Microwave Assisted Synthesis of Antioxidant Dihydro-Pyrazole Hybrids as Possible Lipoxygenase Inhibitors
Abstract
1. Introduction
2. Results
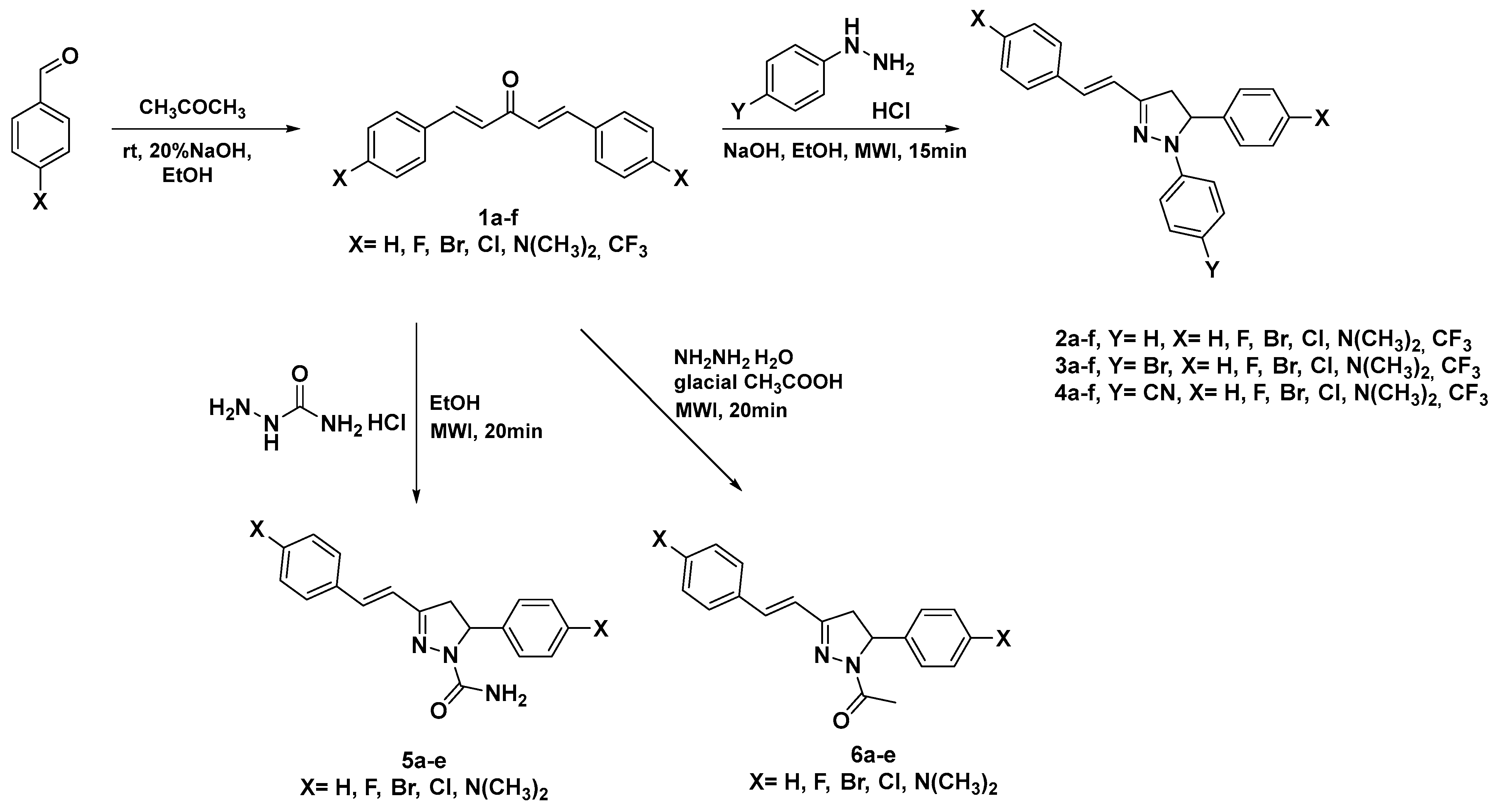
2.1. Chemistry
2.2. Physicochemical Studies
2.2.1. Determination of Lipophilicity
2.2.2. Theoretical Calculation of Physicochemical Properties
2.3. Biological Evaluation
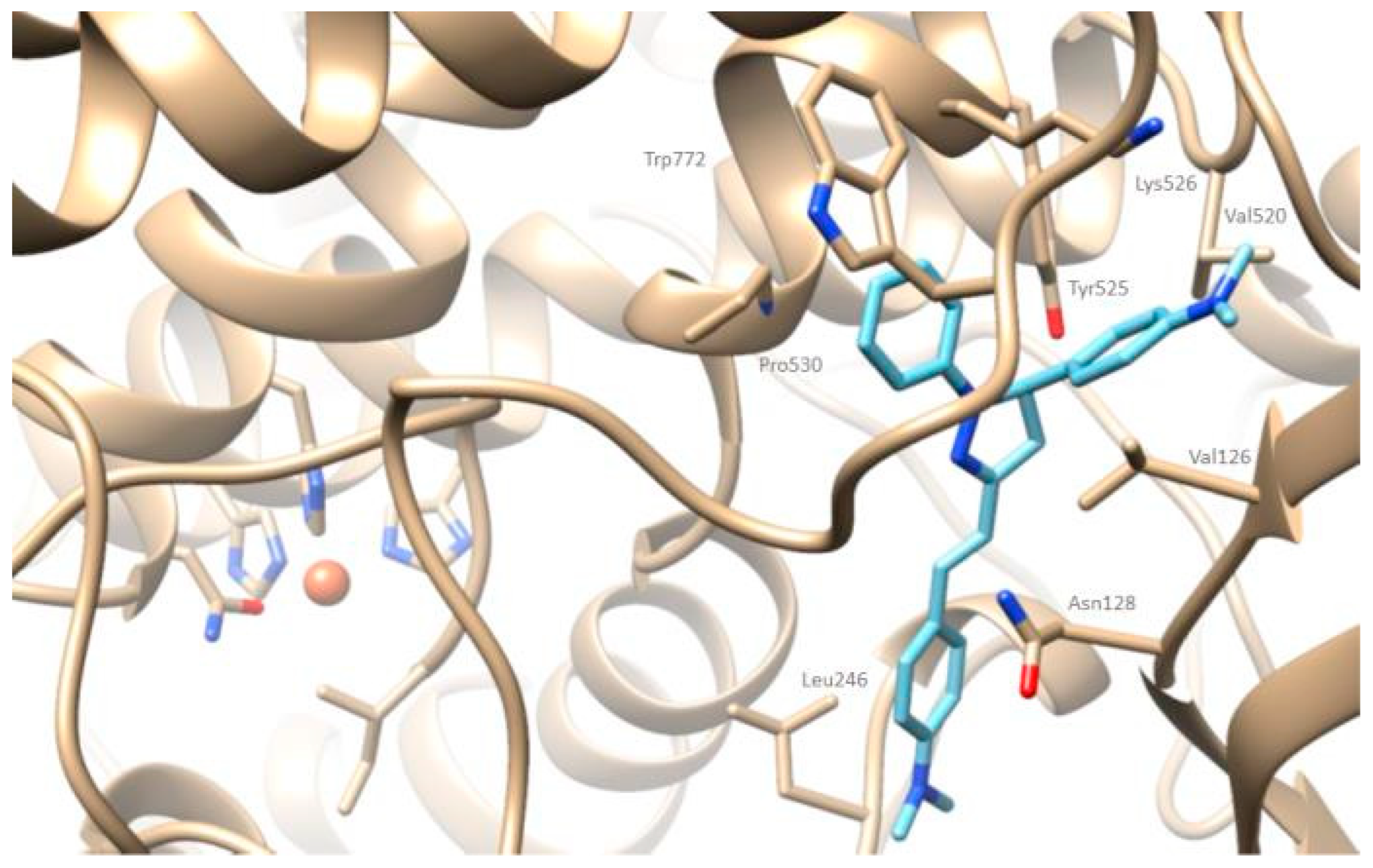
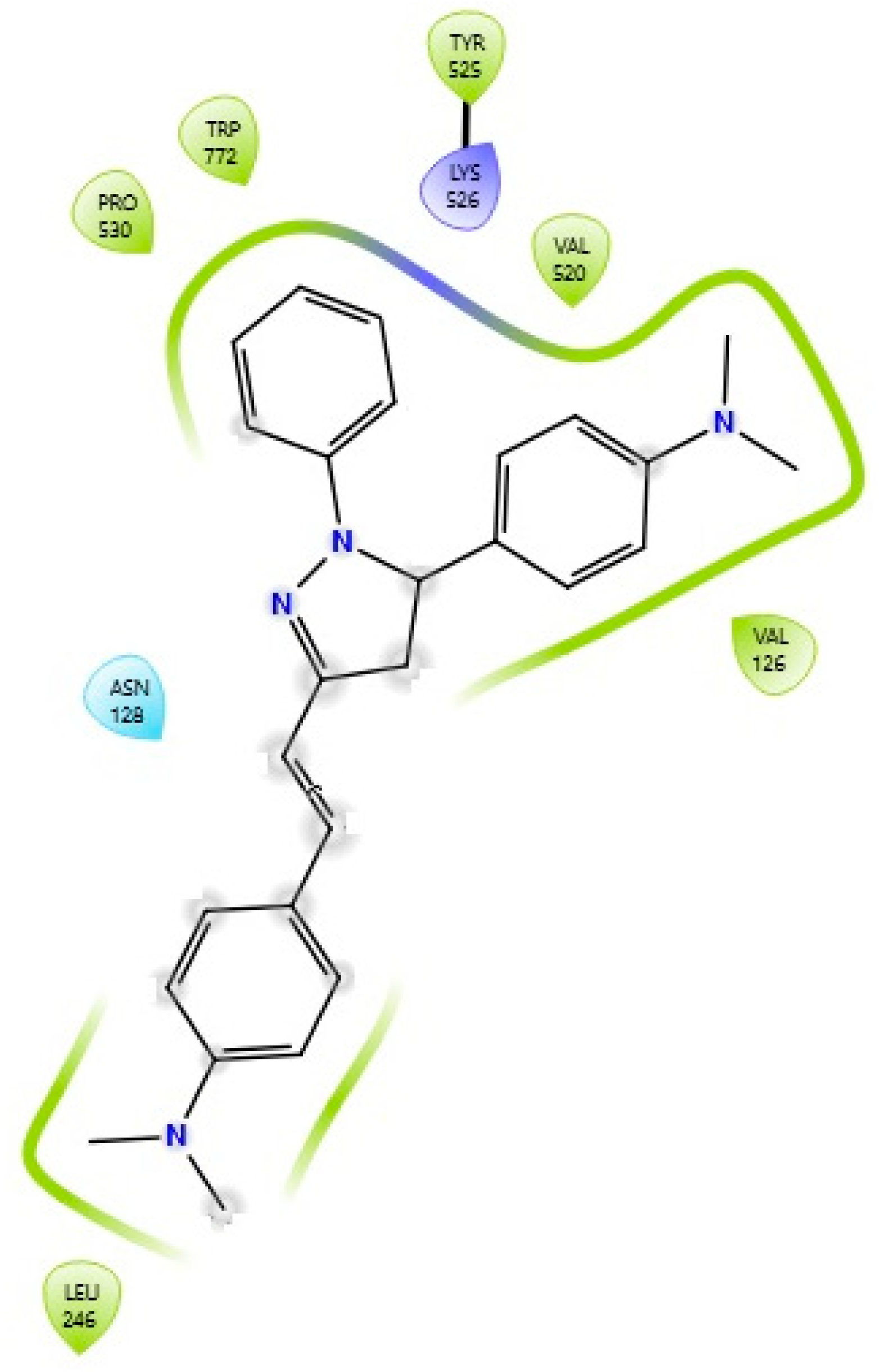
2.4. Docking Simulation Soybean Lipoxygenase Studies
3. Experimental Section
3.1. Materials and Instruments
3.2. Chemistry General Procedure
3.2.1. Synthesis of Dibenzalacetones (1a–f)
3.2.2. Synthesis of Dihydro-Pyrazoles (2a–f, 3a–f, 4a–f)
3.2.3. Synthesis of Pyrazole-Carboxamides (5a–e)
3.2.4. Synthesis of Dihydro-Pyrazol-Ethanones (6a–e)
3.3. Experimental of Physicochemical Studies
Experimental Determination of Lipophilicity by Reverse Phase Thin Layer Chromatography (RP-TLC)—Determination of RM Values
3.4. Biological In Vitro Tests
3.4.1. Determination of the Reducing Activity of the Stable Radical DPPH
3.4.2. Anti-Lipid Peroxidation (AAPH) Activity
3.4.3. Inhibition of Soybean Lipoxygenase
3.5. Molecular Docking and Pharmaco-Similarity Studies
3.5.1. Molecular Docking on Soybean Lipoxygenase
3.5.2. Study of Drug-Likeness of Molecules with the Molinspiration Tool
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratik, K.; Arun, K.; Neha, S.; Bhumika, Y.; Anshuman, S.; Kumar, G.S. Synthesis, Characterization of Ethyl 5-(Substituted)-1H-Pyrazole-3-Carboxylate Derivative as Potent Anti-Inflammatory Agents. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2018, 17, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Gias, Z.T.; Afsana, F.; Debnath, P.; Alam, M.S.; Ena, T.N.; Hossain, M.H.; Jain, P.; Reza, H.M. A Mechanistic Approach to HPLC Analysis, Antinociceptive, Anti-Inflammatory and Postoperative Analgesic Activities of Panch Phoron in Mice. BMC Complement Med. Ther. 2020, 20, 102. [Google Scholar] [CrossRef]
- Benaroyo, L. How do we define inflammation? Praxis 1994, 83, 1343–1347. [Google Scholar]
- McCord, J.M. The Evolution of Free Radicals and Oxidative Stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Singh, R.; Devi, S.; Gollen, R. Role of Free Radical in Atherosclerosis, Diabetes and Dyslipidaemia: Larger-than-Life. Diabetes/Metab. Res. Rev. 2015, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-Y.; Jung, Y.-J.; Surh, Y.-J.; Lee, S.-S.; Park, K.-K. Antioxidative and Antitumor Promoting Effects of [6]-Paradol and Its Homologs. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2001, 496, 199–206. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Girão da Cruz, M.T.; Cordeiro, M.N.D.S.; Milhazes, N.; Borges, F.; Marques, M.P.M. Phenolic Acid Derivatives with Potential Anticancer Properties—A Structure–Activity Relationship Study. Part 1: Methyl, Propyl and Octyl Esters of Caffeic and Gallic Acids. Bioorganic Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P. New Insights on the Anticancer Properties of Dietary Polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef]
- Silva, F.A.M.; Borges, F.; Ferreira, M.A. Effects of Phenolic Propyl Esters on the Oxidative Stability of Refined Sunflower Oil. J. Agric. Food Chem. 2001, 49, 3936–3941. [Google Scholar] [CrossRef]
- Gomes, C.A.; Girão da Cruz, T.; Andrade, J.L.; Milhazes, N.; Borges, F.; Marques, M.P. Anticancer Activity of Phenolic Acids of Natural or Synthetic Origin: A Structure—Activity Study. J. Med. Chem. 2003, 46, 5395–5401. [Google Scholar] [CrossRef]
- Caillon, A.; Schiffrin, E.L. Role of Inflammation and Immunity in Hypertension: Recent Epidemiological, Laboratory, and Clinical Evidence. Curr. Hypertens Rep. 2016, 18, 21. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Todd, I.; Spickett, G.P.; Fairclough, L. Immunology; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-1-118-45166-3. [Google Scholar]
- Jacob P, J.; Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer Anti-Inflammatory Therapy through Dual COX-2/5-LOX Inhibitors: A Structure-Based Approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar] [CrossRef]
- Baysal, T.; Demirdöven, A. Lipoxygenase in Fruits and Vegetables: A Review. Enzym. Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Tuncer, S.; Banerjee, S. Eicosanoid Pathway in Colorectal Cancer: Recent Updates. World J. Gastroenterol. 2015, 21, 11748–11766. [Google Scholar] [CrossRef]
- Qu, T.; Uz, T.; Manev, H. Inflammatory 5-LOX mRNA and Protein Are Increased in Brain of Aging Rats. Neurobiol. Aging 2000, 21, 647–652. [Google Scholar] [CrossRef]
- Kulkarni, P.; Totre, G. Synthesis of Dibenzylidene Acetone via Aldol Condensation of Diacetone Alcohol with Substituted Benzaldehydes. Orbital Electron. J. Chem. 2019, 11, 292–296. [Google Scholar] [CrossRef]
- Ballesteros, J.F.; Sanz, M.J.; Ubeda, A.; Miranda, M.A.; Iborra, S.; Paya, M.; Alcaraz, M.J. Synthesis and Pharmacological Evaluation of 2′-Hydroxychalcones and Flavones as Inhibitors of Inflammatory Mediators Generation. J. Med. Chem. 1995, 38, 2794–2797. [Google Scholar] [CrossRef]
- Go, M.L.; Wu, X.; Liu, X.L. Chalcones: An Update on Cytotoxic and Chemoprotective Properties. Curr. Med. Chem. 2005, 12, 483–499. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, V.; Prasad, A.K.; Raj, H.G.; Bracke, M.E.; Olsen, C.E.; Jain, S.C.; Parmar, V.S. Synthetic and Biological Activity Evaluation Studies on Novel 1,3-Diarylpropenones. Bioorganic Med. Chem. 2001, 9, 337–345. [Google Scholar] [CrossRef]
- Liu, M.; Wilairat, P.; Croft, S.L.; Tan, A.L.-C.; Go, M.-L. Structure–Activity Relationships of Antileishmanial and Antimalarial Chalcones. Bioorganic Med. Chem. 2003, 11, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.M.; Babu, S.K.G.; Mukesh, D. QSAR Studies on Chalcones and Flavonoids as Anti-Tuberculosis Agents Using Genetic Function Approximation (GFA) Method. Chem. Pharm. Bull. 2007, 55, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Viana, G.S.B.; Bandeira, M.A.M.; Matos, F.J.A. Analgesic and Antiinflammatory Effects of Chalcones Isolated from Myracrodruon Urundeuva Allemão. Phytomedicine 2003, 10, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, C.; Kawasaki, N.; Miyataka, H.; Jayachandran, E.; Kim, I.H.; Kirk, K.L.; Taguchi, T.; Takeuchi, Y.; Hori, H.; Satoh, T. Synthesis and Biological Activities of Fluorinated Chalcone Derivatives. Bioorganic Med. Chem. 2002, 10, 699–706. [Google Scholar] [CrossRef]
- Perrin, D.D. Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-1-4832-8461-3. [Google Scholar]
- Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef]
- Mykhailiuk, P.K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2021, 121, 1670–1715. [Google Scholar] [CrossRef]
- Neto, J.S.S.; Zeni, G. Alkynes and Nitrogen Compounds: Useful Substrates for the Synthesis of Pyrazoles. Chem. A Eur. J. 2020, 26, 8175–8189. [Google Scholar] [CrossRef]
- Janin, Y.L. Preparation and Chemistry of 3/5-Halogenopyrazoles. Chem. Rev. 2012, 112, 3924–3958. [Google Scholar] [CrossRef]
- Nájera, C.; Sydnes, L.K.; Yus, M. Conjugated Ynones in Organic Synthesis. Chem. Rev. 2019, 119, 11110–11244. [Google Scholar] [CrossRef]
- Keiko, N.A.; Vchislo, N.V. Synthesis of Diheteroatomic Five-Membered Heterocyclic Compounds from α,β-Unsaturated Aldehydes. Asian J. Org. Chem. 2016, 5, 1169–1197. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, D.; Zhong, Y.; Guo, X.-A.; Guo, J.; Gou, J.; Gao, Z.; Yu, B. Fe(III)-Catalyzed Aerobic Intramolecular N–N Coupling of Aliphatic Azides with Amines. Org. Lett. 2019, 21, 4960–4965. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.M.; Pearson, C.M.; Kennedy, J.M. A Clinical Trial of Indomethacin in Rheumatoid Arthritis. Clin. Pharmacol. Ther. 1965, 6, 25–30. [Google Scholar] [CrossRef]
- Machado, V.; Cenci, A.R.; Teixeira, K.F.; Sens, L.; Tizziani, T.; Nunes, R.J.; Ferreira, L.L.G.; Yunes, R.A.; Sandjo, L.P.; Andricopulo, A.D.; et al. Pyrazolines as Potential Anti-Alzheimer’s Agents: DFT, Molecular Docking, Enzyme Inhibition and Pharmacokinetic Studies. RSC Med. Chem. 2022, 13, 1644–1656. [Google Scholar] [CrossRef]
- Ananikov, V.P.; Khemchyan, L.L.; Ivanova, Y.V.; Bukhtiyarov, V.I.; Sorokin, A.M.; Prosvirin, I.P.; Vatsadze, S.Z.; Medved’ko, A.V.; Nuriev, V.N.; Dilman, A.D.; et al. Development of New Methods in Modern Selective Organic Synthesis: Preparation of Functionalized Molecules with Atomic Precision. Russ. Chem. Rev. 2014, 83, 885–985. [Google Scholar] [CrossRef]
- Gulati, H.K.; Bhagat, K.; Singh, A.; Kumar, N.; Kaur, A.; Sharma, A.; Heer, S.; Singh, H.; Singh, J.V.; Bedi, P.M.S. Design, Synthesis and Biological Evaluation of Novel Indolinedione–Coumarin Hybrids as Xanthine Oxidase Inhibitors. Med. Chem. Res. 2020, 29, 1632–1642. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Arora, S.; Attri, S.; Kaur, P.; Kaur Gulati, H.; Bhagat, K.; Kumar, N.; Singh, H.; Vir Singh, J.; et al. New Coumarin-Benzotriazole Based Hybrid Molecules as Inhibitors of Acetylcholinesterase and Amyloid Aggregation. Bioorganic Med. Chem. Lett. 2020, 30, 127477. [Google Scholar] [CrossRef]
- Bhagat, K.; Singh, J.V.; Sharma, A.; Kaur, A.; Kumar, N.; Gulati, H.K.; Singh, A.; Singh, H.; Bedi, P.M.S. Novel Series of Triazole Containing Coumarin and Isatin Based Hybrid Molecules as Acetylcholinesterase Inhibitors. J. Mol. Struct. 2021, 1245, 131085. [Google Scholar] [CrossRef]
- Mantzanidou, M.; Pontiki, E.; Hadjipavlou-Litina, D. Pyrazoles and Pyrazolines as Anti-Inflammatory Agents. Molecules 2021, 26, 3439. [Google Scholar] [CrossRef]
- Nurkenov, O.A.; Ibraev, M.K.; Schepetkin, I.A.; Khlebnikov, A.I.; Seilkhanov, T.M.; Arinova, A.E.; Isabaeva, M.B. Synthesis, Structure, and Anti-Inflammatory Activity of Functionally Substituted Chalcones and Their Derivatives. Russ. J. Gen. Chem. 2019, 89, 1360–1367. [Google Scholar] [CrossRef]
- Eid, N.M.; George, R.F. Facile Synthesis of Some Pyrazoline-Based Compounds with Promising Anti-Inflammatory Activity. Future Med. Chem. 2018, 10, 183–199. [Google Scholar] [CrossRef]
- Nauduri, D.; Reddy, G.B.S. Antibacetrials and Antimycotics: Part 1: Synthesis and Activity of 2-Pyrazoline Derivatives. Chem. Pharm. Bull. 1998, 46, 1254–1260. [Google Scholar] [CrossRef]
- Afonso, C.A.M.; Candeias, N.R.; Simão, D.P.; Trindade, A.F.; Coelho, J.A.S.; Tan, B.; Franzén, R. Comprehensive Organic Chemistry Experiments for the Laboratory Classroom; Royal Society of Chemistry: London, UK, 2016; ISBN 978-1-84973-963-4. [Google Scholar]
- Carapina da Silva, C.; Pacheco, B.S.; das Neves, R.N.; Dié Alves, M.S.; Sena-Lopes, Â.; Moura, S.; Borsuk, S.; de Pereira, C.M.P. Antiparasitic Activity of Synthetic Curcumin Monocarbonyl Analogues against Trichomonas Vaginalis. Biomed. Pharmacother. 2019, 111, 367–377. [Google Scholar] [CrossRef]
- da Silva, A.A.; Maia, P.I.d.S.; Lopes, C.D.; de Albuquerque, S.; Valle, M.S. Synthesis, Characterization and Antichagasic Evaluation of Thiosemicarbazones Prepared from Chalcones and Dibenzalacetones. J. Mol. Struct. 2021, 1232, 130014. [Google Scholar] [CrossRef]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ghias, M.; Ullah, A.; Rahman, S.U.; Kamal, Z.; Khan, F.A.; Khan, N.M.; Muhammad, J.; et al. Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies. Molecules 2021, 26, 7168. [Google Scholar] [CrossRef]
- Daneshfar, Z.; Rostami, A. Cellulose Sulfonic Acid as a Green, Efficient, and Reusable Catalyst for Nazarov Cyclization of Unactivated Dienones and Pyrazoline Synthesis. RSC Adv. 2015, 5, 104695–104707. [Google Scholar] [CrossRef]
- Li, J.-T.; Zhai, X.-L.; Meng, X.-T. Synthesis of 1,5-Diaryl-3-Arylethenyl-2-Pyrazolines Under Ultrasound Irradiation. Asian J. Chem. 2010, 22, 589–592. [Google Scholar]
- George, R.F.; Kandeel, M.; El-Ansary, D.Y.; El Kerdawy, A.M. Some 1,3,5-Trisubstituted Pyrazoline Derivatives Targeting Breast Cancer: Design, Synthesis, Cytotoxic Activity, EGFR Inhibition and Molecular Docking. Bioorg. Chem. 2020, 99, 103780. [Google Scholar] [CrossRef]
- Vijayabaskar, V.; Perumal, S.; Selvaraj, S.; Lycka, A.; Murugan, R.; Balasubramanian, M. Synthesis and Multinuclear NMR Study of (E)-s-Trans -1-Acetyl-5-Aryl-3-Styryl-2-Pyrazolines. Magn. Reson. Chem. 1999, 37, 133–139. [Google Scholar] [CrossRef]
- Pathak, V.N.; Joshi, R.; Sharma, J.; Gupta, N.; Rao, V.M. Mild and Ecofriendly Tandem Synthesis, and Spectral and Antimicrobial Studies of N1-Acetyl-5-Aryl-3-(Substituted Styryl)Pyrazolines. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 1854–1865. [Google Scholar] [CrossRef]
- Sehmi, A.; Ouici, H.B.; Guendouzi, A.; Ferhat, M.; Benali, O.; Boudjellal, F. Corrosion Inhibition of Mild Steel by Newly Synthesized Pyrazole Carboxamide Derivatives in HCl Acid Medium: Experimental and Theoretical Studies. J. Electrochem. Soc. 2020, 167, 155508. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Available online: https://pubs.acs.org/doi/pdf/10.1021/cr00002a004 (accessed on 15 February 2025).
- Hugo, K. QSAR: Hansch Analysis and Related Approaches; Methods and Principles in Medicinal Chemistry; Wiley: Hoboken, NJ, USA, 1993. [Google Scholar] [CrossRef]
- Claisen, L.; Claparède, A. Ueber Verbindungen Des Acetons Und Mesityloxyds Mit Benzaldehyd Und Über Die Constitution Des Acetophorons. Berichte Der Dtsch. Chem. Ges. 1881, 14, 349–353. [Google Scholar] [CrossRef]
- Kurland, B.F.; Peterson, L.M.; Lee, J.H.; Linden, H.M.; Schubert, E.K.; Dunnwald, L.K.; Link, J.M.; Krohn, K.A.; Mankoff, D.A. Between-Patient and Within-Patient (Site-to-Site) Variability in Estrogen Receptor Binding, Measured In Vivo by 18 F-Fluoroestradiol PET. J. Nucl. Med. 2011, 52, 1541–1549. [Google Scholar] [CrossRef]
- Peyrat-Maillard, M.N.; Cuvelier, M.E.; Berset, C. Antioxidant Activity of Phenolic Compounds in 2,2′-Azobis (2-Amidinopropane) Dihydrochloride (AAPH)-Induced Oxidation: Synergistic and Antagonistic Effects. J. Am. Oil Chem. Soc. 2003, 80, 1007. [Google Scholar] [CrossRef]
- Taraporewala, I.B.; Kauffman, J.M. Synthesis and Structure–Activity Relationships of Anti-Inflammatory 9,lO-Dihydro-9-Oxo-2-Acridine-Alkanoic Acids and 4-(2-Carboxyphenyl)Aminobenzenealkanoic Acids. J. Pharm. Sci. 1990, 79, 173–178. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Lipoxygenase Inhibitors: A Comparative QSAR Study Review and Evaluation of New QSARs. Med. Res. Rev. 2008, 28, 39–117. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Tzani, A.; Polyzos, N.-I.; Karadendrou, M.-A.; Kritsi, E.; Pontiki, E.; Liargkova, T.; Hadjipavlou-Litina, D.; Zoumpoulakis, P.; Detsi, A. Exploring the 2′-Hydroxy-Chalcone Framework for the Development of Dual Antioxidant and Soybean Lipoxygenase Inhibitory Agents. Molecules 2021, 26, 2777. [Google Scholar] [CrossRef]
- El Khatabi, K.; El-Mernissi, R.; Aanouz, I.; Ajana, M.A.; Lakhlifi, T.; Khan, A.; Wei, D.-Q.; Bouachrine, M. Identification of Novel Acetylcholinesterase Inhibitors through 3D-QSAR, Molecular Docking, and Molecular Dynamics Simulation Targeting Alzheimer’s Disease. J. Mol. Model. 2021, 27, 302. [Google Scholar] [CrossRef]
- Mavridis, E.; Bermperoglou, E.; Pontiki, E.; Hadjipavlou-Litina, D. 5-(4H)-Oxazolones and Their Benzamides as Potential Bioactive Small Molecules. Molecules 2020, 25, 3173. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Diassakou, A.; Kavetsou, E.; Kritsi, E.; Zoumpoulakis, P.; Pontiki, E.; Hadjipavlou-Litina, D.; Detsi, A. Novel Quinolinone-Pyrazoline Hybrids: Synthesis and Evaluation of Antioxidant and Lipoxygenase Inhibitory Activity. Mol. Divers. 2021, 25, 723–740. [Google Scholar] [CrossRef]
- Schrödinger Release Notes—Release 2023-4. Available online: https://www.schrodinger.com/life-science/download/release-notes/release-2023-4/ (accessed on 20 February 2025).
- Venkateswarlu, V.; Kour, J.; Kumar, K.A.A.; Verma, P.K.; Reddy, G.L.; Hussain, Y.; Tabassum, A.; Balgotra, S.; Gupta, S.; Hudwekar, A.D.; et al. Direct N-Heterocyclization of Hydrazines to Access Styrylated Pyrazoles: Synthesis of 1,3,5-Trisubstituted Pyrazoles and Dihydropyrazoles. RSC Adv. 2018, 8, 26523–26527. [Google Scholar] [CrossRef]
- Rekker, R.F.; Roelof, F. The Hydrophobic Fragmental Constant, Its Derivation and Application: A Means of Characterizing Membrane Systems; Elsevier Scientific Pub. Co.: Amsterdam, The Netherlands; New York, NY, USA, 1977. [Google Scholar]
- Myriagkou, M.; Papakonstantinou, E.; Deligiannidou, G.E.; Patsilinakos, A.; Kontogiorgis, C.; Pontiki, E. Novel Pyrimidine Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Molecular Modeling Studies. Molecules 2023, 28, 3913. [Google Scholar] [CrossRef]
- Kouzi, O.; Pontiki, E.; Hadjipavlou-Litina, D. 2-Arylidene-1-Indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities. Molecules 2019, 24, 4411. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, e360438. [Google Scholar] [CrossRef]
- Peperidou, A.; Kapoukranidou, D.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Multitarget Molecular Hybrids of Cinnamic Acids. Molecules 2014, 19, 20197–20226. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Antioxidant and Anti-Inflammatory Activity of Aryl-Acetic and Hydroxamic Acids as Novel Lipoxygenase Inhibitors. Med. Chem. 2006, 2, 251–264. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Fiser, A.; Šali, A. Modeller: Generation and Refinement of Homology-Based Protein Structure Models. Methods Enzymol. 2003, 374, 461–491. [Google Scholar]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N.; Giese, T.J.; Götz, A.W.; Gohlke, H.; et al. AmberTools. J. Chem. Inf. Model. 2023, 63, 6183–6191. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved Side-Chain Torsion Potentials for the Amber ff99SB Protein Force Field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Li, P.; Roberts, B.P.; Chakravorty, D.K.; Merz, K.M., Jr. Rational Design of Particle Mesh Ewald Compatible Lennard-Jones Parameters for +2 Metal Cations in Explicit Solvent. J. Chem. Theory Comput. 2013, 9, 2733–2748. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck Molecular Force Field. I. Basis, Form, Scope, Parameterization, and Performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic Atom Type and Bond Type Perception in Molecular Mechanical Calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
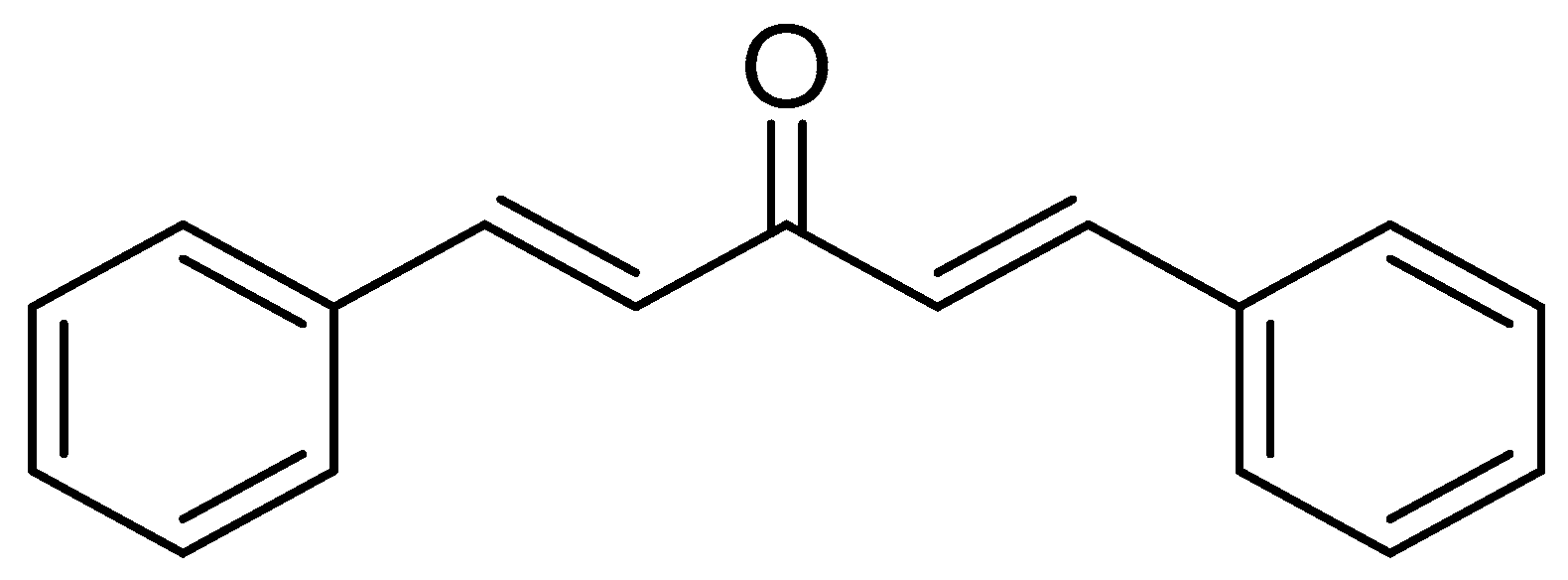
| Compd | General Structure | X | Y | RM ± SD | clog P | πx | MRx |
|---|---|---|---|---|---|---|---|
| 1a | 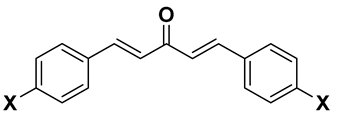 | H | - | 0.10 ± 0.003 | 4.66 | 0 | 0.103 |
| 1b | F | - | 0.19 ± 0.001 | 4.94 | 0.14 | 0.092 | |
| 1c | Br | - | 0.65 ± 0.034 | 6.38 | 0.86 | 0.888 | |
| 1d | Cl | - | 0.50 ± 0.042 | 6.08 | 0.71 | 0.603 | |
| 1e | N(CH3)2 | - | −0.14 ± 0.009 | 4.99 | 0.18 | 1.555 | |
| 1f | CF3 | - | 0.57 ± 0.031 | 6.47 | 0.88 | 0.502 | |
| 2a | 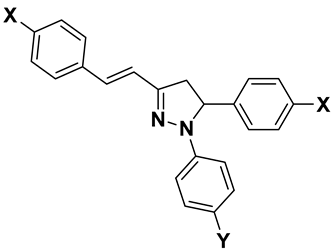 | H | H | 1.00 ± 0.084 | 6.90 | 0 | 0.103 |
| 2b | F | H | 0.78 ± 0.065 | 7.19 | 0.14 | 0.092 | |
| 2c | Br | H | 2.1 ± 0.17 | 8.63 | 0.86 | 0.888 | |
| 2d | Cl | H | 1.07 ± 0.067 | 8.33 | 0.71 | 0.603 | |
| 2e | N(CH3)2 | H | 0.95 ± 0.092 | 7.23 | 0.18 | 1.555 | |
| 2f | CF3 | H | 1.31 ± 0.031 | 8.67 | 0.88 | 0.502 | |
| 3a | H | Br | 1.11 ± 0.068 | 7.76 | 0 | 0.103 | |
| 3b | F | Br | 1.03 ± 0.057 | 8.05 | 0.14 | 0.092 | |
| 3c | Br | Br | 1.46 ± 0.049 | 9.49 | 0.86 | 0.888 | |
| 3d | Cl | Br | 1.39 ± 0.069 | 9.19 | 0.71 | 0.603 | |
| 3e | N(CH3)2 | Br | 1.25 ± 0.086 | 8.09 | 0.18 | 1.555 | |
| 3f | CF3 | Br | 1.47 ± 0.044 | 9.53 | 0.88 | 0.502 | |
| 4a | H | CN | −0.31 ± 0.023 | 6.43 | 0 | 0.103 | |
| 4b | F | CN | 0.61 ± 0.053 | 6.62 | 0.14 | 0.092 | |
| 4c | Br | CN | 0.94 ± 0.069 | 8.06 | 0.86 | 0.888 | |
| 4d | Cl | CN | 0.85 ± 0.068 | 7.76 | 0.71 | 0.603 | |
| 4e | N(CH3)2 | CN | 0.49 ± 0.029 | 6.66 | 0.18 | 1.555 | |
| 4f | CF3 | CN | 0.8 ± 0.031 | 8.10 | 0.88 | 0.502 | |
| 5a |  | H | - | 0.06 ± 0.005 | 3.24 | 0 | 0.103 |
| 5b | F | - | 0.06 ± 0.004 | 3.52 | 0.14 | 0.092 | |
| 5c | Br | - | 0.60 ± 0.055 | 4.96 | 0.86 | 0.888 | |
| 5d | Cl | - | 0.50 ± 0.043 | 4.66 | 0.71 | 0.603 | |
| 5e | N(CH3)2 | - | 0.28 ± 0.019 | 3.57 | 0.18 | 1.555 | |
| 6a | 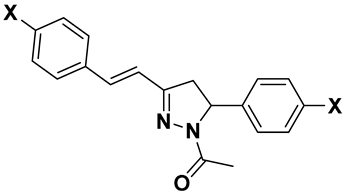 | H | - | 0.17 ± 0.014 | 4.11 | 0 | 0.103 |
| 6b | F | - | 0.24 ± 0.021 | 4.40 | 0.14 | 0.092 | |
| 6c | Br | - | 0.78 ± 0.051 | 5.84 | 0.86 | 0.888 | |
| 6d | Cl | - | 0.65 ± 0.048 | 5.54 | 0.71 | 0.603 | |
| 6e | N(CH3)2 | - | 0.47 ± 0.036 | 4.45 | 0.18 | 1.555 |
| Compd | miLogP | TPSA | Natoms | MW | nON | nOHNH | Nviolations | Nrotb | Volume |
|---|---|---|---|---|---|---|---|---|---|
| 1a | 4.18 | 17.07 | 18 | 234.30 | 1 | 0 | 0 | 4 | 229.27 |
| 1b | 4.51 | 17.07 | 20 | 270.28 | 1 | 0 | 0 | 4 | 239.13 |
| 1c | 5.80 | 17.07 | 20 | 392.09 | 1 | 0 | 1 | 4 | 265.04 |
| 1d | 5.54 | 17.07 | 20 | 303.19 | 1 | 0 | 1 | 4 | 256.34 |
| 1e | 4.39 | 23.55 | 24 | 320.44 | 3 | 0 | 0 | 6 | 321.08 |
| 1f | 5.97 | 17.07 | 26 | 370.29 | 1 | 0 | 1 | 6 | 291.86 |
| 2a | 6.02 | 15.6 | 25 | 324.43 | 2 | 0 | 1 | 4 | 313.11 |
| 2b | 6.34 | 15.6 | 27 | 360.41 | 2 | 0 | 1 | 4 | 322.97 |
| 2c | 7.63 | 15.6 | 27 | 482.22 | 2 | 0 | 1 | 4 | 348.88 |
| 2d | 7.37 | 15.6 | 27 | 393.32 | 2 | 0 | 1 | 4 | 340.18 |
| 2e | 6.22 | 22.08 | 31 | 410.56 | 4 | 0 | 1 | 6 | 404.92 |
| 2f | 7.81 | 15.6 | 33 | 460.42 | 2 | 0 | 1 | 6 | 375.50 |
| 3a | 6.83 | 15.6 | 26 | 403.32 | 2 | 0 | 1 | 4 | 331.00 |
| 3b | 7.15 | 15.6 | 28 | 439.3 | 2 | 0 | 1 | 4 | 340.86 |
| 3c | 8.37 | 15.6 | 28 | 561.12 | 2 | 0 | 2 | 4 | 366.77 |
| 3d | 8.17 | 15.6 | 28 | 472.21 | 2 | 0 | 1 | 4 | 358.07 |
| 3e | 7.03 | 22.08 | 32 | 489.46 | 4 | 0 | 1 | 6 | 422.81 |
| 3f | 8.48 | 15.6 | 34 | 539.32 | 2 | 0 | 2 | 6 | 393.59 |
| 4a | 5.77 | 39.39 | 27 | 349.44 | 3 | 0 | 1 | 4 | 329.97 |
| 4b | 6.10 | 39.39 | 29 | 385.42 | 3 | 0 | 1 | 4 | 339.83 |
| 4c | 7.39 | 39.39 | 29 | 507.23 | 3 | 0 | 2 | 4 | 365.74 |
| 4d | 7.13 | 39.39 | 29 | 418.33 | 3 | 0 | 1 | 4 | 357.04 |
| 4e | 5.97 | 45.87 | 33 | 435.57 | 5 | 0 | 1 | 6 | 421.78 |
| 4f | 7.56 | 39.39 | 35 | 485.43 | 3 | 0 | 1 | 6 | 392.56 |
| 5a | 3.59 | 58.70 | 22 | 291.35 | 4 | 2 | 0 | 3 | 271.97 |
| 5b | 4.95 | 58.70 | 24 | 360.24 | 4 | 2 | 0 | 3 | 299.05 |
| 5c | 5.21 | 58.70 | 24 | 449.15 | 4 | 2 | 1 | 3 | 307.74 |
| 5d | 3.92 | 58.70 | 24 | 327.33 | 4 | 2 | 0 | 3 | 281.84 |
| 5e | 3.80 | 65.17 | 28 | 377.49 | 6 | 2 | 0 | 5 | 363.79 |
| 6a | 3.68 | 32.67 | 22 | 290.37 | 3 | 0 | 0 | 3 | 277.25 |
| 6b | 5.04 | 32.67 | 24 | 359.26 | 3 | 0 | 1 | 3 | 304.32 |
| 6c | 5.30 | 32.67 | 24 | 448.16 | 3 | 0 | 1 | 3 | 313.02 |
| 6d | 4.01 | 32.67 | 24 | 326.35 | 3 | 0 | 0 | 3 | 287.11 |
| 6e | 3.77 | 39.15 | 28 | 376.50 | 5 | 0 | 0 | 5 | 369.06 |
| Compd |
RA%, 100 μM, 20 min (±SD) |
RA%, 100 μM,
60 min (±SD) |
AAPH% Inhibition, 100 μM
(±SD) |
LOX% Inhibition,
100 μM or IC50 (μM) (±SD) |
|---|---|---|---|---|
| 1a | 5 ± 0.5 | 10 ± 0.5 | 69 ± 0.3 | 10 μΜ ± 0.4 |
| 1b | 13 ± 0.6 | 100 ± 1.1 | 37 ± 0.1 | 30 ± 0.1 |
| 1c | n.a. | 44 ± 1.6 | 61 ± 0.5 | 28 ± 0.6 |
| 1d | 86 ± 4.5 | 95 ± 4.8 | 53 ± 0.6 | 38 ± 0.7 |
| 1e | n.a. | 100 ± 8.7 | 50 ± 0.5 | 24 ± 0.6 |
| 1f | 13 ± 0.8 | 26 ± 0.9 | 32 ± 1.4 | 24 ± 1.4 |
| 2a | n.a. | 17 ± 1.2 | 44 ± 0.7 | 42 ± 2.4 |
| 2b | 2 ± 0.1 | 15 ± 0.6 | 39 ± 0.3 | 24 ± 1.9 |
| 2c | 21 ± 0.6 | n.a. | 68 ± 0.6 | n.a |
| 2d | 6 ± 0.4 | 10 ± 0.9 | 75 ± 0.1 | 2.5 μΜ ± 0.2 |
| 2e | 24 ± 0.9 | 45 ± 1 | 78 ± 0.4 | 0.35 μΜ ± 0.02 |
| 2f | 5 ± 0.2 | n.a. | 52 ± 2.5 | 14 ± 0.8 |
| 3a | 10 ± 0.3 | 22 ± 0.9 | 75 ± 1.4 | 51 ± 2.2 |
| 3b | 2 ± 0.1 | 6 ± 0.6 | 63 ± 2.6 | 17 ± 0.8 |
| 3c | 2 ± 0.1 | 24 ± 0.9 | 40 ± 1.8 | 30 μΜ ± 2.9 |
| 3d | 6 ± 0.5 | 11 ± 0.9 | 52 ± 2.5 | 11 ± 0.8 |
| 3e | 30 ± 0.9 | 53 ± 0.9 | 80 ± 2.9 | 20 μΜ ± 0.1 |
| 3f | 5 ± 0.4 | 14 ± 1.2 | 75 ± 2.7 | 38 ± 2.6 |
| 4a | n.a. | n.a. | 98 ± 0.4 | 39 ± 0.4 |
| 4b | 2 ± 0.1 | n.a. | 97 ± 0.6 | n.a |
| 4c | 2 ± 0.1 | n.a. | 59 ± 0.2 | 17 ± 1.2 |
| 4d | 23 ± 0.3 | 2 ± 0.1 | 51 ± 0.2 | 85 μΜ ± 4.1 |
| 4e | 2 ± 0.1 | 2 ± 0.2 | 74 ± 0.9 | 6 ± 0.5 |
| 4f | n.a. | n.a. | 12 ± 1.1 | 22 ± 1.8 |
| 5a | n.a. | n.a. | 29 ± 2.2 | 4 ± 0.3 |
| 5b | 19 ± 0.1 | n.a. | 8 ± 0.2 | 39 ± 0.7 |
| 5c | n.a. | n.a. | 85 ± 0.6 | 37 ± 0.6 |
| 5d | n.a. | 34 ± 0.5 | 33 ± 0.5 | 80 μΜ ± 3.5 |
| 5e | 20 ± 0.1 | n.a. | 61 ± 0.9 | 19 ± 0.4 |
| 6a | 11 ± 0.6 | n.a. | 22 ± 1 | 24 ± 0.8 |
| 6b | n.a. | n.a. | 46 ± 0.6 | 26± 0.3 |
| 6c | n.a. | n.a. | 71 ± 0.4 | 1± 0.07 |
| 6d | n.a. | 8 ± 0.7 | 67 ± 0.4 | 42 ± 2.7 |
| 6e | 7 ± 0.5 | 12 ± 1 | 56 ± 0.3 | 40 μΜ ± 0.6 |
| NDGA | 87 | 93 | - | 93 (0.45 μM) |
| Trolox | - | - | 92 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peitzika, S.-C.; Tsiampakari, E.; Pontiki, E. Microwave Assisted Synthesis of Antioxidant Dihydro-Pyrazole Hybrids as Possible Lipoxygenase Inhibitors. Molecules 2025, 30, 2224. https://doi.org/10.3390/molecules30102224
Peitzika S-C, Tsiampakari E, Pontiki E. Microwave Assisted Synthesis of Antioxidant Dihydro-Pyrazole Hybrids as Possible Lipoxygenase Inhibitors. Molecules. 2025; 30(10):2224. https://doi.org/10.3390/molecules30102224
Chicago/Turabian StylePeitzika, Stergiani-Chrysovalanti, Eirini Tsiampakari, and Eleni Pontiki. 2025. "Microwave Assisted Synthesis of Antioxidant Dihydro-Pyrazole Hybrids as Possible Lipoxygenase Inhibitors" Molecules 30, no. 10: 2224. https://doi.org/10.3390/molecules30102224
APA StylePeitzika, S.-C., Tsiampakari, E., & Pontiki, E. (2025). Microwave Assisted Synthesis of Antioxidant Dihydro-Pyrazole Hybrids as Possible Lipoxygenase Inhibitors. Molecules, 30(10), 2224. https://doi.org/10.3390/molecules30102224






