Clove, Cinnamon, and Peppermint Essential Oils as Antibiofilm Agents Against Alicyclobacillus acidoterrestris
Abstract
1. Introduction
2. Results
2.1. Essential Oils’ Chemical Composition
2.2. Essential Oils’ Activity Against A. acidoterrestris Plaktonic Cells
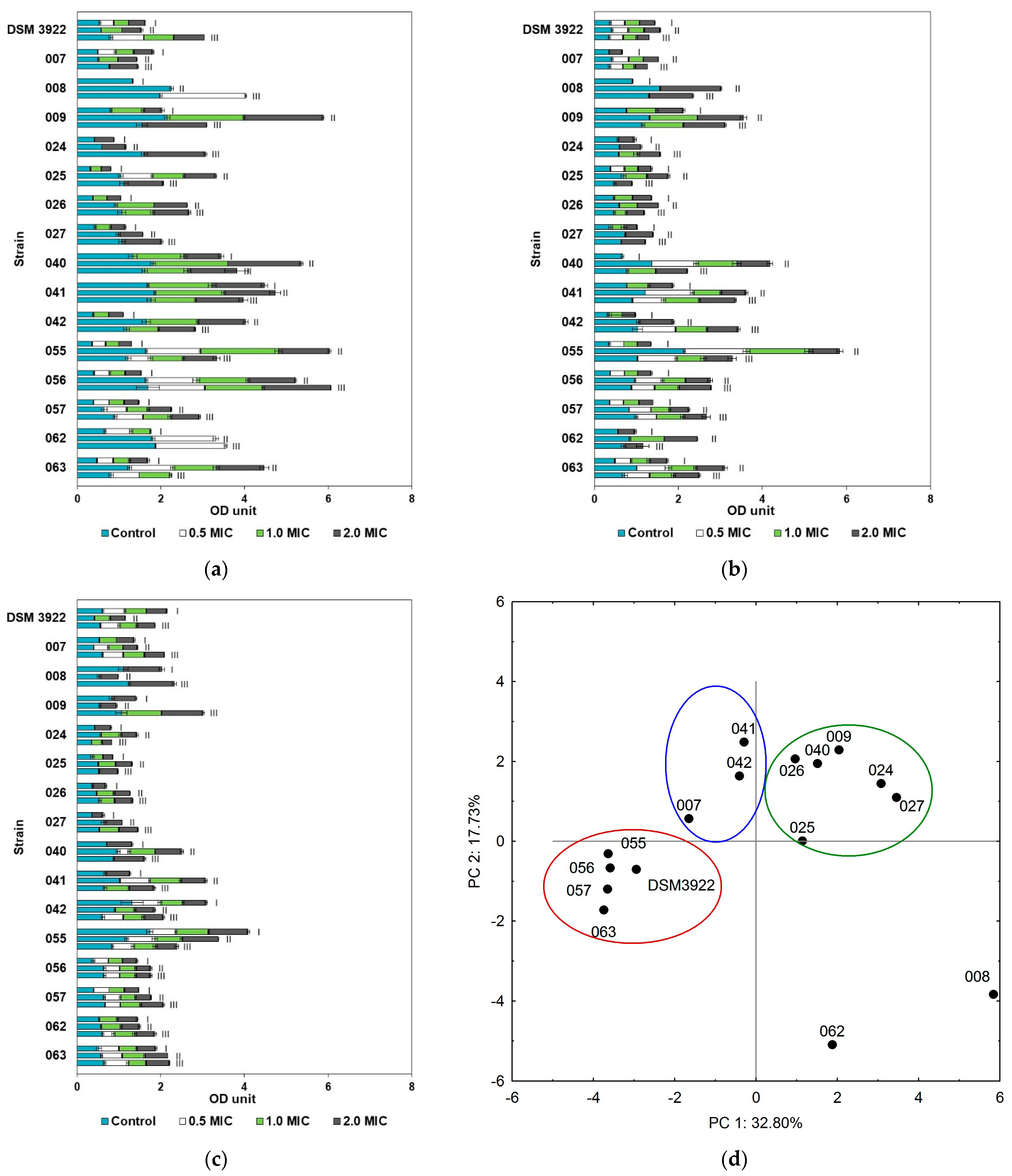
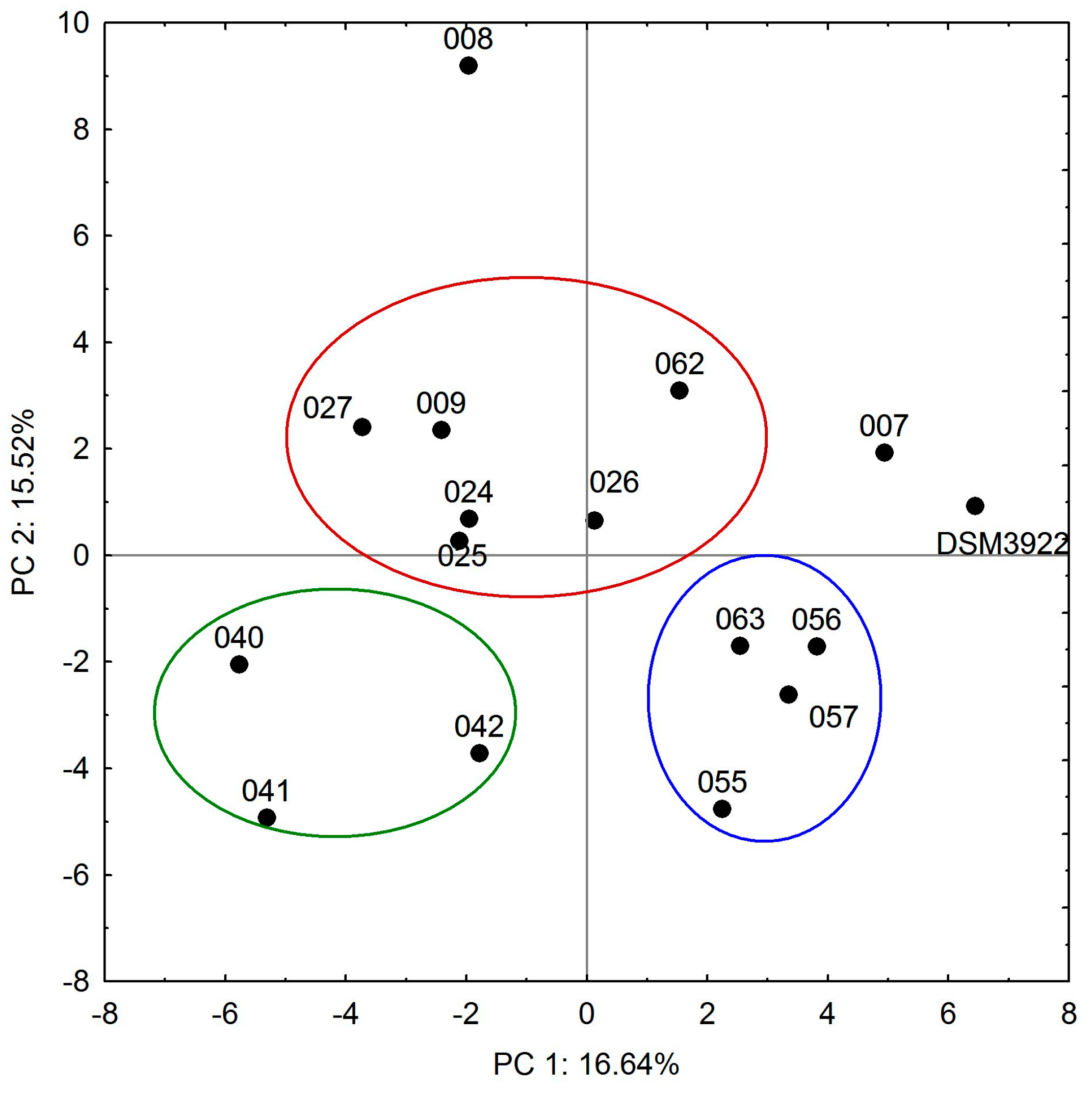
2.3. Biofilm Formation and Eradication with Essential Oils
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. Essential Oils and Their Chemical Analysis
4.3. Determination of Antibacterial Activity of Essential Oils
4.4. Bacterial Biofilm Production Assay
4.5. Bacterial Biofilm Production in Presence of Essential Oils
4.6. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerny, G.; Hennlich, W.; Poralla, K. Fruchtsaftverderb durch bacillen: Isolierung und charakterisierung des verderbserregers. Z. Lebensm. Unters. Forsch. 1984, 179, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Teduka, H.; Shinano, H. Isolation and identification of Alicyclobacillus acidoterrestris from acidic beverages. Biosci. Biotechnol. Biochem. 1996, 60, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, B.; Połaska, M.; Dekowska, A. Alicyclobacillus—Still current issues in the beverage industry. In Safety Issues in Beverage Production; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 18, pp. 105–146. [Google Scholar] [CrossRef]
- Roth, K.; Rana, Y.S.; Daeschel, D.; Kovac, J.; Worobo, R.; Snyder, A.B. Alicyclobacillus mali sp. nov., Alicyclobacillus suci sp. nov. and Alicyclobacillus fructus sp. nov., thermoacidophilic sporeforming bacteria isolated from fruit beverages. Int. J. Syst. Evol. Microbiol. 2021, 71, 005016. [Google Scholar] [CrossRef]
- Ding, H.; Wang, T.; Sun, Y.; Zhang, Y.; Wei, J.; Cai, R.; Guo, C.; Yuan, Y.; Yue, T. Role and mechanism of cold plasma in inactivating Alicyclobacillus acidoterrestris in apple juice. Foods 2023, 12, 1531. [Google Scholar] [CrossRef]
- Sokołowska, B.; Niezgoda, J.; Bytońska, M.; Frankiel, A. Bioróżnorodność szczepów Alicyclobacillus acidoterrestris. Pr. Inst. I Lab. Badaw. Przem. Spożywczego 2010, 65, 29–38. [Google Scholar]
- Deinhard, G.; Blanz, P.; Poralla, K.; Altan, E. Bacillus acidoterrestris sp. nov., a new thermotolerant acidophile isolated from different soils. Syst. Appl. Microbiol. 1987, 10, 47–53. [Google Scholar] [CrossRef]
- Jensen, N.; Whitfield, F.B. Role of Alicyclobacillus acidoterrestris in the development of a disinfectant taint in shelf-stable fruit juice. Lett. Appl. Microbiol. 2003, 36, 9–14. [Google Scholar] [CrossRef]
- Chang, S.-S.; Kang, D.-H. Alicyclobacillus spp. in the fruit juice industry: History, characteristics, and current isolation/detection procedures. Crit. Rev. Microbiol. 2004, 30, 55–74. [Google Scholar] [CrossRef]
- Walls, I.; Chuyate, R. Alicyclobacillus—Historical perspective and preliminary characterization study. Dairy Food Environ. Sanit. 1998, 18, 499–503. [Google Scholar]
- Duong, H.-A.; Jensen, N. Spoilage of iced tea by Alicyclobacillus: Alicyclobacillus in the food industry. Food Aust. 2000, 52, 281–295. [Google Scholar]
- Bahçeci, K.S.; Gökmen, V.; Acar, J. Occurrence of Alicyclobacillus acidoterrestris on apples and in apple juice concentrates and effect of process technology on A. acidoterrestris spores in apple juice. Fruit Process. 2005, 10, 328–331. [Google Scholar]
- Lee, S.-Y.; Chang, S.-S.; Shin, J.-H.; Kang, D.-H. Membrane filtration method for enumeration and isolation of Alicyclobacillus spp. from apple juice. Lett. Appl. Microbiol. 2007, 45, 540–546. [Google Scholar] [CrossRef]
- McKnight, I.C.; Eiroa, M.N.U.; Sant’Ana, A.S.; Massaguer, P.R. Alicyclobacillus acidoterrestris in pasteurized exotic Brazilian fruit juices: Isolation, genotypic characterization and heat resistance. Food Microbiol. 2010, 27, 1016–1022. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Corbo, M.R. Characterization of wild strain of Alicyclobacillus acidoterrestris: Heat resistance and implications for tomato juice. J. Food Sci. 2011, 76, M130–M136. [Google Scholar] [CrossRef] [PubMed]
- Danyluk, M.D.; Friedrich, L.M.; Jouquand, C.; Goodrich-Schneider, R.; Parish, M.E.; Rouseff, R. Prevalence, concentration, spoilage, and mitigation of Alicyclobacillus spp. in tropical and subtropical fruit juice concentrates. Food Microbiol. 2011, 28, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yue, T.; Yuan, Y. Alicyclobacillus contamination in the production line of kiwi products in China. PLoS ONE 2013, 8, e67704. [Google Scholar] [CrossRef]
- Tayefe, M.; Zade, A.N.; Asl, M.S.; Hashemi, S.A. Isolation of Alicyclobacillus acidoterrestris from commercial spoiled apple juice and study on some influence parameters on its growth in apple juice. Biol. Forum Int. J. 2014, 6, 41–45. [Google Scholar] [CrossRef]
- Ciuffreda, E.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M. Alicyclobacillus spp.: New insights on ecology and preserving food quality through new approaches. Microorganisms 2015, 3, 625–640. [Google Scholar] [CrossRef]
- Shayanfar, S.; Harzman, C.; Pillai, S.D. Fruit juice and puree characteristics influence enrichment requirements for real-time PCR detection of Alicyclobacillus acidoterrestris. Int. J. Food Contam. 2015, 2, 2. [Google Scholar] [CrossRef][Green Version]
- Sokołowska, B.; Niezgoda, J.; Dekowska, A.; Porębska, I.; Nasiłowska, J.; Waldon-Wiewióra, E.; Kowalska, M. Incidence of Alicyclobacillus spp. in Polish apple and dark berry juice concentrates and the ability of isolated A. acidoterrestris strains to spoilage of these juices. Postep. Nauk. I Technol. Przem. Rolno-Spożywczego 2016, 71, 5–20. [Google Scholar]
- da Silva, D.A.M.; Fernandes, M.S.; Endo, E.H.; Vital, A.C.P.; Britta, E.A.; Favero, M.E.; Castro, J.C.; Matumoto-Pintro, P.T.; Dias Filho, B.P.; Nakamura, C.V.; et al. Control of the growth of Alicyclobacillus acidoterrestris in industrialized orange juice using rosemary essential oil and nisin. Lett. Appl. Microbiol. 2020, 72, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Sourri, P.; Tassou, C.C.; Nychas, G.-J.E.; Panagou, E.Z. Fruit juice spoilage by Alicyclobacillus: Detection and control methods—A comprehensive review. Foods 2022, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Shemesh, M.; Ostrov, I. Role of Bacillus species in biofilm persistence and emerging antibiofilm strategies in the dairy industry. J. Sci. Food Agric. 2020, 100, 2327–2336. [Google Scholar] [CrossRef]
- Ban, G.-H.; Kim, S.-H.; Kang, D.-H.; Park, S.-H. Comparison of the efficacy of physical and chemical strategies for the inactivation of biofilm cells of foodborne pathogens. Food Sci. Biotechnol. 2023, 32, 1679–1702. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ordonez, A.; Coughlan, L.M.; Briandet, R.; Cotter, P.D. Biofilms in food processing environments: Challenges and opportunities. Annu. Rev. Food Sci. Technol. 2019, 10, 173–195. [Google Scholar] [CrossRef]
- Podolak, R.; Elliott, P.H.; Taylor, B.J.; Khurana, A.; Black, D.G. Destruction of Alicyclobacillus acidoterrestris spores in apple juice on stainless steel surfaces by chemical disinfectants. J. Food Prot. 2009, 72, 510–514. [Google Scholar] [CrossRef]
- dos Anjos, M.M.; Ruiz, S.P.; Nakamura, C.V.; de Abreu Filho, B.A. Resistance of Alicyclobacillus acidoterrestris spores and biofilm to industrial sanitizers. J. Food Prot. 2013, 76, 1408–1413. [Google Scholar] [CrossRef]
- Shemesh, M.; Pasvolsky, R.; Zakin, V. External pH is a cue for the behavioral switch that determines surface motility and biofilm formation of Alicyclobacillus acidoterrestris. J. Food Prot. 2014, 77, 1418–1423. [Google Scholar] [CrossRef]
- Tyfa, A.; Kunicka-Styczyńska, A.; Zabielska, J. Evaluation of hydrophobicity and quantitative analysis of biofilm formation by Alicyclobacillus sp. Acta Biochim. Pol. 2015, 62, 785–790. [Google Scholar] [CrossRef]
- Kunicka-Styczyńska, A.; Tyfa, A.; Laskowski, D.; Plucińska, A.; Rajkowska, K.; Kowal, K. Clove oil (Syzygium aromaticum L.) activity against Alicyclobacillus acidoterrestris biofilm on technical surfaces. Molecules 2020, 25, 3334. [Google Scholar] [CrossRef] [PubMed]
- Romeu, M.J.; Rodrigues, D.; Azeredo, J. Effect of sub-lethal chemical disinfection on the biofilm forming ability, resistance to antibiotics and expression of virulence genes of Salmonella Enteritidis biofilm-surviving cells. Biofouling 2020, 36, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Lories, B.; Roberfroid, S.; Dieltjens, L.; De Coster, D.; Foster, K.R.; Steenackers, H.P. Biofilm bacteria use stress responses to detect and respond to competitors. Curr. Biol. 2020, 30, 1231–1244. [Google Scholar] [CrossRef]
- Ben Akacha, B.; Michalak, M.; Generalić Mekinić, I.; Kačániová, M.; Chaari, M.; Brini, F.; Ben Saad, R.; Mnif, W.; Garzoli, S.; Ben Hsouna, A. Mixture design of α-pinene, α-terpineol, and 1,8-cineole: A multiobjective response followed by chemometric approaches to optimize the antibacterial effect against various bacteria and antioxidant activity. Food Sci. Nutr. 2024, 12, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.; Carvalho, F.; Duarte, A.P.; Ferreira, S. Antimicrobial activity of Thymus zygis essential oil against Listeria monocytogenes and its application as food preservative. Innov. Food Sci. Emerg. Technol. 2022, 80, 103077. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant antimicrobials for food quality and safety: Recent views and future challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.-P.; Pei, R.-S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, H.; Zhang, W.; Mu, H.; Sun, C.; Duan, J. Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett. Appl. Microbiol. 2015, 60, 421–430. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Olkiewicz, D.; Tarnawska, P.; Warżyńska, O. Potential of carvacrol and thymol in reducing biofilm formation on technical surfaces. Molecules 2021, 26, 2723. [Google Scholar] [CrossRef]
- Huang, X.; Lao, Y.; Pan, Y.; Chen, Y.; Zhao, H.; Gong, L.; Xie, N.; Mo, C.-H. Synergistic antimicrobial effectiveness of plant essential oil and its application in seafood preservation: A review. Molecules 2021, 26, 307. [Google Scholar] [CrossRef]
- Li, Y.-X.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Piotrowska-Cyplik, A.; Cyplik, P. Analysis of the effect of various potential antimicrobial agents on the quality of the unpasteurized carrot juice. Molecules 2023, 28, 6297. [Google Scholar] [CrossRef] [PubMed]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Evaluation of antibiofilm efficacy of essential oil components β-caryophyllene, cinnamaldehyde and eugenol alone and in combination against biofilm formation and preformed biofilms of Listeria monocytogenes and Salmonella typhimurium. Lett. Appl. Microbiol. 2020, 71, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Somrani, M.; Inglés, M.-C.; Debbabi, H.; Abidi, F.; Palop, A. Garlic, onion, and cinnamon essential oil anti-biofilms’ effect against Listeria monocytogenes. Foods 2020, 9, 567. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Adams, T.B.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Munro, I.C.; Portoghese, P.S.; Smith, R.L.; Waddell, W.J.; et al. The FEMA GRAS assessment of cinnamyl derivatives used as flavor ingredients. Food Chem. Toxicol. 2004, 42, 157–185. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Kim, S.I.; Baek, K.H.; Lee, J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int. J. Food Microbiol. 2015, 195, 30–39. [Google Scholar] [CrossRef]
- Ferreira, L.R.; Rosário, D.K.A.; Silva, P.I.; Carneiro, J.C.S.; Pimentel Filho, N.J.; Bernardes, P.C. Cinnamon essential oil reduces adhesion of food pathogens to polystyrene. Int. Food Res. J. 2019, 26, 1103–1110. [Google Scholar]
- de Oliveira, M.M.M.; Brugnera, D.F.; do Nascimento, J.A.; Batista, N.N.; Piccoli, R.H. Cinnamon essential oil and cinnamaldehyde in the control of bacterial biofilms formed on stainless steel surfaces. Eur. Food Res. Technol. 2012, 234, 821–832. [Google Scholar] [CrossRef]
- Apolonio, J.; Faleiro, M.L.; Miguel, M.G.; Neto, L. No induction of antimicrobial resistance in Staphylococcus aureus and Listeria monocytogenes during continuous exposure to eugenol and citral. FEMS Microbiol. Lett. 2014, 354, 92–101. [Google Scholar] [CrossRef]
- Perez-Conesa, D.; Cao, J.; Chen, L.; McLandsborough, L.; Weiss, J. Inactivation of Listeria monocytogenes and Escherichia coli O157:H7 biofilms by micelle-encapsulated eugenol and carvacrol. J. Food Prot. 2011, 74, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Millezi, A.F.; Costa, K.A.D.; Oliveira, J.M.; Lopes, S.P.; Pereira, M.O.; Piccoli, R.H. Antibacterial and anti-biofilm activity of cinnamon essential oil and eugenol. Cienic. Rural 2019, 49, e20180314. [Google Scholar] [CrossRef]
- Piovezan, M.; Uchida, N.S.; da Silva, A.F.; Grespan, P.R.; Silva, E.L.; Cuman, R.K.N.; Machinski, M., Jr.; Mikcha, M.J.G. Effect of cinnamon essential oil and cinnamaldehyde on Salmonella Saintpaul biofilm on a stainless steel surface. J. Gen. Appl. Microbiol. 2014, 60, 119–121. [Google Scholar] [CrossRef]
- Hosseini, M.H.; Razavi, S.H.; Mousavi, M.A. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process. Preserv. 2008, 33, 727–743. [Google Scholar] [CrossRef]
- Sharma, D.; Dhanjal, D.S.; Mittal, B. Development of edible biofilm containing cinnamon to control food-borne pathogen. J. Appl. Pharm. Sci. 2017, 7, 160–164. [Google Scholar] [CrossRef]
- Maldonado, M.C.; Aban, M.P.; Navarro, A.R. Chemicals and lemon essential oil effect on Alicyclobacillus acidoterrestris viability. Braz. J. Microbiol. 2013, 44, 1133–1137. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Corbo, M.R. Control of Alicyclobacillus acidoterrestris in apple juice by citrus extracts and a mild heat-treatment. Food Control 2013, 31, 553–559. [Google Scholar] [CrossRef]
- Huertas, J.P.; Esteban, M.D.; Antolinos, V.; Palop, A. Combined effect of natural antimicrobials and thermal treatments on Alicyclobacillus acidoterrestris spores. Food Control 2014, 35, 73–78. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Inhibition of Alicyclobacillus acidoterrestris spores by natural compounds. Int. J. Food Sci. Technol. 2008, 43, 1271–1275. [Google Scholar] [CrossRef]
- Lim, H.W.; Kim, H.; Kim, J.; Bae, D.; Song, K.Y.; Chon, J.W.; Lee, J.M.; Kim, S.H.; Kim, D.H.; Seo, K.H. Antimicrobial effect of Mentha piperita (Peppermint) oil against Bacillus cereus, Staphylococcus aureus, Cronobacter sakazakii, and Salmonella Enteritidis in various dairy foods: Preliminary study. J. Milk Sci. Biotechnol. 2018, 36, 146–154. [Google Scholar] [CrossRef]
- Horváth, P.; Koščová, J. In Vitro antibacterial activity of mentha essential oils against Staphylococcus aureus. Folia Vet. 2017, 61, 71–77. [Google Scholar] [CrossRef][Green Version]
- Mancuso, M. The antibacterial activity of mentha. In Herbs and Spices; Akram, M., Shabir Ahmad, R., Eds.; IntechOpen: London, UK, 2020; pp. 83–92. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT Food Sci. Technol. 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Mevo, S.I.U.; Song, M.; Chowdhury, M.A.H.; Shaila, S.; Kim, D.H.; Nahar, S.; Toushik, S.H.; Park, S.H.; Ha, S.-D. Antibiofilm mechanism of peppermint essential oil to avert biofilm developed by foodborne food spoilage pathogens on food contact surfaces. J. Food Sci. 2023, 88, 3935–3955. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N. Chemical composition and antimicrobial activity of peppermint (Mentha piperita L.) essential oil. Songklanakarin. J. Sci. Technol. 2014, 36, 83–87. [Google Scholar]
- Gandova, V.; Fidan, H.; Iliev, I.; Lasheva, V.; Stankov, S.; Stoyanova, A.; Yavorov, N. Comparative analysis of antibacterial activity and physicochemical properties of peppermint and cornmint essential oils and their main compound menthol. J. Chem. Technol. Metall. 2023, 58, 664–671. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia, 5th ed.; European Union: Brussels, Belgium, 2005. [Google Scholar]
- Nowak, K.; Ogonowski, J.; Jaworska, M.; Grzesik, K. Olejek goździkowy—Właściwości i zastosowanie. Chemik 2012, 66, 145–148. [Google Scholar]
- Razafimamonjison, G.; Jahiel, M.; Duclos, T.; Ramanoelina, P.; Fawbush, F.; Danthu, P. Bud, leaf and stem essential oil composition of clove (Syzygium aromaticum L.) from Indonesia, Madagascar and Zanzibar. Int. J. Basic Appl. Sci. 2014, 3, 224–233. [Google Scholar] [CrossRef][Green Version]
- do Prado, D.B.; dos Anjos Szczerepa, M.M.; Capeloto, O.A.; Astrath, N.G.C.; dos Santos, N.C.A.; Previdelli, I.T.S.; Nakamura, C.V.; Mikcha, J.M.G.; de Abreu Filho, B.A. Effect of ultraviolet (UV-C) radiation on spores and biofilms of Alicyclobacillus spp. in industrialized orange juice. Int. J. Food Microbiol. 2019, 305, 108238. [Google Scholar] [CrossRef]
- Dutra, T.V.; do Prado, D.B.; dos Anjos, M.M.; Machinski, M.J.; Mikcha, J.M.G.; de Abreu Filho, B.A. Contribution of environmental factors in the formation of biofilms by Alicyclobacillus acidoterrestris on surfaces of the orange juice industry. Cienic. Rural 2020, 50, e20190790. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, C.; Gao, Z.; Hu, Z.; Wang, Z.; Xiao, J.; Yuan, Y.; Yue, T. Inactivation effect of thymoquinone on Alicyclobacillus acidoterrestris vegetative cells, spores, and biofilms. Front. Microbiol. 2021, 12, 679808. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, N.; Meng, X.; Li, J.; Zhang, T.; Xu, R.; Wei, X.; Fan, M. Transcriptomic and metabolomic profiling uncovers response mechanisms of Alicyclobacillus acidoterrestris DSM 3922T to acid stress. Microbiol. Spectr. 2023, 11, e00022-23. [Google Scholar] [CrossRef] [PubMed]
- Mevo, S.I.U.; Ashrafudoulla, M.; Furkanur Rahaman Mizan, M.; Park, S.H.; Ha, S.D. Promising strategies to control persistent enemies: Some new technologies to combat biofilm in the food industry—A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5938–5964. [Google Scholar] [CrossRef]
- Bansal, M.; Nannapaneni, R.; Kode, D.; Chang, S.; Sharma, C.S.; McDaniel, C.; Kiess, A. Rugose Morphotype in Salmonella Typhimurium and Salmonella Heidelberg induced by sequential exposure to subinhibitory sodium hypochlorite aids in biofilm tolerance to lethal sodium hypochlorite on polystyrene and stainless steel surfaces. Front. Microbiol. 2019, 10, 2704. [Google Scholar] [CrossRef]
- Orr, R.V.; Beuchat, L.R. Efficacy of disinfectants in killing spores of Alicyclobacillus acidoterrestris and performance of media for supporting colony development by survivors. J. Food Prot. 2000, 63, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Combining eugenol and cinnamaldehyde to control the growth of Alicyclobacillus acidoterrestris. Food Control 2010, 21, 172–177. [Google Scholar] [CrossRef]
- Takahashi, T.; Kokubo, R.; Sakaino, M. Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett. Appl. Microbiol. 2004, 39, 60–64. [Google Scholar] [CrossRef]
- de Pascoli, I.C.; dos Anjos, M.M.; da Silva, A.A.; Lorenzetti, F.B.; Cortez, D.A.G.; Mikcha, J.M.G.; Nakamura, T.U.; Nakamura, C.V.; de Abreu Filho, B.A. Piperaceae extracts for controlling Alicyclobacillus acidoterrestris growth in commercial orange juice. Ind. Crops Prod. 2018, 116, 224–230. [Google Scholar] [CrossRef]
- Molva, C.; Baysal, A.H. Antimicrobial activity of grape seed extract on Alicyclobacillus acidoterrestris DSM 3922 vegetative cells and spores in apple juice. Food Sci. Technol. 2015, 60, 238–245. [Google Scholar] [CrossRef]
- Piskernik, S.; Klančnik, A.; Demšar, L.; Smole Možina, S.; Jeršek, B. Control of Alicyclobacillus spp. vegetative cells and spores in apple juice with rosemary extracts. Food Control 2016, 60, 205–214. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, M.; Cui, L.; Yuan, Y.; Yang, Y.; Wang, Z.; Yue, T. Antibacterial activity and mechanism of thymol against Alicyclobacillus acidoterrestris vegetative cells and spores. LWT Food Sci. Technol. 2019, 105, 377–384. [Google Scholar] [CrossRef]
- Friedrich, L.M.; Goodrich-Schneider, R.; Parish, M.E.; Danyluk, M.D. Mitigation of Alicyclobacillus spp. spores on food contact surfaces with aqueous chlorine dioxide and hypochlorite. Food Microbiol. 2009, 26, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Alberice, J.V.; Funes-Huacca, M.E.; Guterres, S.B.; Carrilho, E. Inactivation of Alicyclobacillus acidoterrestris in orange juice by saponin extracts combined with heat-treatment. Int. J. Food Microbiol. 2012, 159, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Smigielski, K.; Raj, A.; Krosowiak, K.; Gruska, R. Chemical composition of the essential oil of Lavandula angustifolia cultivated in Poland. J. Essent. Oil Bear. Plants 2009, 12, 338–347. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) M07. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 12th ed.; CLSI: Berwyn, PA, USA, 2024. [Google Scholar]


| Component | Clove Oil | Cinnamon Oil | Peppermint Oil | |||
|---|---|---|---|---|---|---|
| % | RI 1 | % | RI 1 | % | RI | |
| α-Pinene | - | - | - | - | 0.83 | 928 |
| Sabinene | - | - | - | - | 0.33 | 963 |
| β-Pinene | - | - | - | - | 1.20 | 967 |
| p-Cymene | - | - | 0.11 | 1009 | - | - |
| 1,8-Cineole | - | - | 0.28 | 1017 | 6.00 | 1017 |
| Limonene | - | - | 0.05 | 1019 | 2.03 | 1019 |
| Linalool | - | - | 6.59 | 1087 | - | - |
| (−)-Menthone | - | - | - | - | 22.46 | 1136 |
| Isomenthone | - | - | - | - | 3.31 | 1143 |
| Menthofuran | - | - | - | - | 2.30 | 1148 |
| (+)-Menthol | - | - | - | - | 2.59 | 1151 |
| (−)-Menthol | - | - | - | - | 46.41 | 1164 |
| Terpinen-4-ol | - | - | - | - | 0.43 | 1170 |
| α-Terpineol | - | - | - | - | 0.40 | 1174 |
| cis-Cinnamaldehyde | - | - | 0.24 | 1181 | - | - |
| Pulegone | - | - | - | - | 1.67 | 1214 |
| Piperitone | - | - | - | - | 0.61 | 1226 |
| trans-Cinnamaldehyde | - | - | 72.49 | 1279 | - | - |
| Menthyl acetate | - | - | - | - | 4.01 | 1577 |
| Eugenol | 86.99 | 1346 | 5.17 | 1332 | - | - |
| α-Copaene | 0.07 | 1377 | 0.15 | 1375 | - | - |
| trans-Cinnamyl acetate | - | - | 4.57 | 1412 | - | - |
| β-Caryophyllene | 8.76 | 1422 | 8.12 | 1421 | 2.03 | 1417 |
| α-Humulene | 1.91 | 1452 | 0.24 | 1450 | 0.24 | 1449 |
| Cadina-1(6),4-diene | 0.04 | 1468 | - | - | - | - |
| γ-Muurolene | 0.02 | 1470 | - | - | - | - |
| Eugenyl acetate | - | - | 0.18 | 1480 | - | - |
| β-Selinene | 0.02 | 1482 | - | - | - | - |
| α-Selinene | 0.05 | 1491 | - | - | - | - |
| β-Farnesene | 0.06 | 1494 | - | - | - | - |
| cis-Calamenene | 0.11 | 1509 | - | - | - | - |
| δ-Cadinene | 0.27 | 1513 | - | - | - | - |
| Cadina-1,4-diene | 0.03 | 1524 | - | - | - | - |
| Caryophyllene oxide | - | - | 0.16 | 1569 | - | - |
| Caryophyllene epoxide | 0.62 | 1571 | - | - | - | - |
| Humulene epoxide | 0.11 | 1594 | - | - | - | - |
| Benzyl benzoate | - | 1.05 | 1723 | - | ||
| Total | 99.06 | - | 99.40 | - | 96.85 | - |
| Strain | MIC (MBC) (% v/v) | ||
|---|---|---|---|
| Cinnamon | Clove | Peppermint | |
| DSM 3922 | 3.0 (5.0) | 0.05 (0.06) | 0.05 (0.06) |
| 007 | >5.0 | >5.0 | 1.0 (1.5) |
| 008 | 3.0 (5.0) | >5.0 | 1.5 (3.0) |
| 009 | >5.0 | >5.0 | 1.0 (1.5) |
| 024 | 5.0 (>5.0) | >5.0 | 1.0 (2.0) |
| 025 | >5.0 | >5.0 | 2.0 (3.0) |
| 026 | 3.0 (>5.0) | >5.0 | 2.0 (3.0) |
| 027 | >5.0 | >5.0 | 3.0 (>5.0) |
| 040 | >5.0 | >5.0 | 2.0 (3.0) |
| 041 | >5.0 | >5.0 | 1.0 (2.0) |
| 042 | >5.0 | >5.0 | 1.5 (3.0) |
| 055 | <0.05 (0.05) | 0.05 (0.06) | 1.5 (3.0) |
| 056 | <0.05 (0.05) | 0.05 (0.06) | 0.05 (0.06) |
| 057 | <0.05 (0.05) | 0.05 (0.06) | 0.05 (0.06) |
| 062 | 5.0 (>5.0) | (5.0) | 1.0 (1.5) |
| 063 | <0.05 (0.05) | <0.05 (0.06) | 0.06 (0.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyfa, A.; Kunicka-Styczyńska, A.; Molska, M.; Gruska, R.M.; Baryga, A. Clove, Cinnamon, and Peppermint Essential Oils as Antibiofilm Agents Against Alicyclobacillus acidoterrestris. Molecules 2025, 30, 2312. https://doi.org/10.3390/molecules30112312
Tyfa A, Kunicka-Styczyńska A, Molska M, Gruska RM, Baryga A. Clove, Cinnamon, and Peppermint Essential Oils as Antibiofilm Agents Against Alicyclobacillus acidoterrestris. Molecules. 2025; 30(11):2312. https://doi.org/10.3390/molecules30112312
Chicago/Turabian StyleTyfa, Agnieszka, Alina Kunicka-Styczyńska, Magdalena Molska, Radosław Michał Gruska, and Andrzej Baryga. 2025. "Clove, Cinnamon, and Peppermint Essential Oils as Antibiofilm Agents Against Alicyclobacillus acidoterrestris" Molecules 30, no. 11: 2312. https://doi.org/10.3390/molecules30112312
APA StyleTyfa, A., Kunicka-Styczyńska, A., Molska, M., Gruska, R. M., & Baryga, A. (2025). Clove, Cinnamon, and Peppermint Essential Oils as Antibiofilm Agents Against Alicyclobacillus acidoterrestris. Molecules, 30(11), 2312. https://doi.org/10.3390/molecules30112312








