The Influence Mechanism of Dissolved Organic Matter on the Photocatalytic Oxidation of Pharmaceuticals and Personal Care Products
Abstract
1. Introduction
2. Effect of DOM on the Photocatalytic Oxidation of PPCPs
2.1. Metal Oxide-Based Photocatalysts
2.1.1. TiO2
2.1.2. ZnO
2.1.3. WO3
2.2. Carbon-Based Photocatalysts
2.2.1. Graphitic Carbon Nitride (g-C3N4)
2.2.2. Graphene
2.2.3. Other Carbon-Based Photocatalysts
2.3. Metal Sulfide-Based Photocatalysts
2.4. Bismuth-Based Photocatalysts
2.5. Composite Photocatalysts
2.6. Novel Photocatalysts
2.7. Chapter Summary
3. Effect of DOM on the Migration and Transformation of PPCPs
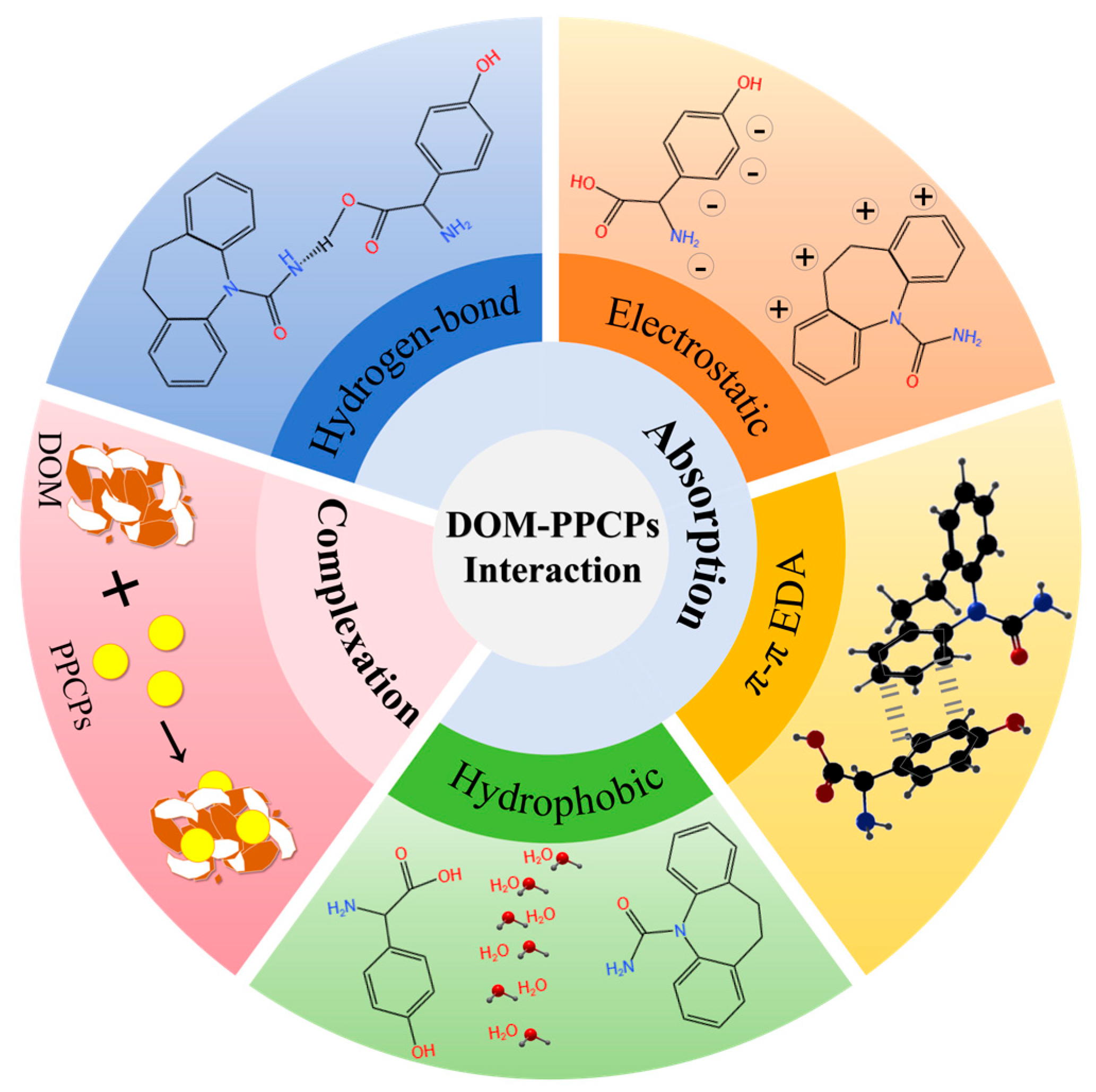
3.1. Adsorption
3.2. Complexation
4. Interference Mechanism of DOM on the Surface and Interface Structure of Photocatalytic Materials
4.1. Effect of DOM on the Adsorption of PPCPs by Photocatalytic Materials
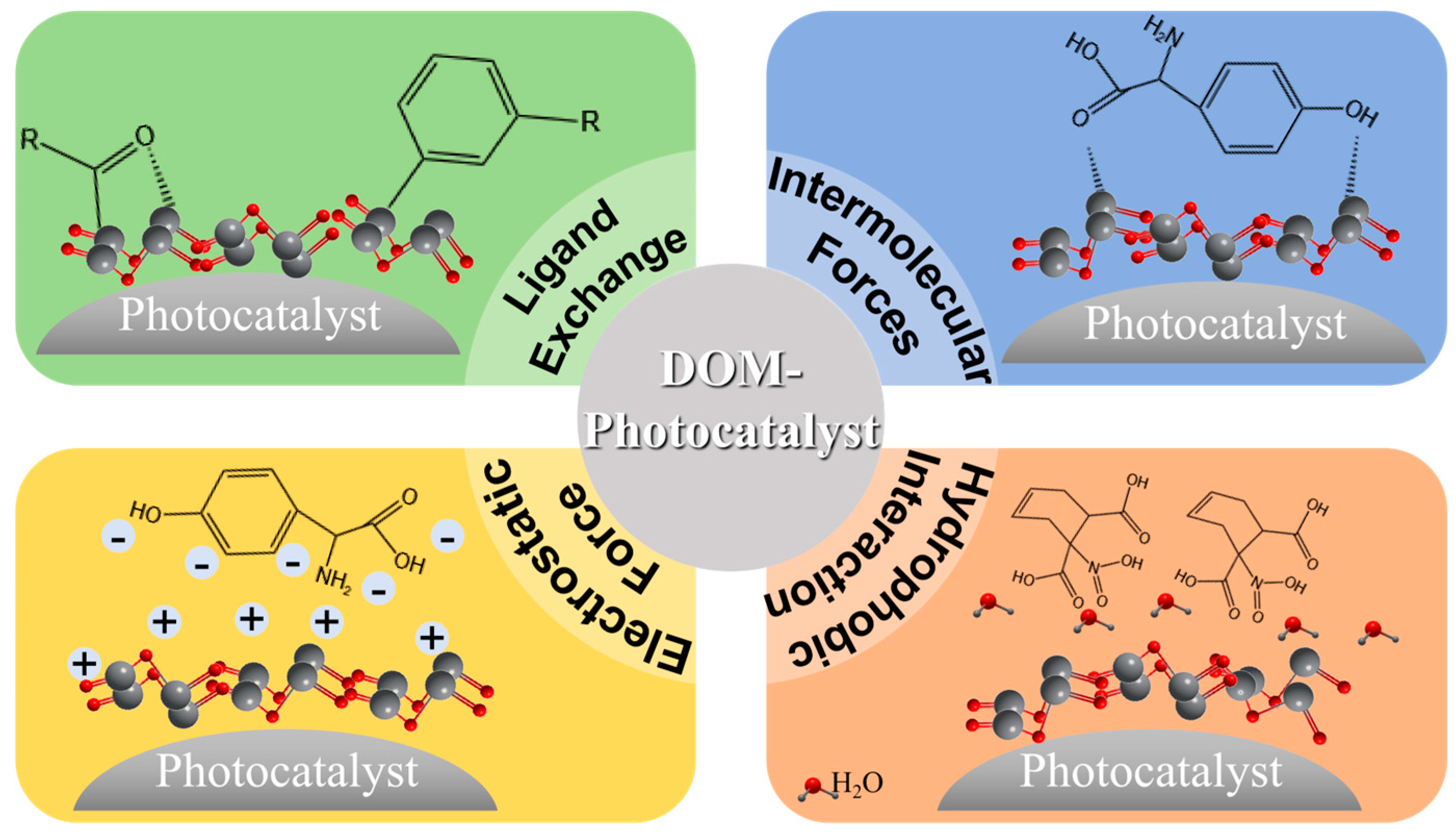
4.2. Effect of DOM on the Apparent Structure of Photocatalysts
4.2.1. Ligand Exchange
4.2.2. Intermolecular Forces
4.2.3. Electrostatic Force
4.2.4. Hydrophobic Interaction
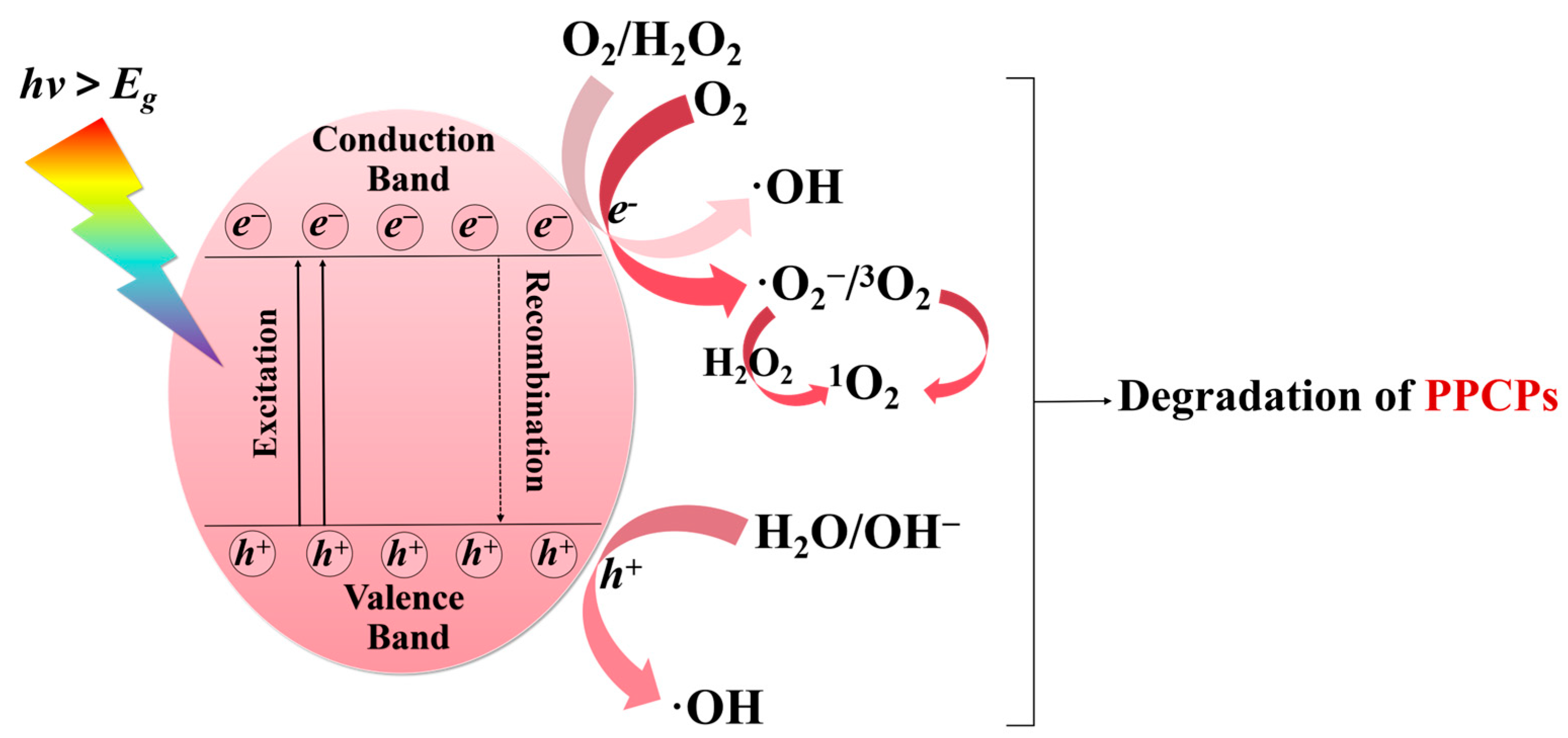
5. Effect of DOM on Active Species
5.1. Generation of Active Species
5.1.1. Light Attenuation/Shielding of DOM
5.1.2. DOM Facilitated Electron Transfer
5.1.3. Photosensitization of DOM
5.2. Quenching of Active Species
6. Conclusions and Future Perspectives
- Most of the existing studies focus on the influence of DOM on the degradation efficiency of PPCPs, but there are few studies on how DOM regulates key processes such as the generation pathway of reactive oxygen species and the separation efficiency of photogenerated electron–hole pairs.
- The types of DOM are complex, such as HA, polysaccharides, and proteins, and the influence of different components on photocatalysis varies significantly. However, most of the current studies use a single standard DOM, such as HA, for simulation experiments, which has a large deviation from the DOM composition in the actual environment, resulting in limited universality of the conclusions.
- DOM participating in the photocatalytic process may generate more toxic intermediate products, such as halogenated by-products, but most of the related studies focus on the short-term degradation effect, and there is insufficient evaluation of the stability of the catalyst and the ecological risk of degradation products during long-term operation.
- Future research should combine in situ characterization techniques, such as in situ fluorescence spectroscopy, electron paramagnetic resonance, and theoretical calculations, such as density functional theory, and other methods to systematically explore the influence of DOM on the generation pathway of reactive oxygen species and the mechanism of DOM in the separation of photogenerated electron–hole pairs.
- Using high-throughput sequencing, mass spectrometry analysis, and other technical means to accurately quantify different components in DOM and evaluate their respective influences on the photocatalytic process. By comparing the results of simulation experiments with a single standard DOM, such as HA, and the actual DOM composition, improve the universality and accuracy of the results.
- By extending the experimental cycle and using various ecological toxicity testing methods, comprehensively evaluate the potential impact of DOM participating in the photocatalytic process on the aquatic ecosystem.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APAP | Acetaminophen (paracetamol) |
| BC | Benzocaine |
| BDOM | Biochar dissolved organic matter |
| Bi-TNB | Bismuth titanate nanobulk |
| BP-2 | 2,2′,4,4′-Tetrehydroxybenzophenone |
| BPA | Bisphenol A |
| BUP | Bupropion |
| CA | Citric acid |
| CBZ | Carbamazepine |
| CDOM | Chromophoric dissolved organic matter |
| CHA | Coal humic acid |
| COFs | Covalent organic frameworks |
| CPX | Cephalexin |
| CTD | Clothianidin |
| DCF | Diclofenac |
| DEET | N,N-Diethyl-3-methyl benzoyl amide |
| DOC | Dissolved organic carbon |
| DOM | Dissolved organic matter |
| FA | Fulvic acid |
| FQNs | Fluoroquinolones |
| GO | Graphene oxide |
| HA | Humic acid |
| IDM | Indomethacin |
| LOAs | Low-molecular-weight organic acids |
| MWCNTs | Multi-walled carbon nanotubes |
| NOM | Natural organic matter |
| NOR | Norfloxacin |
| NPX | Naproxen |
| NPs | Nanoparticles |
| OFX | Ofloxacin |
| PHE | Phenanthrene |
| PPCPs | Pharmaceuticals and personal care products |
| PPRIs | Photoproduction reaction intermediates |
| rGO | Reduced graphene oxide |
| RIs | Reactive intermediates |
| ROS | Reactive oxidative species |
| SHA | Soil humic acid |
| SMX | Sulfamethoxazole |
| SSZ | Sulfasalazine |
| STZ | Sulfathiazole |
| TBBPA | Tetrabromobisphenol A |
| TC | Tetracycline |
| TCS | Triclosan |
| THM | Trimethoprim |
| UV | Ultraviolet |
| VEN | Venlafaxine |
| vis | Visible light |
| 17β-E2 | 17β-Estradiol |
| 4-CP | 4-chlorophenol |
| π-π EDA | π-π electron donor–acceptor |
References
- Chen, X.; Lu, X.; Zhao, R.; Su, G.; Meng, J.; Li, Q.; Hua, Y.; Shi, B. Occurrence and risks of PPCPs of a typical mountainous region: Implications for sustainable urban water systems. Sci. Total Environ. 2024, 951, 175714. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Vymazal, J.; Březinová, T.; Koželuh, M.; Kule, L.; Huang, J.; Chen, Z. Occurrence, removal and environmental risk assessment of pharmaceuticals and personal care products in rural wastewater treatment wetlands. Sci. Total Environ. 2016, 566–567, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Sudaryanto, A.; Witama, R.O.; Nosaki, K.; Tanoue, R.; Suciati, F.; Sachoemar, S.I.; Hayami, Y.; Morimoto, A.; Nomiyama, K.; Kunisue, T. Occurrence of emerging contaminants in Jakarta Bay, Indonesia: Pharmaceuticals and personal care products. Iop Conf. Series Earth Environ. Sci. 2023, 1137, 12050. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, J.; Liu, Y.; Chen, Z.; Yang, Y.; Zhang, Q.; Ying, G. Biocides in the Yangtze River of China: Spatiotemporal distribution, mass load and risk assessment. Environ. Pollut. 2015, 200, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; He, L.; Hu, L.; Chen, J.; Yang, Y.; He, L.; Bai, H.; Liu, Y.; Zhao, J.; Ying, G. The variations of antibiotics and antibiotic resistance genes in two subtropical large river basins of south China: Anthropogenic impacts and environmental risks. Environ. Pollut. 2022, 312, 119978. [Google Scholar] [CrossRef]
- Yi, J.; Huang, X.; Hou, J.; Xiong, J.; Qian, Z.; Liu, S.; Zhang, J.; Yin, D.; Li, J.; Su, Q.; et al. Occurrence and distribution of PPCPs in water from two largest urban lakes of China: First perspective from DGT in-situ measurement. Sci. Total Environ. 2023, 904, 166656. [Google Scholar] [CrossRef]
- Roshan, R.; Nahak, B.K.; Mahata, D.; Yadav, P.; Panda, S.; Patra, S.; Mahato, S.S.; Tiwari, A.; Mahata, S. Photocatalytic waste-to-renewable energy nexus using solar light induced quantum dots. Energy Conv. Manag. 2023, 283, 116917. [Google Scholar] [CrossRef]
- Muhammad, E.; Jan, T.; Jalil, A. Experimental and computational study of Bi2O3/WO3 heterostructures as efficient solar driven photocatalyst. Inorg. Chem. Commun. 2025, 171, 113581. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, N.; Li, Y.; Zhu, Z.; Chen, X.; Zhang, L. S doped g-C3N5 for highly efficient photocatalytic H2O2 production and PPCPs degradation via directional transfer of electrons. J. Environ. Chem. Eng. 2023, 11, 111491. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Xi, X.; Nie, Z.; Lim, F.Y.; Ong, S.L.; Hu, J. Design of dual-LSPR Bi/W18O49 composite and its red-light driven photocatalytic property in the photocatalytic degradation of PPCPs. Chem. Eng. J. 2024, 481, 148119. [Google Scholar] [CrossRef]
- Feng, Q.; Guo, S.; Bai, Z.; Pu, Y.; Zhang, H.; Wang, J. Highly efficient photocatalytic degradation of tetracycline antibiotic enabled by TiO2 nanodispersion. J. Ind. Eng. Chem. 2024, 145, 755–763. [Google Scholar] [CrossRef]
- Guo, Y.; Peng, B.; Liao, J.; Cao, W.; Liu, Y.; Nie, X.; Li, Z.; Ouyang, R. Recent advances in the role of dissolved organic matter during antibiotics photodegradation in the aquatic environment. Sci. Total Environ. 2024, 916, 170101. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Schaumann, G.E. Interactions of dissolved organic matter with natural and engineered inorganic colloids: A review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef]
- Yang, X.; Rosario-Ortiz, F.L.; Lei, Y.; Pan, Y.; Lei, X.; Westerhoff, P. Multiple Roles of Dissolved Organic Matter in Advanced Oxidation Processes. Environ. Sci. Technol. 2022, 56, 11111–11131. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Cheng, X.; Zhang, Y.; Li, W.; Wang, J.; Guo, H. Probing the roles of pH and ionic strength on electrostatic binding of tetracycline by dissolved organic matters: Reevaluation of modified fitting model. Env. Sci. Ecotechnol. 2021, 8, 100133. [Google Scholar] [CrossRef]
- Canonica, S.; Laubscher, H. Inhibitory effect of dissolved organic matter on triplet-induced oxidation of aquatic contaminants. Photochem. Photobiol. Sci. 2008, 7, 547–551. [Google Scholar] [CrossRef]
- Wenk, J.; von Gunten, U.; Canonica, S. Effect of Dissolved Organic Matter on the Transformation of Contaminants Induced by Excited Triplet States and the Hydroxyl Radical. Environ. Sci. Technol. 2011, 45, 1334–1340. [Google Scholar] [CrossRef]
- Maeng, S.K.; Cho, K.; Jeong, B.; Lee, J.; Lee, Y.; Lee, C.; Choi, K.J.; Hong, S.W. Substrate-immobilized electrospun TiO2 nanofibers for photocatalytic degradation of pharmaceuticals: The effects of pH and dissolved organic matter characteristics. Water Res. 2015, 86, 25–34. [Google Scholar] [CrossRef]
- Haroune, L.; Salaun, M.; Ménard, A.; Legault, C.Y.; Bellenger, J. Photocatalytic degradation of carbamazepine and three derivatives using TiO2 and ZnO: Effect of pH, ionic strength, and natural organic matter. Sci. Total Environ. 2014, 475, 16–22. [Google Scholar] [CrossRef]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.H.; Langenhoff, A.A.M. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Tran, M.L.; Fu, C.; Juang, R. Effects of water matrix components on degradation efficiency and pathways of antibiotic metronidazole by UV/TiO2 photocatalysis. J. Mol. Liq. 2019, 276, 32–38. [Google Scholar] [CrossRef]
- Subramani, K.; Ganeshan, J.K.S.; Srinivasan, S.; Mohanasundaram, N.V.T.; Incharoensakdi, A. Hydrothermally and green synthesized TiO2 nanoparticles with high photocatalytic textile industry dye degradation, antibacterial and antioxidant activities. J. Alloy. Compd. 2025, 1017, 179068. [Google Scholar] [CrossRef]
- Yan, C.; Abed, A.M.; Shaban, M.; Li, X.; Zhou, X.; Lei, G.; Abdullaev, S.; Mahariq, I. Superb photocatalytic H2 production/tetracycline pollutant degradation by synthesizing novel and recyclable ternary g-C3N4-based photocatalyst: Characterization/optimization/mechanism/toxicity assessment. J. Water Process. Eng. 2025, 69, 106642. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, C.; Yuan, G. Design of CdS/Covalent Organic Framework as an efficient heterojunction photocatalyst for enhanced photocatalytic H2 evolution. Mater. Lett. 2024, 365, 136417. [Google Scholar] [CrossRef]
- Eghbali, S.; Boroujerdnia, M.; Haghighatzadeh, A. Visible-light-active Ag-doped BiVO4 nanostructures: Preparation and optical and photocatalytic studies. Phys. B 2025, 703, 416990. [Google Scholar] [CrossRef]
- Abdoli, M.; Cigeroglu, Z.; Acikbas, Y. Eco-friendly synthesis of ZnO/g-C3N4 using Salix aegyptiaca extract: A high-performance photocatalyst for tetracycline degradation under visible light, response surface methodology. J. Mol. Struct. 2025, 1332, 141719. [Google Scholar] [CrossRef]
- Jiang, S.; Zheng, G.; Sun, J.; Zhang, F.; Yu, H. SPR-promoted Bi/Bi2WO6 heterojunction with effectively enhanced visible light photocatalytic degradation of pollutants. J. Alloy Compd. 2025, 1017, 179057. [Google Scholar] [CrossRef]
- Zhou, P.; Han, X.; Wu, L.; Qi, M.; Shen, Y.; Nie, J.; Liu, X.; Wang, F.; Bian, L.; Liang, J. Facile construction of novel BiOBr/black-TiO2/tourmaline composites for the synergistic degradation of tetracycline in aqueous. Sep. Purif. Technol. 2025, 362, 131976. [Google Scholar] [CrossRef]
- Karamifar, M.; Sabbaghi, S.; Mohtaram, M.S.; Rasouli, K.; Mohsenzadeh, M.; Kamyab, H.; Derakhshandeh, A.; Dolatshah, L.; Moradi, H.; Chelliapan, S. Ultrasonic-assisted synthesis of TiO2/MWCNT/Pani nanocomposite: Photocatalyst characterization and optimization of efficient variables in the degradation of benzene via RSM-CCD. Powder Technol. 2024, 432, 119176. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Jiao, Y.; Yu, J.; Wang, L.; Chen, J. SnO2-x/CD-MOF S-Scheme heterostructure photocatalysts synergized by oxygen vacancies and highly porous for rhodamine B and tetracycline degradation: Process, and mechanism analysis. Colloid. Surf. A-Physicochem. Eng. Asp. 2025, 705, 135625. [Google Scholar] [CrossRef]
- Evgenidou, E.; Rapti, A.; Koronaiou, L.; Petromelidou, S.; Anagnostopoulou, K.; Lambropoulou, D. Photocatalytic degradation of the antidepressant drug bupropion. Performance, water matrix effect and identification of transformation products. Sustain. Chem. Environ. 2023, 3, 100028. [Google Scholar] [CrossRef]
- Lee, Y.; Jae Jeong, Y.; Sun Cho, I.; Lee, C.; Park, S.; Alvarez, P.J.J. The inhibitory mechanism of humic acids on photocatalytic generation of reactive oxygen species by TiO2 depends on the crystalline phase. Chem. Eng. J. 2023, 476, 146785. [Google Scholar] [CrossRef]
- Ye, Y.; Feng, Y.; Bruning, H.; Yntema, D.; Rijnaarts, H.H.M. Photocatalytic degradation of metoprolol by TiO2 nanotube arrays and UV-LED: Effects of catalyst properties, operational parameters, commonly present water constituents, and photo-induced reactive species. Appl. Catal. B Environ. 2018, 220, 171–181. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Fu, T.; Liu, K.; Sun, J.; Yan, J.; Jiang, L.; Tong, Z.; Zhou, Z.; Zhang, H. Different water matrixes leading to noticeable differences of tetracycline removal by ZnO. Mater. Today Commun. 2023, 37, 106967. [Google Scholar] [CrossRef]
- Chandran, P.; Netha, S.; Sudheer, K.S. Effect of humic acid on photocatalytic activity of ZnO nanoparticles. J. Photochem. Photobiol. B 2014, 138, 155–159. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Guo, Y.; Rhodes, G.; Yeom, J.; Li, H.; Zhang, W. Photocatalytic degradation of cephalexin by ZnO nanowires under simulated sunlight: Kinetics, influencing factors, and mechanisms. Environ. Int. 2019, 132, 105105. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.; Eslami, A.; Massoudinejad, M.; Gholami, Z.; Sarafraz, M.; Noorimotlagh, Z.; Adiban, M.; Mirzaee, S.A. Photocatalytic degradation and dechlorination mechanism of diclofenac using heterojunction Mn-doped tungsten trioxide (Mn-WO3) nanoparticles under LED visible light from aqueous solutions. Sci. Rep. 2024, 14, 29583. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.; Tan, J.; Li, J.; Wu, J.; Zhang, T.; Wang, X. Oxygen-doped porous graphitic carbon nitride in photocatalytic peroxymonosulfate activation for enhanced carbamazepine removal: Performance, influence factors and mechanisms. Chem. Eng. J. 2022, 429, 130860. [Google Scholar] [CrossRef]
- Qiao, M.; Fu, L.; Barcelo, D. Removal of polycyclic aromatic hydrocarbons by g-C3N4 nanosheets under visible light irradiation and effect of typical co-existence substances in river water. Process Saf. Environ. Protect. 2022, 159, 376–381. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, W.; Wang, H.; Pan, C.; Xu, J.; Pozdnyakov, I.P.; Wu, F.; Li, J. Interaction between graphene oxide and acetaminophen in water under simulated sunlight: Implications for environmental photochemistry of PPCPs. Water Res. 2023, 228, 119364. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, Z.; Ouyang, Y.; Ji, X.; Hu, X.; Li, T.; Ouyang, K.; Wang, P.; Wang, H.; Hu, X. Multi-phase CdS loaded on biochar for photocatalytic activation of peroxymonosulfate for thiamethoxam degradation: π-conjugation improves PMS adsorption. Sep. Purif. Technol. 2023, 326, 124842. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, X.; Liu, Y.; Wang, F.; Li, D.; Liu, H.; Li, R.; Chen, T.; Lv, W.; Liu, G. Integration of oxygen vacancies into BiOI via a facile alkaline earth ion-doping strategy for the enhanced photocatalytic performance toward indometacin remediation. J. Hazard. Mater. 2021, 412, 125147. [Google Scholar] [CrossRef]
- Liu, L.; Zhi, S.; Chen, R.; Yang, Y.; Luo, C.; Liang, P.; Liu, Y.; Zhu, G. Highly selective and rapid sequential degradation of venlafaxine-class antidepressants using a novel BiOCl-based two-dimensional molecularly imprinted photocatalyst. Sep. Purif. Technol. 2025, 362, 131777. [Google Scholar] [CrossRef]
- Fan, G.; Ning, R.; Luo, J.; Zhang, J.; Hua, P.; Guo, Y.; Li, Z. Visible-light-driven photocatalytic degradation of naproxen by Bi-modified titanate nanobulks: Synthesis, degradation pathway and mechanism. J. Photochem. Photobiol. A Chem. 2020, 386, 112108. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Czech, B.; Göktaş, A.C.; Yubuta, K.; Kadirova, Z.C. SnO2@ZnS photocatalyst with enhanced photocatalytic activity for the degradation of selected pharmaceuticals and personal care products in model wastewater. J. Alloy. Compd. 2020, 827, 154339. [Google Scholar] [CrossRef]
- Guo, C.; Gao, S.; Lv, J.; Hou, S.; Zhang, Y.; Xu, J. Assessing the photocatalytic transformation of norfloxacin by BiOBr/iron oxides hybrid photocatalyst: Kinetics, intermediates, and influencing factors. Appl. Catal. B Environ. 2017, 205, 68–77. [Google Scholar] [CrossRef]
- Sun, J.; Wang, L.; Huang, T.; Liu, K.; Fu, T.; Xu, Z.; Yang, W.; Tong, Z.; Zhang, H. Floating BiOBr/Ti3C2 aerogel spheres for efficient degradation of quinolone antibiotics: Rapid oxygen transfer via triphase interface and effective charges separation by internal electric field. J. Colloid. Interface. Sci. 2025, 685, 813–825. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, S.; Li, G.; Li, J.; Chen, F.; Chen, R. Chlorine-enhanced photocatalytic degradation of PPCPs over Bi2MoO6/(BiO)2CO3 heterostructures. J. Environ. Chem. Eng. 2021, 9, 106597. [Google Scholar] [CrossRef]
- Du, B.; Fan, G.; Yang, S.; Luo, J.; Wu, J.; Xu, K. Mechanistic insight into humic acid-enhanced sonophotocatalytic removal of 17β-estradiol: Formation and contribution of reactive intermediates. Environ. Res. 2023, 231, 116249. [Google Scholar] [CrossRef]
- Lykos, C.; Bairamis, F.; Efthymiou, C.; Konstantinou, I. Synthesis and Characterization of Composite WO3 Fibers/g-C3N4 Photocatalysts for the Removal of the Insecticide Clothianidin in Aquatic Media. Nanomaterials 2024, 14, 1045. [Google Scholar] [CrossRef]
- Chen, R.; Ding, S.; Fu, N.; Ren, X. Preparation of a g-C3N4/Ag3PO4 composite Z-type photocatalyst and photocatalytic degradation of Ofloxacin: Degradation performance, reaction mechanism, degradation pathway and toxicity evaluation. J. Environ. Chem. Eng. 2023, 11, 109440. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Shen, L.; Li, R.; Tan, C.; Chen, T.; Liu, H.; Liu, Y.; Cai, Z.; Liu, G.; et al. Insights into the synergetic mechanism of a combined vis-RGO/TiO2/peroxodisulfate system for the degradation of PPCPs: Kinetics, environmental factors and products. Chemosphere 2019, 216, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, J.; Shi, J.; Deng, H. Bi2Fe4O9/rGO nanocomposite with visible light photocatalytic performance for tetracycline degradation. Environ. Res. 2024, 249, 118361. [Google Scholar] [CrossRef]
- Gao, P.; Li, Z.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Construction of novel MWCNTs/Bi4O5I2 nanosheets with enhanced adsorption and photocatalytic performance for the degradation of tetracycline: Efficiency, mechanism and regeneration. Chem. Eng. J. 2022, 429, 132398. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Mahdipour, F.; Wacławek, S.; Lin, K.A.; Ghanbari, F. Insights into the synergistic role of photocatalytic activation of peroxymonosulfate by UVA-LED irradiation over CoFe2O4-rGO nanocomposite towards effective Bisphenol A degradation: Performance, mineralization, and activation mechanism. Chem. Eng. J. 2023, 453, 139556. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Liu, Y.; Li, R.; Chen, P.; Liu, H.; Lv, W.; Liu, G.; Feng, Y. Ultrathin Ag2WO4-coated P-doped g-C3N4 nanosheets with remarkable photocatalytic performance for indomethacin degradation. J. Hazard. Mater. 2020, 392, 122355. [Google Scholar] [CrossRef]
- Gao, X.; Niu, J.; Wang, Y.; Ji, Y.; Zhang, Y. Solar photocatalytic abatement of tetracycline over phosphate oxoanion decorated Bi2WO6/polyimide composites. J. Hazard. Mater. 2021, 403, 123860. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, J.; Liu, Y.; Li, D.; Tan, C.; Chen, P.; Liu, H.; Zheng, X.; Wen, C.; Lv, W.; et al. Construction of double-functionalized g-C3N4 heterojunction structure via optimized charge transfer for the synergistically enhanced photocatalytic degradation of sulfonamides and H2O2 production. J. Hazard. Mater. 2022, 422, 126868. [Google Scholar] [CrossRef]
- Xu, B.; An, Z.; Li, M.; Zhang, B.; Guo, L. Photocatalytic degradation of tetrabromobisphenol A by Z-scheme WO3/ZnIn2S4 heterojunction: Efficiency, mechanism and toxicity evaluation. J. Environ. Chem. Eng. 2023, 11, 111522. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, X.; Yang, G.; Qin, L.; Pan, Y.; Guo, Y. Porous cyclopentadiene unit-incorporated graphitic carbon nitride nanosheets for efficient photocatalytic oxidation of recalcitrant organic micropollutants in wastewater. J. Hazard. Mater. 2023, 460, 132365. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, G.; Yan, Z.; Wu, D.; Zhou, R.; Bao, X. Insights into a CQD-SnNb2O6/BiOCl Z-scheme system for the degradation of benzocaine: Influence factors, intermediate toxicity and photocatalytic mechanism. Chem. Eng. J. 2019, 374, 79–90. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Y.; Dong, K.; Wang, C.; Li, S. A novel S-scheme heterojunction of Cd0.5Zn0.5S/BiOCl with oxygen defects for antibiotic norfloxacin photodegradation: Performance, mechanism, and intermediates toxicity evaluation. J. Colloid. Interface. Sci. 2023, 629, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, T.; Che, H.; Tang, C.; Liu, B.; Ao, Y. In-situ growing Ni2P on CdS@g-C3N4 composites for highly efficient synergistically photocatalytic H2 evolution and antibiotic degradation. Surf. Interfaces 2024, 47, 104205. [Google Scholar] [CrossRef]
- Qiu, P.; Liu, H.; Wang, G.; Wang, C.; Huang, Z.; Huang, C.; Lai, Y.; Xing, C.; Liang, K. Dual vacancies and S-scheme BiOBr/Bi2WO6 heterojunction synergistically boost the directional transfer of photogenerated electrons for efficient photocatalytic degradation of norfloxacin. J. Water Process. Eng. 2024, 68, 106372. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, C.; Liu, R.; Fang, Q.; Zheng, Y.; He, Z.; Song, S.; Shen, Y. Elucidating the charge redistribution in bimetallic metal–organic frameworks: High-electron-density reactive center for removal of organic micropollutants in water. Chem. Eng. J. 2023, 468, 143723. [Google Scholar] [CrossRef]
- Yang, P.; Wu, C.; Zhang, X.; Sun, Q.; Hou, X.; Wang, T. Enhanced photocatalytic degradation of sulfathiazole via dual-ligand Zr-MOFs: A porphyrin and pyrene-based energy transfer. Sep. Purif. Technol. 2025, 354, 128727. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Wang, L.; Wang, Q.; Zhang, Q.; Cui, F.; Jiang, G. Synthesis of S-type heterostructure π-COF for photocatalytic tetracycline degradation. Chem. Eng. J. 2024, 479, 147534. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, H.; Wu, M.; Zhang, Y.; Zhu, U.; Xu, J.; Deng, J.; Zhou, T. Rational fabrication of Z-scheme heterojunction ZnFe2O4-seed@TpTt-COF for the visible-light-driven photocatalytic degradation of bisphenol A in food waste leachate boosted by primitive humic acid. Chem. Eng. J. 2024, 487, 150684. [Google Scholar] [CrossRef]
- Luo, Y.; Shi, G.; Yu, S.; Liu, Z.; Yin, J.; Xue, M.; Sun, Q.; Shen, F.; Li, X.; Yin, Z.; et al. Novel MIL-88B(Fe)/ZnTi-LDH high-low junctions for adsorption and photodegradation of tetracycline: Characteristics, performance, and mechanisms. Chem. Eng. J. 2023, 473, 145198. [Google Scholar] [CrossRef]
- Jiangbo, Y.; Jing, Y.; Jie, L.; Zhanchao, W.; Shaoping, K. Photocatalytic Removal of Antibiotics from Water. Prog. Chem. 2024, 36, 95–105. [Google Scholar]
- Hua, Q.; Cheng, L.; Qu, W.; Wu, D.; Li, Y.; Xu, Z.; Cao, X.; Xie, M.; Yan, X.; Dong, C.; et al. Tailoring reactant adsorption on WO3 via surface bismuth incorporation for enhanced photocatalytic degradation of acetaldehyde. Appl. Catal. A Gen. 2025, 700, 120303. [Google Scholar] [CrossRef]
- Parchami, K.; Habibzadehnesami, N.; Pirkarami, A.; Haghighat-Nezhad, S.; Gheitasi, N.; Nabavi, M.S.; Ghasemi, E. Enhancing NiAl-LDH@TiO2-Tiv-S photoelectrocatalyst performance for textile dye degradation and oxygen production: S-doped Ti vacancies. Environ. Res. 2025, 279, 121741. [Google Scholar] [CrossRef] [PubMed]
- Safaralizadeh, E.; Mahjoub, A.; Janitabardarzi, S. Visible light-induced degradation of phenolic contaminants utilizing nanoscale TiO2 and ZnO impregnated with SR 7B (SR) dye as advanced photocatalytic systems. Ceram. Int. 2025, 51, 1958–1969. [Google Scholar] [CrossRef]
- Chang, L.; Ahmad, N.; Zeng, G.; Ray, A.; Zhang, Y. N, S co-doped carbon quantum dots/TiO2 composite for visible-light-driven photocatalytic reduction of Cr (VI). J. Environ. Chem. Eng. 2022, 10, 108742. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, W.; Liu, Z.; Zhang, X.; Wang, X. Construction of CoFe2O4/TiO2@C S-scheme heterojunction and removal of phenol by activated peroxymonosulfate. J. Alloy Compd. 2025, 1022, 179992. [Google Scholar] [CrossRef]
- Hernandez-Laverde, M.; Morante, N.; Gutierrez, B.L.; Murcia, J.J.; Monzillo, K.; Sannino, D.; Vaiano, V. Solar Light Elimination of Bacteria, Yeast and Organic Pollutants by Effective Photocatalysts Based on Ag/Cr-TiO2 and Pd/Cr-TiO2. Nanomaterials 2024, 14, 1730. [Google Scholar] [CrossRef]
- Rath, P.C.; Mishra, M.; Saikia, D.; Chang, J.K.; Perng, T.; Kao, H. Facile fabrication of titania-ordered cubic mesoporous carbon composite: Effect of Ni doping on photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2019, 44, 19255–19266. [Google Scholar] [CrossRef]
- Katz, A.; Mcdonagh, A.; Tijing, L.; Shon, H.K. Fouling and Inactivation of Titanium Dioxide-Based Photocatalytic Systems. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1880–1915. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2 -assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.U.H. Modification strategies of TiO2 based photocatalysts for enhanced visible light activity and energy storage ability: A review. J. Environ. Chem. Eng. 2023, 11, 111532. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Jin, P.; Peng, A.; Zhao, X.; Yang, K.; He, J. Highly efficient photocatalytic degradation of microcystin-LR with permonosulfate activated by visible-light over Z-scheme ZnO/g-C3N4: Degradation pathways and mechanism. J. Environ. Chem. Eng. 2023, 11, 111088. [Google Scholar] [CrossRef]
- Bai, X.; Sun, C.; Liu, D.; Luo, X.; Li, D.; Wang, J.; Wang, N.; Chang, X.; Zong, R.; Zhu, Y. Photocatalytic degradation of deoxynivalenol using graphene/ZnO hybrids in aqueous suspension. Appl. Catal. B Environ. 2017, 204, 11–20. [Google Scholar] [CrossRef]
- Mahesha, A.; Nagaraja, M.; Madhu, A.; Suriyamurthy, N.; Satyanarayana Reddy, S.; Al-Dossari, M.; El-Gawaad, N.S.A.; Manjunatha, S.O.; Gurushantha, K.; Srinatha, N. Chromium-doped ZnO nanoparticles synthesized via auto-combustion: Evaluation of concentration-dependent structural, band gap-narrowing effect, luminescence properties and photocatalytic activity. Ceram. Int. 2023, 49, 22890–22901. [Google Scholar] [CrossRef]
- Li, Y.; Niu, J.; Shang, E.; Crittenden, J.C. Influence of dissolved organic matter on photogenerated reactive oxygen species and metal-oxide nanoparticle toxicity. Water Res. 2016, 98, 9–18. [Google Scholar] [CrossRef]
- Jiang, C.; Aiken, G.R.; Hsu-Kim, H. Effects of Natural Organic Matter Properties on the Dissolution Kinetics of Zinc Oxide Nanoparticles. Environ. Sci. Technol. 2015, 49, 11476–11484. [Google Scholar] [CrossRef]
- Ghattavi, S.; Nezamzadeh-Ejhieh, A. Nanoscale AgI-WO3 binary photocatalyst: Synthesis, brief characterization, and investigation of its photocatalytic activity. Mater. Res. Bull. 2023, 158, 112085. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Xiong, B.; Li, J.; Li, Y.; Zhou, Y.; Yang, A.; Zhang, Q. Constructing WO3/TiO2 heterojunction with solvothermal-sintering for enhanced photocatalytic activity under visible light irradiation. Solid State Sci. 2022, 131, 106963. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S. MoS2/WO3 heterojunction with the intensified photocatalytic performance for decomposition of organic pollutants under the broad array of solar light. J. Clean. Prod. 2021, 324, 129290. [Google Scholar] [CrossRef]
- Chai, Q.; Dong, J.; Yu, X.; Zhang, X.; Li, J.; Guo, S.; Yang, Y. Photocatalytic activation of oxalic acid over FeOOH loaded FeWO4/WO3 heterojunction for high-efficient degradation of tetracycline. J. Environ. Chem. Eng. 2024, 12, 111728. [Google Scholar] [CrossRef]
- Javaid, A.; Latif, S.; Imran, M.; Hussain, N.; Rajoka, M.S.R.; Iqbal, H.M.N.; Bilal, M. Nanohybrids-assisted photocatalytic removal of pharmaceutical pollutants to abate their toxicological effects—A review. Chemosphere 2022, 291, 133056. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, G.; Li, Y.; Li, H.; Deng, N. Carboxymethyl-β-cyclodextrin functionalized TiO2@Fe3O4@RGO magnetic photocatalyst for efficient photocatalytic degradation of tetracycline under visible light irradiation. J. Environ. Chem. Eng. 2024, 12, 113303. [Google Scholar] [CrossRef]
- Mcallister, M.J.; Li, J.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’Homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mat. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, S.; Zheng, W.; Xue, X.; Liu, H.; Chen, S.; Zhu, Y. Preparation of N-TiO2/RGO nanocomposites through sol-gel method. Korean J. Chem. Eng. 2021, 38, 1913–1922. [Google Scholar] [CrossRef]
- Duan, M.; Jiang, L.; Shao, B.; Feng, C.; Yu, H.; Guo, H.; Chen, H.; Tang, W. Enhanced visible-light photocatalytic degradation activity of Ti3C2/PDIsm via π–π interaction and interfacial charge separation: Experimental and theoretical investigations. Appl. Catal. B Environ. 2021, 297, 120439. [Google Scholar] [CrossRef]
- Alhowyan, A.; Mahdi, W.A.; Obaidullah, A. DFT-based study of doped C20 nanostructures for efficient detection of the narcotic drug ecstasy. Diam. Relat. Mat. 2025, 152, 111873. [Google Scholar] [CrossRef]
- Ray, A.; Passiu, C.; Nasuda, M.; Ramakrishna, S.N.; Rossi, A.; Kuzuya, A.; Spencer, N.D.; Yamakoshi, Y. Reactive-Oxygen-Species-Mediated Surface Oxidation of Single-Molecule DNA Origami by an Atomic Force Microscope Tip-Mounted C60 Photocatalyst. ACS Nano 2021, 15, 19256–19265. [Google Scholar] [CrossRef]
- Cho, E.; Ciou, J.; Zheng, J.; Pan, J.; Hsiao, Y.; Lee, K.; Huang, J. Fullerene C70 decorated TiO2 nanowires for visible-light-responsive photocatalyst. Appl. Surf. Sci. 2015, 355, 536–546. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; He, Q.; Yuan, X.; Huang, D.; et al. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B Environ. 2020, 265, 118579. [Google Scholar] [CrossRef]
- Hou, J.; Lan, X.; Shi, J.; Yu, S.; Zhang, Y.; Wang, H.; Ren, C. A mild and simple method to fabricate commercial TiO2 (P25) and C60 composite for highly enhancing H2 generation. Int. J. Hydrogen Energy 2020, 45, 2852–2861. [Google Scholar] [CrossRef]
- Sardar, S.; Munawar, T.; Mukhtar, F.; Nadeem, M.S.; Khan, S.A.; Koc, M.; Manzoor, S.; Ashiq, M.N.; Iqbal, F. Fullerene trigged energy storage and photocatalytic ability of La2O3-ZnO@C60 core-shell nanocomposite. Mater. Sci. Eng. B 2023, 288, 116151. [Google Scholar] [CrossRef]
- Murakami, N.; Tango, Y.; Miyake, H.; Tajima, T.; Nishina, Y.; Kurashige, W.; Negishi, Y.; Takaguchi, Y. SWCNT Photocatalyst for Hydrogen Production from Water upon Photoexcitation of (8, 3) SWCNT at 680-nm Light. Sci. Rep. 2017, 7, 43445. [Google Scholar] [CrossRef] [PubMed]
- Sobahi, T.R.; Amin, M.S. Photocatalytic oxidation of atrazine using BaTiO3 -MWCNT nanocomposites under visible light. Ceram. Int. 2021, 47, 14366–14374. [Google Scholar] [CrossRef]
- Xun, L.; Xuehua, L.; Jingwen, C. Synergistic Photogeneration of 1O2 and ·OH by Humid Acid and Carbon Nanomaterial in Aqueous Phase. Asian J. Ecotoxicol. 2016, 11, 49–56. (In Chinese) [Google Scholar]
- Zhang, L.; Zhang, Y.; Lin, X.; Yang, K.; Lin, D. The role of humic acid in stabilizing fullerene (C60) suspensions. J. Zhejiang Univ. Sci. A 2014, 15, 634–642. [Google Scholar] [CrossRef]
- Akram, M.Y.; Hu, B.; Jia, J.; Li, C.; Dong, H.; Lu, H. The science behind the scene: Theoretical and experimental foundations of metal sulfide photocatalyst modification. Int. J. Hydrogen Energy 2024, 93, 21–42. [Google Scholar] [CrossRef]
- Abdullah, U.; Ali, M.; Pervaiz, E. An Inclusive Review on Recent Advancements of Cadmium Sulfide Nanostructures and its Hybrids for Photocatalytic and Electrocatalytic Applications. Mol. Catal. 2021, 508, 111575. [Google Scholar] [CrossRef]
- Chen, Q.; Cheng, X.; Long, H.; Rao, Y. A short review on recent progress of Bi/semiconductor photocatalysts: The role of Bi metal. Chin. Chem. Lett. 2020, 31, 2583–2590. [Google Scholar] [CrossRef]
- Ghosh, S.; Sahu, M. Biochar Supported Oxygen Rich TiO2-CuO Heterojunction Photocatalyst for the Degradation of Dimethyl Phthalate: Optimization, Degradation Kinetics and Mechanisms. Int. J. Environ. Res. 2025, 19, 58. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Farzadkia, M.; Rezaei, K.R.; Sobhi, H.R.; Yeganeh, M.; Esrafili, A. Efficient removal of oxytetracycline antibiotic from aqueous media using UV/g-C3N4/Fe3O4 photocatalytic process. Heliyon 2024, 10, e30604. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, T.; Xu, Y.; Li, A.; Xu, H.; Gao, Y.; Wang, X.; Fujii, R.; Sasaki, S. β-carotene assisted chlorin-modified Pt/TiO2 photocatalysts for enhanced hydrogen evolution. Appl. Surf. Sci. 2025, 684, 161943. [Google Scholar] [CrossRef]
- Ahmad, I.; Athar, M.S.; Muneer, M.; Altass, H.M.; Felemban, R.; Ahmed, S.A. Synergistic design of a graphene oxide-mediated polyaniline/α-Fe2O3 ternary heterostructure: Advancing photocatalytic degradation and adsorption efficiency. Nanoscale 2024, 17, 3822–3836. [Google Scholar] [CrossRef] [PubMed]
- Awang, H.; Peppel, T.; Strunk, J. Photocatalytic Degradation of Diclofenac by Nitrogen-Doped Carbon Quantum Dot-Graphitic Carbon Nitride (CNQD). Catalysts 2023, 13, 735. [Google Scholar] [CrossRef]
- Dong, W.; Qi, K.; Zhang, L.; Xie, X.; Hu, J.; Mao, M.; Wang, Z.; Yang, B. Synthesis of hydrochar-based AgBr/Bi2WO6 heterojunction photocatalyst using dopant element and its loading on PVDF membrane: Study on degradation of sulfadiazine. Sep. Purif. Technol. 2025, 360, 131132. [Google Scholar] [CrossRef]
- Zhan, M.; Hong, Y.; Fang, Z.; Qiu, D. Magnetic recyclable visible light-driven Bi2WO6/Fe3O4/RGO for photocatalytic degradation of Microcystin-LR: Mechanism, pathway, and influencing factors. Environ. Res. 2024, 252, 118885. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Rauf, S.; Al Alwan, B.; El Jery, A.; Almuqati, N.; Melhi, S.; Amin, M.A.; Al-Hadeethi, Y.; Sohail, M.; Orooji, Y.; et al. Recent advance in MOFs and MOF-based composites: Synthesis, properties, and applications. Mater. Today Energy 2024, 41, 101542. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Eghbali, T. Design of bifunctional sandwich-like Co@Si/Ox-MXene nanocomposite to increase the supercapacitor properties and removal of pollutants from wastewater. J. Alloy Compd. 2024, 983, 173920. [Google Scholar] [CrossRef]
- Wang, X.; Muhmood, A.; Lyu, T.; Dong, R.; Liu, H.; Wu, S. Mechanisms of genuine humic acid evolution and its dynamic interaction with methane production in anaerobic digestion processes. Chem. Eng. J. 2021, 408, 127322. [Google Scholar] [CrossRef]
- Aolin, H.; Qin, L.; Zhu, S.; Hu, X.; Yin, D. Combined effects of pH and dissolved organic matter on the availability of pharmaceuticals and personal care products in aqueous environment. Sci. Total Environ. 2024, 929, 172637. [Google Scholar] [CrossRef]
- Yu, M.; He, X.; Liu, J.; Wang, Y.; Xi, B.; Li, D.; Zhang, H.; Yang, C. Characterization of isolated fractions of dissolved organic matter derived from municipal solid waste compost. Sci. Total Environ. 2018, 635, 275–283. [Google Scholar] [CrossRef]
- Sun, H.; Shi, X.; Mao, J.; Zhu, D. Tetracycline sorption to coal and soil humic acids: An examination of humic structural heterogeneity. Environ. Toxicol. Chem. 2010, 29, 1934–1942. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, S.; Gao, P.; Ren, X.; Li, F.; Liu, Z.; Wang, L.; Peng, H.; Ju, S. Photo-transformation of biochar-derived dissolved organic matter and its binding with phenanthrene/9-phenanthrol: The role of functional group and pyrolysis temperature. Bioresour. Technol. 2024, 413, 131547. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, J.; Xie, Y.; Ren, M. Effects of DOM and cations on the diffusion migration of PPCPs through ion exchange membranes. Sep. Purif. Technol. 2025, 359, 130508. [Google Scholar] [CrossRef]
- Yao, W.; Qi, Y.; Han, Y.; Ge, J.; Dong, Y.; Wang, J.; Yi, Y.; Volmer, D.A.; Li, S.; Fu, P. Seasonal variation and dissolved organic matter influence on the distribution, transformation, and environmental risk of pharmaceuticals and personal care products in coastal zone: A case study of Tianjin, China. Water Res. 2024, 249, 120881. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ye, Z.; Cai, J.; Lin, L. Quantification of DOM effects on tetracyclines transport during struvite recovery from swine wastewater. Water Res. 2021, 206, 117756. [Google Scholar] [CrossRef]
- Ma, D.; Gao, B.; Sun, S.; Wang, Y.; Yue, Q.; Li, Q. Effects of dissolved organic matter size fractions on trihalomethanes formation in MBR effluents during chlorine disinfection. Bioresour. Technol. 2013, 136, 535–541. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, J.; Zhong, Y.; Ding, T.; Dissanayake, P.D.; Yang, Y.; Tsang, Y.F.; Ok, Y.S. Sorption of pharmaceuticals and personal care products (PPCPs) from water and wastewater by carbonaceous materials: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 727–766. [Google Scholar] [CrossRef]
- Kun, Y.; Lizhong, Z.; Xing, B. Sorption of sodium dodecylbenzene sulfonate by montmorillonite. Environ. Pollut. 2007, 145, 571–576. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Ghosh, S.; Xing, B. Phenanthrene binding by humic acid-protein complexes as studied by passive dosing technique. Environ. Pollut. 2014, 184, 145–153. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Novel Magnetic Carbon Nanotube-TiO2 Composites for Solar Light Photocatalytic Degradation of Pharmaceuticals in the Presence of Natural Organic Matter. J. Water Process. Eng. 2019, 31, 100836. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, J.; Wang, S.; Du, P.; Xing, B. Effects of Solution Chemistry on Adsorption of Selected Pharmaceuticals and Personal Care Products (PPCPs) by Graphenes and Carbon Nanotubes. Environ. Sci. Technol. 2014, 48, 13197–13206. [Google Scholar] [CrossRef]
- Liao, X.; Chen, C.; Liang, Z.; Zhao, Z.; Cui, F. Selective adsorption of antibiotics on manganese oxide-loaded biochar and mechanism based on quantitative structure–property relationship model. Bioresour. Technol. 2023, 367, 128262. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Bruning, H.; Liu, W.; Rijnaarts, H.; Yntema, D. Effect of dissolved natural organic matter on the photocatalytic micropollutant removal performance of TiO2 nanotube array. J. Photochem. Photobiol. A Chem. 2019, 371, 216–222. [Google Scholar] [CrossRef]
- Gora, S.L.; Andrews, S.A. Adsorption of natural organic matter and disinfection byproduct precursors from surface water onto TiO2 nanoparticles: pH effects, isotherm modelling and implications for using TiO2 for drinking water treatment. Chemosphere 2017, 174, 363–370. [Google Scholar] [CrossRef]
- Mwaanga, P.; Carraway, E.R.; Schlautman, M.A. Preferential sorption of some natural organic matter fractions to titanium dioxide nanoparticles: Influence of pH and ionic strength. Environ. Monit. Assess. 2014, 186, 8833–8844. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Tang, L.; Mei, J.; Fu, J. Unveiling combined ecotoxicity: Interactions and impacts of engineered nanoparticles and PPCPs. Sci. Total Environ. 2024, 921, 170746. [Google Scholar] [CrossRef]
- Prarat, P.; Hongsawat, P.; Punyapalakul, P. Amino-functionalized mesoporous silica-magnetic graphene oxide nanocomposites as water-dispersible adsorbents for the removal of the oxytetracycline antibiotic from aqueous solutions: Adsorption performance, effects of coexisting ions, and natural organic matter. Environ. Sci. Pollut. Res. Int. 2020, 27, 6560–6576. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Li, Y.; Chen, L.; Zhao, Z.; Zuo, X.; Wang, D.; Zhao, L.; Feng, X. Interaction between multi-walled carbon nanotubes and propranolol. Sci. Rep. 2020, 10, 10259. [Google Scholar] [CrossRef]
- Lin, D.; Tian, X.; Li, T.; Zhang, Z.; He, X.; Xing, B. Surface-bound humic acid increased Pb2+ sorption on carbon nanotubes. Environ. Pollut. 2012, 167, 138–147. [Google Scholar] [CrossRef]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of Dissolved Organic Matter on the Environmental Fate of Metals, Nanoparticles, and Colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef]
- Huixian, Z.; Ping, J.; Qiurong, Z. Influence of Dissolved Organic Matter on Environmental Behavior and Biological Effects of Titanium Dioxide Nanoparticles. Asian J. Ecotoxicol. 2024, 19, 195–205. [Google Scholar]
- Lu, L.; Rijian, L.; Qiucen, S.; Jing, Z.; Guipeng, Y. Research progress on photodegradation behavior of emerging pollutants in water containing dissolved organic matter. Environ. Chem. 2024, 43, 1429–1444. [Google Scholar]
- Li, Y.; Yang, C.; Guo, X.; Dang, Z.; Li, X.; Zhang, Q. Effects of humic acids on the aggregation and sorption of nano-TiO2. Chemosphere 2015, 119, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Gong, Y.; Zeng, Z.; Chen, S.; Ye, J.; Wang, Z.; Dionysiou, D.D. Dissolved organic matter promotes photocatalytic degradation of refractory organic pollutants in water by forming hydrogen bonding with photocatalyst. Water Res. 2023, 242, 120297. [Google Scholar] [CrossRef]
- Yang, L.; Liu, M.; Liu, Y.; Luo, S.; Luo, Y.; Luo, X.; Li, G.; Peng, P. Theoretical analyses of organic acids assisted surface-catalyzed reduction of Cr VI on TiO2 nanowire arrays. Appl. Catal. B Environ. 2016, 198, 508–515. [Google Scholar] [CrossRef]
- Loosli, F.; Le Coustumer, P.; Stoll, S. TiO2 nanoparticles aggregation and disaggregation in presence of alginate and Suwannee River humic acids. pH and concentration effects on nanoparticle stability. Water Res. 2013, 47, 6052–6063. [Google Scholar] [CrossRef]
- Sun, D.D.; Lee, P.F. TiO2 microsphere for the removal of humic acid from water: Complex surface adsorption mechanisms. Sep. Purif. Technol. 2012, 91, 30–37. [Google Scholar] [CrossRef]
- Luo, M.; Huang, Y.; Zhu, M.; Tang, Y.; Ren, T.; Ren, J.; Wang, H.; Li, F. Properties of different natural organic matter influence the adsorption and aggregation behavior of TiO2 nanoparticles. J. Saudi Chem. Soc. 2018, 22, 146–154. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.; Tu, C.; Luo, Y. The limited facilitating effect of dissolved organic matter extracted from organic wastes on the transport of titanium dioxide nanoparticles in acidic saturated porous media. Chemosphere 2019, 237, 124529. [Google Scholar] [CrossRef]
- Carlos, L.; Cipollone, M.; Soria, D.B.; Sergio Moreno, M.; Ogilby, P.R.; García Einschlag, F.S.; Mártire, D.O. The effect of humic acid binding to magnetite nanoparticles on the photogeneration of reactive oxygen species. Sep. Purif. Technol. 2012, 91, 23–29. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Zang, M.; Cheng, Y.; Qi, H. Graphene-based photocatalysts for degradation of organic pollution. Chemosphere 2023, 341, 140038. [Google Scholar] [CrossRef] [PubMed]
- Brame, J.; Long, M.; Li, Q.; Alvarez, P. Inhibitory effect of natural organic matter or other background constituents on photocatalytic advanced oxidation processes: Mechanistic model development and validation. Water Res. 2015, 84, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, Y.; Mao, L.; Zhang, X. Significant changes in the photo-reactivity of TiO2 in the presence of a capped natural dissolved organic matter layer. Water Res. 2017, 110, 233–240. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Zhang, M.; Lian, F.; Wang, M.; Sun, B.; Li, Y.; Li, M.; Lu, Y.; Sun, H. Citric acid improved photocatalytic performance of iron-enriched sludge hydrochar towards organic Pollutants: Roles of iron species and dissolved organic matter. Sep. Purif. Technol. 2025, 354, 128661. [Google Scholar] [CrossRef]
- Wu, H.; Sun, Q.; Chen, J.; Wang, G.; Wang, D.; Zeng, X.; Wang, J. Citric acid-assisted ultrasmall CeO2 nanoparticles for efficient photocatalytic degradation of glyphosate. Chem. Eng. J. 2021, 425, 130640. [Google Scholar] [CrossRef]
- Liu, S.; Edara, P.C.; Schäfer, A.I. Influence of organic matter on the photocatalytic degradation of steroid hormones by TiO2-coated polyethersulfone microfiltration membrane. Water Res. 2023, 245, 120438. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, D.; Wan, S.; Yu, Z.; Li, M. Role of small molecular weight organic acids with different chemical structures as electron donors in the photocatalytic degradation of ronidazole: Synergistic performance and mechanism. Chem. Eng. J. 2017, 326, 1030–1039. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, J.; Yang, Z.; Liu, D.; Xu, J.; Ning, Y.; Jiang, F.; Yang, S.; Li, Y. Fe-Mn bimetallic sulfide enhanced persulfate activation for monochlorobenzene degradation in groundwater: Performance, mechanism and applicability. Chem. Eng. J. 2025, 513, 162790. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, L.; Ye, J.; Li, N.; Yan, B.; Chen, G. Influence and mechanism of water matrices on H2O2-based Fenton-like oxidation processes: A review. Sci. Total Environ. 2023, 888, 164086. [Google Scholar] [CrossRef]
- Cao, R.; Liu, X.; Duan, J.; Gao, B.; He, X.; Nanthi, B.; Li, Y. Opposite impact of DOM on ROS generation and photoaging of aromatic and aliphatic nano- and micro-plastic particles. Environ. Pollut. 2022, 315, 120304. [Google Scholar] [CrossRef]
- Coppola, A.I.; Seidel, M.; Ward, N.D.; Viviroli, D.; Nascimento, G.S.; Haghipour, N.; Revels, B.N.; Abiven, S.; Jones, M.W.; Richey, J.E.; et al. Marked isotopic variability within and between the Amazon River and marine dissolved black carbon pools. Nat. Commun. 2019, 10, 4018. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; He, Y.; Fan, J.; Fu, H.; Zheng, S.; Xu, Z.; Qu, X.; Kong, A.; Zhu, D. Predicting apparent singlet oxygen quantum yields of dissolved black carbon and humic substances using spectroscopic indices. Chemosphere 2018, 194, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Zhang, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Quantitative structure-activity relationship models for the reaction rate coefficients between dissolved organic matter and PPCPs. J. Hazard. Mater. 2023, 458, 131845. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, Z.; Ding, D.; Bai, Y.; Chen, J.; Cui, H.; Su, R.; Qu, K. Effect of the molecular weight of DOM on the indirect photodegradation of fluoroquinolone antibiotics. J. Environ. Manag. 2023, 348, 119192. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cooper, W.J.; Jung, J.; Song, W. Photosensitized degradation of amoxicillin in natural organic matter isolate solutions. Water Res. 2011, 45, 632–638. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, T.; Yang, H.; Liu, Y.; Qu, J.; Zhang, Y.; Peijnenburg, W.J.G.M. Effects of dissolved organic matter and halogen ions on phototransformation of pharmaceuticals and personal care products in aquatic environments. J. Hazard. Mater. 2024, 469, 134033. [Google Scholar] [CrossRef]
- Shang, E.; Li, Y.; Niu, J.; Zhou, Y.; Wang, T.; Crittenden, J.C. Relative importance of humic and fulvic acid on ROS generation, dissolution, and toxicity of sulfide nanoparticles. Water Res. 2017, 124, 595–604. [Google Scholar] [CrossRef]
- Goldstone, J.V.; Pullin, M.J.; Bertilsson, S.; Voelker, B.M. Reactions of hydroxyl radical with humic substances: Bleaching, mineralization, and production of bioavailable carbon substrates. Environ. Sci. Technol. 2002, 36, 364–372. [Google Scholar] [CrossRef]

| Photocatalysts | Types of Light Sources | Catalyst Dosage (g·L−1) | PPCPs | DOM and Its Concentration | Level of Influence | Reference |
|---|---|---|---|---|---|---|
| TiO2 | UV | 1.0 | BUP | HA, 10 mg·L−1 | −, 80% | [31] |
| TiO2 (anatase) | UV | 0.2 | 4-CP | HA, 1–30 mg·L−1 | − | [32] |
| TiO2 (rutile) | HA, <20 mg·L−1 | + | ||||
| HA, 30 mg·L−1 | − | |||||
| TiO2 TNAs | UV | / | MTL | NOM, 15 mg·L−1 | −, 48.16% | [33] |
| ZnO | vis | 0.8 | TC | HA, 5 mg·L−1 | −, 19% | [34] |
| ZnO NPs | vis | 0.01 | MB | HA, 10 mg·L−1 | −, 79.4% | [35] |
| ZnO nanowires | vis | 0.02 | CPX | SRNOM, 10 mg·L−1 | −, 59.1% | [36] |
| Mn-WO3 | LED | 2.2 | DCF | HA | − | [37] |
| Oxygen-doped porous g-C3N4 | vis | 1.0 | CBZ | HA, 20 mM | + | [38] |
| g-C3N4 nanosheets | vis | 1.0 | PHE | HA, 10 mg·L−1 | −, 18% | [39] |
| GO | vis | 0.1 | APAP | FA, 20 mg·L−1 | −, 84.8% | [40] |
| CdS@BC | vis | 1.0 | THM | HA | − | [41] |
| SrBiOI | vis | 0.4 | IDM | DOM, 10 mg·L−1 | −, 30% | [42] |
| MI-BiOCl | vis | 0.4 | VEN | HA, 20 mg·L−1 | o | [43] |
| Bi-TNB | vis | 0.5 | NPX | HA, 5 mg·L−1 | +, 2 times | [44] |
| FA, 10 mg·L−1 | − | |||||
| SnO2@ZnS | vis | / | MTL | DOM | −, 52% | [45] |
| BiOBr/Fe3O4 | vis | 0.5 | NOR | HA, 10 mM | −, 44.49% | [46] |
| BiOBr/Ti3C2 | vis | 2.4 | FQNs | HA | o | [47] |
| Bi2MoO6/(BiO)2CO3 | vis | 0.5 | APAP | NOM, 10 mg·L−1 | −, 21% | [48] |
| Er3+-CdS/MoS2 | vis | 0.125 | 17β-E2 | HA | + | [49] |
| WO3 Fibers/g-C3N4 | vis | 0.1 | CTD | HA, 20 mg·L−1 | −, 12.9% | [50] |
| g-C3N4/Ag3PO4 | vis | 0.5 | OFX | HA | − | [51] |
| rGO/TiO2 | Blue light | 0.3 | DCF | FA, ≤10 mg·L−1 | + | [52] |
| FA > 10 mg·L−1 | − | |||||
| Bi2Fe4O9/rGO | vis | 0.2 | TC | HA | − | [53] |
| MWCNTs/Bi4O5I2 nanosheets | vis | 0.2 | TC | HA, 8 mg·L−1 | −, 5% | [54] |
| CoFe2O4-rGO | UVA- LED | 0.4 | BPA | HA, 10 mg·L−1 | − | [55] |
| Ag2WO4/PCN | vis | 0.2 | IDM | DOM, 10 mg·L−1 | −, 26% | [56] |
| PO43−-Bi2WO6/PI | vis | 1.0 | TC | HA | − | [57] |
| Benzene-ring doped CN/Phosphorus-doped CN | Blue light (LED) | 0.2 | SSZ | DOM, 10 mg·L−1 | −, 74.8% | [58] |
| WO3/ZnIn2S4-3 | LED | 1.6 | TBBPA | HA, 1 mmol·L−1 | −, 53.9% | [59] |
| CCPD-g-C3N4 | vis | 1.0 | MBP | SRHA, 50 mg·L−1 | −, 20% | [60] |
| CQD-SnNb2O6/BiOCl | vis | 0.5 | BC | HA, 10 mg·L−1 | −, 18% | [61] |
| Cd0.5Zn0.5S/BiOCl | vis | 0.2 | NOR | HA, 20 mg·L−1 | −, 35% | [62] |
| IS-Ni2P/CdS/CN | vis | 0.1 | TC | HA | + | [63] |
| VW+Br-BiOBr/Bi2WO6 | vis | 0.3 | NOR | HA, 10 mg·L−1 | −, 21.9% | [64] |
| BMOF-Ti/Zr6% | UV | / | BP-2 | DOM, 20 μg·L−1 | − | [65] |
| Zr-MOFs | vis | / | STZ | HA | − | [66] |
| π-COF | vis | 0.2 | TC | HA | − | [67] |
| ZnFe2O4-seed@TpTt-COF | vis | 0.1 | BPA | HA | + | [68] |
| MIL-88B(Fe)/ZnTi-LDH high-low junction | vis | 0.2 | TC | HA | + | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhu, M.; Sun, A.; Yuan, R.; Chen, H.; Zhou, B. The Influence Mechanism of Dissolved Organic Matter on the Photocatalytic Oxidation of Pharmaceuticals and Personal Care Products. Molecules 2025, 30, 2266. https://doi.org/10.3390/molecules30112266
Wang J, Zhu M, Sun A, Yuan R, Chen H, Zhou B. The Influence Mechanism of Dissolved Organic Matter on the Photocatalytic Oxidation of Pharmaceuticals and Personal Care Products. Molecules. 2025; 30(11):2266. https://doi.org/10.3390/molecules30112266
Chicago/Turabian StyleWang, Jie, Minyi Zhu, Anli Sun, Rongfang Yuan, Huilun Chen, and Beihai Zhou. 2025. "The Influence Mechanism of Dissolved Organic Matter on the Photocatalytic Oxidation of Pharmaceuticals and Personal Care Products" Molecules 30, no. 11: 2266. https://doi.org/10.3390/molecules30112266
APA StyleWang, J., Zhu, M., Sun, A., Yuan, R., Chen, H., & Zhou, B. (2025). The Influence Mechanism of Dissolved Organic Matter on the Photocatalytic Oxidation of Pharmaceuticals and Personal Care Products. Molecules, 30(11), 2266. https://doi.org/10.3390/molecules30112266






