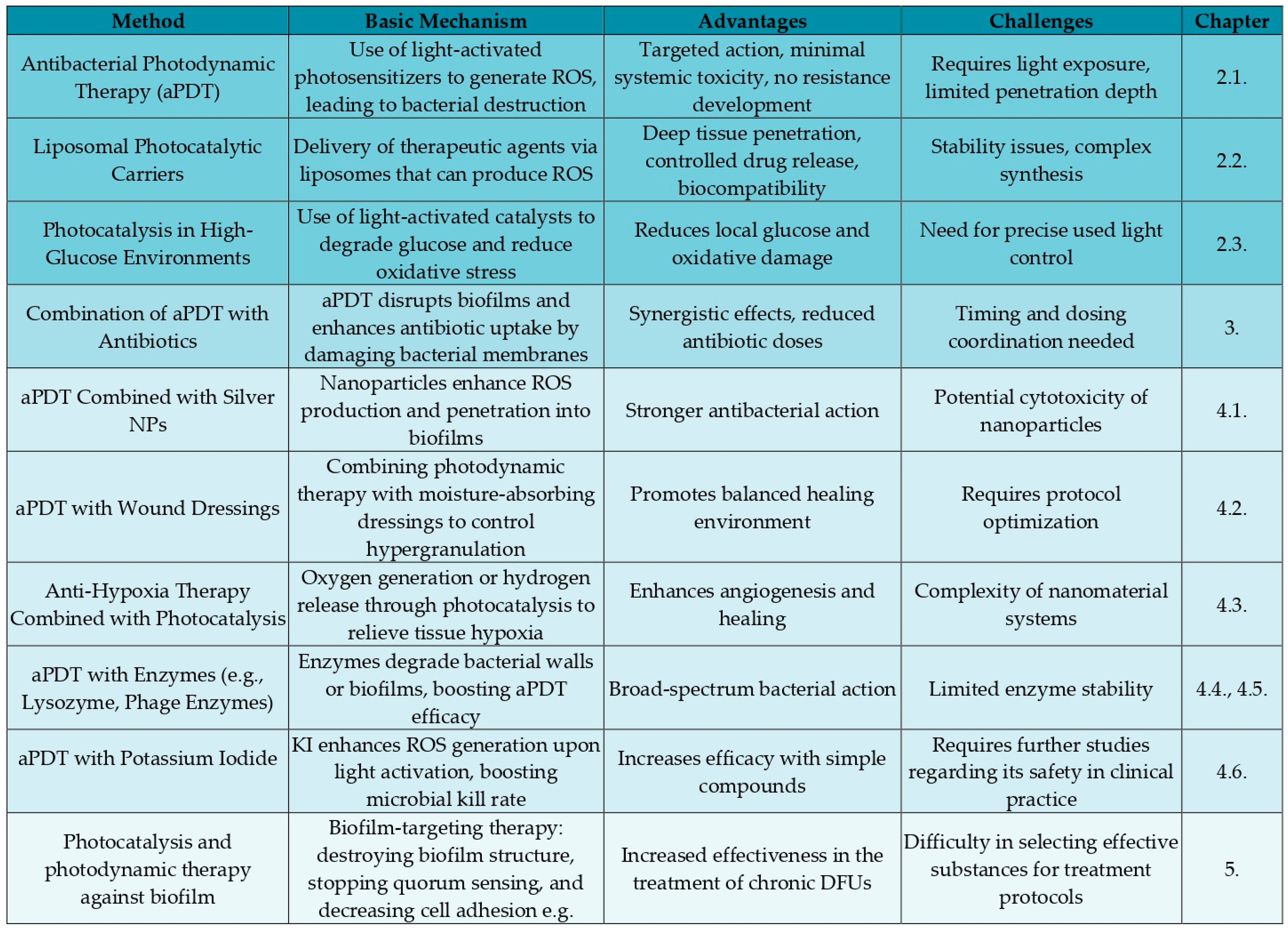
2. Novel Types of DFUs Therapy
2.1. Antibacterial Photodynamic Therapy
2.2. Liposomal Photocatalytic Carriers
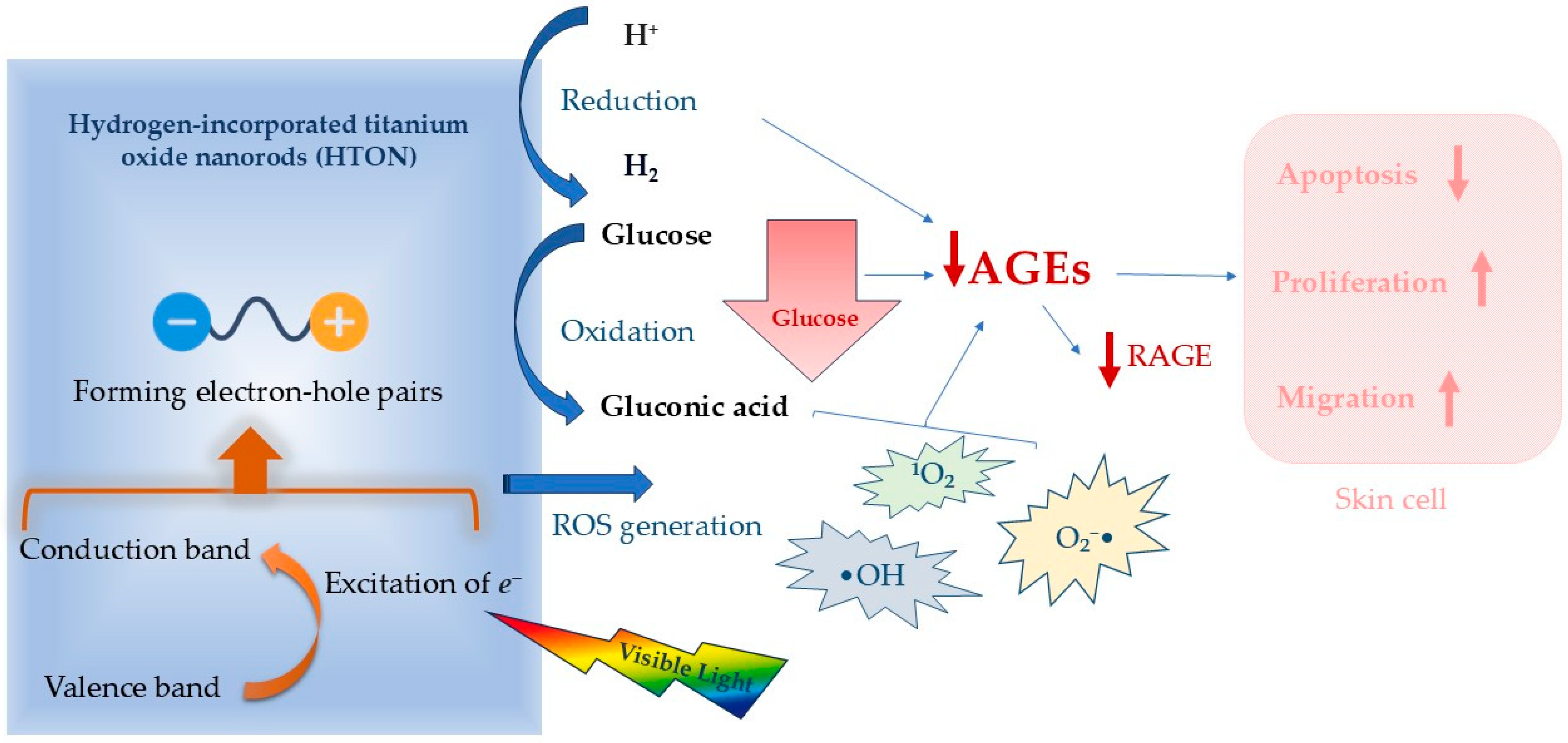
2.3. Photocatalysis Against High Glucose Level Environment
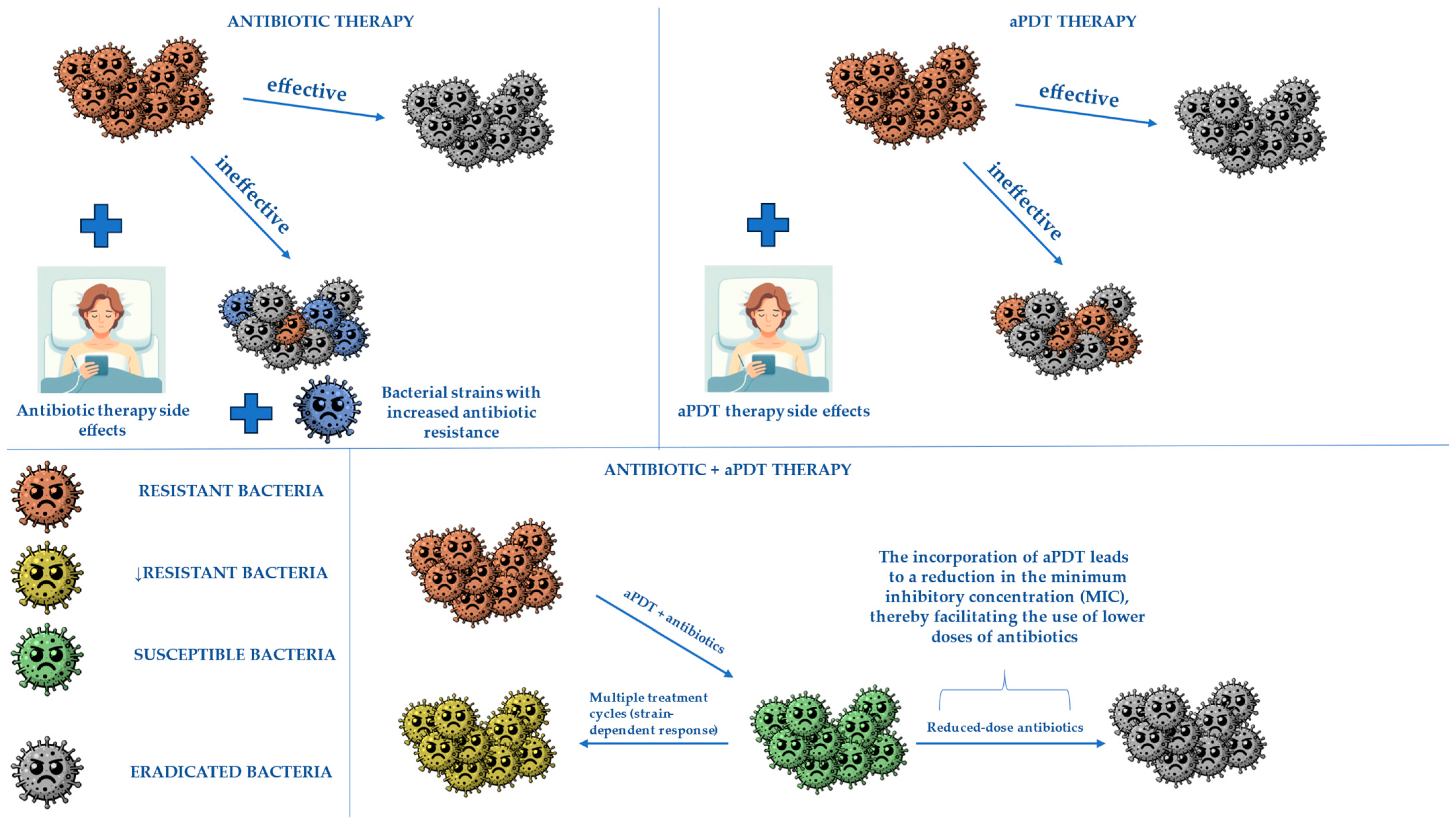
3. Combining aPDT with Antibiotics to Obtain Better Treatment Effects
3.1. Gentamicin
3.2. Ciprofloxacin
3.3. Imipenem
3.4. Vancomycin
3.5. Ceftriaxone
4. Other Potential Combination Therapies with Photodynamic Therapy to Enhance Treatment and Receive Better Healing Outcomes
4.1. Antimicrobial Photodynamic Therapy Combined with Silver Nanoparticles
4.2. Antimicrobial Photodynamic Therapy Combined with Wound Dressings
4.3. Antimicrobial Photocatalytic Therapy Combined with Anti-Hipoxia Mechanism
4.4. Antimicrobial Photodynamic Therapy Combined with Lysozyme
4.5. Antimicrobial Photodynamic Therapy Combined with Phage Enzymes
4.6. Antimicrobial Photodynamic Therapy Combined with Potassium Iodide
5. Photocatalysis and Photodynamic Therapy as an Opportunity for Effective Biofilm Eradication in Diabetic Foot Ulcers—A Novel Approach
5.1. Biofilm Formation in Diabetic Wounds
5.2. Biofilm Eradication—The Use of Photocatalysis and aPDT
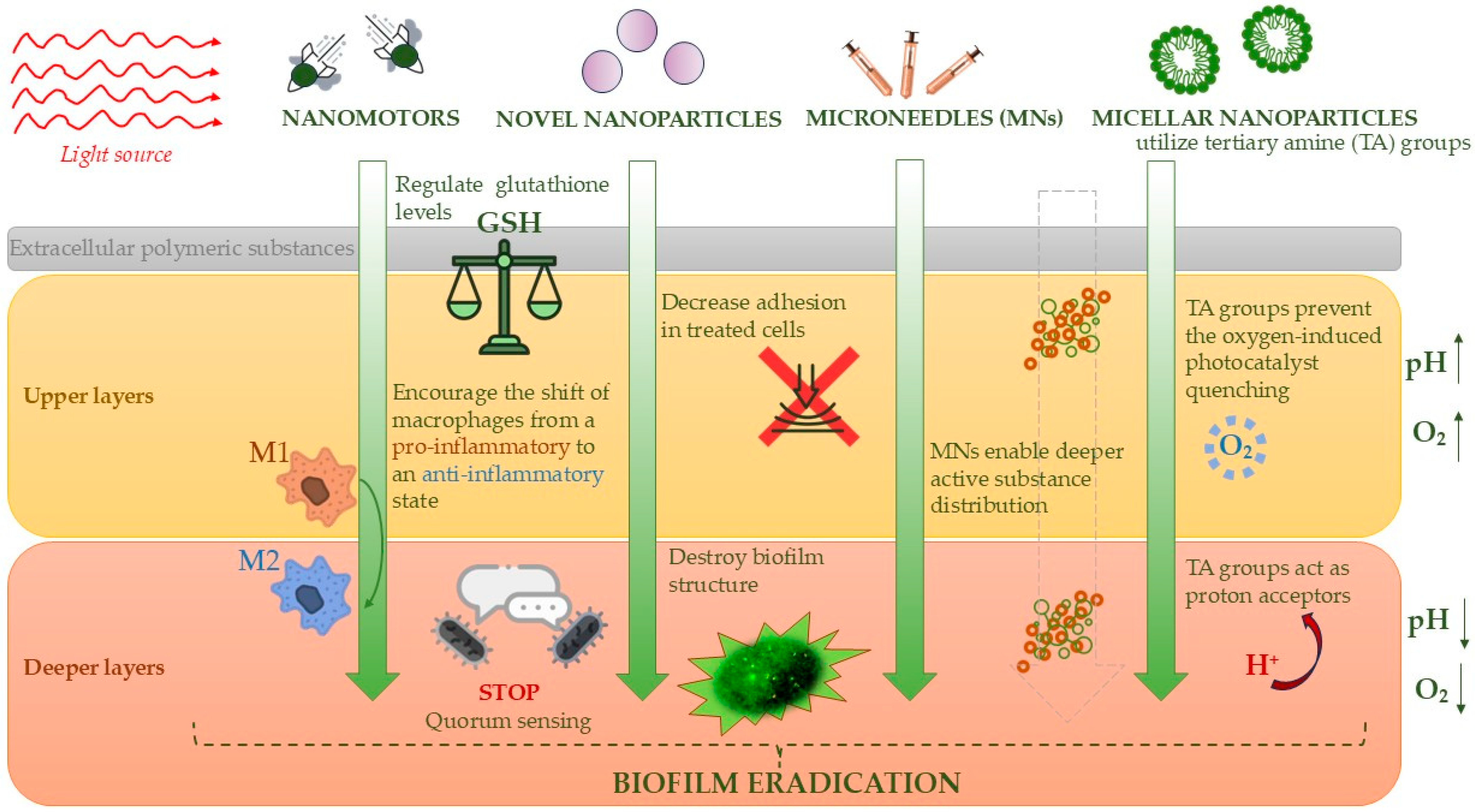
5.3. Practical Modern Approaches to Biofilm Destruction in the Treatment of Diabetic Foot Ulcers
5.4. Eradication of Fungal Biofilm on the Example of the Problem of Mature Candida spp. Biofilm
5.5. Conclusion of Possible Biofilm Novel Treatment
6. Materials and Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapra, A.; Bhandari, P. Diabetes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Soyoye, D.O.; Abiodun, O.O.; Ikem, R.T.; Kolawole, B.A.; Akintomide, A.O. Diabetes and Peripheral Artery Disease: A Review. World J. Diabetes 2021, 12, 827–838. [Google Scholar] [CrossRef]
- Bandyk, D.F. The Diabetic Foot: Pathophysiology, Evaluation, and Treatment. Semin. Vasc. Surg. 2018, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Raja, J.M.; Maturana, M.A.; Kayali, S.; Khouzam, A.; Efeovbokhan, N. Diabetic Foot Ulcer: A Comprehensive Review of Pathophysiology and Management Modalities. World J. Clin. Cases 2023, 11, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Matheson, E.M.; Bragg, S.W.; Blackwelder, R.S. Diabetes-Related Foot Infections: Diagnosis and Treatment. Am. Fam. Physician 2021, 104, 386–394. [Google Scholar] [PubMed]
- Akkus, G.; Sert, M. Diabetic Foot Ulcers: A Devastating Complication of Diabetes Mellitus Continues Non-Stop in Spite of New Medical Treatment Modalities. World J. Diabetes 2022, 13, 1106–1121. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Amani, A.M. Photocatalytic Systems: Reactions, Mechanism, and Applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The Initiation of Oxidative Stress and Therapeutic Strategies in Wound Healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Su, Y.; Ye, B.; Zhang, Z.; Gao, Q.; Zeng, L.; Wan, Y.; Sun, W.; Chen, S.; Quan, D.; Yu, J.; et al. Photocatalytic oxygen evolution and antibacterial biomimetic repair membrane for diabetes wound repair via HIF1-α pathway. Mater. Today Bio 2023, 20, 100616. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A.; Madkour, F.F. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef] [PubMed]
- Widyastuti, E.; Chiu, C.-T.; Hsu, J.-L.; Lee, Y.C. Photocatalytic Antimicrobial and Photostability Studies of TiO2/ZnO Thin Films. Arab. J. Chem. 2023, 16, 105010. [Google Scholar] [CrossRef]
- Gengan, S.; Ananda Murthy, H.C.; Sillanpää, M.; Nhat, T. Carbon Dots and Their Application as Photocatalyst in Dye Degradation Studies—Mini Review. Results Chem. 2022, 4, 100674. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Bian, H.; Zhang, Z.; Xu, X.; Gao, Y.; Wang, T. Photocatalytic Activity of Ag/ZnO/AgO/TiO2 Composite. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 124, 114236. [Google Scholar] [CrossRef]
- Dai, S.; Yao, L.; Liu, L.; Cui, J.; Su, Z.; Zhao, A.; Yang, P. Carbon Dots-Supported Zn Single Atom Nanozymes for the Catalytic Therapy of Diabetic Wounds. Acta Biomater. 2024, 186, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Lopez Goerne, T.; Padilla-Godínez, F.J.; Perez Davalos, L.; Ramírez, P.; Arellano, D. Nanobiocatalysts: Cu/TiO2-SiO2 Nanoparticles as Tissue-Regeneration Treatment for Diabetic Foot Ulcers: In Vivo Studies. Curr. Biotechnol. 2020, 9, 230–239. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad spectrum microbicidal activity of photocatalysis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef]
- Paluch, E.; Seniuk, A.; Plesh, G.; Widelski, J.; Szymański, D.; Wiglusz, R.J.; Motola, M.; Dworniczek, E. Mechanism of Action and Efficiency of Ag3PO4-Based Photocatalysts for the Control of Hazardous Gram-Positive Pathogens. Int. J. Mol. Sci. 2023, 24, 13553. [Google Scholar] [CrossRef]
- He, J.; Zheng, Z.; Lo, I.M.C. Different responses of gram-negative and gram-positive bacteria to photocatalytic disinfection using solar-light-driven magnetic TiO2-based material, and disinfection of real sewage. Water Res. 2021, 207, 117816. [Google Scholar] [CrossRef]
- Paluch, E.; Sobierajska, P.; Okińczyc, P.; Widelski, J.; Duda-Madej, A.; Krzyżanowska, B.; Krzyżek, P.; Ogórek, R.; Szperlik, J.; Chmielowiec, J.; et al. Nanoapatites Doped and Co-Doped with Noble Metal Ions as Modern Antibiofilm Materials for Biomedical Applications against Drug-Resistant Clinical Strains of Enterococcus faecalis VRE and Staphylococcus aureus MRSA. Int. J. Mol. Sci. 2022, 23, 1533. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Czech, S.; Dynarowicz, K.; Misiołek, M.; Komosińska-Vassev, K.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Photodynamic therapy: Past, current, and future. Int. J. Mol. Sci. 2024, 25, 11325. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Hoorijani, M.N.; Rostami, H.; Pourhajibagher, M.; Chiniforush, N.; Heidari, M.; Pourakbari, B.; Kazemian, H.; Davari, K.; Amini, V.; Raoofian, R.; et al. The effect of antimicrobial photodynamic therapy on the expression of novel methicillin resistance markers determined using cDNA-AFLP approach in Staphylococcus aureus. Photodiagnosis Photodyn. Ther. 2017, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ziganshyna, S.; Guttenberger, A.; Lippmann, N.; Schulz, S.; Bercker, S.; Kahnt, A.; Rüffer, T.; Voigt, A.; Gerlach, K.; Werdehausen, R. Tetrahydroporphyrin-tetratosylate (THPTS)-based photodynamic inactivation of critical multidrug-resistant bacteria in vitro. Int. J. Antimicrob. Agents 2020, 55, 105976. [Google Scholar] [CrossRef] [PubMed]
- Schastak, S.; Ziganshyna, S.; Gitter, B.; Wiedemann, P.; Claudepierre, T. Efficient photodynamic therapy against gram-positive and gram-negative bacteria using THPTS, a cationic photosensitizer excited by infrared wavelength. PLoS ONE 2010, 5, e11674. [Google Scholar] [CrossRef]
- Tim, M. Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J. Photochem. Photobiol. B 2015, 150, 2–10. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar]
- Muniz, I.P.R.; Galantini, M.P.L.; Ribeiro, I.S.; Gonçalves, C.V.; dos Santos, D.P.; Moura, T.C.; Silva, E.S.; Silva, N.R.; Cipriano, B.P.; Correia, T.M.L.; et al. Antimicrobial photodynamic therapy (aPDT) with curcumin controls intradermal infection by Staphylococcus aureus in mice with type 1 diabetes mellitus: A pilot study. J. Photochem. Photobiol. B Biol. 2021, 224, 112325. [Google Scholar] [CrossRef]
- Cláudio, M.M.; Nuernberg, M.A.A.; Rodrigues, J.V.S.; Belizário, L.C.G.; Batista, J.A.; Duque, C.; Garcia, V.G.; Theodoro, L.H. Effects of multiple sessions of antimicrobial photodynamic therapy (aPDT) in the treatment of periodontitis in patients with uncompensated type 2 diabetes: A randomized controlled clinical study. Photodiagnosis Photodyn. Ther. 2021, 35, 102451. [Google Scholar] [CrossRef]
- Cunha, P.O.; Gonsales, I.R.; Greghi, S.L.A.; Sant’ana, A.C.P.; Honório, H.M.; Negrato, C.A.; Zangrando, M.S.R.; Damante, C.A. Adjuvant antimicrobial photodynamic therapy improves periodontal health and reduces inflammatory cytokines in patients with type 1 diabetes mellitus. J. Appl. Oral Sci. 2024, 32, e20240258. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.Q.M.; da Rocha, T.E.; Toro, L.F.; Guiati, I.Z.; Ervolino, E.; Garcia, V.G.; Wainwright, M.; Theodoro, L.H. Antimicrobial photodynamic therapy compared to systemic antibiotic therapy in non-surgical treatment of periodontitis: Systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2020, 31, 101808. [Google Scholar] [CrossRef]
- Ai, R.; Nie, M.; Yang, J.; Deng, D. Effects of Antibiotics Versus Repeated Applications of Photodynamic Therapy as an Adjunctive Treatment for Periodontitis: A Systematic Review and Meta-Analysis. Photobiomodulation Photomed. Laser Surg. 2021, 39, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Morley, S.; Griffiths, J.; Philips, G.; Moseley, H.; O’Grady, C.; Mellish, K.; Lankester, C.L.; Faris, B.; Young, R.J.; Brown, S.B.; et al. Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: A new approach to antimicrobial therapy. Br. J. Dermatol. 2013, 168, 617–624. [Google Scholar] [CrossRef]
- Carrinho, P.M.; Andreani, D.I.K.; Morete, V.A.; Iseri, S.; Navarro, R.S.; Villaverde, A.B. A study on the macroscopic morphometry of the lesion area on diabetic ulcers in humans treated with photodynamic therapy using two methods of measurement. Photomed. Laser Surg. 2018, 36, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; Cecatto, R.B.; Perez, S.T.; Mesquita-Ferrari, R.A.; Bussadori, S.K.; Duran, C.C.; Horliana, A.C.T.; Fernandes, K.P.S. Adjuvant effect of antimicrobial photodynamic therapy (aPDT) in the treatment of diabetic foot ulcers: A case series. J. Biophotonics 2024, 17, e202300412. [Google Scholar] [CrossRef]
- Martinelli, N.; Curci, V.; Quarantiello, A.; Saldalamacchia, G. The benefits of antimicrobial photodynamic therapy with RLP068 in the management of diabetic foot ulcers. Drugs Context 2019, 8, 212610. [Google Scholar] [CrossRef]
- Li, X.; Kou, H.; Zhao, C.; Zhu, F.; Yang, Y.; Lu, Y. Efficacy and safety of ALA-PDT in treatment of diabetic foot ulcer with infection. Photodiagnosis Photodyn. Ther. 2022, 38, 102822. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, L.; Wang, L.; Zhao, S.; Nie, J.; Lv, M.; Zhang, W.; Su, X.; Tian, S.; Li, Y. A meta-analysis and systematic review of photodynamic therapy for diabetic foot ulcers. Photodiagnosis Photodyn. Ther. 2024, 48, 104228. [Google Scholar] [CrossRef]
- Kandregula, B.; Narisepalli, S.; Chitkara, D.; Mittal, A. Exploration of lipid-based nanocarriers as drug delivery systems in diabetic foot ulcer. Mol. Pharm. 2022, 19, 1977–1998. [Google Scholar] [CrossRef]
- Li, H.; Lin, Z.; Ouyang, L.; Lin, C.; Zeng, R.; Liu, G.; Zhou, W. Lipid nanoparticle: Advanced drug delivery systems for promotion of angiogenesis in diabetic wounds. J. Liposome Res. 2024, 35, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Wang, J.; Guo, Z.; Zhang, Y.; Wang, Y.; Dong, S.; Liu, C.; Xing, H.; Li, X. Efficient delivery of VEGF-A mRNA for promoting diabetic wound healing via ionizable lipid nanoparticles. Int. J. Pharm. 2023, 632, 122565. [Google Scholar] [CrossRef]
- Deol, P.K.; Kaur, I.P.; Dhiman, R.; Kaur, H.; Sharma, G.; Rishi, P.; Ghosh, D. Investigating wound healing potential of sesamol loaded solid lipid nanoparticles: Ex-vivo, in vitro and in-vivo proof of concept. Int. J. Pharm. 2024, 654, 123974. [Google Scholar] [CrossRef] [PubMed]
- Motsoene, F.; Abrahamse, H.; Dhilip Kumar, S.S. Multifunctional lipid-based nanoparticles for wound healing and antibacterial applications: A review. Adv. Colloid Interface Sci. 2023, 321, 103002. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-C.; Nain, A.; Lin, Y.-F.; Wu, R.-S.; Srivastva, P.; Chang, L.; Huang, Y.-F.; Chang, H.-T.; Huang, C.-C. Light triggered programmable states of carbon dot liposomes accelerate chronic wound healing via photocatalytic cascade reaction. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Wan, W.L.; Tian, B.; Lin, Y.J.; Korupalli, C.; Lu, M.Y.; Cui, Q.; Wan, D.; Chang, Y.; Sung, H.W. Photosynthesis-inspired H2 generation using a chlorophyll-loaded liposomal nanoplatform to detect and scavenge excess ROS. Nat. Commun. 2020, 11, 534. [Google Scholar] [CrossRef]
- Xiong, Y.; Knoedler, S.; Alfertshofer, M.; Kim, B.-S.; Jiang, D.; Liu, G.; Rinkevich, Y.; Mi, B. Mechanisms and Therapeutic Opportunities in Metabolic Aberrations of Diabetic Wounds: A Narrative Review. Cell Death Dis. 2025, 16, 341. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Y.; Xu, Q.; Jiang, Q.; Chen, D.; Chen, T.; Xu, X.; Jin, Z.; He, Q. Photocatalytic glucose depletion and hydrogen generation for diabetic wound healing. Nat. Commun. 2022, 13, 5684. [Google Scholar] [CrossRef]
- El-Dafrawy, S.M.; Tarek, M.; Samra, S.; Hassan, S.M. Synthesis, photocatalytic and antidiabetic properties of ZnO/PVA nanoparticles. Sci. Rep. 2021, 11, 11404. [Google Scholar] [CrossRef]
- Buchovec, I.; Vyčaitė, E.; Badokas, K.; Sužiedelienė, E.; Bagdonas, S. Application of antimicrobial photodynamic therapy for inactivation of Acinetobacter baumannii biofilms. Int. J. Mol. Sci. 2022, 24, 722. [Google Scholar] [CrossRef]
- Martins Antunes de Melo, W.C.; Celiešiūtė-Germanienė, R.; Šimonis, P.; Stirkė, A. Antimicrobial photodynamic therapy (aPDT) for biofilm treatments. Possible synergy between aPDT and pulsed electric fields. Virulence 2021, 12, 2247–2272. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.A.; Cheburkanov, V.; Chen, S.; Soares, J.M.; Kassab, G.; Blanco, K.C.; Bagnato, V.S.; de Figueiredo, P.; Yakovlev, V.V. Breaking down antibiotic resistance in methicillin-resistant Staphylococcus aureus: Combining antimicrobial photodynamic and antibiotic treatments. Proc. Natl. Acad. Sci. USA 2022, 119, e2208378119. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, K.E.; Boeckh, S.; Stacey, H.J.; Jones, J.D. The microbiology of diabetic foot infections: A meta-analysis. BMC Infect. Dis. 2021, 21, 770. [Google Scholar] [CrossRef]
- Nieves, I.; Hally, C.; Viappiani, C.; Agut, M.; Nonell, S. A porphycene-gentamicin conjugate for enhanced photodynamic inactivation of bacteria. Bioorganic Chem. 2020, 97, 103661. [Google Scholar] [CrossRef]
- Barra, F.; Roscetto, E.; Soriano, A.A.; Vollaro, A.; Postiglione, I.; Pierantoni, G.M.; Palumbo, G.; Catania, M.R. Photodynamic and antibiotic therapy in combination to fight biofilms and resistant surface bacterial infections. Int. J. Mol. Sci. 2015, 16, 20417–20430. [Google Scholar] [CrossRef] [PubMed]
- Ronqui, M.R.; de Aguiar Coletti, T.M.; de Freitas, L.M.; Miranda, E.T.; Fontana, C.R. Synergistic antimicrobial effect of photodynamic therapy and ciprofloxacin. J. Photochem. Photobiol. B 2016, 158, 122–129. [Google Scholar] [CrossRef]
- Feng, Y.; Palanisami, A.; Ashraf, S.; Bhayana, B.; Hasan, T. Photodynamic inactivation of bacterial carbapenemases restores bacterial carbapenem susceptibility and enhances carbapenem antibiotic effectiveness. Photodiagnosis Photodyn. Ther. 2020, 30, 101693. [Google Scholar] [CrossRef]
- Mills, B.; Kiang, A.; Mohanan, S.M.P.C.; Bradley, M.; Klausen, M. Riboflavin-Vancomycin Conjugate Enables Simultaneous Antibiotic Photo-Release and Photodynamic Killing against Resistant Gram-Positive Pathogens. JACS Au 2023, 3, 3014–3023. [Google Scholar] [CrossRef]
- Costa Magacho, C.; Pinto, J.G.; Souza, B.M.N.; Pereira, A.H.C.; Ferreira-Strixino, J. Comparison of Photodynamic Therapy with Methylene Blue Associated with Ceftriaxone in Gram-Negative Bacteria; An In Vitro Study. Photodiagnosis Photodyn. Ther. 2020, 30, 101691. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Yang, S.; Hu, L.; Zhao, Y.; Meng, G.; Xu, S.; Han, R. Prevalence of multidrug-resistant bacterial infections in diabetic foot ulcers: A meta-analysis. Int. Wound J. 2024, 21, e14864. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.S.; Shah, R.; Khajavi, R.; Alizadeh, M.; Ghadiri, M.; Nasiri, E.; Dastan, D.; Farahani, M.; Zeynali, M.; Ebrahimi, M. Therapeutic Effect of Silver Nanoparticles in the Management of Diabetic Ulcers: A Systematic Review and Meta-Analysis on RCTs. Int. J. Low. Extrem. Wounds 2024, 15347346241241836. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, M.; Rizk, M.; Mahmoud, A.; Mohamed, H. The Efficacy of Silver Nanoparticles (Tetrasilver Tetroxide) in Treatment of Diabetic Foot Ulcer. QJM Int. J. Med. 2024, 117, hcae175.1022. [Google Scholar] [CrossRef]
- Akhtar, F.; Khan, A.U.; Qazi, B.; Kulanthaivel, S.; Mishra, P.; Akhtar, K.; Ali, A. A Nano Phototheranostic Approach of Toluidine Blue Conjugated Gold-Silver Core Shells Mediated Photodynamic Therapy to Treat Diabetic Foot Ulcer. Sci. Rep. 2021, 11, 24464. [Google Scholar] [CrossRef]
- Parasuraman, P.; R.Y, T.; Shaji, C.; Sharan, A.; Bahkali, A.H.; Al-Harthi, H.F.; Syed, A.; Anju, V.T.; Dyavaiah, M.; Siddhardha, B. Biogenic silver nanoparticles decorated with methylene blue potentiated the photodynamic inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Pharmaceutics 2020, 12, 709. [Google Scholar] [CrossRef]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
- Li, H.; Wen, T.; Wang, T.; Ji, Y.; Shen, Y.; Chen, J.; Xu, H.; Wu, X. In Vivo Metabolic Response upon Exposure to Gold Nanorod Core/Silver Shell Nanostructures: Modulation of Inflammation and Upregulation of Dopamine. Int. J. Mol. Sci. 2020, 21, 384. [Google Scholar] [CrossRef]
- Al-Doaiss, A.; Jarrar, Q.; Moshawih, S. Hepatic Histopathological and Ultrastructural Alterations Induced by 10 nm Silver Nanoparticles. IET Nanobiotechnol. 2020, 14, 405–411. [Google Scholar] [CrossRef]
- Nosrati, H.; Hajipour, M.J.; Dehghan Ghanbari, F.; Mahjoub, S.; Ghasemzadeh, M.; Mahmoudi, M. The Potential Renal Toxicity of Silver Nanoparticles after Repeated Oral Exposure and Its Underlying Mechanisms. BMC Nephrol. 2021, 22, 228. [Google Scholar] [CrossRef]
- den Hollander, D. Invited Commentary: Management of Hypergranulation Requires a Multimodal Approach. World J. Surg. 2023, 47, 3105–3106. [Google Scholar] [CrossRef]
- Brandão, M.G.S.A.; Ximenes, M.A.M.; Strachan, R.; Costa, I.G. Photodynamic Therapy Associated with Mesalt® in the Treatment of Hypergranulation in Diabetic Foot Ulcer. Wound Care Can. 2024, 22, 2. [Google Scholar] [CrossRef]
- Li, H.; Jiang, J.; Lv, X.; Xu, Y.; Wang, W.; Yang, D.; Dong, X. Enzyme-Like Photocatalytic Octahedral Rh/Ag2MoO4 Accelerates Diabetic Wound Healing by Photo-Eradication of Pathogen and Relieving Wound Hypoxia. Small 2024, 20, e2402723. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Wang, T.Y. Study on antibacterial activity and structure of chemically modified lysozyme. Molecules 2022, 28, 95. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- Okamoto, I.; Miyaji, H.; Miyata, S.; Shitomi, K.; Sugaya, T.; Ushijima, N.; Akasaka, T.; Enya, S.; Saita, S.; Kawasaki, H. Antibacterial and Antibiofilm Photodynamic Activities of Lysozyme-Au Nanoclusters/Rose Bengal Conjugates. ACS Omega 2021, 6, 9279–9290. [Google Scholar] [CrossRef]
- Li, Z.; Lu, S.; Liu, W.; Dai, T.; Ke, J.; Li, X.; Li, R.; Zhang, Y.; Chen, Z.; Chen, X. Synergistic lysozyme-photodynamic therapy against resistant bacteria based on an intelligent upconversion nanoplatform. Angew. Chem. Int. Ed. 2021, 60, 19201–19206. [Google Scholar] [CrossRef] [PubMed]
- Bispo, M.; Santos, S.B.; Melo, L.D.R.; Azeredo, J.; van Dijl, J.M. Targeted antimicrobial photodynamic therapy of biofilm-embedded and intracellular Staphylococci with a phage endolysin’s cell binding domain. Microbiol. Spectr. 2022, 10, e0146621. [Google Scholar] [CrossRef]
- Petrosino, A.; Saporetti, R.; Starinieri, F.; Sarti, E.; Ulfo, L.; Boselli, L.; Cantelli, A.; Morini, A.; Zadran, S.K.; Zuccheri, G.; et al. A modular phage vector platform for targeted photodynamic therapy of Gram-negative bacterial pathogens. iScience 2023, 26, 108032. [Google Scholar] [CrossRef]
- Frei, B.; Stocker, R.; Ames, B.N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc. Natl. Acad. Sci. USA 1988, 85, 9748–9752. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Inorganic Salts and Antimicrobial Photodynamic Therapy: Mechanistic Conundrums? Molecules 2018, 23, 3190. [Google Scholar] [CrossRef]
- Bispo, M.; Suhani, S.; van Dijl, J.M. Empowering antimicrobial photodynamic therapy of Staphylococcus aureus infections with potassium iodide. J. Photochem. Photobiol. B Biol. 2021, 225, 112334. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Beltran, P.A.; Levine, H.; Altamirano, D.S.; Martinez, J.D.; Durkee, H.; Mintz, K.; Leblanc, R.; Tóthová, J.D.; Miller, D.; Parel, J.M.; et al. Rose Bengal photodynamic antimicrobial therapy: A review of the intermediate-term clinical and surgical outcomes. Am. J. Ophthalmol. 2022, 243, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.D.; Arrieta, E.; Naranjo, A.; Monsalve, P.; Mintz, K.J.; Peterson, J.; Arboleda, A.; Durkee, H.; Aguilar, M.C.; Pelaez, D.; et al. Rose Bengal photodynamic antimicrobial therapy: A pilot safety study. Cornea 2021, 40, 1036–1043. [Google Scholar] [CrossRef]
- Greczek-Stachura, M.; Różanowski, B.; Kania, A. The effect of rose bengal activated with green diode laser light on selected Gram-positive and Gram-negative bacterial strains. Ann. Univ. Paedagog. Cracoviensis Stud. Nat. 2023, 8, 53–67. [Google Scholar] [CrossRef]
- Wei, D.; Hamblin, M.R.; Wang, H.; Fekrazad, R.; Wang, C.; Wen, X. Rose Bengal diacetate-mediated antimicrobial photodynamic inactivation: Potentiation by potassium iodide and acceleration of wound healing in MRSA-infected diabetic mice. BMC Microbiol. 2024, 24, 246. [Google Scholar] [CrossRef] [PubMed]
- Mikziński, P.; Kraus, K.; Widelski, J.; Paluch, E. Modern Microbiological Methods to Detect Biofilm Formation in Orthopedy and Suggestions for Antibiotic Therapy, with Particular Emphasis on Prosthetic Joint Infection (PJI). Microorganisms 2024, 12, 1198. [Google Scholar] [CrossRef]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.P. Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms 2020, 8, 1580. [Google Scholar] [CrossRef]
- Afonso, A.C.; Oliveira, D.; Saavedra, M.J.; Borges, A.; Simões, M. Biofilms in Diabetic Foot Ulcers: Impact, Risk Factors and Control Strategies. Int. J. Mol. Sci. 2021, 22, 8278. [Google Scholar] [CrossRef]
- Dong, H.; Yang, K.; Zhang, Y.; Li, Q.; Xiu, W.; Ding, M.; Shan, J.; Mou, Y. Photocatalytic Cu2WS4 Nanocrystals for Efficient Bacterial Killing and Biofilm Disruption. Int. J. Nanomed. 2022, 17, 2735–2750. [Google Scholar] [CrossRef]
- Oliveira, R.I.S.; de Oliveira, I.N.; de Conto, J.F.; de Souza, A.M.; Batistuzzo de Medeiros, S.R.; Egues, S.M.; Padilha, F.F.; Hernández-Macedo, M.L. Photocatalytic Effect of N-TiO2 Conjugated with Folic Acid against Biofilm-Forming Resistant Bacteria. Heliyon 2023, 9, e22108. [Google Scholar] [CrossRef]
- Warrier, A.; Mazumder, N.; Prabhu, S.; Satyamoorthy, K.; Murali, T.S. Photodynamic Therapy to Control Microbial Biofilms. Photodiagnosis Photodyn. Ther. 2021, 33, 102090. [Google Scholar] [CrossRef]
- Taraszkiewicz, A.; Fila, G.; Grinholc, M.; Nakonieczna, J. Innovative strategies to overcome biofilm resistance. Biomed Res Int. 2013, 2013, 150653. [Google Scholar] [CrossRef] [PubMed]
- Maric, T.; Løvind, A.; Zhang, Z.; Geng, J.; Boisen, A. Near-Infrared Light-Driven Mesoporous SiO2/Au Nanomotors for Eradication of Pseudomonas aeruginosa Biofilm. Adv. Healthc. Mater. 2023, 12, e2203018. [Google Scholar] [CrossRef]
- Deng, Y.; Zheng, J.; Li, J.; Liu, B.; Chen, K.; Xu, Y.; Deng, L.; Liu, H.; Liu, Y.N. NIR Light-Driven Nanomotor with Cascade Photodynamic Therapy for MRSA Biofilm Eradication and Diabetic Wound Healing. Theranostics 2025, 15, 3474–3489. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G.; Yin, H.; An, T. Bacterial Response Mechanism during Biofilm Growth on Different Metal Material Substrates: EPS Characteristics, Oxidative Stress and Molecular Regulatory Network Analysis. Environ. Res. 2020, 185, 109451. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, D.; Li, W.; Lin, H.; Ding, C.; Liu, Q.; Wang, L.; Li, Z.; Mei, L.; Chen, H.; et al. Biofilm Microenvironment Triggered Self-Enhancing Photodynamic Immunomodulatory Microneedle for Diabetic Wound Therapy. Nat. Commun. 2023, 14, 7658. [Google Scholar] [CrossRef]
- Cheng, J.; Gan, G.; Zheng, S.; Zhang, G.; Zhu, C.; Liu, S.; Hu, J. Biofilm Heterogeneity-Adaptive Photoredox Catalysis Enables Red Light-Triggered Nitric Oxide Release for Combating Drug-Resistant Infections. Nat. Commun. 2023, 14, 7510. [Google Scholar] [CrossRef]
- Gupta, A.K.; Shemer, A.; Economopoulos, V.; Talukder, M. Diabetic Foot and Fungal Infections: Etiology and Management from a Dermatologic Perspective. J. Fungi 2024, 10, 577. [Google Scholar] [CrossRef]
- Bansal, E.; Garg, A.; Bhatia, S.; Attri, A.K.; Chander, J. Spectrum of Microbial Flora in Diabetic Foot Ulcers. Indian J. Pathol. Microbiol. 2008, 51, 204–208. [Google Scholar] [CrossRef]
- Zubair, M.; Husain, F.M.; Al-Amri, M.; Hasan, I.; Hassan, I.; Albalawi, T.; Fatima, F.; Khan, A.; Arshad, M.; Alam, P.; et al. In Vitro Inhibition of Biofilm and Virulence Factor Production in Azole-Resistant Strains of Candida albicans Isolated from Diabetic Foot by Artemisia vulgaris Stabilized Tin (IV) Oxide Nanoparticles. Front. Cell. Infect. Microbiol. 2024, 13, 1322778. [Google Scholar] [CrossRef]
- Tang, N.; Yuan, S.; Luo, Y.; Wang, A.J.; Sun, K.; Liu, N.N.; Tao, K. Nanoparticle-Based Photodynamic Inhibition of Candida albicans Biofilms with Interfering Quorum Sensing. ACS Omega 2023, 8, 4357–4368. [Google Scholar] [CrossRef] [PubMed]

| Feature | Gram-Positive Bacteria | Gram-Negative Bacteria |
|---|---|---|
| Cell Wall Thickness | 20–80 nm | 10 nm |
| Peptidoglycan Content | >50% | 10–20% |
| Lipid and Lipoprotein Content | 0–3% | 58% |
| Presence of Lipopolysaccharides (LPSs) | Absent | 13% |
| Permeability to Reactive Oxygen Species (ROS) | High—the cell wall is porous, facilitating ROS penetration | Low—the outer lipid membrane restricts ROS access |
| Effectiveness of Photocatalysis | Higher—ROS easily penetrate, causing damage to proteins, lipids, and DNA | Lower—requires outer membrane damage first, demanding longer exposure and higher energy |
| Effect of ROS Damage | Rapid loss of proteins and K+ ions, DNA damage, enzyme denaturation | Slow outer membrane damage first, then cytoplasmic and genetic material effects |
| Additional Defense Mechanisms | Some bacteria produce endospores, biofilm, or a polysaccharide layer | LPS and biofilms provide protection against photocatalysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikziński, P.; Kraus, K.; Seredyński, R.; Widelski, J.; Paluch, E. Photocatalysis and Photodynamic Therapy in Diabetic Foot Ulcers (DFUs) Care: A Novel Approach to Infection Control and Tissue Regeneration. Molecules 2025, 30, 2323. https://doi.org/10.3390/molecules30112323
Mikziński P, Kraus K, Seredyński R, Widelski J, Paluch E. Photocatalysis and Photodynamic Therapy in Diabetic Foot Ulcers (DFUs) Care: A Novel Approach to Infection Control and Tissue Regeneration. Molecules. 2025; 30(11):2323. https://doi.org/10.3390/molecules30112323
Chicago/Turabian StyleMikziński, Paweł, Karolina Kraus, Rafał Seredyński, Jarosław Widelski, and Emil Paluch. 2025. "Photocatalysis and Photodynamic Therapy in Diabetic Foot Ulcers (DFUs) Care: A Novel Approach to Infection Control and Tissue Regeneration" Molecules 30, no. 11: 2323. https://doi.org/10.3390/molecules30112323
APA StyleMikziński, P., Kraus, K., Seredyński, R., Widelski, J., & Paluch, E. (2025). Photocatalysis and Photodynamic Therapy in Diabetic Foot Ulcers (DFUs) Care: A Novel Approach to Infection Control and Tissue Regeneration. Molecules, 30(11), 2323. https://doi.org/10.3390/molecules30112323







