Abstract
Ganoderma capense, a member of the Ganoderma genus within the Polyporaceae family, has long been recognized for its high nutritional value and extensive use in traditional medicine. Its primary distribution is in China and South Africa, with the type locality being South Africa. This species is rich in a diverse array of bioactive compounds, including various polysaccharides, glycopeptide macromolecules, and various small-molecule compounds, such as sesquiterpenes, triterpenes, steroids, and alkaloids. Research indicates that these chemical constituents exhibit numerous pharmacological properties, including antioxidant, anti-inflammatory, and anti-tumor activities, as well as inhibition of acetylcholinesterase, reduction in blood lipids, and promotion of neural synapse growth. Apart from its use in traditional Chinese medicine, the components of G. capense are utilized globally for the treatment of a wide range of diseases, including Alzheimer’s disease, febrile convulsions, HIV, and diabetes. This underscores the extensive medical applications of G. capense, emphasizing its significance in contemporary and traditional healthcare. This review summarizes the latest research findings on the bioactive compounds and pharmacological effects of G. capense, compiled from databases such as PubMed, Web of Science, and Elsevier. This study aimed at providing researchers in this field with in-depth scientific insights and guidance, promoting further application and development in the pharmaceutical and food industries, and serving as a reference for subsequent exploration of active substances and the development of new disease treatments.
1. Introduction
Ganoderma capense, a fungus belonging to the Polyporaceae family, is widely known as the “medicinal mushroom” [1]. In traditional Chinese medicine, its mycelium, fruiting body, and spore powder can be used as medicine, and it has been regarded by generations of traditional Chinese medicine practitioners as a precious tonic that strengthens the body and nourishes the essence [2]. As an important medicinal fungus in the Ganoderma genus, G. capense has the effects of tonifying the kidneys, calming the nerves, boosting the immune system, and fighting inflammation and bacteria [3,4]. These effects are due to its rich content of various active metabolites, such as polysaccharides, triterpenoids, alkaloids, sterols, and other small-molecule compounds. Research conducted in the early stages of the study of G. capense focused on the composition of its compounds. For instance, in 1988, Yu et al. conducted a study in China on the chemical composition of G. capense mycelium following submerged fermentation, thereby ascertaining that it exerts certain therapeutic effects on diseases such as muscular dystrophy and atrophy [5]. Further research identified three new compounds: ganoderpurine and ganoine I and II [6]. Meanwhile, its pharmacological effects have also begun to attract widespread attention. For example, in 1980, Leng, W. et al. studied the pharmacological effects of G. capense fermentation broth and found that it has a certain hepatoprotective and detoxifying effect [7]. Because it is rich in nutrients and has significant medicinal value, it has the potential to be developed into health foods, medicines, etc. [8]. At present, preparations such as Boji injection and Boji tablets made from the fermentation culture products of the fungus have also been used in clinical practice to relieve symptoms such as muscle and joint pain, swelling, and skin edema [9].
This review article provides a comprehensive overview of the chemical structures and biological activities of the polysaccharide macromolecular compounds and small-molecule compounds, including terpenes and steroids, in G. capense. The primary objective of this review is to serve as a reference for subsequent pharmacological research and active compound mining of G. capense.
2. Polysaccharides
Polysaccharides represent a significant bioactive component of G. capense. Ten polysaccharides have been identified in G. capense. Wei, Z.H. et al. determined the content of compounds such as intracellular polysaccharides, hydrolyzed amino acids, and organic acids in G. capense and found that the polysaccharide content in G. capense was as high as 16% [10]. These polysaccharides are usually extracted with water and alkali solutions and are mainly composed of monosaccharides, such as glucose, arabinose, and xylose. These have been shown to possess activities including the treatment of malignant tumors and DPPH-hydroxyl radical activity, as demonstrated in the following experiments.
Initially, the liquid obtained by subjecting dried G. capense mycelial powder to soaking in petroleum ether for 24 h and subsequently refluxing it at 60 °C for 2 h in a round-bottomed flask was utilized as the experimental material for all the subsequent experiments. Li, N. et al. extracted G. capense mycelium by subjecting it to three cycles of boiling in water, each cycle lasting two hours. The mycelium was then deproteinized using a Sevag reagent, and the resulting mixture was further purified by DEAE Sepharose CL-6 and Sephadex G-75 column chromatography. This process resulted in the isolation of GCP50-1 [11]. It was identified that its main chain consisted of α-D-glucan, interspersed with (1→4)-α-D-glucopyranosyl groups, and the side chain is connected to the O-6 position with (1→4,6)-α-D-glucopyranosyl groups. GCPB-1b was also extracted from the mycelium of G. capense with an alkaline solution and purified using ion exchange and gel permeation column chromatography. The main chain is composed of (1→4)-linked α-D-glucopyranose residues and (1→4,6)-linked α-D-glucopyranose residues [12]. Huang Y. extracted a heteropolysaccharide from the mycelium using a sodium hydroxide alkaline solution and then purified it through a series of chromatographic columns (namely, DEAE-52 cellulose, DEAE Sepharose CL-6B, and Sephadex G-75) to yield a new polysaccharide, GCP50-2, whose main chain consists of (1→4)-linked β-D-xylose residues [13]. The structure of the substance is such that two branches are present at residue O-3, each of which constitutes a β-L-arabinopyranosyl residue terminated by a β-D-xylopyranosyl residue (1→3) and a β-D-xylopyranosyl residue terminated by a β-L-arabinopyranosyl residue (1→4). Yi, P. et al. extracted crude polysaccharides from the mycelia and then purified them by Sevag protein removal, anion exchange chromatography, and gel permeation chromatography to obtain polysaccharide GCPB-3, whose main chain is composed of β-L-arabinopyranosyluronic acid linked to β-D-xylose via 1→4 glycosidic bonds [14]. Huang, Y.T. extracted the fungal powder with deionized water (100 °C, 2 h), precipitated it with ethanol, and dialyzed it with deionized water. Finally, multiple polysaccharide components were obtained using DEAE Sepharose CL-6B ion exchange column chromatography and Sephadex G-75 gel columns and were named GCP50-2, GCP50-3, GCP70-1, GCP70-3, GCP 90-2, and GCP 90-3 based on the differences in the main chain and branch chain structures [4].
Furthermore, a plethora of glycopeptides have been identified within the mycelium of G. capense, exhibiting biological activities that encompass adjuvant therapy for tumors, liver protection, immunomodulation, and anti-tumor properties [15]. Lu, Y. et al. used methods such as oxidation with periodate and Smith degradation to confirm that the polysaccharide part of the glycopeptide is mostly composed of glucose, with two connection methods: 1→6 and 1→2 [16]. The glycopeptide was determined to be rich in various amino acids, such as glutamic acid, alanine, aspartic acid, and serine, using pre-column derivatization high-performance liquid chromatography. Lin, Y.Q. et al. used thin-layer chromatography and HPLC-ELSD to determine the monosaccharide composition of the glycopeptides, which was glucose: xylose: galactose: D-mannose = 1:0.0178:0.067:0.074 [17]. For the extraction part, extraction solution, relative molecular mass, and biological activity of G. capense polysaccharides, see Table 1.

Table 1.
Polysaccharide components and bioactivity in G. capense.
3. Small Molecular Compounds
At present, 70 small-molecule compounds have been isolated from G. capense, including 13 sesquiterpenoids, 6 triterpenoids, 24 meroterpenoids, 17 steroids, and 10 other compounds. It has been demonstrated through experimental means that the aforementioned small molecules possess antioxidant, cytotoxic, and anti-cancer properties. In addition, they have been shown to exhibit anti-inflammatory, hypolipidemic, and neurotherapeutic effects, as well as therapeutic benefits in pemphigus vulgaris [16].
3.1. Terpenoid
Terpenoids are an important secondary metabolite in fungi [18]. They are usually classified according to the number of isoprene units in their molecular structure, such as monoterpenes with C10, sesquiterpenes with C15, diterpenes with C20, and triterpenes with C30. They are synthesized by the terpene synthase (TPS) acting on the polyisoprene precursor to form the basic backbone structure and then further modified by hydroxylation, dehydrogenation, acylation, or glycosylation to synthesize terpenoids with complex structures [19]. In addition, meroterpenoids are a distinctive class of compounds present in Ganoderma. Their structure, which is characterized by the presence of 1,2,4-trisubstituted phenyl groups and polyunsaturated terpene components, has been shown to have a variety of beneficial properties, such as antioxidant, immunosuppressive, and antiviral activities [20]. At present, several research teams have conducted in-depth research on the terpenoids in G. capense.
3.1.1. Sesquiterpene
Sesquiterpene compounds are widely found in nature, often in the form of oxygen-containing derivatives, such as alcohols and ketones [21]. Tan, Z. et al. diluted air-dried mycelium with 75% ethanol and then extracted the concentrated crude extract with water, followed by sequential extraction with petroleum ether (60–90 °C), ethyl acetate, and n-butanol. The extract was then purified using a series of petroleum ether–acetone gradients, petroleum ether–ethyl acetate gradient elution, and HPLC purification to obtain ganodermanol A–K (1–11), rel-(+)-(2aR,5R,5aR,8S,8aS,8bR)-decahydro-2,2,5,8-tetramethyl-2H-naphtho[1,8-bc] furan-5-ol (12), and eudesm-1β,6α,11-triol (13) [22]. The structures and absolute configurations of the compounds were identified through a combination of spectroscopic analysis, circular dichroism (CD), and Mo2(AcO)4-induced CD. The structural formulae of compounds 1–13 are shown in Figure 1. Please direct your attention to Table 2 in order to ascertain the biological activity and extraction sites of compounds 1–13.
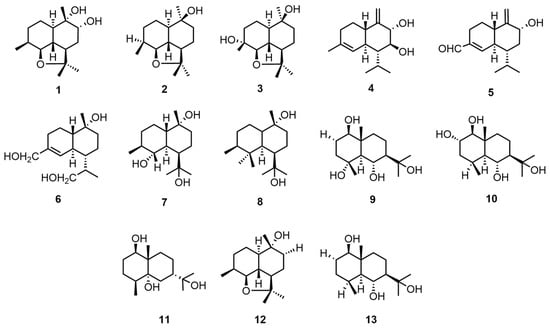
Figure 1.
Structural formulas of compounds 1–13.

Table 2.
Biological activity of small-molecule compounds 1–13.
3.1.2. Triterpenoid
Triterpenoids have diverse structures, including monocyclic, bicyclic, tricyclic, tetracyclic, and pentacyclic types, among which tetracyclic and pentacyclic triterpenoids are the most common. So far, more than 30,000 triterpenoid compounds have been identified [24]. Tan, Z. et al. concentrated the crude extract obtained after air-dried mycelium was extracted by refluxing with 75% ethanol and then sequentially extracted it with petroleum ether (60–90 °C), ethyl acetate, and n-butanol. Then, the extract was eluted through a petroleum ether–acetone and petroleum ether–ethyl acetate silica gel column and then purified by normal-phase semi-preparative HPLC using hexane–ethyl acetate methanol reversed-phase semi-preparative HPLC for further purification and separation to obtain four lanostane triterpenes: (24E)-15α-acetoxy-3-oxolanosta-8, 24-dien-26-oic acid (14), 15α-Acetoxy-3α-hydroxylanosta-8,24-dien-26-oic acid (15), (24E)-15α-acetoxy-3β-hydroxylanosta-8, 24-dien-26-oic acid (16), and 26-Methy-15α,22β-diacetoxy-7,9(11),24-trien-26-oic ester (17) [25]. The structures of these molecules were determined on the basis of extensive spectroscopic data analysis, including HRESIMS, 1D nuclear magnetic resonance (NMR), and 2D NMR.
Similarly, Peng, X.R. et al. used acetone to extract and concentrate the G. capense fruiting bodies and then treated them with macroporous adsorption resin and finally eluted them with a chloroform–acetone gradient to obtain the pentacyclic triterpene betulinic acid (18) [26]. Bean sapogenin-B (19) was extracted by similar means [6]. The structural formulas of triterpenoid compounds 14–19 are shown in Figure 2. Please direct your attention to Table 3 in order to ascertain the biological activity and extraction sites of compounds 14–19.
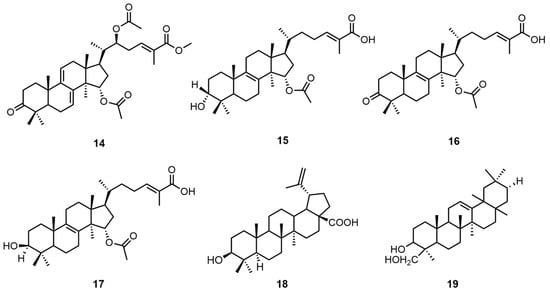
Figure 2.
Structural formulas of compounds 14–19.

Table 3.
Biological activity of small-molecule compounds 14–19.
3.1.3. Meroterpenoid
Meroterpenoid compounds refer to those in the structure that are partially derived from the terpenoid biosynthesis pathway but also contain a non-terpene pathway source [27]. Liao, G.F. et al. used an 80% ethanol extract of mycelium in the ethyl acetate soluble fraction to sequentially perform MCI gel CHP 20 P, Sephadex LH-20, ODS (RP-18), and semi-preparative HPLC to obtain ganocapenoids A–D (20–23), ganoresain B (24), ganocalidin E (25), fornicin B (26), ganomycin I (27), zizhine A (28), amaurosubresin (29), co-chlearin I (30), ganocochlearin B (31), patchiene A (32), gano-mycin K (33), ganocalidin A (34), and spirolingzhine B (35) [28]. Then, the structures of the new compounds were determined through the application of spectroscopic methods, incorporating 1D and 2D NMR analyses, in addition to MS analyses.
Peng, X. et al. then used acetone to wash the chopped G. capense mycelium and then evaporated it under reduced pressure to obtain a crude extract. The extract was then passed through a methanol–water macroporous adsorption resin, eluted with a chloroform–methanol and chloroform–acetone silica gel column, and then was subjected to TLC to obtain ganocapensin A (36), ganomycin F (37), and fornicin E (38). Ganocapensin B (39), ganomycin E (40), and ganomycin C (41) were obtained by further using a reverse-phase C18 column and eluting the extract through a chloroform/methanol silica gel column, LH-20 (methanol) [29]. Peng, X.R. et al. extracted the G. capense fruiting bodies with acetone and concentrated them. They were first treated with a macroporous adsorption resin, followed by a normal-phase silica gel column sequentially using chloroform–methanol and chloroform–acetone to isolate cochlearin A (42). The compound was then treated with methanol–water from a 45% gradient to a 75% reverse-phase silica gel column and finally cut and gel-treated with a normal-phase silica gel column to obtain cochlearin B (43) [26]. The structures of the two meroterpenoids were characterized by 1DNMR, 2DNMR, mass spectrometry, and other spectroscopic techniques. The identification method was developed from earlier experiments of a similar nature [30]. The structural formulas of compounds 20–43 are shown in Figure 3. Please direct your attention to Table 4 in order to ascertain the biological activity and extraction sites of compounds 20–43.
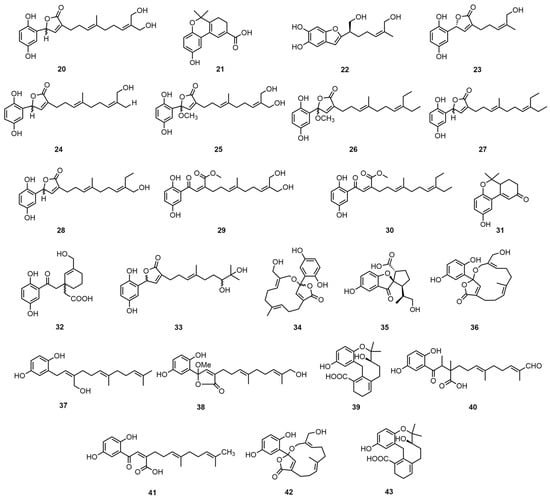
Figure 3.
Structural formulas of compounds 20–43.

Table 4.
Biological activity of small-molecule compounds 20–43.
3.2. Steroid
Steroids are widely occurring natural compounds. Almost all living organisms can biosynthesize steroidal compounds themselves. Their molecular structures all contain a cyclopentane polyhydrophenanthrene carbon skeleton called a steryl nucleus [35]. Tan, Z. et al. extracted the mycelium of G. capense with petroleum ether (60–90 °C), eluted it with a gradient of petroleum ether–acetone and petroleum ether–ethyl acetate, and then separated and purified it by normal hexane–ethyl acetate normal phase semi-preparative HPLC and methanol reversed-phase semi-preparative HPLC to obtain demethylincisterol A3 (44) and 5α,9α-epidioxyergosta-6,8(18),22-triene-3β-ol (45) [25]. They also extracted the mycelium with ethyl acetate and petroleum ether–ethyl acetate silica gel column chromatography, followed by methanol Sephadex LH-20 column chromatography, methanol open MCI column chromatography, and reversed-phase semi-preparative HPLC to obtain 11α-hydroxy-21-hydroxy-demethylincisterol A3 (46) [25].
Peng, X.R. et al. extracted the fruiting body with acetone, then eluted it with macroporous adsorption resin, followed by a normal-phase silica gel column fractionation and a gradient elution with chloroform–acetone. Then, it was purified by normal-phase HPLC (acetonitrile–water) with a chloroform–acetone system and P-TLC elution with chloroform–acetone to obtain (22E)-ergosta-7,22-dien-3β, 5α,6β-triol (47), (22E)-ergosta-7,22-dien-3β,5α-diol (48), ergosta-7,22-dien-3β,5α,6β, 5α,14α-pentol (49), (22E)-ergosta-7,22-dien-3β-ol (50), 3β,5α,9α,14β-tetrahydroxy-(22E)-ergosta-7,22-dien-6-one (51), (22E)-ergosta-7,22-dien-3β,5α,6β,9α-tetraol (52), (22E)-ergosta-7,9(11),22-dien-3β, 5α,6β-triol (53), (22E)-ergosta-7,14,22-dien-3β, 5α,6β-triol (54), 3β,5α,9α-trihydroxy-(22E)-ergosta-7,22-dien-6-one (55), and 5α,9α-epidioxy-(22E)-ergosta-6,22-dien-3β-ol (56) [26]. The identification of these compounds was facilitated by spectroscopic techniques, including 1DNMR, 2DNMR, and mass spectrometry. There are also articles mentioning that Yu, J.G. and others first extracted the fruiting body with ethanol and then with ether, and then eluted the extract with petroleum ether, ether, and acetone in sequence to obtain ergosterol (57), ergosterol palmitate (58), ergosterol-7,22-dien-3-one (59), and 5 α -sitostanedione-3,6 (60) [6]. The team then identified these compounds by high-resolution mass spectrometry (HRMS) with infrared spectroscopy (IR). The structural formulas of compounds 44–60 are shown in Figure 4. For information pertaining to the biological activity and extraction sites of compounds 44–60, please refer to Table 5.
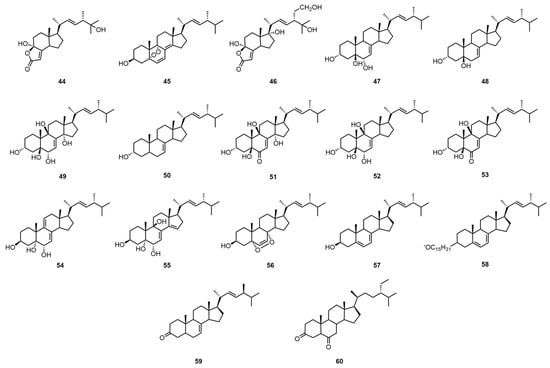
Figure 4.
Structural formulas of compounds 44–60.

Table 5.
Biological activity of small-molecule compounds 44–60.
3.3. Others
Small-molecule compounds, such as amides, ganoderic alkaloids, and furan derivatives, were also isolated from the thin-skinned G. capense fruiting bodies. Peng, X.R. et al. utilized microporous adsorption resin for the extraction of the fruiting bodies, followed by normal-phase silica gel column fractionation. A chloroform–acetone gradient elution and acetonitrile–water HPLC were then employed to obtain aurantiamide (61) [26]. Yu, J.G. et al. used 95% ethanol to extract the mycelium and then successively extracted with ether, chromatographed with methanol–petroleum ether on a silica gel column, and eluted with a gradient of petroleum ether–acetone to obtain ganoine I (62) by silica gel column chromatography [30]. Alternatively, the extracted liquid was acetylated and then successively subjected to silica gel thin-layer separation, petroleum ether–ethyl acetate development, and petroleum ether–dichloromethane recrystallization to obtain a white crystalline ganoine II (63). In addition, Yu, J.G. et al. treated mycelium with cationic resin, alkalized it with hydroxylamine, and eluted it with ethanol, then performed multiple silica gel column chromatography and finally recrystallized it with acetone to obtain ganoderpurine (64) [30]. In addition, articles have been published that report on the use of 95% ethanol reflux for the extraction of mycelium by Yu, J.G. and colleagues. Successive extraction methods employed included ether, dichloromethane–methanol, hexane–ethyl acetate elution, and vacuum distillation. The final product obtained was 5-hydroxymethyl furfuraldehyde (65). The process of elution was concentrated, and the resulting solid was allowed to stand in order to obtain the dehydrated disulfide 1,1′-di-α-glyoxal dimethyl ether (66), 5-acetyloxymethyl furfuraldehyde (67), or 5-butoxymethyl furfuraldehyde (68). These were obtained by thin-layer preparation using a silica gel column and hexane–ethyl acetate. Finally, nicotinic acid (69) was obtained through a series of chemical processes, including ethanol extraction, treatment with cationic resin, alkalization with ammonia, heating, and elution with ac-acetone. The product was then subjected to multiple silica gel column chromatography steps, followed by recrystallization [6]. Tan, Z. et al. extracted an ethyl acetate extract (8.9 g) with a petroleum ether–ethyl acetate gradient and then sequentially eluted with methanol. Sephadex LH-20 chromatography column fractionation and methanol reversed-phase semi-preparative HPLC were used to obtain bluemenol A (70) [22]. The structural formulas of compounds 61–70 are shown in Figure 5. For information pertaining to the biological activity and extraction sites of compounds 61–70, please refer to Table 6.
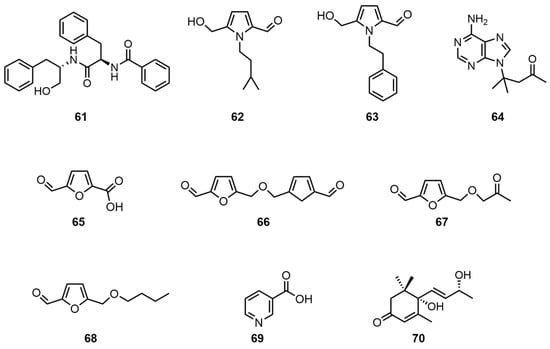
Figure 5.
Structural formulas of compounds 61–70.

Table 6.
Biological activity of small-molecule compounds 61–70.
4. Pharmacological Effect
4.1. Antioxidant
Reactive oxygen species (ROS) have a strong damaging effect on DNA and proteins, and this damage can lead to aging and the development of many other important diseases, such as cancer, cardiovascular disease, and neurodegeneration [46]. Terpenoids have the potential to act as antioxidants by scavenging ROS in cells [47].
The majority of studies on scavenging effects against oxidants have been conducted in vitro, as Peng, X. et al. found when they added heterocyclic compounds 26, 27, 37, 38, 39, 40, and 41 to a DPPH radical methanol solution. The half-inhibitory concentrations (IC50) values exhibited a range from 6.00 ± 0.11 to 8.20 ± 0.30 µg/mL, and it was found that these had a stronger DPPH radical scavenging effect than the positive control (Trolox antioxidant, IC50 value of 3.63 ± 0.17 μg/mL) [29]. Yan, C. et al. dissolved the polysaccharides in distilled water at concentrations of 0.125 to 25 mg/mL, added 0.5 mL 0.1% H2O2, and used DPPH free radical and hydroxyl radical activity assays with ascorbic acid as a positive control. It was found that the polysaccharides in the mycelium all had concentration-dependent scavenging abilities [48]. For example, the antioxidant capacity of GCPB-1b was measured using this DPPH assay, and the effective concentration (EC50) value for the inhibition of DPPH radicals was 3.23 μM, which removed 60.2% of the DPPH radicals [12]. Huang, Y. et al. and Yi, P. et al. used the same method to determine that GCP50-2 and GCPB-3 had DPPH radical scavenging rates of 10.9% and 15.0%, respectively [13]. In addition, polysaccharides can also improve the cell proliferation index, alter the expression levels of genes related to skin cell aging, and have an anti-epidermal aging effect, so they can be used to make anti-aging creams and other cosmetics [49].
4.2. Cytotoxicity and Anti-Cancer Effect
Cytotoxic anti-tumor drugs are currently one of the main treatments for malignant tumors. They are a type of chemotherapy drug that can directly kill tumor cells or inhibit tumor cell growth and proliferation [50]. Research in this area has achieved certain results. For example, Tan, Z. et al. extracted sesquiterpene compound 3 from the mycelium, which was cytotoxic to the human liver cancer cell line HCT 116. In vitro experiments were performed, with paclitaxel and leflunomide used as positive controls. The IC50 values for HCT116, NCI-H1650, BGC823, Daoy, and HepG2 cells were found to be 0.000809, 0.945, 0.00177, 0.00933, and 0.0249 µM, respectively. Cell viability was measured by the conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to purple formazan crystals. The IC50 of the antagonists were 24.5 and 12.2 μM. Sesquiterpene compound 4 is cytotoxic to human colon cancer cells HCT 116, human gastric adenocarcinoma cells BGC 823, human medulloblastoma cells Daoy, and human liver cancer cells HepG2, with IC50 values of 16.6, 49.9, 31.1, 27.9, and 37.9 μM, respectively. The IC50 values of sesquiterpene compound 6 for the human cancer cell lines NCI-H1650 and Daoy were 28.9 and 35.5 μM, respectively [22]. Later, they used the same method to measure triterpene compound 14, which showed moderate cytotoxic activity against NCI-H1650, with an IC50 value of 22.3 μM, and the cytotoxic activity of steroidal compound 44 against the human cancer cell line HCT 116, with an IC50 value of 17.4 μM [25]. Huang, Y.T. also used the MTT method to measure the same polysaccharide GCP70-3, which has a strong inhibitory effect on the proliferation of human gastric cancer cells SGC-7901, human cervical cancer cells Hela, and human nasopharyngeal cancer cells CNE-1. At a concentration of 3200 μg/mL, the inhibition rates were 50.44%, 41.69%, and 52.30%, respectively [4].
Song, A.R. et al. measured the toxicity of sesquiterpene compound 13 on canine renal epithelial cells MDCK. After treating the monolayer of MDCK cells with compound 13 for 48 h, the OD492 value was measured in a microplate reader, and the cytotoxic concentration 50% (CC50) was determined to be 85.39 μM. The MDCK cells were then infected with TCID 50, which is related to the pathogenicity of the virus, and zanamivir was designated as the positive control, and its EC50 value was determined to be 15 μM. The virus inhibition rate was determined to calculate the 50% effective concentration. The EC50 of compound 13 was 0.14 nM, indicating that it has extremely strong anti-influenza virus activity [23]. Tran, P.T. et al. used bone marrow cells that were isolated from the femurs and tibias of male mice and then treated them with heterocyclic compound 27, which was found to inhibit the phosphorylation of the extracellular signal-regulated kinase signaling pathway (ERK), c-Jun N-terminal kinase (JNK), and mitogen-activated protein kinase p38 (p38 MAPK), induced by the receptor activator of nuclear factor-κB ligand (RANKL) pathway, and the expression of cellular oncogene fos (c-Fos) and nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1 (NFATc 1) transcription factors, thereby inhibiting osteoclast formation [51]. This mechanism can be used in anti-rheumatic drugs. Zhou, J. et al. mentioned that compound 70 is an anti-tumor compound with anti-proliferative effects. It showed an effective dose 50% (ED50) of 20 μg/mL in an in vitro test of quinone reductase inducers in cultured Hepa1c1c7 mouse liver cancer cells [43].
Kitagawa, K. et al. studied the anti-tumor and anti-epithelial–mesenchymal transition inhibitory effects of heterocyclic compound 33 through an in vivo xenograft study, in which mice were inoculated with bladder cancer cells, and the compound was administered intratumorally. They found that it significantly inhibited the non-muscle-invasive KK 47 tumor cells (p < 0.01) and muscle-invasive T24 tumor cells (p < 0.01). Even when the compound was used at a high dose (1.0 mg/individual) intra-tumorally, it not only inhibited tumor growth in KK 47 and T24 cells (p = 0.009 and p = 0.003, respectively) but also did so without significant adverse events [34].
4.3. Anti-Inflammatory
Inflammation is an adaptive response of the immune system to harmful stimuli. Inflammation can be caused by infections, pathogen invasion, cell damage, excessive autoimmunity, and toxic compounds. It is a complex and important physiological and pathological response [52]. The main physical manifestations of inflammation are redness, swelling, heat, pain, and loss of function in the affected area. Inflammation has a significant impact on human health, so research on inflammation is currently very important [53]. Zhou, Y. et al. stimulated macrophages with Baozi polysaccharide peptide complexes and found that it can regulate the production of NO and the normal expression of inducible nitric oxide synthase (iNOS) through the nuclear factor kappa-light-chain-enhancer of the activated B cells (NFκ-B) signaling pathway and the phosphorylation and degradation of inhibitor of NF-κB (IκB), thus demonstrating its inflammatory regulatory effect on macrophages [54].
Peng, X.R. discovered amide compound 61 from the fruiting body, and then He, R.B. et al. used the CCK 8 technique to evaluate cell viability in vitro and found that the compound restored the viability of human proximal tubular epithelial cells in a dose-dependent manner at concentrations of 25, 50, and 100 μM. In subsequent in vivo experiments, by ligating the ceca of mice with a puncture needle, then suturing them to induce sepsis, and administering the compound orally, it was found that it could improve renal tubular damage and inflammation induced by ischemia and the cecal ligation and puncture by acting on the gastrin-releasing peptide receptor [38].
4.4. Lower Blood Sugar and Lipids
Diseases caused by high blood sugar levels have always been a great challenge for mankind. As posited by Yu, J.G., a furan derivative, designated 65, was identified in mycelium. Zhang, X. and Shimizu, A. utilized this compound on Balb/c 3T3 A31 clone 19 cells, which demonstrated sensitivity to insulin or insulin-like growth factor stimulation. This resulted in a glucose absorption rate of 50.68%, thereby confirming that both the compound and its derivatives possess hypoglycemic activity [40,41]. In addition, Liao, G.F. et al. assessed the effect of heterocyclic compound 34 on lipid accumulation in HepG2 cells by oil red O staining. When it reached 20 μM, it could reduce liver lipid accumulation by increasing cholesterol efflux and reducing cholesterol synthesis [28].
Wang et al. subsequently demonstrated in vitro that heterocyclic compound 27 exhibited a superior dual inhibitory effect on α-glucosidase and HMG-CoA reductase in comparison to the positive control atorvastatin. Furthermore, it was observed that 27 effectively reduced blood glucose and lipid levels. In comparison, the medication was found to be comparable to rosiglitazone, with a reduction in blood glucose levels of 15 mM on the first day and maintenance of this level subsequently. In vivo experiments using an insulin-resistant male mouse model demonstrated that the liver glycogen concentration of the experimental group was significantly higher than that of the untreated control group (p < 0.01). These findings suggest that this compound may improve insulin resistance and serve as a potential therapeutic agent for diabetes [55].
4.5. Neurotherapeutic Effect
Etukudo, E. M.’s paper systematically discusses that sesquiterpenoids 22, 25, 29, and 30 all have acetylcholinesterase (Ache) inhibitory effects, which can repair neuronal degeneration and treat Alzheimer’s disease associated with decreased Ache levels, as well as treat myasthenia gravis [56,57]. Liao, G.F. et al. also found that heterocyclic compound 30 has an effective Ache inhibitory effect, with an IC50 value of 8.2 μM, and when the rate of neural differentiation was examined after 72 h of action at 10 μM, it was found to promote moderate neurite growth [28].
Zheng, F.X. et al. conducted an in vivo experiment by inducing a mouse model of thermal convulsions and found that the incidence of severe convulsions in the experimental group was lower than that in the saline control group after intraperitoneal injection of G. capense. The content of cytokines in the hippocampal homogenates was then determined by ELISA; it was found that G. capense can reduce excitotoxic brain damage caused by interleukin-1 beta (IL-1β) and reduce the expression level of the apoptosis protein caspase-3 in the hippocampus of rats, thereby reducing the incidence of epilepsy in rats with recurrent febrile seizures [58]. In addition, Wang, Z.Y. observed family members with hereditary cerebellar ataxia who were injected intramuscularly with G. capense mycelium extract and found that their symptoms were relieved [59].
4.6. Antibacterial Activity
Given the prevalence of antibiotic-resistant microorganisms, there is an urgent need for the development of novel antibiotics. G. capense contains substances with corresponding functions.
Vazirian, M. dissolved compounds 50 and 59 in chloroform at concentrations of 100–3.125 mg/mL and 10–1.25 mg/mL, respectively, and then inoculated them into culture media containing Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans. The compounds (50 and 59) were subjected to an incubation process at a temperature of 37 °C for a duration of 24 h. Thereafter, the average zone diameter (in millimeters) of the inhibition zone was measured at a concentration of 10 milligrams per milliliter. The minimum concentration at which an inhibition zone was observed was defined as the MIC. The minimum inhibitory concentration (MIC) values for compound 50 were determined to be 5, 5, >10, >10, and 5 milligrams per milliliter (mg/mL), suggesting the absence of an inhibition zone in both Escherichia coli and Pseudomonas aeruginosa. A similar pattern was observed for compound 59, with MIC values of 5, 5, >10, >10, and 5 mg/mL, consistent with the aforementioned findings. However, when the concentration of compound 50 was 10 mg/mL, the inhibition zone diameters of Staphylococcus aureus, Bacillus subtilis, and Candida albicans were 9.7 ± 0.3, 9.6 ± 0.7, and 9.6 ± 0.9, respectively. The mean values ± standard deviation for the inhibition zone diameters of compounds 59 were 9.7 ± 0.6, 9.5 ± 0.5, and 9.6 ± 0.1 (millimeters ± standard deviation), respectively [37].
The antibacterial effect may be attributable to two distinct mechanisms: the inhibitory effect of active oxygen and the induction of intracellular protein leakage in bacterial cells [60]. To date, a paucity of studies has been published on the antimicrobial activity of G. capense. Moreover, the majority of these studies have been conducted in vitro, and the mechanisms underlying the antimicrobial and antiviral activities of G. capense remain to be elucidated. Despite the high concentration of bioactive compounds (carbohydrates, glycosides, triterpenoids, phenolic compounds, and tannins) present in the extracts, these compounds primarily demonstrate antimicrobial activity when present in a mixed form [61].
4.7. Other Pharmacological Activities
Stuart, K.L. and Mogana, R. et al. found that compound 70 is a compound with activity comparable to that of abscisic acid, has Ache activity, and exhibits moderate anti-leishmanial activity [44,45]. Ngai, P.H. isolated lectins from mycelium extracts that have strong mitogenic activity compared to cowpea globulin A and strong antiproliferative activity against leukemia (L1210 and M1) cells and hepatoma (HepG 2) cells [62]. Tan, Z. et al. and Hapuarachchi, K. all detected the concentration of viral capsid protein through in vitro antigen capture enzyme-linked immunosorbent assays and found that sesquiterpenoid compound 4, triterpenoid compounds 14, 15, 16, heterocyclic compound 27, and sterol compound 45 had anti-HIV capabilities, and the IC50 values were determined to be 37.9, 23.5, 46.7, 21.6, 30.1, and 46.7 μM, respectively [22,25,31]. In other respects, Liu, Y.M. et al. mentioned that Professor Xuan Guowei often uses Phellinus linteus when treating alopecia areata because it can enhance macrophage activation and thus secrete interleukin, inhibit the proliferation of T lymphocytes and B lymphocytes, and thus promote hair growth [63].
Then, Li, W. used the 2HRZE/4HR regimen on patients with AIDS and secondary tuberculosis and found that the regimen had an effective rate of 91.67% after the addition of low-molecular-weight ganderma glycopeptide, which was higher than the control group’s 77.08% (p < 0.05), confirming that the addition of low-molecular-weight ganoderic acid peptide can effectively boost immunity [64]. Zeng, L.G. used G. capense and acyclovir to treat recurrent genital herpes, and the cure rate reached 81%, which was a significant improvement compared to the 47% cure rate of the control group that took levamisole tablets [65]. For a visual representation of the pharmacological activities corresponding to each small molecule compound, as well as the relationship between small molecule compounds and polysaccharides, please refer to Figure 6.
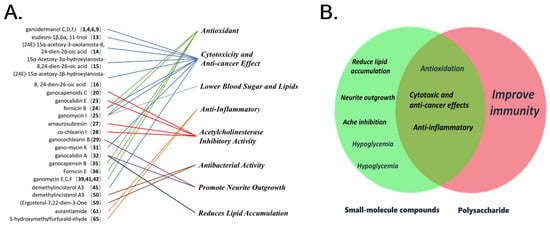
Figure 6.
This chart depicts the pharmacological value of the main compounds (A). This Venn diagram depicts the connection and relationship between the direct pharmacological value of polysaccharides and small-molecule compounds (B).
5. Conclusions and Prospection
In summary, the polysaccharides and small-molecule chemical constituents in G. capense show high medicinal value. G. capense has been widely used to treat various diseases, including alopecia, diabetes [56], and areata [63]. Nevertheless, research on its chemical constituents remains comparatively restricted, with the majority of studies concentrating on terpenes, steroids, and other components. It is evident that the mining and exploration of other active ingredients remains inadequate. Therefore, it is necessary to conduct more in-depth and extensive research on the medicinal value of G. capense. Although the anti-tumor activity of G. capense polysaccharides has been preliminarily explored, its specific mechanism of action and application prospects still require further research in order to fully tap its potential and apply it clinically.
In addition, the triterpenoids in G. capense have a wide range of applications in medicine. To increase the production of triterpenoids, genetic engineering can be used to overexpress key genes in the terpene synthesis pathway, such as HMGR (3-hydroxy-3-methylglutaryl-coenzyme A reductase), SQS (squalene synthase), and LS (lanosterol synthase), and the transcription factor [66], which negatively regulates terpene synthesis, is simultaneously inhibited, thereby promoting the synthesis of triterpenoids. With the development of genome sequencing technology and the gradual improvement of the analysis of natural product biosynthesis pathways, the functional identification of biosynthesis genes for the key active ingredients of this fungus and metabolic pathway reconstruction can be carried out in the future. By transferring these genes to a host, such as Saccharomyces cerevisiae, an efficient heterologous biosynthesis platform can be established that can enable the large-scale production of important components of this fungus, thereby reducing costs and improving the controllability and stability of the product. In addition, synthetic biology methods can further optimize yield and quality, providing a new approach for the clinical drug development of G. capense.
This review aims to provide basic data and strong evidence for future pharmacological mechanism research on this fungus by organizing and summarizing the medicinal activities of the polysaccharides and small-molecule compounds of G. capense. This will not only deepen our understanding of its pharmacological effects but also open up new directions for its clinical application in anti-tumor, anti-inflammatory, and immune regulation. By further optimizing its biosynthesis pathway and exploring effective drug application schemes, the application prospects of G. capense in modern medicine will be even broader.
Author Contributions
L.L., X.S. and L.J.: conceptualization and data curation, writing—reviewing and editing; X.S. and C.L.: writing—reviewing and editing; C.L. and R.W.: conceptualization, writing—reviewing and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. U22A20369 and 32370069) and the Natural Science Foundation of Heilongjiang Province of China (No. LH2023C035).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marek, S.; Piotr, R.; Niedzielski, P.; Budka, A.; Gąsecka, M.; Pavel, K.; Jasińska, A.; Budzyńska, S.; Kozak, L.; Mleczek, M. Comparison of multielemental composition of Polish and Chinese mushrooms (Ganoderma spp.). Eur. Food Res. Technol. 2017, 243, 1555–1566. [Google Scholar] [CrossRef]
- Hanjian, J.; Ning, N. Research Advances of the Chemical Constituents and Pharmacological Effects of Ganoderma lucidum. Guangzhou Chem. Ind. 2014, 42, 3. [Google Scholar]
- Ling, Z.B.; Wang, P.Y. The pharmacological study of Ganoderma spores and their active components. J. Peking Univ. 2006, 38, 541–547. [Google Scholar]
- Huangya, T. Isolation, Purification, Structural Characterization and Antitumor Activity of Polysaccharides from Ganoderma capense. Master’s Thesis, Guangdong Pharmaceutical University, Guangzhou, China, 2016. [Google Scholar]
- Yu, J.; Zhai, Y. Studies on the Constituents of Ganoderma Capens (Part I). Acta Pharm. Sin. 1979, 14, 374–378. [Google Scholar]
- Chen, R.Y. Chemical Research On Ganoderma lucidum. In Proceedings of the First Symposium on Development of China’s Medicinal Fungi Industry, Nantong, China, 6 April 2005; Mycological Society of China: Beijing, China, 2006; pp. 100–105. [Google Scholar]
- Leng, W.; Liugeng, T. Some pharmacological effects of thin-skinned Ganoderma capense fermentation liquid. Chin. Pharm. J. 1980, 15, 1–2. [Google Scholar]
- Chen, T.; Li, K.; Fang, Z.; Zhang, Y.; Lan, L. Components Analysis and Appraisal of Safety Toxicology Chemical of Mycelia Powder of Ganoderma capuse. Edible Fungi China 2004, 23, 39–42. [Google Scholar]
- Yang, Y.M. Clinical Research Progress on Bozhi Glycopeptide Injection. Guangming J. Chin. Med. 2017, 32, 1528. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Duan, Y.-Y.; Qian, Y.-Q.; Guo, X.-F.; Li, Y.-J.; Jin, S.-H.; Zhou, Z.-X.; Shan, S.-Y.; Wang, C.-R.; Chen, X.-J.; et al. Screening of Ganoderma strains with high polysaccharides and ganoderic acid contents and optimization of the fermentation medium by statistical methods. Bioprocess Biosyst. Eng. 2014, 37, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yan, C.; Hua, D. Isolation, purification, and structural characterization of a novel polysaccharide from Ganoderma capense. Int. J. Biol. Macromol. 2013, 57, 285–290. [Google Scholar] [CrossRef]
- Jiang, J.-Y.; Kong, F.-S.; Li, N.-S.; Zhang, D.; Yan, C.; Lv, H. Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi. Carbohydr. Polym. 2016, 147, 365–371. [Google Scholar] [CrossRef]
- Huang, Y.; Li, N.; Wan, J.-B.; Zhang, D.; Yan, C. Structural characterization and antioxidant activity of a novel heteropolysaccharide from the submerged fermentation mycelia of Ganoderma capense. Carbohydr. Polym. 2015, 134, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Li, N.; Wan, J.-B.; Zhang, D.; Li, M.; Yan, C. Structural characterization and antioxidant activity of a heteropolysaccharide from Ganoderma capense. Carbohydr. Polym. 2015, 121, 183–189. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q. Bibliometric Analysis on Clinical Application of Bozhi Glycopeptides. Chin. Pharm. 2016, 6, 6. [Google Scholar]
- Lu, Y.; Jin, Y.-H.; Zhang, H.-H. Research on the Composition and Structure of Glycopeptide from Ganoderma capense. Pharm. Biotechnol. 2007, 14, 364–367. [Google Scholar]
- Lin, Y.-Q.; Fu, X.-Q.; Gai, Y.; Wei, H.-W. Study on Monosaccharides in Bozhi Glycopeptide by TLC and HPLC. Biotechnology 2004, 14, 33–34. [Google Scholar]
- Liu, B. Functional Analysis of HMGR and DXR Genes from Amomum villosum Lour. on Biosynthesis of Terpenoids in Tobacco. Doctoral Dissertation, Guangzhou University of Chinese Medicine, Guangzhou, China, 2012. [Google Scholar]
- Yazaki, K.; Arimura, G.-I.; Ohnishi, T. ‘Hidden’ Terpenoids in Plants: Their Biosynthesis, Localization and Ecological Roles. Plant Cell Physiol. 2017, 58, 1615–1621. [Google Scholar] [CrossRef]
- Peng, S.; Qi, J.Z.; Lin, C.; Xu, Z.C.; Li, Z.H.; Liu, C.W. From natural laboratory to drug discovery: Chemical structures, bioactivities, and biosynthesis of meroterpenoids from Ganoderma species. Chin. Herb. Med. 2025, in press. [CrossRef]
- Huang, T.-R.; Zheng, W.-Y.; Wu, L.-Y.; Gan, Y.-R. Research Progress of the Pharmacological Action of the Effective Components in Centipeda minima on Allergic Rhinitis. Med. Chem. 2022, 10, 292–297. [Google Scholar] [CrossRef]
- Tan, Z.; Zhao, J.; Liu, J. Sesquiterpenoids from the cultured mycelia of Ganoderma capense. Fitoterapia 2017, 118, 73–79. [Google Scholar] [CrossRef]
- Song, A.-R.; Sun, X.-L.; Kong, C.; Zhao, C.; Qin, D.; Huang, F.; Yang, S. Discovery of a new sesquiterpenoid from Phellinus ignarius with antiviral activity against influenza virus. Arch. Virol. 2014, 159, 753–760. [Google Scholar] [CrossRef]
- Tao, L.-Q.; Zhang, H. Research progress of triterpenoids in the prevention and treatment of Alzheimer’s disease. Chin. Bull. Life Sci. 2024, 36, 487–498. [Google Scholar] [CrossRef]
- Tan, Z.; Zhao, J.-L.; Liu, J.-M.; Zhang, M.; Chen, R.-D.; Xie, K.-B.; Chen, D.-W.; Dai, J.-G. Lanostane triterpenoids and ergostane-type steroids from the cultured mycelia of Ganoderma capense. J. Asian Nat. Prod. Res. 2018, 20, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-R. Study on the Chemical Constituents and Bioactivities of Ganoderma. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2015. [Google Scholar]
- Tang, Y.; Liu, Y.; Ruan, Q.; Zhao, M.; Zhao, Z.; Cui, H. Aspermeroterpenes A–C: Three Meroterpenoids from the Marine-Derived Fungus Aspergillus terreus GZU-31-1. Org. Lett. 2020, 22, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.-F.; Wu, Z.-H.; Liu, Y.; Yan, Y.-M.; Lu, R.-M.; Cheng, Y.-X. Ganocapenoids A–D: Four new aromatic meroterpenoids from Ganoderma capense. Bioorganic Med. Chem. Lett. 2019, 29, 143–147. [Google Scholar] [CrossRef]
- Peng, X.; Li, L.; Wang, X.; Zhu, G.; Li, Z.; Qiu, M. Antioxidant farnesylated hydroquinones from Ganoderma capense. Fitoterapia 2016, 111, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.G.; Chen, R.Y. Studies on Constituents of Ganoderma capense IV. The Chemical Structures of Ganoine, Ganodine and Ganoderpurine. Acta Pharm. Sin. 1990, 25, 612–616. [Google Scholar]
- Hapuarachchi, K.; Cheng, C.-R.; Wen, T.-C.; Jeewon, R.; Kakumyan, P. Mycosphere Essays 20: Therapeutic potential of Ganoderma species: Insights into its use as traditional medicine. Mycosphere 2017, 8, 1653–1694. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Liu, L.; Chen, S. The chemistry and biology of fungal meroterpenoids (2009–2019). Org. Biomol. Chem. 2021, 19, 1644–1704. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.-G.; Ma, Q.-Y.; Huang, S.-Z.; Kong, F.-D.; Zhou, L.-M.; Dai, H.-F.; Zhao, Y.-X. Chemical constituents from the fruiting bodies of Amauroderma subresinosum. J. Asian Nat. Prod. Res. 2016, 18, 1030–1035. [Google Scholar] [CrossRef]
- Kitagawa, K.; Shigemura, K.; Ishii, A.; Nakashima, T.; Matsuo, H.; Takahashi, Y.; Omura, S.; Nakanishi, J.; Fujisawa, M. Nanaomycin K inhibited epithelial mesenchymal transition and tumor growth in bladder cancer cells in vitro and in vivo. Sci. Rep. 2021, 11, 9217. [Google Scholar] [CrossRef]
- Guo, R.-X.; Li, L.-G.; Wang, Y.-F.; Wang, L.; Zhang, M.-L.; Zhan, W.-H.; Shi, Q.-W. Historical story on natural medicinal chemistry: Steroids. Chin. Tradit. Herb. Drugs 2016, 47, 14. [Google Scholar] [CrossRef]
- Liu, P.; Liu, X.-L.; Li, J.-Y. Progress in study on biological activities and preparation of ergosterol peroxide. Chin. J. New Drugs 2023, 32, 2058–2065. [Google Scholar] [CrossRef]
- Vazirian, M.; Faramarzi, M.A.; Ebrahimi, S.E.; Esfahani, H.R.; Samadi, N.; Hosseini, S.A.; Asghari, A.; Manayi, A.; Mousazadeh, A.; Asef, M.R.; et al. Antimicrobial effect of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes) and its main compounds. Int. J. Med. Mushrooms 2014, 16, 77–84. [Google Scholar] [CrossRef]
- He, R.-B.; Li, W.; Yao, R.; Xu, M.-Y.; Dong, W.; Chen, Y.; Ni, W.-J.; Xie, S.-S.; Sun, Z.-H.; Li, C.; et al. Aurantiamide mitigates acute kidney injury by suppressing renal necroptosis and inflammation via GRPR-dependent mechanism. Int. Immunopharmacol. 2024, 139, 112745. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-F.; Ma, J.; Chen, Y.-G. Research Progress on Active Ingredients of Ganoderma sp. Heilongjiang Agric. Sci. 2014, 2014, 137–142. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.-W.; Wu, S.; Di, G.-H.; Hao, L.-H. Synthesis and hypoglycemic activity of 5-hydroxylmethyl-2- furfural derivatives. Chin. J. Med. Chem. 2012, 22, 349–355. [Google Scholar] [CrossRef]
- Shimizu, A.; Yano, T.; Saito, Y.; Inada, Y. Isolation of an inhibitor of platelet aggregation from a fungus, Ganoderma lucidum. Chem. Pharm. Bull. 1985, 33, 3012–3015. [Google Scholar] [CrossRef][Green Version]
- Li, Z.-Y.; Li, L.-S. Progress on Research of Nicotinic Acid and Nicotinamide. Chem. Ind. Times 2003, 17, 6–9. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, G.; Yan, X. Encyclopedia of Traditional Chinese Medicines—Molecular Structures, Pharmacological Activities, Natural Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2011; pp. 154–196. [Google Scholar]
- Stuart, K.-L.; Coke, L.-B. The effect of vomifoliol on stomatal aperture. Planta 1975, 122, 307–310. [Google Scholar] [CrossRef]
- Mogana, R.; Adhikari, A.; Debnath, S.; Hazra, S.; Hazra, B.; Teng, J.-K.; Wiart, C. The antiacetylcholinesterase and antileishmanial activities of Canarium patentinervium Miq. BioMed Res. Int. 2014, 2014, 903529. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Bono, A. Phytochemical antioxidants for health and medicine—A move towards nature. Biotechnol. Mol. Biol. Rev. 2007, 1, 97–104. [Google Scholar]
- Luo, J.-W.; Zhang, Y.; Huang, W.; Zhao, X.; Zeng, F.-K. Sources and functions of terpenoids in foods. Food Ferment. Ind. 2019, 45, 267–272. [Google Scholar] [CrossRef]
- Yan, C.; Kong, F.; Zhang, D.; Cui, J. Anti-glycated and antiradical activities in vitro of polysaccharides from Ganoderma capense. Pharmacogn. Mag. 2013, 9, 23–27. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Duanc, C.-X.; Yang, M.-H. An Anti-Aging Ganoderma lucidum Face Cream and a Method for Preparing the Same. CN Patent 115887327A, 11 November 2024. [Google Scholar]
- National Medical Products Administration. Technical guidelines for non-clinical research on cytotoxic antitumor drugs. Chin. J. New Drugs Clin. Remedies 2008, 27, 462–465. [Google Scholar] [CrossRef]
- Tran, P.T.; Dat, N.T.; Dang, N.H.; Van, C.P.; Lee, S.; Hwangbo, C.; Van, M.C.; Lee, J.H. Ganomycin I from Ganoderma lucidum attenuates RANKL-mediated osteoclastogenesis by inhibiting MAPKs and NFATc1. Phytomed. Int. J. Phytother. Phytopharm. 2019, 55, 1–8. [Google Scholar] [CrossRef]
- Palanisamy, A.; Tangestani, F.M.; Sean, T.W.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the Crossroads of Cell Signalling and Inflammatory Disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, S.; Ding, R.; Yao, W.; Gao, X. Inflammatory Modulation Effect of Glycopeptide from Ganoderma capense (Lloyd) Teng. Mediat. Inflamm. 2014, 2014, 691285. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Bao, L.; Ma, K.; Zhang, J.; Chen, B.; Han, J.; Ren, J.; Luo, H.; Liu, H. A novel class of α-glucosidase and HMG-CoA reductase inhibitors from Ganoderma leucocontextum and the anti-diabetic properties of ganomycin I in KK-Ay mice. Eur. J. Med. Chem. 2017, 127, 1035–1046. [Google Scholar] [CrossRef]
- Etukudo, E.M.; Mwabaleke, J.A.; Makeri, D.; Archibong, V.B.; Ifie, J.; Usman, I.M. A review on the neuroprotective potential of tamarindus indica: Evidence from preclinical studies done between 2016 to 2023. KIU J. Health Sci. 2024, 4, 119–131. [Google Scholar]
- Young, S.; Chgun, E.; Chen, M.A. Cardiovascular Complications of Acetylcholinesterase Inhibitors in Patients with Alzheimer’s Disease: A Narrative Review. Ann. Geriatr. Med. Res. 2021, 25, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.X.; Shi, L.M.; Hu, Y.; Lin, Z.D. The Effect of Ganoderma capense on IL-1Β and Caspase-3 Expressions in the Hippocampus of Rats. Zhejiang Clin. Med. J. 2017, 19, 1208–1210. [Google Scholar]
- Wang, Z.Y.; Fu, H.T. Treatment of Hereditary Cerebellar Ataxia with Ganoderma capense Report of 4 Cases. J. Tradit. Chin. Med. 1981, 1, 47–50. [Google Scholar] [PubMed]
- Mishra, J.; Rajput, R.; Singh, K. Antibacterial Natural Peptide Fractions from Indian Ganoderma lucidum. Int. J. Pept. Res. Ther. 2017, 24, 543–554. [Google Scholar] [CrossRef]
- Cör, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, Antimicrobial, Antioxidant and Antiacetylcholinesterase Effect of Ganoderma lucidum Terpenoids and Polysaccharides: A Review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef]
- Ngai, P.H.; Ng, T.B. A mushroom (Ganoderma capense) lectin with spectacular thermostability, potent mitogenic activity on splenocytes, and antiproliferative activity toward tumor cells. Biochem. Biophys. Res. Commun. 2004, 314, 988–993. [Google Scholar] [CrossRef]
- Liu, Y.M.; Li, H.Y. XUAN Guowei’s Clinical Experience in Treating Alopecia Areata from Liver and Kidney Yin Deficiency. J. Tradit. Chin. Med. 2020, 61, 13–16. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y. The Role of the Ganoderma capense Glycopeptide in Treatment of AIDS Complicated with Pulmonary Tuberculosis. China Health Stand. Manag. 2018, 9, 74–76. [Google Scholar] [CrossRef]
- Zeng, L.-J. The therapeutic effect’s analysis of the internal use of Gcapense and Acyclovir for curing recurrent Genital Herpes. In Proceedings of the 2011 International Meeting on Ganoderma Research, Beijing, China, 16 August 2011; Dermatovenereology Branch of the No.1 People’s Hospital Xiantao City Hubei Province: Xiantao, China, 2011; pp. 87–88. [Google Scholar]
- Li, L.; Yang, Y.L.; Yang, X.F.; He, W.; Yuan, K.; Zhu, W.Y.; Peng, C.; He, Y.F.; Dong, Y.M.; Zhou, W.Q. Advances on process regulation of Ganoderma triterpene acids production by liquid fermentation. Food Ferment. Ind. Editor. Staff 2021, 47, 304–312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).