Porphyrin-Based Sorbents for the Enrichment and Removal of Metal Ions
Abstract
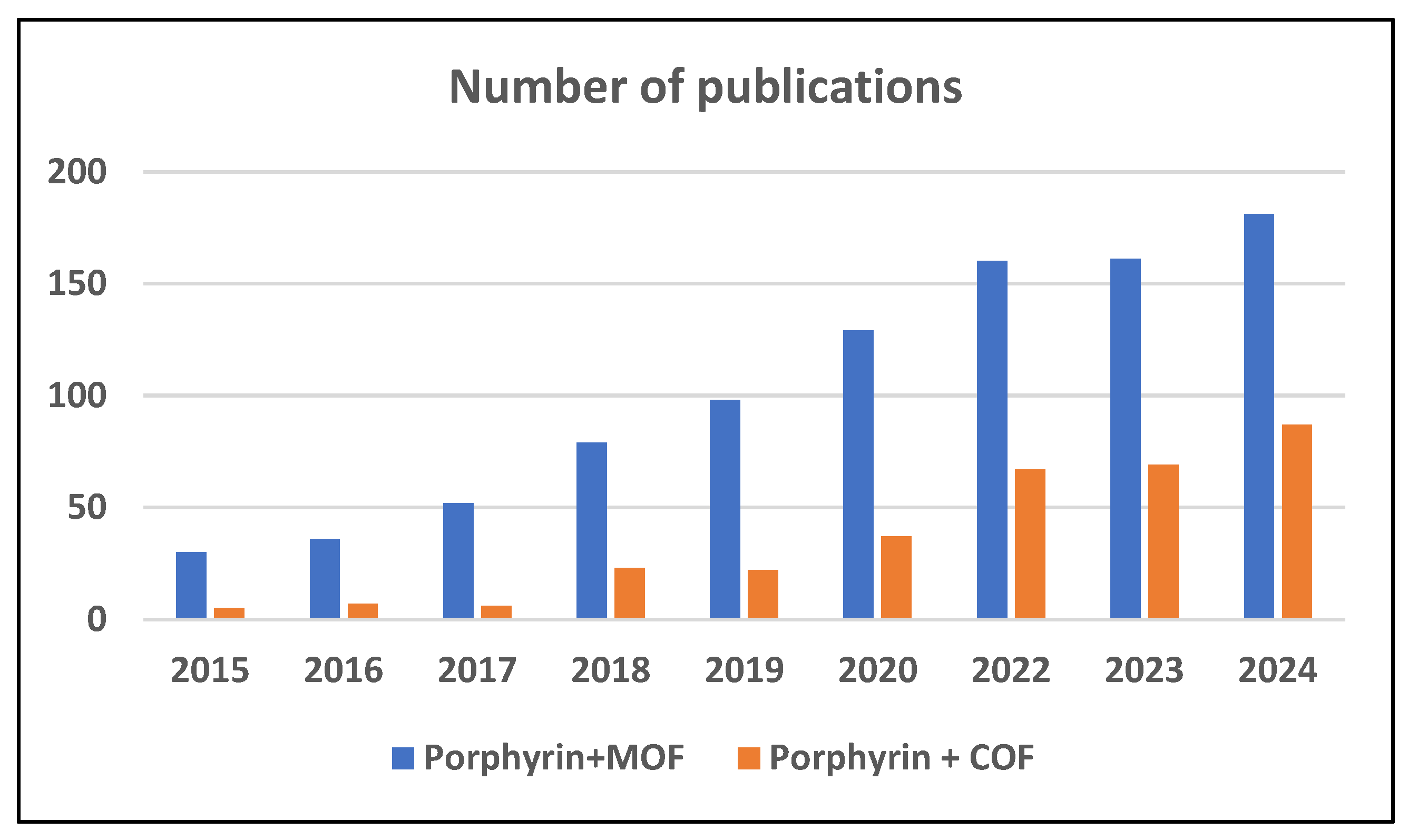
1. Introduction
2. Porphyrin-Based Sorbents
2.1. Characterization of Porphyrin-Based Materials
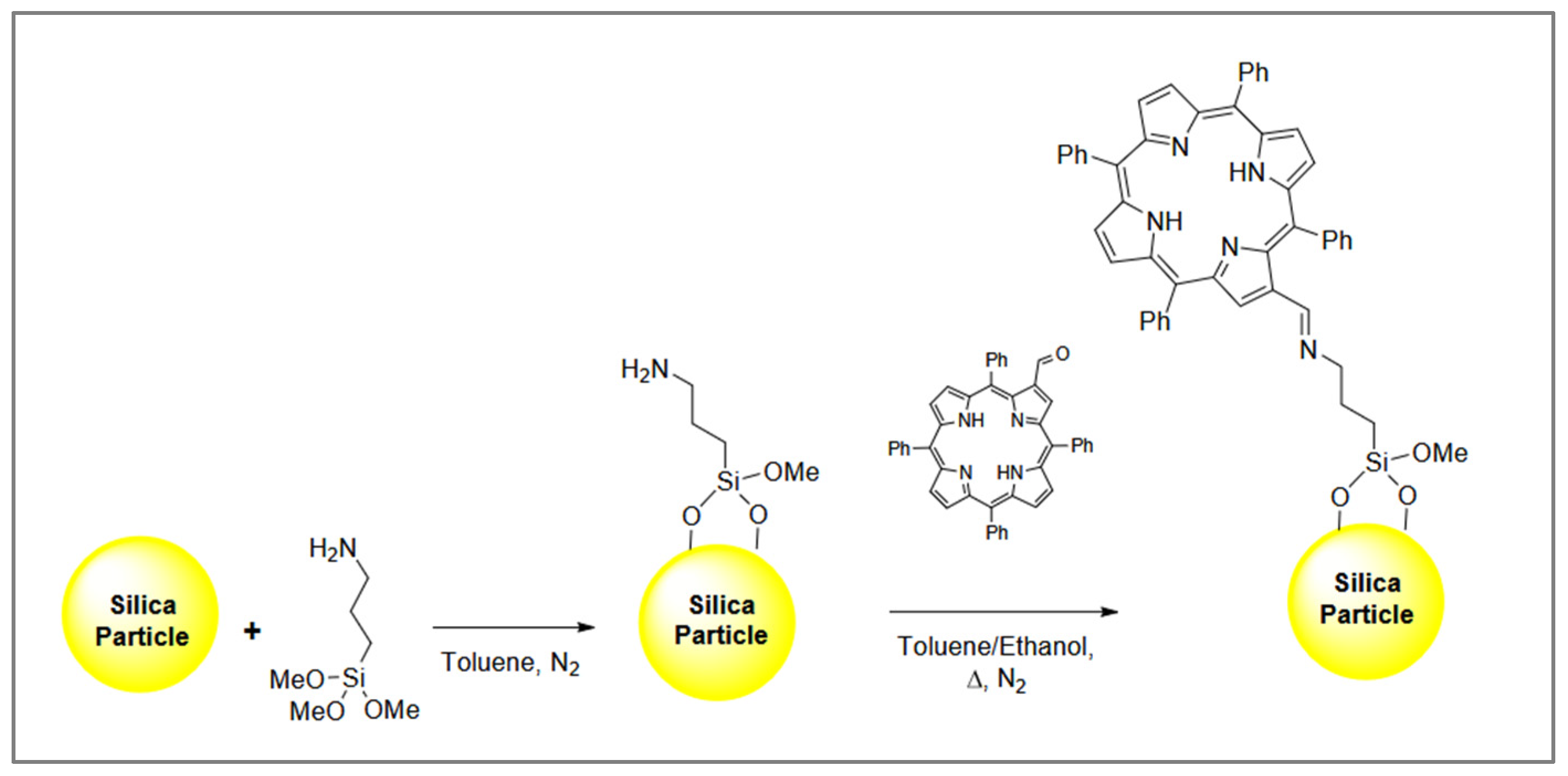
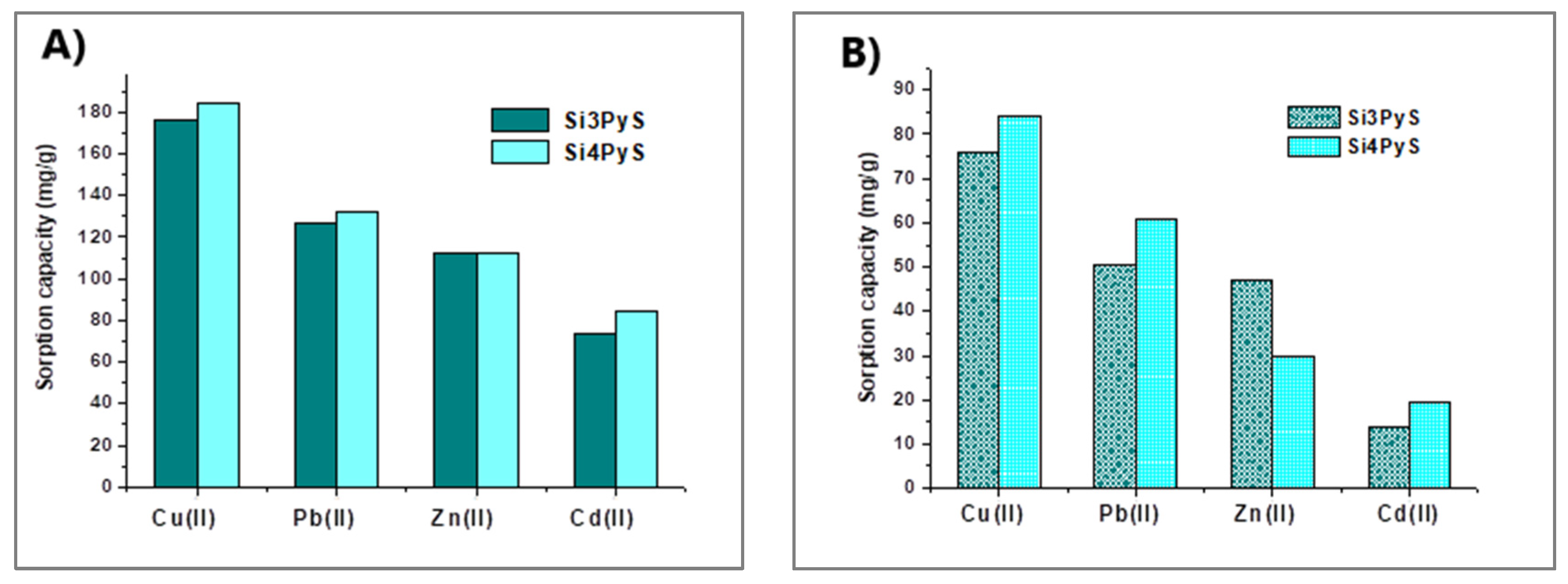
2.2. Porphyrin–Silica Materials
2.3. Porphyrin-Immobilized Resins
2.4. Porphyrin-Based Porous Organic Polymers

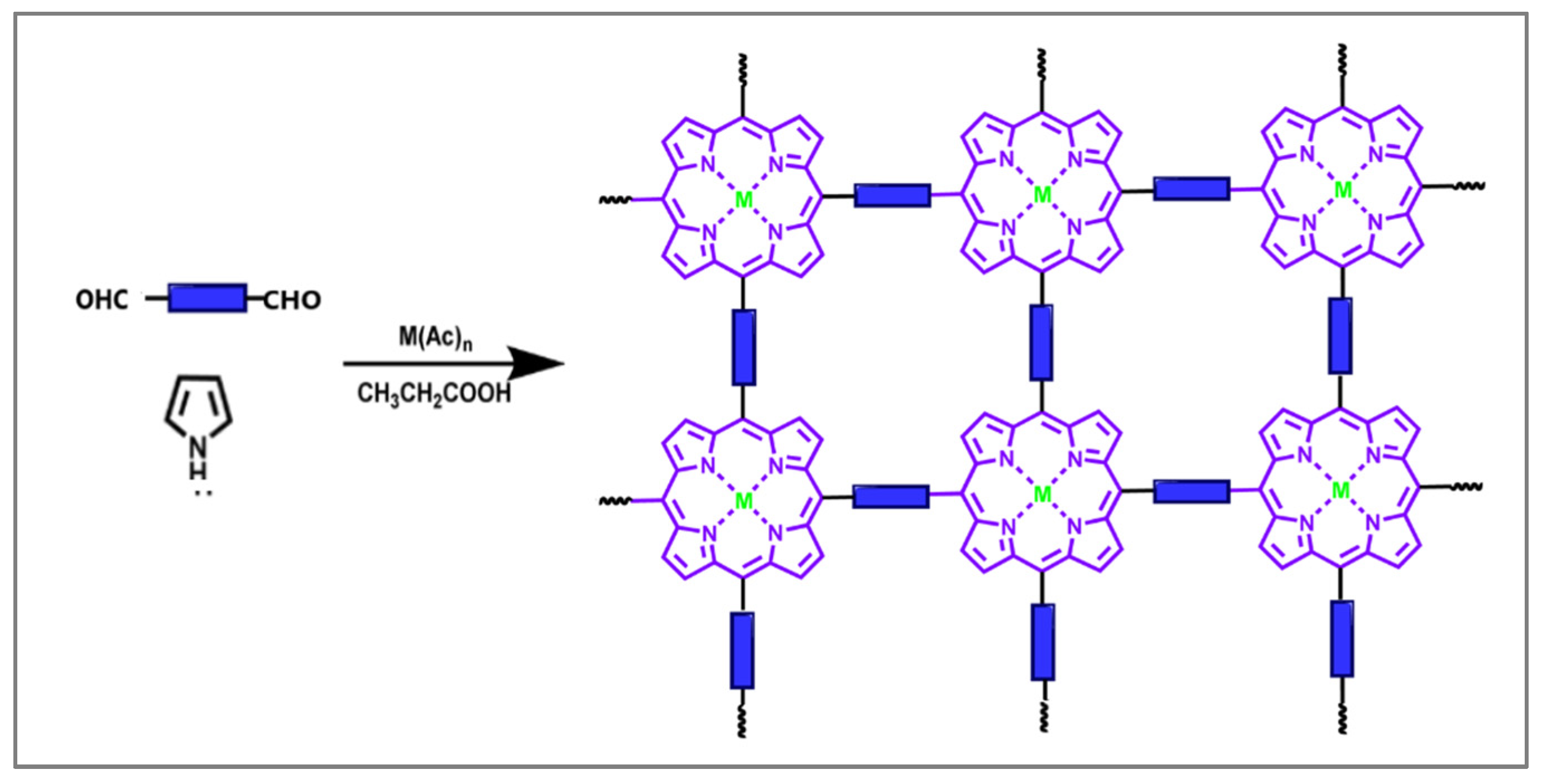
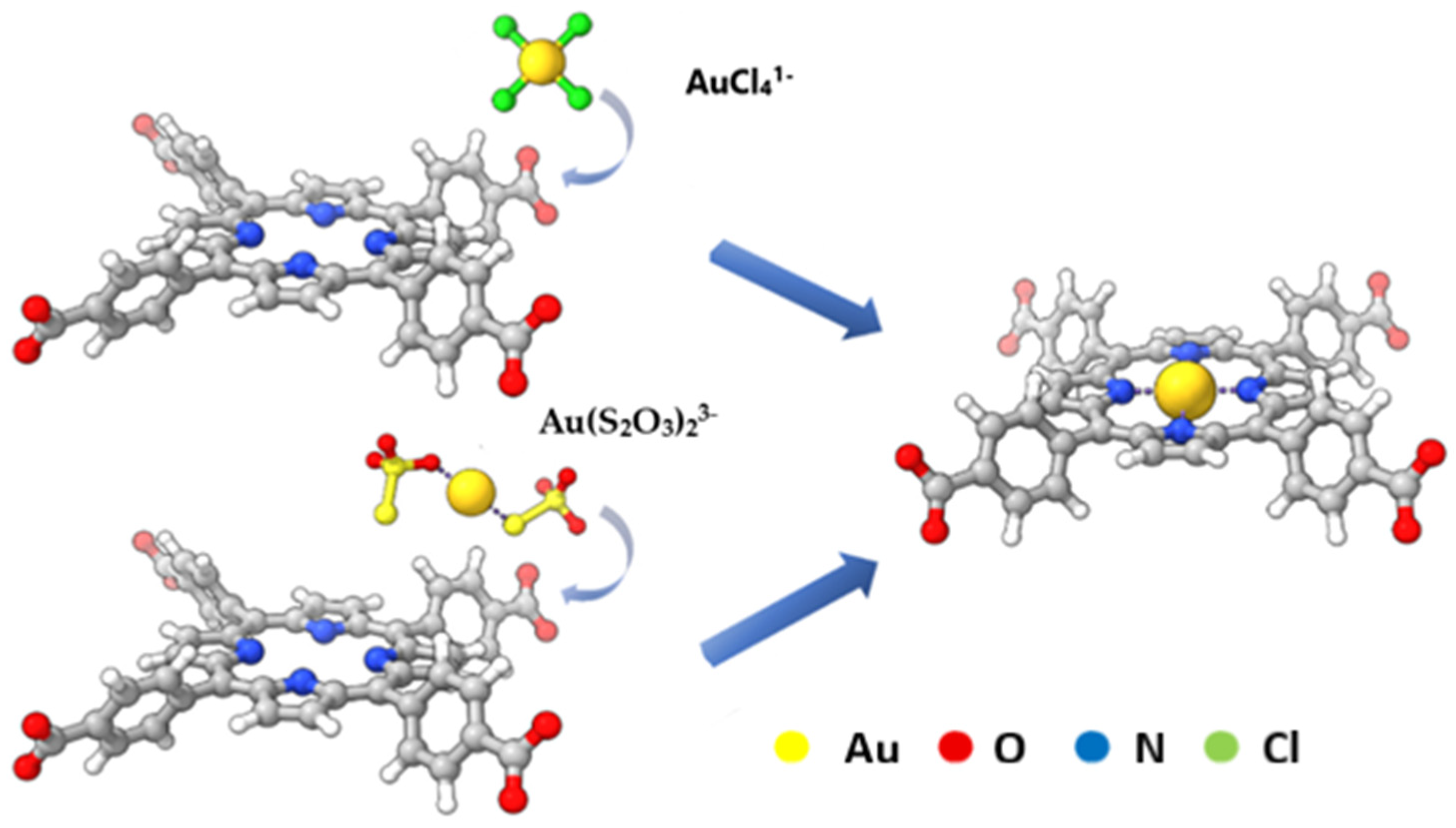
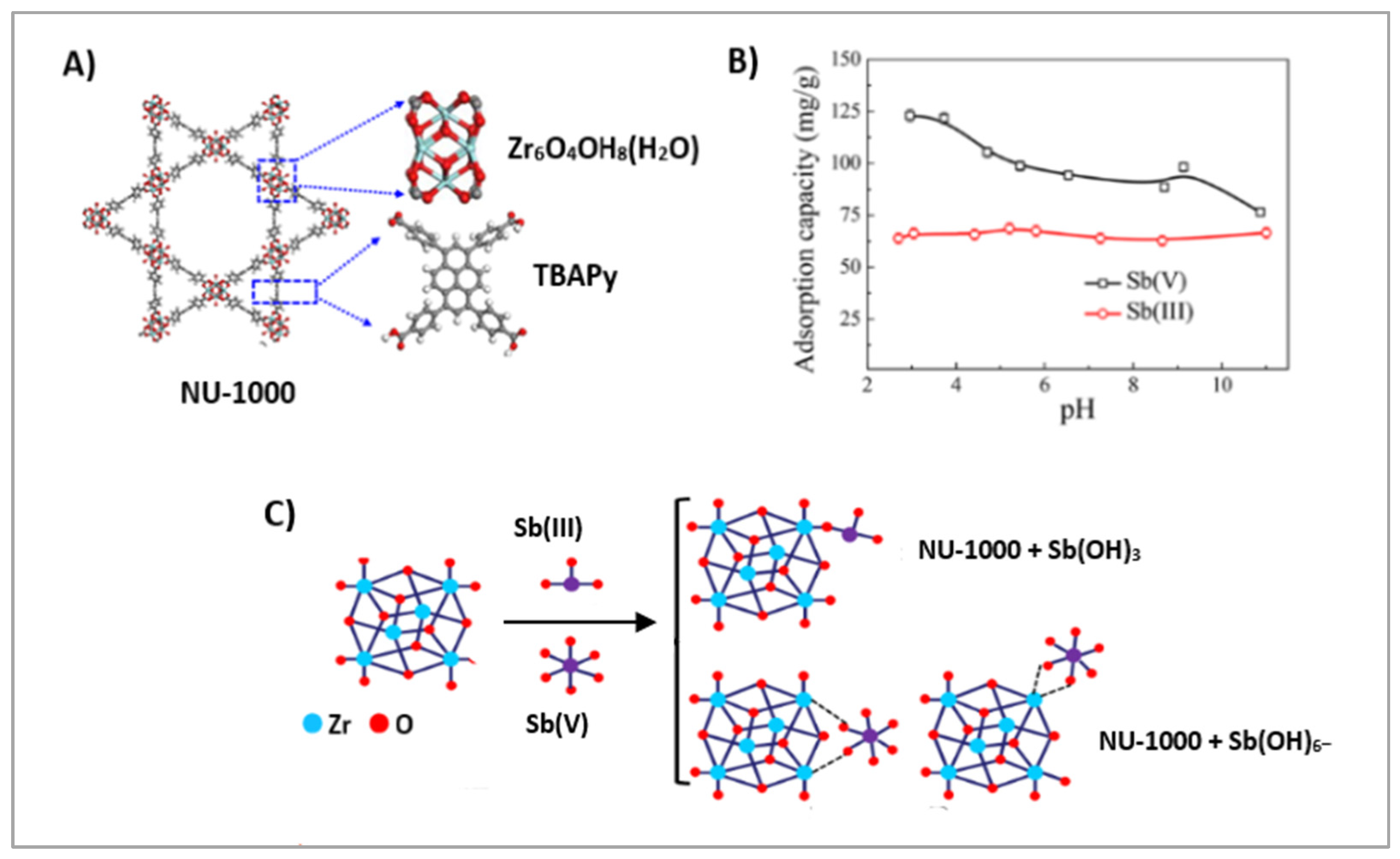
2.5. Porphyrin-Based Meta–Organic Frameworks
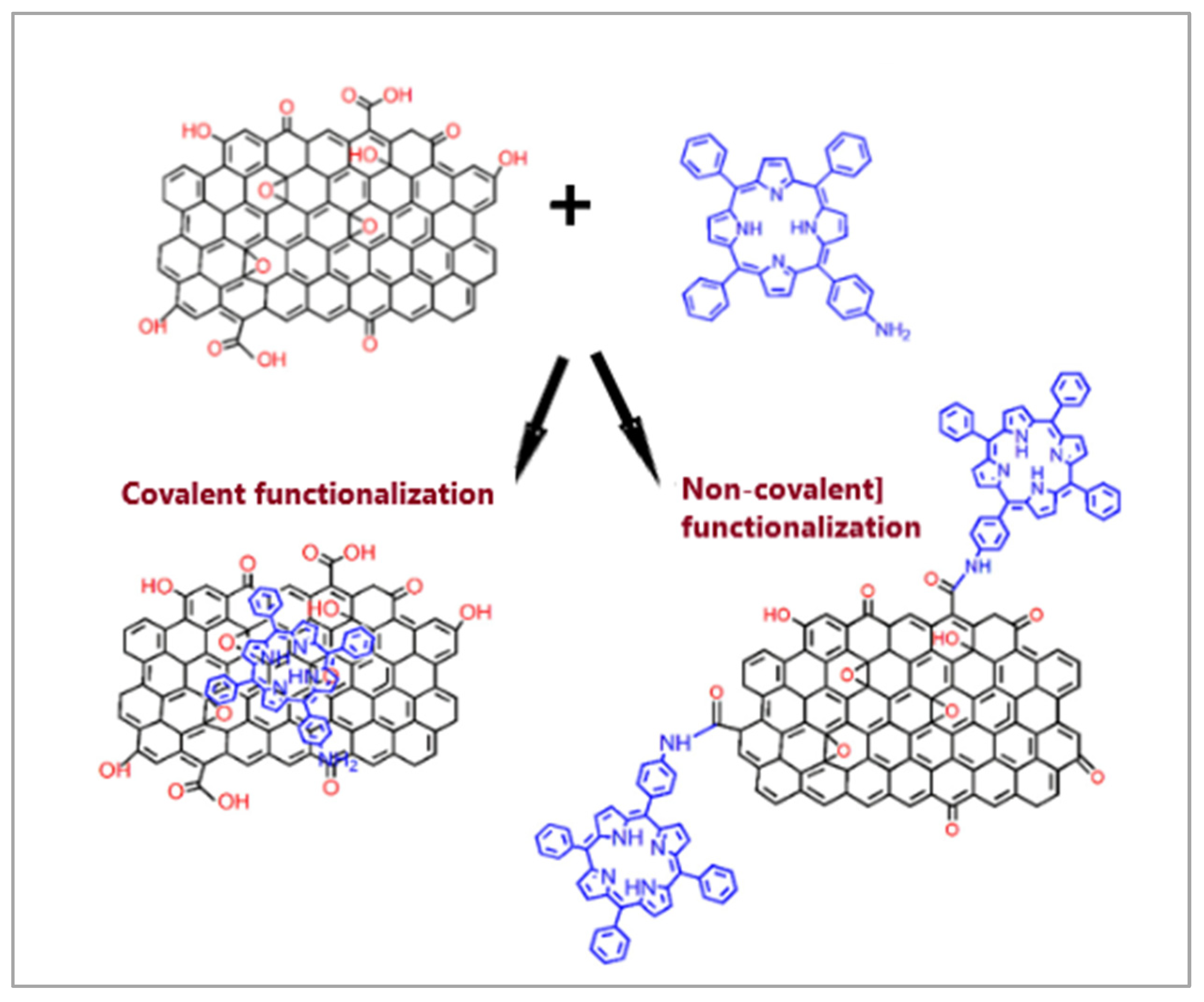
2.6. Carbon Nanostructures Modified with Porphyrins
3. Comparison of Porphyrin-Based Sorbents for the Enrichment and Removal of Metal Ions
| Metal Ions | Sorbent | Sorption Conditions | qmax (mg/g) | Desorption/ Regeneration | Ref. | ||
|---|---|---|---|---|---|---|---|
| Dose (mg) | pH | Time | |||||
| Au(III) | TNPPH2-phenazine COP | 10 | 2 | 48 h | 1354 | 0.1 M Th + 0.1 M H2SO4, 6 h | [60] |
| Imi-P-POP | 2 | 6 | 48 h | 1543 | 10% Th + 5% HCl | [62] | |
| TCPP-MOP in dark +light glow +acetic acid | 1 | 3 | 4 h | 885 2613 4996 | na | [80] | |
| Au(I), Au(III) | (Fe(III)-TCPP)n-MOF | 2 | 3 | 5 min | Au(I)-2026 Au(III)-2296 | 36% HNO3 + 4% HCl | [81] |
| Cd(II) | TAPP-COF | 40 | 8 | 60 min | 181 | 0.1 M HCl | [64] |
| PS@TAPP-COF | 40 | 8 | 35 min | 166 | 0.1 M HCl, 0.1 M EDTA | ||
| CF@TAPP-Tp-COF | 80 | 8 | 15 min | 75.2 | 0.1 M HNO3 | [65] | |
| Cu(II) | SiO2@4TF5PP | 10 | 6 | 15 min | 184.15 | 6 M HCl | [51] |
| Cr(VI) | TPP-COF | 2 | na | 4 h | 293 | 0.3 M NaBr, 5 h | [66] |
| T4MPP-CMP | 0.15 mg/L | 2 | 17 min | 339.17 | 0.4 M NaOH, 2 h | [67] | |
| Imi-TPP-COF | 50 | 2 | 5 min | 373.14 | na | [68] | |
| TCPP-COF | 15 | 2 | 5 h | 246.76 | na | [70] | |
| Zn-TCPP-COF | 15 | 2 | 60 min | 273.09 | na | ||
| Hg(II) | Thiophene-P-POP | na | 7 | 30 min | 1049 | na | [71] |
| DA-P-POP | 5 | 6 | 5 min | 384.6 | 1 M HCl | [69] | |
| Pb(II) | SiO2@TCPP | 10 | 6 | 25 min | 182.16 | 6 M HCl | [50] |
| FeO@SiO2@TCPP | 6 | 25 min | 798.34 | na | [54] | ||
| Sb(V) | Zr-TCPP-MOF | 3 | 180 min | 250.22 | na | [82] | |
| Sb(III) | Zr-TCPP-MOF | 0.7 g/L | 2 | 3 h | 175.17 | 0.5 M HCl | [83] |
| Sb(III), Sb(V) | Zr-TCPP-MOF | 8 | 2 | 9 min | Sb(III)-136.97 Sb(V)-287.88 | na | [84] |
| U(VI) | CNT@COF-OH TPP-AO-HCP | 5 5 | 5 6 | 12 h 6 h | 518.2 110 | na 2 M HCl | [90,91] |
| V(V) | SiO2@TCPP | 10 | 6–8 | 60 min | 35.0 | 2 M HNO3 | [52] |
3.1. Thermodynamic and Kinetic Parameters
3.2. Selectivity
3.3. Desorption of Metal Ions and Regeneration/Stability of Sorbents
3.4. Comparison of Porphyrin-Based Sorbents with Other Types of Sorbents
| Metal Ions | Sorbent | qmax (mg/g) | Equilibrium Time | No. of Cycles | Selectivity | Ref. |
|---|---|---|---|---|---|---|
| Au(III) | TNPPH2-phenazine COP | 1354 | 30 min | >3 | >93.4% of Au | [60] |
| Zr UiO-66-NH2 MOF | 650 | 12.2 h | >5 | Selective vs. Co, Ni | [100] | |
| Fe-BTC/PpPDA MOF | 934 | <2 min | >10 | >99.9% of Au | [101] | |
| Cd(II) | PS@TAPP COF | 166 | 40 min | >5 | >85% of Cd | [64] |
| PANH | 156 | 180 min | >3 | >70% of Cd | [102] | |
| N-riched COF | 396 | 40 min | na | na | [103] | |
| Cu(II) | SiO2@4TF5PP SiO2@NP2 SiO2@NNN | 184.15 131.82 121.6 | 10 min 25 min 20 min | >5 na >5 | Co-sorption of Cd, Pb, Zn Co-sorption of Cd, Pb, Zn >80% of Cu | [51,104,105] |
| Cr(VI) | Imi-TPP-COF | 373.14 | 40 min | >5 | >90% of Cr | [68] |
| PTAPDAC | 273.17 | 60 min | na | na | [106] | |
| CS-PVA hydrogel | 320 | 200 min | 3 | na | [107] | |
| CCGP | 179.2 | 30 min | 3 | na | [108] | |
| Hg(II) | Thiophene-P-POP | 1049 | 30 min | 5 | Significantly selective | [71] |
| S-SH COF S-CX4P | 1350 1686 | 10 min 5 min | na 4 | Co-sorption of Cu, Pb Co-sorption of Cu, Zn, Mg, Ca | [109,110] | |
| Sb(V) | Zr-TCPP MOF | 250.22 | 100 min | 5 | Strong negative impact of SO42− | [82] |
| RGO@Mn3O4 | 105.50 | 25 min | na | Negative impact of PO43− | [111] | |
| TiO2 | 156 | 60 min | na | Negative impact of PO43− and SO42− | [112] |
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AO | Amidooxime group |
| APTMS | 3-aminopropyltrimethoxysilane |
| CF | Carbon fiber |
| CMPs | Conjugated microporous polymers |
| COFs | Covalent organic frameworks |
| COPs | Covalent organic polymers |
| DA | Dialdehyde |
| DLS | Dynamic light scattering |
| DMSPE | Dispersive micro-solid phase extraction |
| FTR | Fourier-transform infrared |
| HPC | Hyper cross-linked polymers |
| MOFs | Metal-organic frameworks |
| P-MOFs | Porphyrin-based MOFs |
| P-POPs | Porphyrin-based porous organic polymers |
| PS | Urethane sponge |
| SEM | Scanning electron microscopy |
| SPE | Solid-phase extraction |
| TAPP | 5,10,15,20-tetrakis(4-aminophenyl)porphyrin |
| TCPP | 5,10,15,20-tetrakis5,10,15,20-tetrakis |
| TEM | Transmission electron microscopy |
| TF5PP | 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin |
| T4MPP | 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrin |
| TNPPH2 | 5,10,15,20-tetrakis(4-nitrophenyl)-21H,23H-porphyrin |
| TPP | 5,10,15,20-tetrakis(4-pyridyl)porphyrin |
| T4MPP | 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrin |
| ZnTCPP | [5,10,15,20-tetrakis(5,10,15,20-tetrakis)porphyrinato]zinc |
References
- Andleeb, S.; Ur Rehman, K.; Mahmood, A.; Elsadek, M.F.; Ul Safa, N.; Hussein, D.S.; Essam El-Din, M.M. Human Health Risk Hazards by Heavy Metals through Consumption of Vegetables Cultivated by Wastewater. J. King Saud Univ. Sci. 2023, 35, 102467. [Google Scholar] [CrossRef]
- Oladimeji, T.E.; Oyedemi, M.; Emetere, M.E.; Agboola, O.; Adeoye, J.B.; Odunlami, O.A. Review on the Impact of Heavy Metals from Industrial Wastewater Effluent and Removal Technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy Metals: Toxicity and Human Health Effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef] [PubMed]
- Wiater, J.; Sulewska, M. Heavy Metal Speciation in Municipal Sewage Sludge Depending on Treatment Method. Desalin. Water Treat. 2023, 301, 159–172. [Google Scholar] [CrossRef]
- Qiu, C.; Xie, S.; Liu, N.; Meng, K.; Wang, C.; Wang, D.; Wang, S. Removal Behavior and Chemical Speciation Distributions of Heavy Metals in Sewage Sludge during Bioleaching and Combined Bioleaching/Fenton-like Processes. Sci. Rep. 2021, 11, 14879. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lee, S.M.; Jang, Y.; Lee, J.; Lee, C.M.; Cho, E.-M.; Seo, Y.R. Adverse Human Health Effects of Chromium by Exposure Route: A Comprehensive Review Based on Toxicogenomic Approach. Int. J. Mol. Sci. 2023, 24, 3410. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Fang, M.; Tan, X.; Hu, B.; Wang, X. Highly Efficient Selective Elimination of Heavy Metals from Solutions by Different Strategies. Sep. Purif. Technol. 2024, 350, 127975. [Google Scholar] [CrossRef]
- Gahrouei, A.E.; Rezapour, A.; Pirooz, M.; Pourebrahimi, S. From Classic to Cutting-Edge Solutions: A Comprehensive Review of Materials and Methods for Heavy Metal Removal from Water Environments. Desalin. Water Treat. 2024, 319, 100446. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Khatun, M.H.; Kobir, M.; Miah, A.R.; Sarkar, A.K.; Alam, A. Technologies for Remediation of Heavy Metals in Environment and Ecosystem: A Critical Overview of Comparison Study. Asian J. Environ. Ecol. 2024, 23, 61–80. [Google Scholar] [CrossRef]
- Sathya, K.; Nagarajan, K.; Carlin Geor Malar, G.; Rajalakshmi, S.; Raja Lakshmi, P. A Comprehensive Review on Comparison among Effluent Treatment Methods and Modern Methods of Treatment of Industrial Wastewater Effluent from Different Sources. Appl. Water Sci. 2022, 12, 70. [Google Scholar] [CrossRef]
- Ayach, J.; El Malti, W.; Duma, L.; Lalevée, J.; Al Ajami, M.; Hamad, H.; Hijazi, A. Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies. Polymers 2024, 16, 1959. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Hou, J.; Wang, Y.; Lin, D. A Review on the Industrial Waste Based Adsorbents for the Removal of Pollutants from Water: Modification Methods and Adsorption Study. Resour. Environ. Sustain. 2025, 19, 100183. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Ali, S.; Zaman, W. Innovative Adsorbents for Pollutant Removal: Exploring the Latest Research and Applications. Molecules 2024, 29, 4317. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetics and Isotherm Models of Heavy Metals by Various Adsorbents: An Overview. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1837–1865. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A Review of the Modern Principles and Applications of Solid-Phase Extraction Techniques in Chromatographic Analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Pyrzynska, K. Nanomaterials in Speciation Analysis of Metals and Metalloids. Talanta 2020, 212, 120784. [Google Scholar] [CrossRef]
- Khan, W.A.; Arain, M.B.; Soylak, M. Nanomaterials-Based Solid Phase Extraction and Solid Phase Microextraction for Heavy Metals Food Toxicity. Food Chem. Toxicol. 2020, 145, 111704. [Google Scholar] [CrossRef]
- Hu, C.; Jiang, D.; Zhang, Y.; Gao, H.; Zeng, Y.; Khaorapapong, N.; Liu, Z.; Yamauchi, Y.; Pan, M. Porphyrins-Based Multidimensional Nanomaterials: Structural Design, Modification and Applications. Coord. Chem. Rev. 2025, 523, 216264. [Google Scholar] [CrossRef]
- Zhang, K.; Song, X.; Liu, M.; Chen, M.; Li, J.; Han, J. Review on the Use of Magnetic Nanoparticles in the Detection of Environmental Pollutants. Water 2023, 15, 3077. [Google Scholar] [CrossRef]
- Nikolaou, V.; Nikoloudakis, E.; Ladomenou, K.; Charalambidis, G.; Coutsolelos, A.G. Porphyrins—Valuable Pigments of Life. Front. Chem. Biol. 2024, 2, 1346465. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Kilian, K.; Pęgier, M. Porphyrins as Chelating Agents for Molecular Imaging in Nuclear Medicine. Molecules 2022, 27, 3311. [Google Scholar] [CrossRef]
- Nemeth, T.; Pallier, A.; Çelik, Ç.; Garda, Z.; Yoshizawa-Sugata, N.; Masai, H.; Tóth, É.; Yamakoshi, Y. Water-Soluble Mn(III)-Porphyrins with High Relaxivity and Photosensitization. Chem. Biomed. Imaging 2025, 3, 5–14. [Google Scholar] [CrossRef]
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Khan, M.A.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef]
- Oyim, J.; Omolo, C.A.; Amuhaya, E.K. Photodynamic Antimicrobial Chemotherapy: Advancements in Porphyrin-Based Photosensitize Development. Front. Chem. 2021, 9, 635344. [Google Scholar] [CrossRef] [PubMed]
- Chino, M.; Leone, L.; Zambrano, G.; Pirro, F.; D’Alonzo, D.; Firpo, V.; Aref, D.; Lista, L.; Maglio, O.; Nastri, F.; et al. Oxidation Catalysis by Iron and Manganese Porphyrins within Enzyme-like Cages. Biopolymers 2018, 109, e23107. [Google Scholar] [CrossRef]
- Gao, P.; Mukherjee, S.; Zahid Hussain, M.; Ye, S.; Wang, X.; Li, W.; Cao, R.; Elsner, M.; Fischer, R.A. Porphyrin-Based MOFs for Sensing Environmental Pollutants. Chem. Eng. J. 2024, 492, 152377. [Google Scholar] [CrossRef]
- Saadh, M.J.; Ahmed Mustafa, M.; Kamil Ghadir, G.; Kaur, M.; Kaur, H.; Mohammed, F.; Abed Jawad, I.; Mahtab Alam, M.; Hassan, Z.F.; Jasim Mohammed, I.; et al. Porphyrin-Based Nanoarchitectures in Sensing: Characterization, and Applications in Detecting Gases, Biomolecules, and Environmental Contaminants. Inorg. Chem. Commun. 2024, 163, 112352. [Google Scholar] [CrossRef]
- Negut, C.C.; Stefan-Van Staden, R.-I.; Van Staden, J.F. Porphyrins-as Active Materials in the Design of Sensors. An Overview. ECS J. Solid State Sci. Technol. 2020, 9, 051005. [Google Scholar] [CrossRef]
- Xie, M.; Liu, J.; Dai, L.; Peng, H.; Xie, Y. Advances and Prospects of Porphyrin Derivatives in the Energy Field. RSC Adv. 2023, 13, 24699–24730. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Q.; Peng, Q.; Yang, X.; Zhao, H.; Fan, H.; Liu, H.; Cao, X. A Novel Strategy to Improve Gas Capture Performance of Metal-Free Azo-Bridged Porphyrin Porous Organic Polymers: The Design of Traps. Eur. Polym. J. 2022, 175, 111359. [Google Scholar] [CrossRef]
- Gu, S.; Marianov, A.N.; Lu, T.; Zhong, J. A Review of the Development of Porphyrin-Based Catalysts for Electrochemical CO2 Reduction. Chem. Eng. J. 2023, 470, 144249. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.; Oh, S.; Jang, W.-D. Porphyrin-Based Nanoporous Materials for Photocatalytic Applications. Appl. Phys. Rev. 2024, 11, 031319. [Google Scholar] [CrossRef]
- Gkika, D.A.; Ladomenou, K.; Bououdina, M.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption and Photocatalytic Applications of Porphyrin-Based Materials for Environmental Separation Processes: A Review. Sci. Total Environ. 2024, 908, 168293. [Google Scholar] [CrossRef]
- Qi, Z.-L.; Cheng, Y.-H.; Xu, Z.; Chen, M.-L. Recent Advances in Porphyrin-Based Materials for Metal Ions Detection. Int. J. Mol. Sci. 2020, 21, 5839. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Porphyrin-Based Nanomaterials for the Photocatalytic Remediation of Wastewater: Recent Advances and Perspectives. Molecules 2024, 29, 611. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Zhang, P.; Wang, Y.; Lu, W. Synthesis of Porphyrin Porous Organic Polymers and Their Application of Water Pollution Treatment: A Review. Environ. Technol. Innov. 2023, 29, 102972. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, W.; Zhang, J.; Yang, M.; Chen, Q.; Liu, F.; Li, J.; Wei, S.; Zhu, G. Porphyrin-Based Porous Organic Polymers Synthesized Using the Alder–Longo Method: The Most Traditional Synthetic Strategy with Exceptional Capacity. RSC Adv. 2024, 14, 20837–20855. [Google Scholar] [CrossRef]
- Ji, W.; Wang, T.-X.; Ding, X.; Lei, S.; Han, B.-H. Porphyrin- and Phthalocyanine-Based Porous Organic Polymers: From Synthesis to Application. Coord. Chem. Rev. 2021, 439, 213875. [Google Scholar] [CrossRef]
- Li, X.-G.; Li, J.; Chen, J.; Rao, L.; Zheng, L.; Yu, F.; Tang, Y.; Zheng, J.; Ma, J. Porphyrin-Based Covalent Organic Frameworks from Design, Synthesis to Biological Applications. Biomater. Sci. 2024, 12, 2766–2785. [Google Scholar] [CrossRef]
- Chen, M.; Li, H.; Liu, C.; Liu, J.; Feng, Y.; Wee, A.G.H.; Zhang, B. Porphyrin- and Porphyrinoid-Based Covalent Organic Frameworks (COFs): From Design, Synthesis to Applications. Coord. Chem. Rev. 2021, 435, 213778. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef]
- Dhir, R.; Kaur, M.; Malik, A.K. Porphyrin Metal-Organic Framework Sensors for Chemical and Biological Sensing. J. Fluoresc. 2024, 35, 1895–1917. [Google Scholar] [CrossRef]
- Biesaga, M. Porphyrins in Analytical Chemistry. A Review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Hoskins, B.F.; Michail, D.M.; Robson, R. Assembly of Porphyrin Building Blocks into Network Structures with Large Channels. Nature 1994, 369, 727–729. [Google Scholar] [CrossRef]
- Pourret, O.; Bollinger, J.-C.; Hursthouse, A.; Van Hullebusch, E.D. Sorption vs Adsorption: The Words They Are a-Changin’, Not the Phenomena. Sci. Total Environ. 2022, 838, 156545. [Google Scholar] [CrossRef]
- Jayawardena, H.S.N.; Liyanage, S.H.; Rathnayake, K.; Patel, U.; Yan, M. Analytical Methods for Characterization of Nanomaterial Surfaces. Anal. Chem. 2021, 93, 1889–1911. [Google Scholar] [CrossRef]
- El Abiad, C.; Radi, S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Moura, N.M.M. Supramolecular Hybrid Material Based on Engineering Porphyrin Hosts for an Efficient Elimination of Lead(II) from Aquatic Medium. Molecules 2019, 24, 669. [Google Scholar] [CrossRef]
- El Abiad, C.; Radi, S.; El Massaoudi, M.; Lamsayah, M.; Figueira, F.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Moura, N.M.M. Porphyrin-Silica Gel Hybrids as Effective and Selective Copper(II) Adsorbents from Industrial Wastewater. J. Environ. Chem. Eng. 2023, 11, 110097. [Google Scholar] [CrossRef]
- Pyrzyńska, K.; Wierzbicki, T. Sorption Behavior of Vanadium on Silica Gel Modified with Tetrakis(4-Carboxyphenyl)Porphyrin. Anal. Sci. 2005, 21, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Radi, S.; Abiad, C.E.; Moura, N.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S. New Hybrid Adsorbent Based on Porphyrin Functionalized Silica for Heavy Metals Removal: Synthesis, Characterization, Isotherms, Kinetics and Thermodynamics Studies. J. Hazard. Mater. 2019, 370, 80–90. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, S.; Chen, P.; Zhu, G.-T.; Jiang, X.; Di, S. Adsorption Behavior and Mechanism of Pb(II) on a Novel and Effective Porphyrin-Based Magnetic Nanocomposite. Appl. Surf. Sci. 2019, 484, 124–134. [Google Scholar] [CrossRef]
- Uche, C.C.; Mukaba, J.-L.M.; Bode-Aluko, C.A.; Pereao, O.; Petrik, L. Immobilization of Metal Selective Ligands upon Polymer Nanofibers: Successes and Challenges. Proceedings 2020, 57, 67. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Kilian, K. Application of Porphyrin Ligands for Preconcentration and Determination of Metal Ions. Chem. Anal. 2002, 47, 439. [Google Scholar]
- Mohamed, M.G.; EL-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.-W. Advances in Porous Organic Polymers: Syntheses, Structures, and Diverse Applications. Mater. Adv. 2022, 3, 707–733. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Tran, C.H.; Choi, H.K.; Yu, D.-G.; Kim, I. Porous Organic Polymers with Defined Morphologies: Synthesis, Assembly, and Emerging Applications. Prog. Polym. Sci. 2023, 142, 101691. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.; Ryu, D.Y.; Jang, W.-D. Porphyrin-Based Supramolecular Polymers. Chem. Soc. Rev. 2023, 52, 1947–1974. [Google Scholar] [CrossRef]
- Nguyen, T.S.; Hong, Y.; Dogan, N.A.; Yavuz, C.T. Gold Recovery from E-Waste by Porous Porphyrin–Phenazine Network Polymers. Chem. Mater. 2020, 32, 5343–5349. [Google Scholar] [CrossRef]
- Son, J.; Hong, Y.; Han, G.; Nguyen, T.S.; Yavuz, C.T.; Han, J.-I. Gold Recovery Using Porphyrin-Based Polymer from Electronic Wastes: Gold Desorption and Adsorbent Regeneration. Sci. Total Environ. 2020, 704, 135405. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Liu, J.; Wang, T.; Zhang, X. Bottom-up Synthesis of Cationic Porphyrin-Based Porous Organic Polymers for Highly Efficient and Selective Recovery of Gold. Chem. Eng. J. 2022, 449, 137758. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, Z.; Xia, J. 2D/3D Porphyrin-Based Porous Polyaniline Derivatives for Highly Efficient Adsorption of Au3+ Ions. Sep. Purif. Technol. 2023, 327, 124820. [Google Scholar] [CrossRef]
- Jin, W.-L.; Li, W.; Wang, H.-X.; Liu, X.-W.; Jiang, H.-X.; Zhu, L.-N.; Kong, D.-M. Sponge-Supported Monolithic Materials of Porphyrin Covalent Organic Frameworks for Selective Recognition, Convenient Removal and Extraction of Cd2+. J. Environ. Chem. Eng. 2022, 10, 107662. [Google Scholar] [CrossRef]
- Jin, W.-L.; Ji, X.; Hou, X.-L.; Ji, S.-Y.; Li, W.; Yu, X.; Liu, X.-W.; Zhu, L.-N.; Jiang, H.-X.; Kong, D.-M. Porphyrin COF and Its Mechanical Pressing-Prepared Carbon Fiber Hybrid Membrane for Ratiometric Detection, Removal and Enrichment of Cd2+. J. Hazard. Mater. 2022, 439, 129574. [Google Scholar] [CrossRef]
- Li, Z.-J.; Xue, H.-D.; Zhang, Y.-Q.; Hu, H.-S.; Zheng, X.-D. Construction of a Cationic Organic Network for Highly Efficient Removal of Anionic Contaminants from Water. New J. Chem. 2019, 43, 11604–11609. [Google Scholar] [CrossRef]
- Lone, I.A.; Beig, S.U.R.; Kumar, R.; Shah, S.A. Porphyrin-Based Conjugated Microporous Adsorbent Material for the Efficient Remediation of Hexavalent Chromium from the Aquatic Environment. Environ. Sci. Pollut. Res. 2023, 30, 81055–81072. [Google Scholar] [CrossRef]
- Ding, R.; Liu, J.; Meng, Q.; Wang, T.; Zhang, X. Dual-Functional Flexible Cationic Porphyrin-Based Covalent Organic Frameworks for Selective Adsorption and Sensitive Detection of Cr (VI). Sep. Purif. Technol. 2025, 359, 130544. [Google Scholar] [CrossRef]
- Zare, A.H.; Khajeh, M.; Oveisi, A.R.; Daliran, S.; Ghaffari-Moghaddam, M. A New Porphyrin-Porous Organic Polymer for Effective Adsorption of Mercury Ions. J. Polym. Environ. 2024, 32, 5771–5782. [Google Scholar] [CrossRef]
- Song, Z.; Xu, Y.; Zhang, M.; Zhu, W.; Yang, X.; Hao, D.; Li, Q. Efficient Removal of Cr (VI) by Bifunction Zinc Porphyrin COF: Coupling Adsorption with Photocatalysis, Performance Evaluation, and Mechanism Analysis. J. Colloid Interface Sci. 2025, 677, 346–358. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Wang, J.; Zhang, D.; Huang, J. Thiophene-Based Porphyrin Polymers for Mercury (II) Efficient Removal in Aqueous Solution. J. Colloid Interface Sci. 2024, 653, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Hassan, A.A.; Bilal, M.; Hussain, T.; Rizwan, K. Metal-Organic Frameworks Based Adsorbents: A Review from Removal Perspective of Various Environmental Contaminants from Wastewater. Chemosphere 2020, 259, 127369. [Google Scholar] [CrossRef]
- Mosca, L.P.L.; Gapan, A.B.; Angeles, R.A.; Lopez, E.C.R. Stability of Metal–Organic Frameworks: Recent Advances and Future Trends. Eng. Proc. 2023, 56, 146. [Google Scholar] [CrossRef]
- Ding, G.; Li, C.; Chen, L.; Liao, G. Porphyrin-Based Metal–Organic Frameworks for Photo(Electro)Catalytic CO2 Reduction. Energy Environ. Sci. 2024, 17, 5311–5335. [Google Scholar] [CrossRef]
- Hassanzadeh Goji, N.; Ramezani, M.; Saljooghi, A.S.; Alibolandi, M. Porphyrin-Based Metal–Organic Frameworks: Focus on Diagnostic and Therapeutic Applications. J. Nanostruct. Chem. 2024, 14, 167–208. [Google Scholar] [CrossRef]
- Tang, C.; Li, X.; Hu, Y.; Du, X.; Wang, S.; Chen, B.; Wang, S. Porphyrin-Based Metal-Organic Framework Materials: Design, Construction, and Application in the Field of Photocatalysis. Molecules 2024, 29, 467. [Google Scholar] [CrossRef]
- Harvey, P.D. Sustainable Development in the Removal and Photocatalytic Reduction of Heavy Metals in Wastewaters Using Environmentally Friendly and Health Benign Porphyrin-Based Metal-Organic Frameworks. Sep. Purif. Technol. 2023, 322, 124214. [Google Scholar] [CrossRef]
- Cai, W.-R.; Zeng, H.-B.; Xue, H.-G.; Marks, R.S.; Cosnier, S.; Zhang, X.-J.; Shan, D. Enhanced Electrochemiluminescence of Porphyrin-Based Metal–Organic Frameworks Controlled via Coordination Modulation. Anal. Chem. 2020, 92, 1916–1924. [Google Scholar] [CrossRef]
- Hibbard, H.A.J.; Burnley, M.J.; Rubin, H.N.; Miera, J.A.; Reynolds, M.M. Porphyrin-Based Metal-Organic Framework and Polyvinylchloride Composites for Fluorescence Sensing of Divalent Cadmium Ions in Water. Inorg. Chem. Commun. 2020, 115, 107861. [Google Scholar] [CrossRef]
- Shu, Y.; Chen, Y.; Han, Q.; Liu, X.; Liu, B.; Wang, Z. Selective and Light-Enhanced Au(III) Recovery by a Porphyrin-Based Metal–Organic Framework: Performance and Underlying Mechanisms. ACS EST Eng. 2023, 3, 1042–1052. [Google Scholar] [CrossRef]
- Ren, H.; Zhu, S.; Gao, H.; Song, S.; Sun, C.; Hu, Y.; Ding, J. Highly Selective Adsorption of Au(III) and Au(I) by a Porphyrin Metal Organic Framework Material. Colloids Surf. Physicochem. Eng. Asp. 2025, 705, 135549. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, R.; Guo, Y.; Ai, Y.; Jiang, T.; Wu, G.; Wang, Y.; Wang, Y.; Ju, N.; Niu, S.; et al. Sustainable Antimony Management via Porphyrin Ligand-Based MOFs: From Enrichment of Antimony from Water to the Utilization in SbO2–Based Aqueous Batteries. J. Environ. Chem. Eng. 2023, 11, 111148. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Xie, N.; Guo, R.; Wang, Y.; Sun, Z.; Li, H.; Jia, H.; Niu, D.; Sun, H. Investigation of Antimony Adsorption on a Zirconium-Porphyrin-Based Metal–Organic Framework. Dalton Trans. 2021, 50, 13932–13942. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Hayat, T.; Alsaedi, A.; Chen, C. Screening of Zirconium-Based Metal–Organic Frameworks for Efficient Simultaneous Removal of Antimonite (Sb(III)) and Antimonate (Sb(V)) from Aqueous Solution. ACS Sustain. Chem. Eng. 2017, 5, 11496–11503. [Google Scholar] [CrossRef]
- Behbahani, A.; Eghbali, H.; Ardjmand, M.; Noufal, M.M.M.; Williamson, H.C.; Sayar, O. A Novel Bio-Compatible Sorbent Based on Carbon Nanostructure Modified by Porphyrin for Heavy Metal Separation from Industrial Wastewaters. J. Environ. Chem. Eng. 2016, 4, 398–404. [Google Scholar] [CrossRef]
- Manouchehri, M.; Seidi, S.; Rouhollahi, A.; Shanehsaz, M. Porphyrin-Functionalized Graphene Oxide Sheets: An Efficient Nanomaterial for Micro Solid Phase Extraction of Non-Steroidal Anti-Inflammatory Drugs from Urine Samples. J. Chromatogr. A 2019, 1607, 460387. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Neves, M.G.P.M.S.; Trindade, T. Functionalization of Graphene Oxide with Porphyrins: Synthetic Routes and Biological Applications. ChemPlusChem 2020, 85, 1857–1880. [Google Scholar] [CrossRef]
- Lindner, A.; Lesniewicz, A.; Kolman, A.; Larowska-Zarych, D.; Marciniak, B.; Lewandowska-Andralojc, A. When Porphyrins Meet 2D Materials: Spectroscopic and Photocatalytic Properties. J. Mater. Chem. C 2024, 12, 9012–9067. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Y.; Dong, W.; Wu, S.; Xing, G.; Duan, Q. Porous Carbon Nanofiber/Porphyrin-Based Metal Organic Frameworks/TiO2 Nanoparticles Ternary Materials for Selective Detection and Photoreduction of Cr(VI). J. Environ. Chem. Eng. 2024, 12, 113238. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Jiang, W.; Zhang, C.-R.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. Covalent Organic Framework Modified Carbon Nanotubes for Removal of Uranium (VI) from Mining Wastewater. Chem. Eng. J. 2022, 450, 138062. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Liu, X.; Liu, J.; Shi, Z.; Zang, Y.; Aoki, T. An Amidoxime Porphyrin-Based Porous Hyper-Cross-Linked Polymer for Efficient Uranium Extraction from Aqueous Solution. ACS Appl. Polym. Mater. 2024, 6, 15332–15344. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Fang, Z.; Pei, J.; Yin, K.; Fu, K.; Luo, J. Selective Gold Extraction from E-Waste Leachate via Sulfur-Redox Mechanisms Using Sulfhydryl-Functionalized MOFs. Water Res. 2025, 275, 123170. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm Models for Adsorption of Heavy Metals from Water—A Review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wang, Y.; Wei, Z.; Yang, W.; Yu, Y.; Sun, Y. Amino-Assisted AHMT Anchored on Graphene Oxide as High Performance Adsorbent for Efficient Removal of Cr(VI) and Hg(II) from Aqueous Solutions under Wide pH Range. J. Hazard. Mater. 2021, 416, 125825. [Google Scholar] [CrossRef] [PubMed]
- Mohammadmoradi, P.; Taheri, S.; Bryant, S.L.; Kantzas, A. Solvent Diffusion and Dispersion in Partially Saturated Porous Media: An Experimental and Numerical Pore-Level Study. Chem. Eng. Sci. 2018, 191, 300–317. [Google Scholar] [CrossRef]
- Shen, Y.; Ryde, U. The Structure of Sitting-atop Complexes of Metalloporphyrins Studied by Theoretical Methods. J. Inorg. Biochem. 2004, 98, 878–895. [Google Scholar] [CrossRef]
- Dar, U.A.; Shahnawaz, M.; Taneja, P.; Dar, M.A. Recent Advances in Main Group Coordination Driven Porphyrins: A Comprehensive Review. ChemistrySelect 2024, 9, e202304817. [Google Scholar] [CrossRef]
- Gode, F.; Pehlivan, E. A Comparative Study of Two Chelating Ion-Exchange Resins for the Removal of Chromium(III) from Aqueous Solution. J. Hazard. Mater. 2003, 100, 231–243. [Google Scholar] [CrossRef]
- Malla, M. Evaluation of Sorption and Desorption Characteristics of Cadmium, Lead and Zinc on Amberlite IRC-718 Iminodiacetate Chelating Ion Exchanger. Talanta 2002, 57, 277–287. [Google Scholar] [CrossRef]
- Chang, Z.; Li, F.; Qi, X.; Jiang, B.; Kou, J.; Sun, C. Selective and Efficient Adsorption of Au (III) in Aqueous Solution by Zr-Based Metal-Organic Frameworks (MOFs): An Unconventional Way for Gold Recycling. J. Hazard. Mater. 2020, 391, 122175. [Google Scholar] [CrossRef]
- Sun, D.T.; Gasilova, N.; Yang, S.; Oveisi, E.; Queen, W.L. Rapid, Selective Extraction of Trace Amounts of Gold from Complex Water Mixtures with a Metal–Organic Framework (MOF)/Polymer Composite. J. Am. Chem. Soc. 2018, 140, 16697–16703. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, P.; Chatterjee, S.; Rudra, P.; De, S. Chelating Polyacrylonitrile Beads for Removal of Lead and Cadmium from Wastewater. Sep. Purif. Technol. 2018, 193, 202–213. [Google Scholar] [CrossRef]
- Dinari, M.; Hatami, M. Novel N-Riched Crystalline Covalent Organic Framework as a Highly Porous Adsorbent for Effective Cadmium Removal. J. Environ. Chem. Eng. 2019, 7, 102907. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Garcia, Y. New Hybrid Material Based on a Silica-Immobilised Conjugated β-Ketoenol-Bipyridine Receptor and Its Excellent Cu(II) Adsorption Capacity. Anal. Methods 2016, 8, 6923–6931. [Google Scholar] [CrossRef]
- El-Massaoudi, M.; Radi, S.; Lamsayah, M.; Tighadouini, S.; Séraphin, K.K.; Kouassi, L.K.; Garcia, Y. Ultra-Fast and Highly Efficient Hybrid Material Removes Cu(II) from Wastewater: Kinetic Study and Mechanism. J. Clean. Prod. 2021, 284, 124757. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.; Zhao, L.; Liu, J.; Liu, X.; Xue, J.; Tang, A. Removal Performance and Mechanism of Poly(N1,N1,N3,N3 -Tetraallylpropane-1,3-Diaminium Chloride) toward Cr(VI). Environ. Technol. 2020, 41, 2450–2463. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V.; Olariu, R.I. Kinetics, Equilibrium Modeling, and Thermodynamics on Removal of Cr(VI) Ions from Aqueous Solution Using Novel Composites with Strong Base Anion Exchanger Microspheres Embedded into Chitosan/Poly(Vinyl Amine) Cryogels. Chem. Eng. J. 2017, 330, 675–691. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Facile Synthesis of Cross Linked-Chitosan–Grafted-Polyaniline Composite and Its Cr(VI) Uptake Studies. Int. J. Biol. Macromol. 2014, 67, 210–219. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Perman, J.; Earl, L.D.; Abney, C.W.; Cheng, Y.; Wei, H.; Nguyen, N.; Wojtas, L.; Ma, S. Postsynthetically Modified Covalent Organic Frameworks for Efficient and Effective Mercury Removal. J. Am. Chem. Soc. 2017, 139, 2786–2793. [Google Scholar] [CrossRef]
- Shetty, D.; Boutros, S.; Eskhan, A.; De Lena, A.M.; Skorjanc, T.; Asfari, Z.; Traboulsi, H.; Mazher, J.; Raya, J.; Banat, F.; et al. Thioether-Crown-Rich Calix [4]Arene Porous Polymer for Highly Efficient Removal of Mercury from Water. ACS Appl. Mater. Interfaces 2019, 11, 12898–12903. [Google Scholar] [CrossRef]
- Zou, J.-P.; Liu, H.-L.; Luo, J.; Xing, Q.-J.; Du, H.-M.; Jiang, X.-H.; Luo, X.-B.; Luo, S.-L.; Suib, S.L. Three-Dimensional Reduced Graphene Oxide Coupled with Mn3 O4 for Highly Efficient Removal of Sb(III) and Sb(V) from Water. ACS Appl. Mater. Interfaces 2016, 8, 18140–18149. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Song, J.; Chan, T.; Jing, C. Insights into Antimony Adsorption on {001} TiO2: XAFS and DFT Study. Environ. Sci. Technol. 2017, 51, 6335–6341. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrzynska, K.; Kilian, K. Porphyrin-Based Sorbents for the Enrichment and Removal of Metal Ions. Molecules 2025, 30, 2238. https://doi.org/10.3390/molecules30102238
Pyrzynska K, Kilian K. Porphyrin-Based Sorbents for the Enrichment and Removal of Metal Ions. Molecules. 2025; 30(10):2238. https://doi.org/10.3390/molecules30102238
Chicago/Turabian StylePyrzynska, Krystyna, and Krzysztof Kilian. 2025. "Porphyrin-Based Sorbents for the Enrichment and Removal of Metal Ions" Molecules 30, no. 10: 2238. https://doi.org/10.3390/molecules30102238
APA StylePyrzynska, K., & Kilian, K. (2025). Porphyrin-Based Sorbents for the Enrichment and Removal of Metal Ions. Molecules, 30(10), 2238. https://doi.org/10.3390/molecules30102238









