Chitosan-Oligosaccharide-Bearing Biphasic Calcium Phosphate Bone Cement: Preparation and Angiogenic Activity In Vitro
Abstract
1. Introduction
2. Results
2.1. Material Properties
2.1.1. XRD Patterns
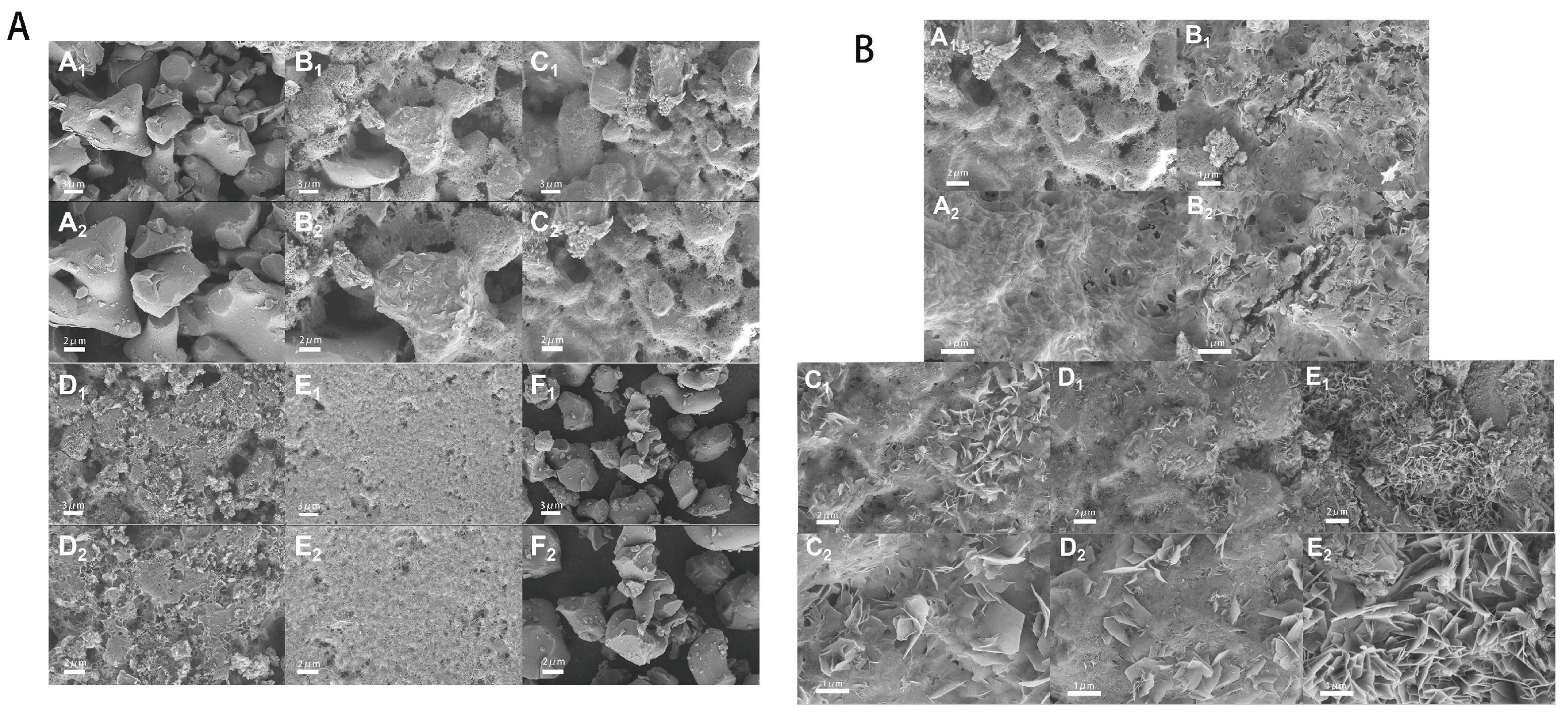
2.1.2. SEM Results
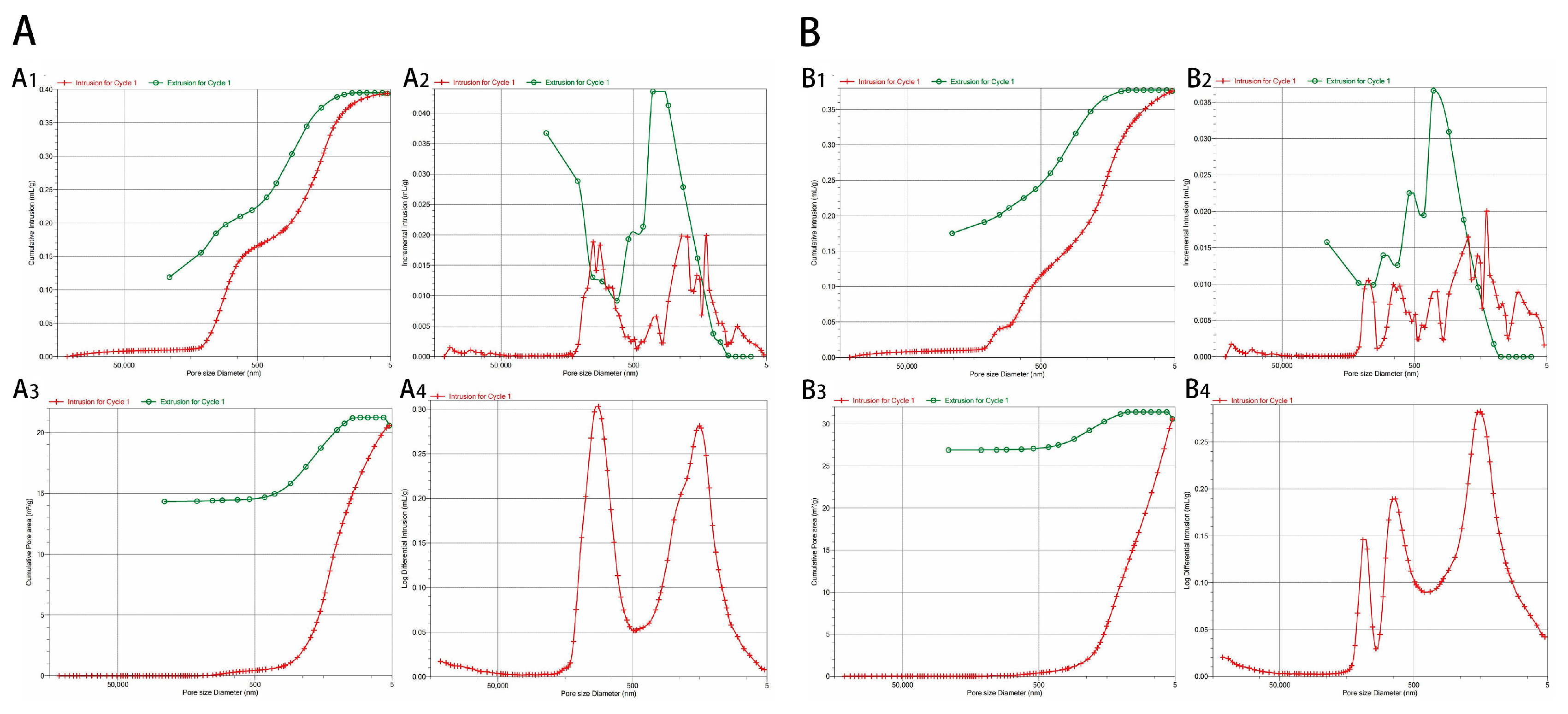
2.1.3. MIP Results
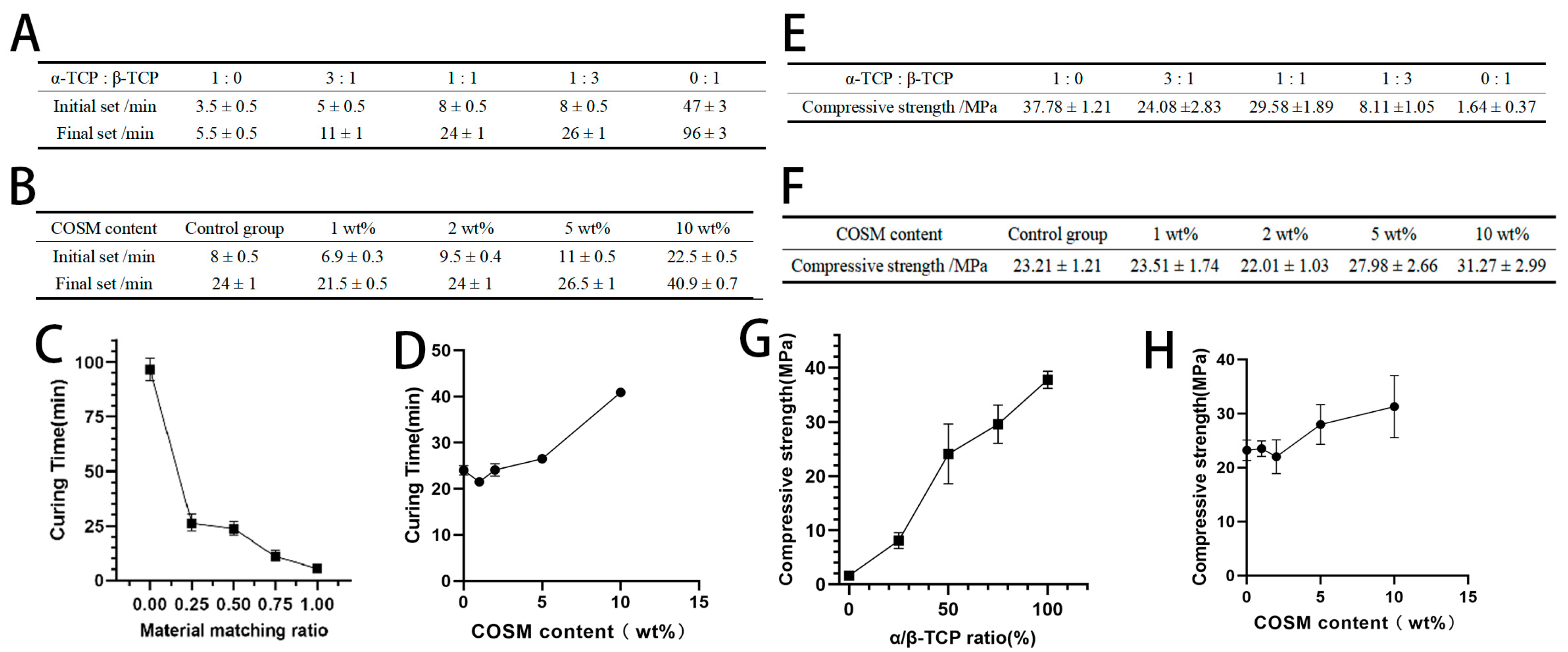
2.2. Analysis of Curing Time and Compressive Strength Results
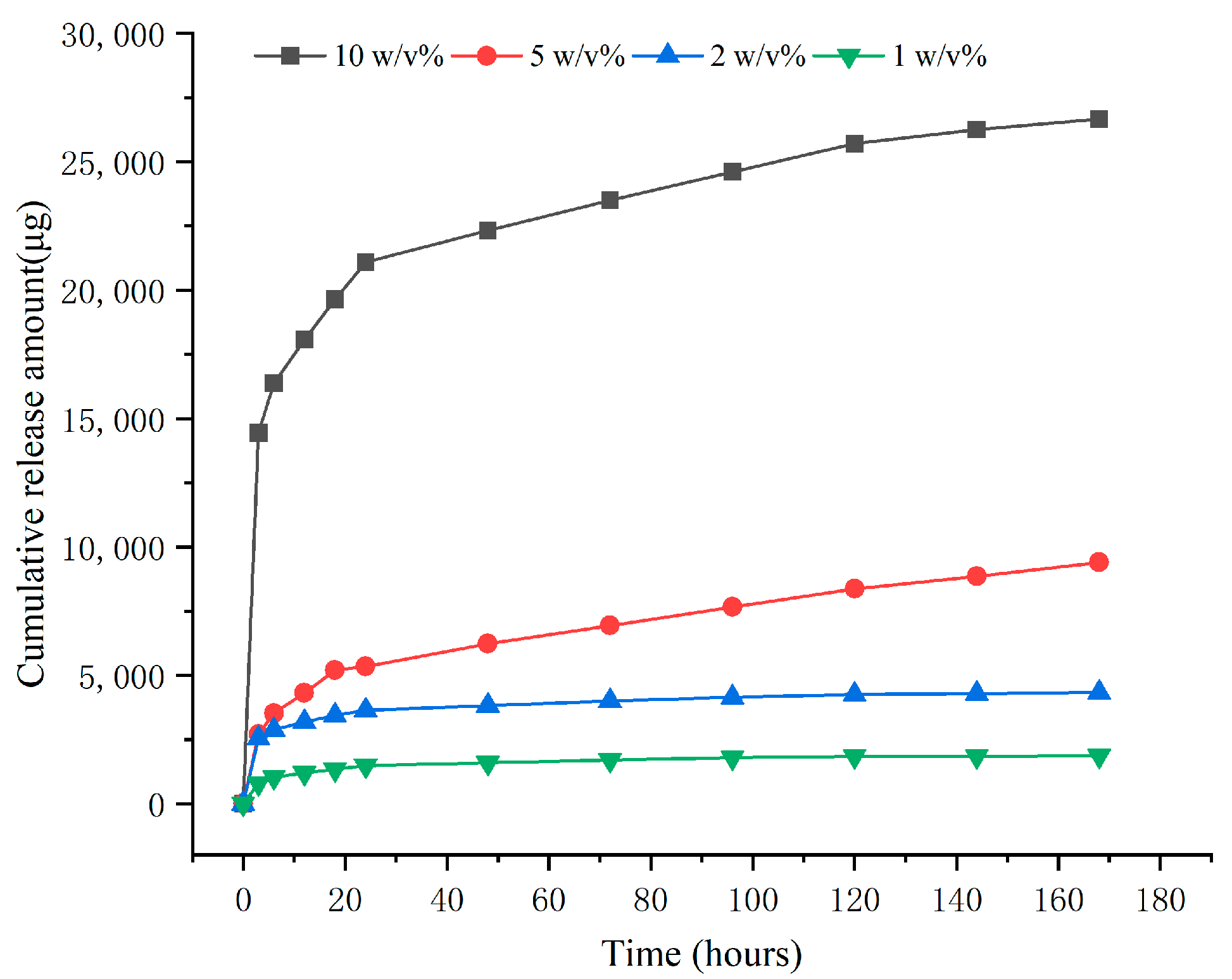
2.3. In Vitro Drug Release Results
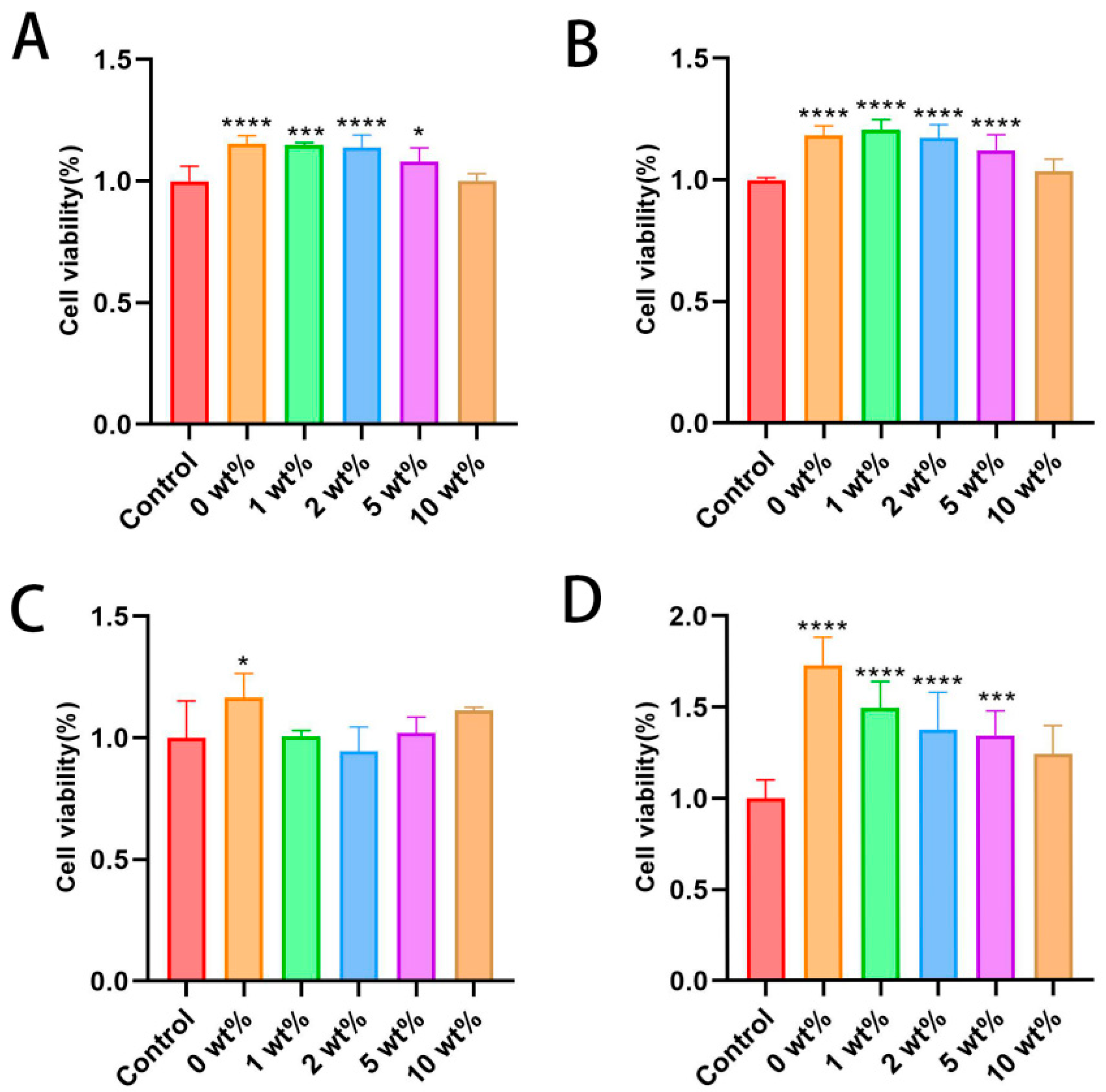
2.4. In Vitro Cell Proliferation
2.5. In Vitro Cell Migration Ability
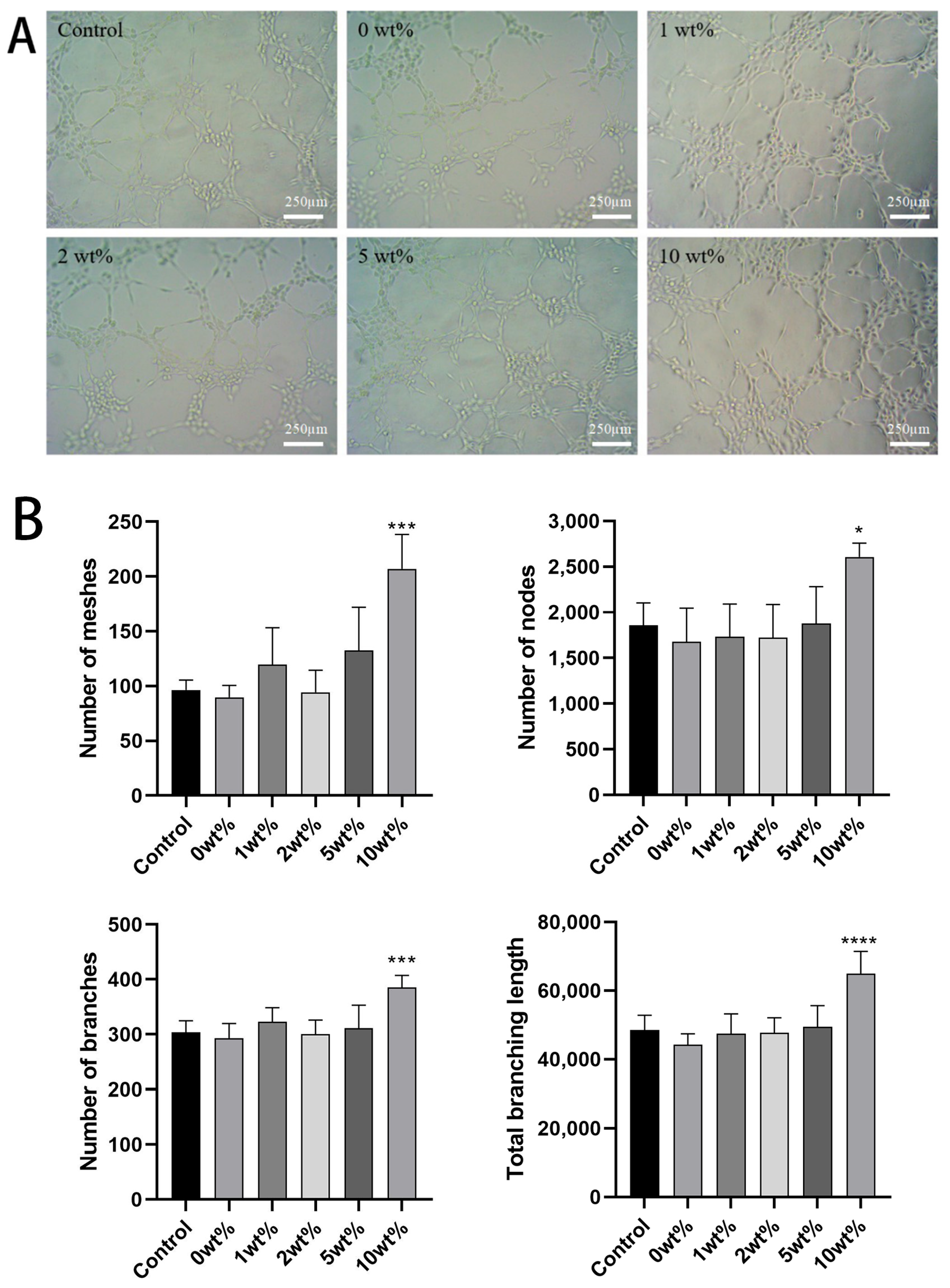
2.6. In Vitro Angiogenesis Ability Results
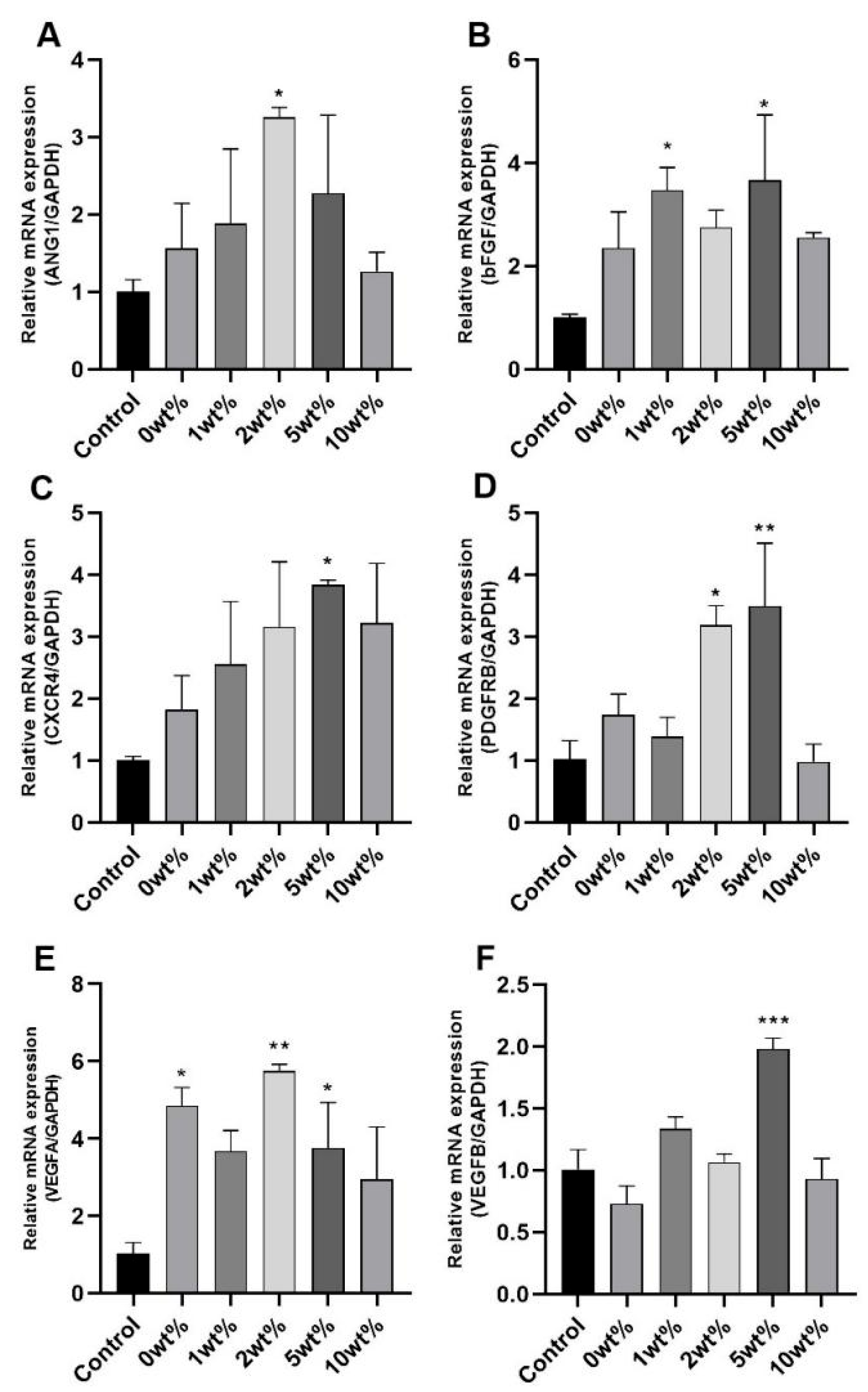
2.7. Expression Results of Angiogenesis-Related Genes in HUVECs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Biphasic Calcium Phosphate Cement Loaded with COSM
4.2.1. Preparation of Biphasic Calcium Phosphate (BCP) Powder
4.2.2. Preparation of Bone Cement Pastes
4.2.3. Chitosan Loading
4.3. Physical and Chemical Properties Analysis
4.3.1. Characterization of BCP
4.3.2. Determination of Curing Time and Compressive Strength
4.3.3. In Vitro Release of COSM
4.4. In Vitro Cell Culture Study
4.4.1. Preliminary Study on the Toxicity of Osteoblasts and Vascular Cells
4.4.2. Cell Scratch Test In Vitro
4.4.3. In Vitro Tubule Formation Experiment
4.4.4. RNA Isolation and Real-Time PCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| α-TCP | α-tricalcium phosphate |
| ACP | Amorphous calcium phosphate |
| ADSCs | Adipose-derived stem cells |
| ALP | Alkaline Phosphatase |
| β-TCP | β-tricalcium phosphate |
| bFGF | Fibroblast Growth Factor |
| BMP | Bone Morphogenetic Protein |
| BCP | Biphasic calcium phosphate |
| CD31 | Platelet endothelial cell adhesion molecule-1 |
| CaP | Calcium phosphate |
| CPP | Calcium pyrophosphate |
| CBD | Cannabidiol |
| CPC | Calcium phosphate cement |
| CDHA | Calcium-deficient hydroxyapatite |
| COSM | COS (MW ≤ 3000) |
| CCK-8 | Cell embryo osteoblast precursor cells |
| DCPA | Dicyclopentenyl acrylate |
| DNS | 3,5-dinitrosalicylic acid |
| DMSO | Dimethyl sulfoxide |
| ECM | Extracellular matrix |
| Emcm | Endomucin |
| EC | Endothelial cell |
| ERK | Extracellular regulated protein kinases |
| FBS | Fetal bovine serum |
| Glu | Glucosamine hydrochloride |
| HAP/HA | Hydroxyapatite |
| HIF-1α | Hypoxia inducible factor 1 subunit alpha Gene |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| MAPK | Mitogen-activated protein kinase |
| MIP | Mercury intrusion porosimetry |
| MC3T3-E1s | Mouse embryonic osteoblast precursor cells |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MSC | Mesenchymal stem cells |
| OPG | Osteoclastogenesis inhibitory factor |
| PBS | Phosphate Buffer system |
| PCR | Polymerase Chain Reaction |
| PDGF | Platelet-derived growth factor |
| PLA | Polylactic acid |
| PGA | Polyglycolic acid |
| PLGA | Poly(lactic-co-glycolic acid |
| PMMA | Polymethyl Methacrylate |
| PEEK | Poly(ether-ether-ketone) |
| RANKL | Receptor Activator of Nuclear Factor-κ B Ligand |
| SLIT3 | Slit guidance ligand 3 Gene |
| SEM | Scanning electron microscope |
| TNF-α | Tumor necrosis factor-α |
| TGF-β | Transforming Growth Factor-β |
| TTCP | Tetracalcium phosphate |
| VEGF | Vascular endothelial growth factor |
| XRD | X-ray Diffraction |
References
- Chande, S.; Bergwitz, C. Role of phosphate sensing in bone and mineral metabolism. Nat. Rev. Endocrinol. 2018, 14, 637–655. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ou, K.-L.; Mao, C.; Hosseinkhani, H. Importance of dual delivery systems for bone tissue engineering. J. Control. Release 2016, 225, 152–169. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Bahtiyar, G.; Banerji, M.A.; Sacerdote, A.S. Diabetes and bone health. Maturitas 2013, 76, 253–259. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483. [Google Scholar] [CrossRef]
- Bose, S.; Sarkar, N. Natural Medicinal Compounds in Bone Tissue Engineering. Trends Biotechnol. 2019, 38, 404. [Google Scholar] [CrossRef]
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as bone substitutes: A review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef]
- Amini, Z.; Lari, R. A systematic review of decellularized allograft and xenograft-derived scaffolds in bone tissue regeneration. Tissue Cell 2021, 69, 101494. [Google Scholar] [CrossRef]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Huang, J.; Han, Q.; Cai, M.; Zhu, J.; Li, L.; Yu, L.; Wang, Z.; Fan, G.; Zhu, Y.; Lu, J.; et al. Effect of Angiogenesis in Bone Tissue Engineering. Ann. Biomed. Eng. 2022, 50, 898–913. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, J.; Jing, W. Motivating role of type H vessels in bone regeneration. Cell Prolif. 2020, 53, e12874. [Google Scholar] [CrossRef]
- Menger, M.M.; Laschke, M.W.; Nussler, A.K.; Menger, M.D.; Histing, T. The vascularization paradox of non-union formation. Angiogenesis 2022, 25, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.; Saxon, M.; Bozanich, J.; Tillberg, C.; Luan, X.; Diekwisch, T.G.H. The Glycoprotein/Cytokine Erythropoietin Promotes Rapid Alveolar Ridge Regeneration In Vivo by Promoting New Bone Extracellular Matrix Deposition in Conjunction with Coupled Angiogenesis/Osteogenesis. Int. J. Mol. Sci. 2021, 22, 2788. [Google Scholar] [CrossRef]
- Tanaka, T.; Komaki, H.; Chazono, M.; Kitasato, S.; Kakuta, A.; Akiyama, S.; Marumo, K. Basic research and clinical application of beta-tricalcium phosphate (β-TCP). Morphologie 2017, 101, 164–172. [Google Scholar] [CrossRef]
- Tronco, M.C.; Cassel, J.B.; Dos Santos, L.A. α-TCP-based calcium phosphate cements: A critical review. Acta Biomater. 2022, 151, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Rz, L. Calcium phosphate materials in restorative dentistry: A review. Adv. Dent. Res. 1988, 2, 164–180. [Google Scholar] [CrossRef]
- Horch, H.-H.; Sader, R.; Pautke, C.; Neff, A.; Deppe, H.; Kolk, A. Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb) for bone regeneration in the reconstructive surgery of the jaws. Int. J. Oral Maxillofac. Surg. 2006, 35, 708–713. [Google Scholar] [CrossRef]
- Yamada, S.; Heymann, D.; Bouler, J.M.; Daculsi, G. Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/beta-tricalcium phosphate ratios. Biomaterials 1997, 18, 1037–1041. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012, 8, 963–977. [Google Scholar] [CrossRef]
- Ellinger, R.F.; Nery, E.B.; Lynch, K.L. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: A case report. Int. J. Periodontics Restor. Dent. 1986, 6, 22–33. [Google Scholar]
- Daculsi, G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 1998, 19, 1473–1478. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Ramay, H.R.R.; Zhang, M. Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials 2004, 25, 5171–5180. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Linh, N.T.B.; Min, Y.K.; Lee, B.-T. Bone formation of a porous Gelatin-Pectin-biphasic calcium phosphate composite in presence of BMP-2 and VEGF. Int. J. Biol. Macromol. 2015, 76, 10–24. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ren, W.; Tian, X.; Liu, W.; Wu, S.; Chen, X. Comparative study on in vivo response of porous calcium carbonate composite ceramic and biphasic calcium phosphate ceramic. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 117–123. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Hu, C.-C.; Hsieh, P.-H.; Shih, H.-N.; Ueng, S.W.N.; Chang, Y. Vancomycin and Ceftazidime in Bone Cement as a Potentially Effective Treatment for Knee Periprosthetic Joint Infection. J. Bone Jt. Surg. Am. 2017, 99, 223–231. [Google Scholar] [CrossRef]
- Monavari, M.; Homaeigohar, S.; Fuentes-Chandía, M.; Nawaz, Q.; Monavari, M.; Venkatraman, A.; Boccaccini, A.R. 3D printing of alginate dialdehyde-gelatin (ADA-GEL) hydrogels incorporating phytotherapeutic icariin loaded mesoporous SiO2-CaO nanoparticles for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112470. [Google Scholar] [CrossRef]
- Yu, D.; Feng, J.; You, H.; Zhou, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Microstructure, Antibacterial and Antitumor Activities of Chitosan Oligosaccharides and Derivatives. Mar. Drugs 2022, 20, 69. [Google Scholar] [CrossRef]
- Huang, X.; Chen, M.; Wu, H.; Jiao, Y.; Zhou, C. Macrophage Polarization Mediated by Chitooligosaccharide (COS) and Associated Osteogenic and Angiogenic Activities. ACS Biomater. Sci. Eng. 2020, 6, 1614–1629. [Google Scholar] [CrossRef]
- Vimalraj, S.; Govindarajan, D.; Sudhakar, S.; Suresh, R.; Palanivel, P.; Sekaran, S. Chitosan derived chito-oligosaccharides promote osteoblast differentiation and offer anti-osteoporotic potential: Molecular and morphological evidence from a zebrafish model. Int. J. Biol. Macromol. 2024, 259, 129250. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, C.; Gilbert, S.R.; Clemens, T.L. Oxygen sensing and osteogenesis. Ann. N. Y. Acad. Sci. 2007, 1117, 1–11. [Google Scholar] [CrossRef]
- Kavasi, R.-M.; Coelho, C.C.; Platania, V.; Quadros, P.A.; Chatzinikolaidou, M. In Vitro Biocompatibility Assessment of Nano-Hydroxyapatite. Nanomaterials 2021, 11, 1152. [Google Scholar] [CrossRef] [PubMed]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.; Fierstein, S.; Purkey, L.; DeCicco-Skinner, K. Endothelial Cell Tube Formation Assay: An In Vitro Model for Angiogenesis. Methods Mol. Biol. 2022, 2475, 187–196. [Google Scholar] [CrossRef]
- Li, Y.; Weng, W.; Tam, K.C. Novel highly biodegradable biphasic tricalcium phosphates composed of alpha-tricalcium phosphate and beta-tricalcium phosphate. Acta Biomater. 2007, 3, 251–254. [Google Scholar] [CrossRef]
- Safronova, T.V.; Selezneva, I.I.; Tikhonova, S.A.; Kiselev, A.S.; Davydova, G.A.; Shatalova, T.B.; Larionov, D.S.; Rau, J.V. Biocompatibility of biphasic α,β-tricalcium phosphate ceramics in vitro. Bioact. Mater. 2020, 5, 423–427. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; Aza, A.H.D.; Turrillas, X.; Pena, P.; Aza, S.D. New Approach to the β→α Polymorphic Transformation in Magnesium-Substituted Tricalcium Phosphate and its Practical Implications. J. Am. Ceram. Soc. 2008, 91, 1281–1286. [Google Scholar] [CrossRef]
- Enderle, R.; Götz-Neunhoeffer, F.; Göbbels, M.; Müller, F.A.; Greil, P. Influence of magnesium doping on the phase transformation temperature of beta-TCP ceramics examined by Rietveld refinement. Biomaterials 2005, 26, 3379–3384. [Google Scholar] [CrossRef]
- Destainville, A.; Champion, E.; Bernache-Assollant, D.; Laborde, E. Synthesis, characterization and thermal behavior of apatitic tricalcium phosphate. Mater. Chem. Phys. 2003, 80, 269–277. [Google Scholar] [CrossRef]
- Brazete, D.; Torres, P.M.C.; Abrantes, J.C.C.; Ferreira, J.M.F. Influence of the Ca/P ratio and cooling rate on the allotropic α↔β-tricalcium phosphate phase transformations. Ceram. Int. 2018, 44, 8249–8256. [Google Scholar] [CrossRef]
- Nurse, R.W.; Welch, J.H.; Gutt, W. 220. High-temperature phase equilibria in the system dicalcium silicate–tricalcium phosphate. J. Chem. Soc. 1959, 1077–1083. [Google Scholar] [CrossRef]
- Sinusaite, L.; Renner, A.M.; Schütz, M.B.; Antuzevics, A.; Rogulis, U.; Grigoraviciute-Puroniene, I.; Mathur, S.; Zarkov, A. Effect of Mn doping on the low-temperature synthesis of tricalcium phosphate (TCP) polymorphs. J. Eur. Ceram. Soc. 2019, 39, 3257–3263. [Google Scholar] [CrossRef]
- Morejón-Alonso, L.; Ferreira, O.J.B.; Carrodeguas, R.G.; dos Santos, L.A. Bioactive composite bone cement based on α-tricalcium phosphate/tricalcium silicate. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 94–102. [Google Scholar] [CrossRef]
- Cicek, G.; Aksoy, E.A.; Durucan, C.; Hasirci, N. Alpha-tricalcium phosphate (α-TCP): Solid state synthesis from different calcium precursors and the hydraulic reactivity. J. Mater. Sci. Mater. Med. 2011, 22, 809–817. [Google Scholar] [CrossRef]
- Weichhold, J.; Gbureck, U.; Goetz-Neunhoeffer, F.; Hurle, K. Setting Mechanism of a CDHA Forming α-TCP Cement Modified with Sodium Phytate for Improved Injectability. Materials 2019, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef]
- Ambard, A.J.; Mueninghoff, L. Calcium phosphate cement: Review of mechanical and biological properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef]
- Shen, J.; Sun, Y.; Liu, X.; Zhu, Y.; Bao, B.; Gao, T.; Chai, Y.; Xu, J.; Zheng, X. EGFL6 regulates angiogenesis and osteogenesis in distraction osteogenesis via Wnt/β-catenin signaling. Stem Cell Res. Ther. 2021, 12, 415. [Google Scholar] [CrossRef]
- Silva, L.C.F.D.; Porto, G.G.; Andrade, E.S.D.S.; Laureano Filho, J.R. Demineralized bone matrix and calcium-phosphate cement in bone regeneration in rats. Acta Cir. Bras. 2018, 33, 354–361. [Google Scholar] [CrossRef]
- Alonso-Fernández, I.; Haugen, H.J.; Nogueira, L.P.; López-Álvarez, M.; González, P.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz-Guzón, F. Enhanced Bone Healing in Critical-Sized Rabbit Femoral Defects: Impact of Helical and Alternate Scaffold Architectures. Polymers 2024, 16, 1243. [Google Scholar] [CrossRef]
- Piard, C.; Jeyaram, A.; Liu, Y.; Caccamese, J.; Jay, S.M.; Chen, Y.; Fisher, J. 3D printed HUVECs/MSCs cocultures impact cellular interactions and angiogenesis depending on cell-cell distance. Biomaterials 2019, 222, 119423. [Google Scholar] [CrossRef] [PubMed]
- Bezenah, J.R.; Kong, Y.P.; Putnam, A.J. Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures. Sci. Rep. 2018, 8, 2671. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, P.; Dougherty, J.A.; Weist, J.; Kumar, N.; Angelos, M.G.; Powell, H.M.; Khan, M. Sustained Release of Basic Fibroblast Growth Factor (bFGF) Encapsulated Polycaprolactone (PCL) Microspheres Promote Angiogenesis In Vivo. Nanomaterials 2019, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Ardoña, H.A.M.; Campbell, P.H.; Gonzalez, G.M.; Parker, K.K. Alfalfa Nanofibers for Dermal Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 33535–33547. [Google Scholar] [CrossRef]
- Li, K.; Li, P.; Xing, R.; Guan, X.; Liu, S. Comparison of Determination Methods for Chitosan Oligosaccharide Content. Chin. J. Pharm. Anal. 2011, 31, 1530–1532. [Google Scholar] [CrossRef]
- Wang, H.Y.; Srguleng, Q.; Zhao, Q.C.; Zhang, Z.; Jin, F.X. Determination of Polysaccharide in Ophiopogon japonicus with 3,5-Dinitrosalicylic Acid Colorimeitry. J. Shenyang Agric. Univ. 2005. [Google Scholar]


| Gene Name | Primer Sequence (5′→3′) |
|---|---|
| GAPDH | F: GAAAGCCTGCCGGTGACTAA |
| R: GCCCAATACGACCAAATCAGAG | |
| ANG1 | F: CAGTGGCTGCAAAAACTTGAGA |
| R: AGTCTGAGAGAGGAGGCTGG | |
| bFGF | F: GCGACCCTCACATCAAGCTA |
| R: AGCCAGGTAACGGTTAGCAC | |
| CXCR4 | F: GGGCAGAGGAGTTAGCCAAG |
| R: GGGCTAAGGGCACAAGAGAA | |
| PDGFRB | F: CCATCAGCAGCAAGGCGA |
| R: AGCAGGTCAGAACGAAGGTG | |
| VEGFA | F: ACAAATGTGAATGCAGACCAAA |
| R: ACCAACGTACACGCTCCAG | |
| VEGFB | F: CAAGTCCGGATGCAGATCCT |
| R: TCTGCATTCACACTGGCTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Guo, X.; Che, Q.; Su, Z. Chitosan-Oligosaccharide-Bearing Biphasic Calcium Phosphate Bone Cement: Preparation and Angiogenic Activity In Vitro. Molecules 2025, 30, 2286. https://doi.org/10.3390/molecules30112286
Liu J, Guo X, Che Q, Su Z. Chitosan-Oligosaccharide-Bearing Biphasic Calcium Phosphate Bone Cement: Preparation and Angiogenic Activity In Vitro. Molecules. 2025; 30(11):2286. https://doi.org/10.3390/molecules30112286
Chicago/Turabian StyleLiu, Jianshen, Xinghua Guo, Qishi Che, and Zhengquan Su. 2025. "Chitosan-Oligosaccharide-Bearing Biphasic Calcium Phosphate Bone Cement: Preparation and Angiogenic Activity In Vitro" Molecules 30, no. 11: 2286. https://doi.org/10.3390/molecules30112286
APA StyleLiu, J., Guo, X., Che, Q., & Su, Z. (2025). Chitosan-Oligosaccharide-Bearing Biphasic Calcium Phosphate Bone Cement: Preparation and Angiogenic Activity In Vitro. Molecules, 30(11), 2286. https://doi.org/10.3390/molecules30112286







