Exploring the Photophysical Properties of Some Dextran-Iron Oxide Nanoparticle Composites
Abstract
1. Introduction
2. Results and Discussion
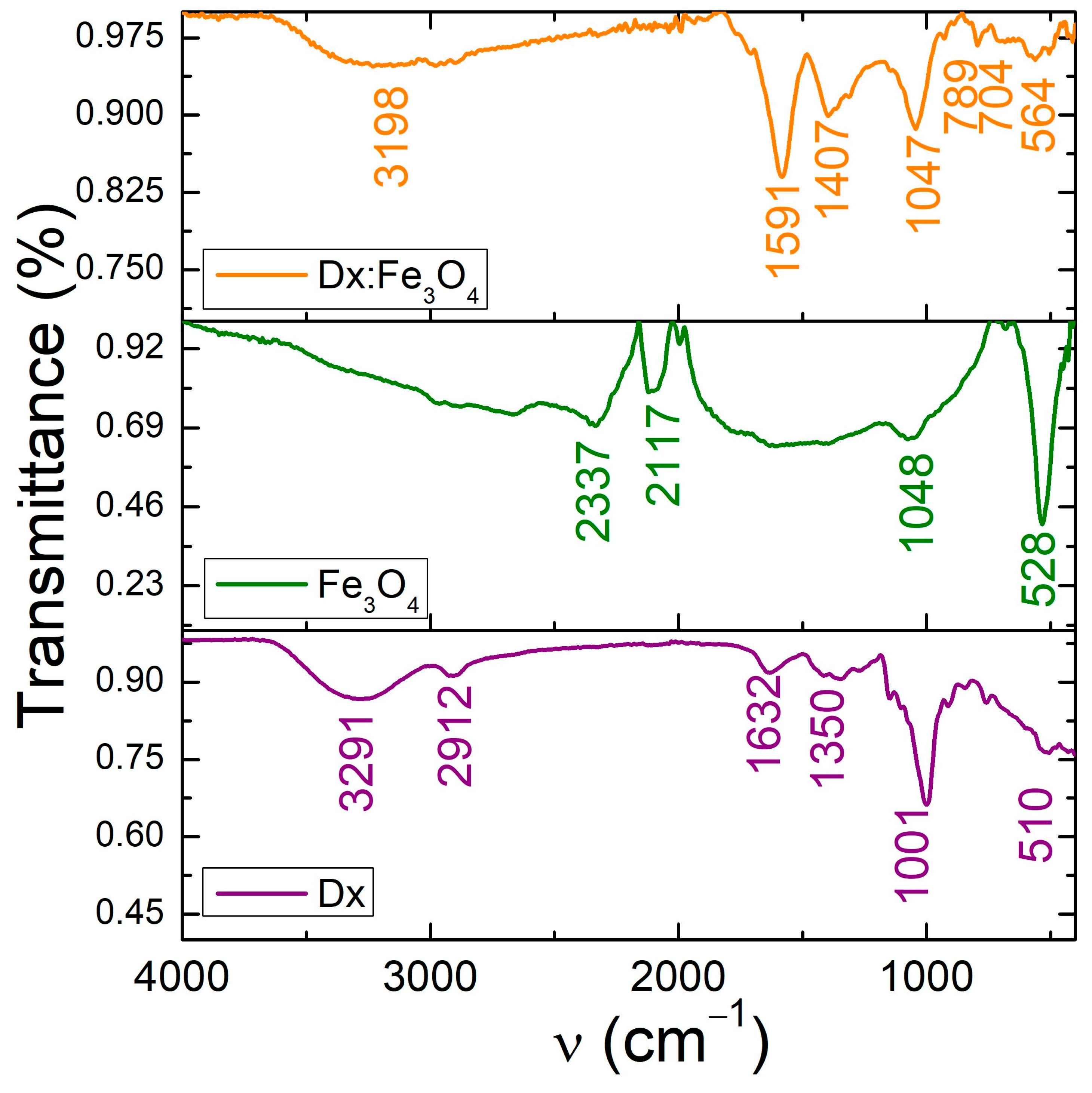
2.1. Structural and Chemical Composition of the Dx:Fe3O4 Composite
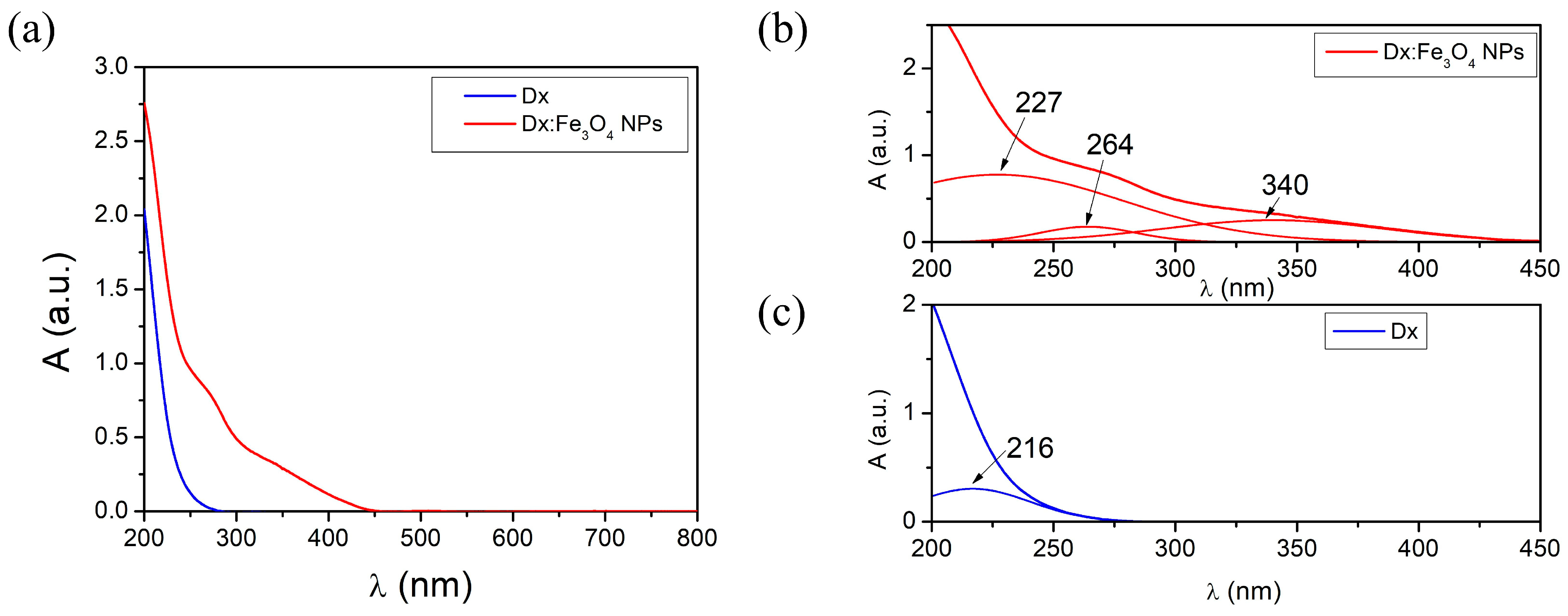
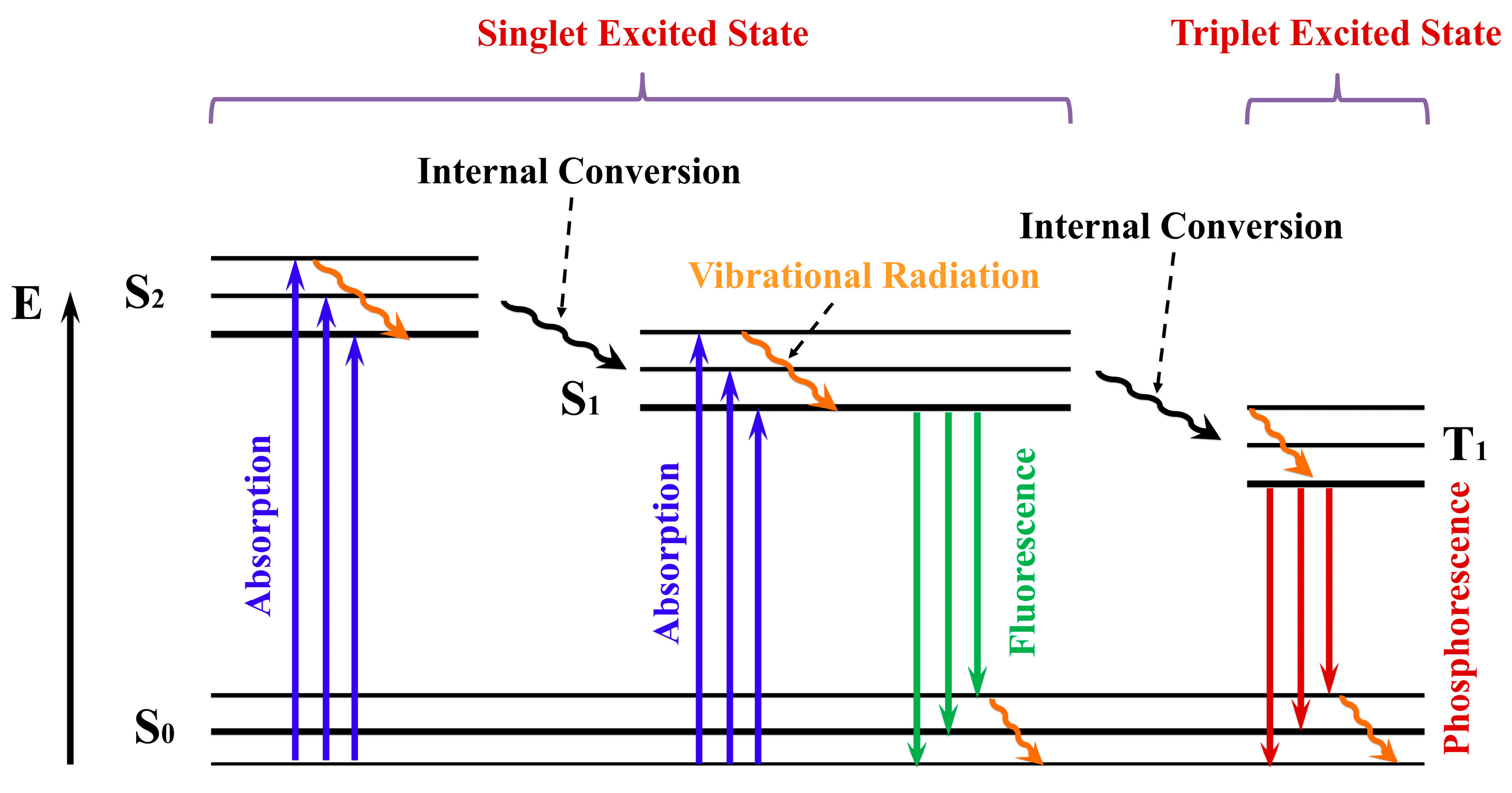
2.2. Electronic Transitions in Dx:Fe3O4 Composite
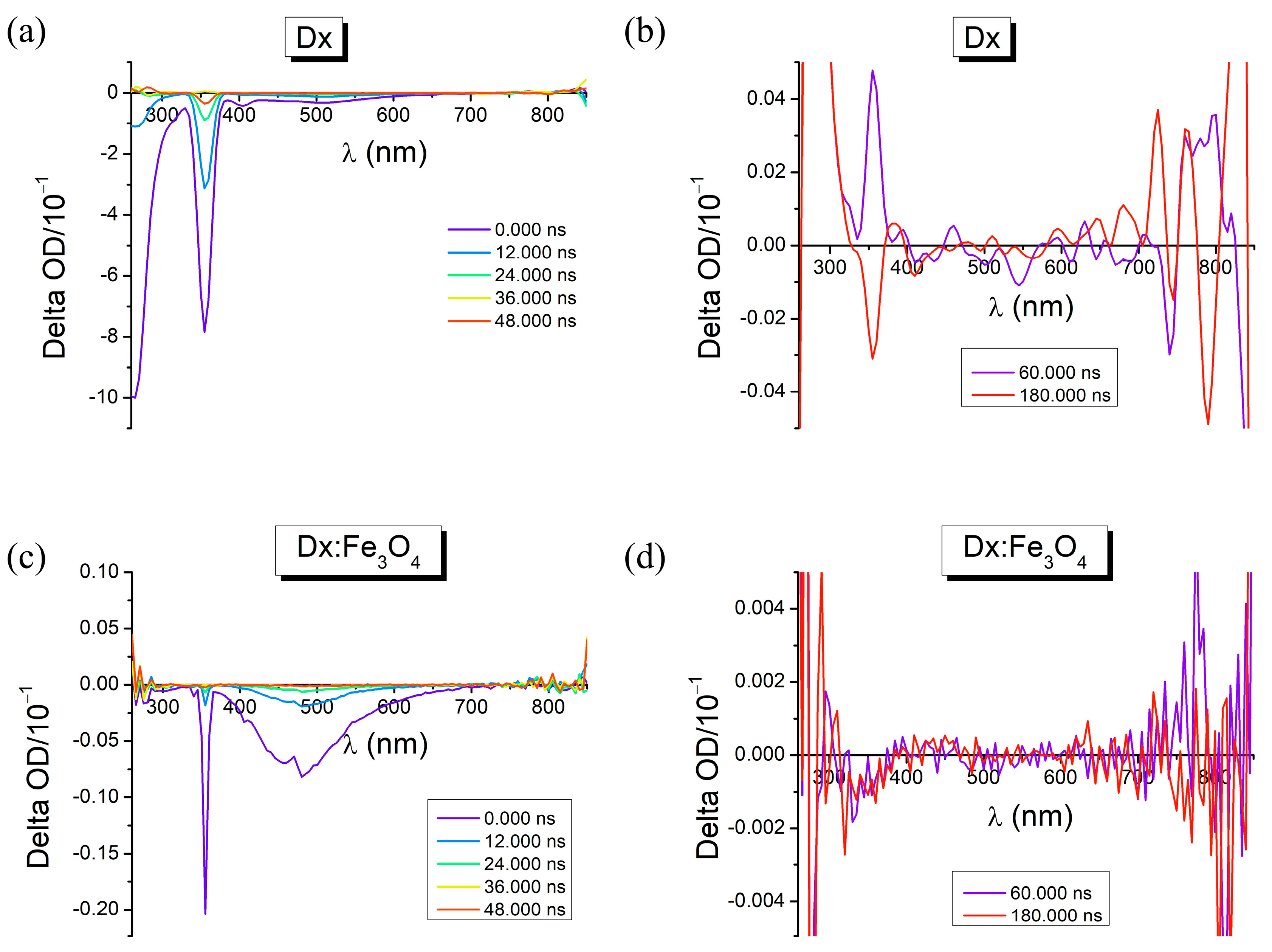
2.3. Photophysical Properties in Dx:Fe3O4 Composite
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 8, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Cheng, H.; Liu, J.; Shao, M.; Wei, M.; Evans, D.G.; Zhang, H.; Duan, X. Confined synthesis of 2D nanostructured materials toward electrocatalysis. Adv. Energy Mater. 2020, 10, 1900486. [Google Scholar] [CrossRef]
- Bao, X.; Ou, Q.; Xu, Z.; Zhang, Y.; Bao, Q.; Zhang, H. Band structure engineering in 2D materials for optoelectronic applications. Adv. Mater. Technol. 2018, 3, 1800072. [Google Scholar] [CrossRef]
- Wang, W.; Qi, L. Light management with patterned micro-and nanostructure arrays for photocatalysis, photovoltaics, and optoelectronic and optical devices. Adv. Funct. Mater. 2019, 29, 1807275. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.; Dufresne, A.; Danquah, M. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Wykowska, U.; Piekut, K.; Satuła, D. Stability of Fe3O4 nanoparticles in various model solutions. Coll. Surf. A Physicochem. Eng. Asp. 2014, 450, 15–24. [Google Scholar] [CrossRef]
- Hong, R.; Feng, B.; Chen, L.; Liu, G.; Li, H. Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem. Eng. J. 2008, 42, 290–300. [Google Scholar] [CrossRef]
- Sheldareh, A.H.A.; Safaeijavan, R.; Taheri, A.; Moniri, E. A comparative study between iron oxide nanoparticles coated glutamic acids as magnetic bio-nanocarriers for cytarabine release. J. Mol. Liq. 2025, 425, 127239. [Google Scholar] [CrossRef]
- Barrow, M.; Taylor, A.; Murray, P.; Rosseinsky, M.; Adams, D. Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chem. Soc. Rev. 2015, 44, 6733–6748. [Google Scholar] [CrossRef]
- Predescu, A.; Matei, E.; Berbecaru, A.; Pantilimon, C.; Drăgan, C.; Vidu, R.; Predescu, C. Synthesis and characterization of dextran-coated iron oxide nanoparticles. R. Soc. Open Sci. 2018, 5, 171525. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali, M.; Sadighian, S.; Rostamizadeh, K.; Khoeini, F.; Naghibi, M.; Bayat, N.; Habibizadeh, M. Synthesis and characterization of dextran coated magnetite nanoparticles for diagnostics and therapy. BioImpacts 2015, 5, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Alnasrani, A.H.F.; Joe, I.H.; Al-Musawi, S. Investigation of dextran-coated magnetic nanoparticles encapsulated by medication and modified with folate for targeted drug delivery: In vitro and docking studies. Mater. Chem. Phys. 2024, 321, 129446. [Google Scholar] [CrossRef]
- Smit, M.; Lutz, M. Polymer-coated magnetic nanoparticles for the efficient capture of Mycobacterium tuberculosis (Mtb). SN Appl. Sci. 2020, 2, 1658. [Google Scholar] [CrossRef]
- Li, Y.; Nie, W.; Chen, Y.; Zhou, P. Preparation and characterization of sulfonated poly (styrene-alt-maleic anhydride) and its selective removal of cationic dyes. Coll. Surf. A Physicochem. Eng. Asp. 2016, 499, 46–53. [Google Scholar] [CrossRef]
- Wulandari, I.; Mardila, V.; Santjojo, D.; Sabarudin, A. Preparation and characterization of chitosan-coated Fe3O4 nanoparticles using ex-situ co-precipitation method and tripolyphosphate/sulphate as dual crosslinkers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 012064. [Google Scholar] [CrossRef]
- Saxer, S.; Erdogan, O.; Paniagua, C.; Chavanieu, A.; Garric, X.; Darcos, V. Protein-polymer bioconjugates prepared by post-polymerization modification of alternating copolymers. Eur. J. Org. Chem. 2022, 2022, e202100576. [Google Scholar] [CrossRef]
- Davaa, E.; Lee, J.; Jenjob, R.; Yang, S.G. MT1-MMP responsive doxorubicin conjugated poly (lactic-co-glycolic acid)/poly(styrene-alt-maleic anhydride) core/shell microparticles for intrahepatic arterial chemotherapy of hepatic cancer. ACS Appl. Mater. Interfaces 2017, 9, 71–79. [Google Scholar] [CrossRef]
- Alex, M.; Nagpal, N.; Kulshreshtha, R.; Koul, V. Synthesis and evaluation of cationically modified poly (styrene-alt-maleic anhydride) nanocarriers for intracellular gene delivery. RSC Adv. 2015, 5, 21931–21944. [Google Scholar] [CrossRef]
- Smit, M. Polymer-Coated Magnetic Nanoparticles and Polymer Nanoparticles for the Treatment of Mycobacterium Tuberculosis (Mtb). Doctoral Dissertation, Stellenbosch University, Stellenbosch, South Africa, 2018. [Google Scholar]
- Rosca, I.; Moleavin, I.A.; Sarghi, A.; Lungoci, A.L.; Varganici, C.D.; Petrovici, A.R.; Fifere, A.; Pinteala, M. Dextran coated iron oxide nanoparticles loaded with protocatechuic acid as multifunctional therapeutic agent. Int. J. Biol. Macromol. 2024, 256, 128314. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.; Huang, H. Preparation and application of dextran and its derivatives as carriers. Int. J. Biol. Macromol. 2020, 145, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Ying, Y.; Zhou, Y.; Yu, J.; Qiao, L.; Zeng, J.; Li, W.; Li, J.; Che, S. Preparation of dextran and carboxymetyl dextran-coated Fe3O4 nanoparticles for breast cancer cell labeling and magnetic hyperthermia. J. Supercond. Novel Magn. 2024, 37, 1453–1463. [Google Scholar] [CrossRef]
- Choi, J.G.; Hasan, M.; Akter, H.; Lee, S.S. Characterization of dextran-coated magnetic nanoparticles (Fe3O4) conjugated with monoclonal antibody through low gradient magnet and centrifugation-based buffer separation process. Curr. Appl. Phys. 2023, 48, 79–83. [Google Scholar] [CrossRef]
- Unterweger, H.; Dézsi, L.; Matuszak, J.; Janko, C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.R.; et al. Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: Evaluation of size-dependent imaging properties, storage stability and safety. Int. J. Nanomed. 2018, 13, 1899–1915. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Makino, M.; Ohura, T.; Yamamoto, K.; Enomoto, Y.; Takase, H. Surface modification of Fe3O4 nanoparticles with dextran via a coupling reaction between naked Fe3O4 mechano-cation and naked dextran mechano-anion: A new mechanism of covalent bond formation. Adv. Powder Technol. 2019, 30, 795–806. [Google Scholar] [CrossRef]
- Altikatoglu, M.; Basaran, Y.; Arioz, C.; Ogan, A.; Kuzu, H. Glucose oxidase-dextran conjugates with enhanced stabilities against temperature and pH. Appl. Biochem. Biotechnol. 2010, 160, 2187. [Google Scholar] [CrossRef]
- Shu, R.; Wang, M.; Yang, Y.; Chang, S.; Zhang, G.; Gan, Y.; Shi, J.; He, J. Solvothermal synthesis of reduced grapheme oxide ferroferric oxide hybrid composite with enhanced microwave absorption properties. Nano 2017, 12, 1750144. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Pearson Education Ltd.: London, UK, 2014. [Google Scholar]
- Li, H.; Wu, Q.; Yuan, X.; Li, Y.; Xu, Y.; Hong, R. Preparation of water-based dextran coated Fe3O4 magnetic fluid for magnetic hyperthermia. Nanotechnol. Rev. 2023, 12, 20220534. [Google Scholar] [CrossRef]
- Lesiak, B.; Rangam, N.; Jiricek, P.; Gordeev, I.; Tóth, J.; Kövér, L.; Mohai, M.; Borowicz, P. Surface study of Fe3O4 nanoparticles functionalized with biocompatible adsorbed molecules. Front. Chem. 2019, 7, 642. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Khelef, A.; Tedjani, M.L.; Guemari, F. Effect of ferric chloride concentration on the type of magnetite (Fe3O4) nanoparticles biosynthesized by aqueous leaves extract of Artemisia and assessment of their antioxidant activities. J. Clust. Sci. 2021, 32, 1033–1041. [Google Scholar] [CrossRef]
- Cakić, M.; Glišić, S.; Nikolić, G.; Nikolić, G.M.; Cakić, K.; Cvetinov, M. Synthesis, characterization and antimicrobial activity of dextran sulphate stabilized silver nanoparticles. J. Mol. Struct. 2016, 1110, 156–161. [Google Scholar] [CrossRef]
- Abdurrahmanoglu, S.; First, Y. Synthesis and characterization of new dextran-acrylamide gels. J. Appl. Polym. Sci. 2007, 106, 3565–3570. [Google Scholar] [CrossRef]
- Du, R.; Yu, L.; Sun, M.; Ye, G.; Yang, Y.; Zhou, B.; Qian, Z.; Ling, H.; Ge, J. Chararcterization of dextran biosynthsized by glucansucrase from Leuconostoc pseudomesenterroides and their potential biotechonological applications. Antioxidants 2023, 12, 275. [Google Scholar] [CrossRef]
- Kobayashi, M.; Utsugi, H.; Matsuda, K. Intensive UV absorption of dextrans and its application to enzyme reactions. Agric. Biol. Chem. 1986, 50, 1051–1053. [Google Scholar] [CrossRef]
- Takai, Z.; Mustafa, M.; Asman, S.; Sekak, K. Preparation and characterization of magnetite (Fe3O4) nanoparticles by sol-gel method. Int. J. Nanoelectron. Mater. 2019, 12, 37–46. [Google Scholar]
- Biswas, S.; Panja, S.; Bose, S. The long-range π-conjugation between electron-rich species and multiwall carbon nanotubes influences the fluorescence lifetime and electromagnetic shielding. Nanoscale Adv. 2020, 2, 4464–4472. [Google Scholar] [CrossRef]
- Jhun, B.H.; Yi, S.Y.; Jeong, D.; Cho, J.; Park, S.Y.; You, Y. Aggregation of an n–π* molecule induces fluorescence turn-on. J. Phys. Chem. C 2017, 121, 11907–11914. [Google Scholar] [CrossRef]
- May, A.; Dempsey, J. A new era of LMCT: Leveraging ligand-to-metal charge transfer excited states for photochemical reactions. Chem. Sci. 2024, 15, 6661–6678. [Google Scholar] [CrossRef]
- Bie, B.; Zhao, X.; Yan, J.; Ke, X.; Liu, F.; Yan, G. Dextran fluorescent probes containing sulfadiazine and rhodamine B groups. Molecules 2022, 27, 6747. [Google Scholar] [CrossRef]
- Moitra, T.; Karak, P.; Chakraborty, S.; Ruud, K.; Chakrabarti, S. Behind the scenes of spin-forbidden decay pathways in transition metal complexes. Phys. Chem. Chem. Phys. 2021, 23, 59–81. [Google Scholar] [CrossRef] [PubMed]


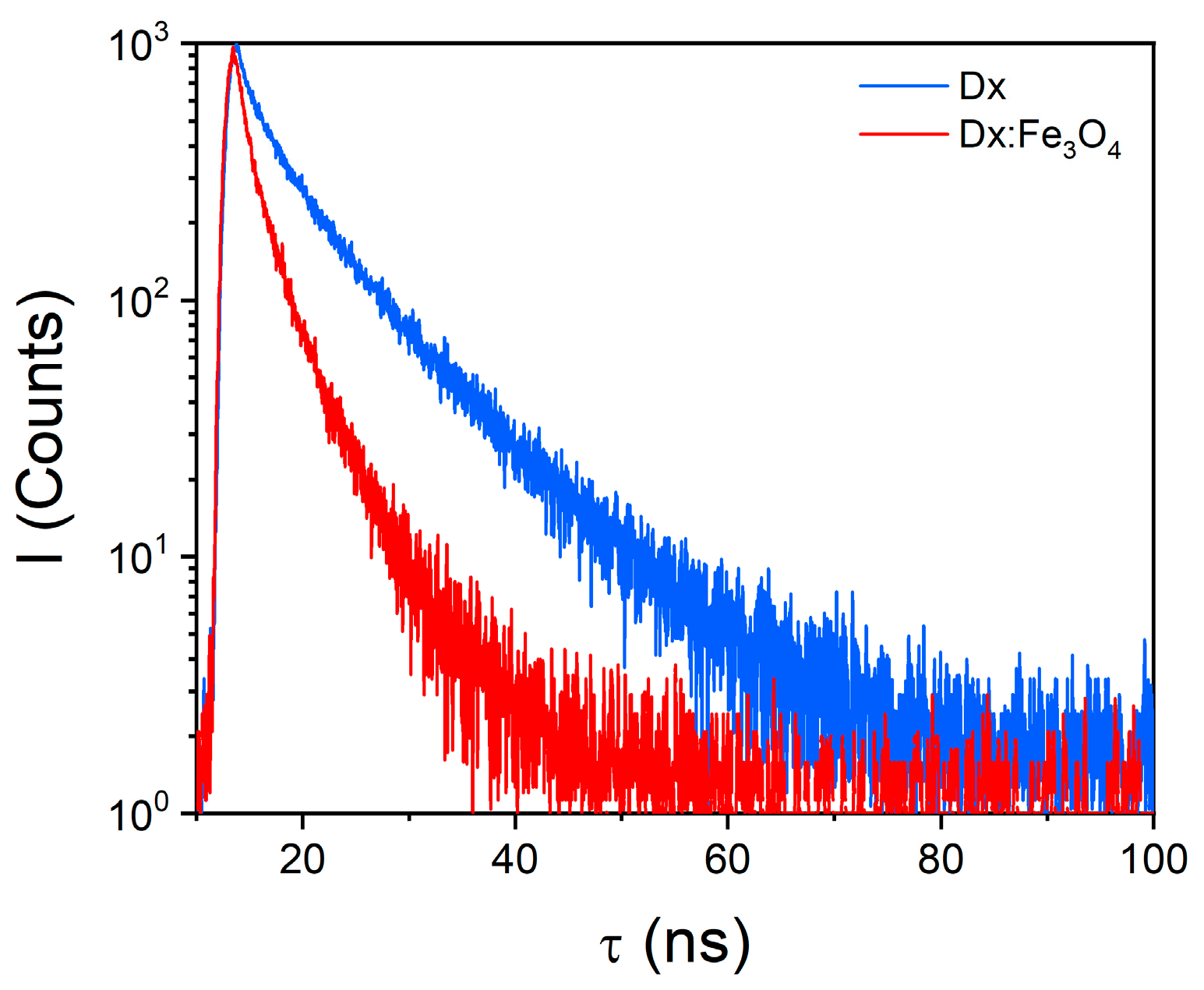




| Sample | τ1 (ns) | τ2 (ns) | a1 (%) | a2 (%) | Φ (%) |
|---|---|---|---|---|---|
| Dx | 2.18 | 9.24 | 27.41 | 72.59 | 0.07 |
| Dx:Fe3O4 | 1.18 | 4.44 | 48.60 | 51.40 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, I.; Potlog, T.; Airinei, A.; Tigoianu, R.; Gherasim, C. Exploring the Photophysical Properties of Some Dextran-Iron Oxide Nanoparticle Composites. Molecules 2025, 30, 2290. https://doi.org/10.3390/molecules30112290
Lungu I, Potlog T, Airinei A, Tigoianu R, Gherasim C. Exploring the Photophysical Properties of Some Dextran-Iron Oxide Nanoparticle Composites. Molecules. 2025; 30(11):2290. https://doi.org/10.3390/molecules30112290
Chicago/Turabian StyleLungu, Ion, Tamara Potlog, Anton Airinei, Radu Tigoianu, and Carmen Gherasim. 2025. "Exploring the Photophysical Properties of Some Dextran-Iron Oxide Nanoparticle Composites" Molecules 30, no. 11: 2290. https://doi.org/10.3390/molecules30112290
APA StyleLungu, I., Potlog, T., Airinei, A., Tigoianu, R., & Gherasim, C. (2025). Exploring the Photophysical Properties of Some Dextran-Iron Oxide Nanoparticle Composites. Molecules, 30(11), 2290. https://doi.org/10.3390/molecules30112290





