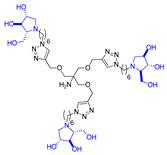
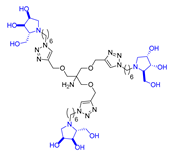
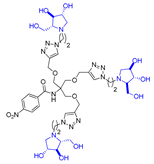
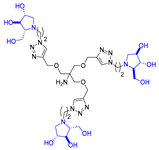
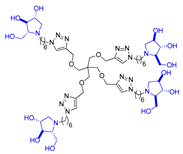
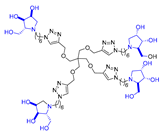
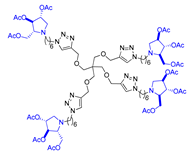
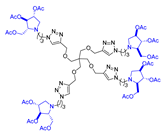
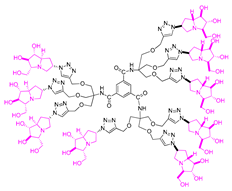
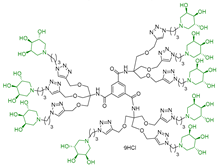
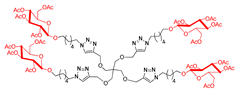
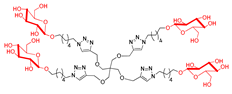
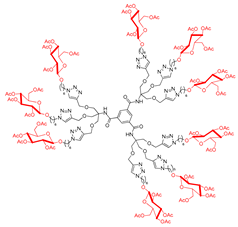
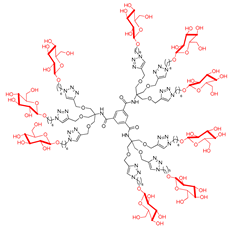
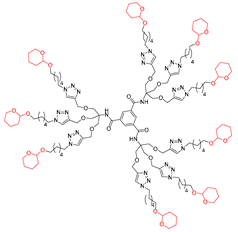
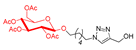
Abstract
Morquio A syndrome is a lysosomal disorder caused by the deficiency of the lysosomal enzyme N-acetylgalactosamine 6-sulfatase (GALNS, EC 3.1.6.4). Currently, enzyme replacement therapy (ERT) is used to treat Morquio A through the infusion of the recombinant enzyme VIMIZIM® (elosulfase alfa, BioMarin). Unfortunately, the recombinant enzyme exhibits low conformational stability in vivo. A promising approach to address this issue is the coadministration of recombinant human GALNS (rhGALNS) with a pharmacological chaperone (PC), a molecule that selectively binds to the misfolded protein, stabilizes its conformation, and assists in the restoration of the impaired function. We report in this work the synthesis of a library of multivalent glycomimetics exploiting the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction between several dendrimeric scaffolds armed with terminal alkynes and azido ending iminosugars of different structures (pyrrolidines, piperidines, and pyrrolizidines) or simple azido ending carbohydrates as bioactive units. The biological evaluation identified pyrrolidine-based nonavalent dendrimers 1 and 36 as the most promising compounds, able both to bind the native enzyme with IC50 in the micromolar range and to act as enzyme stabilizers toward rhGALNS in a thermal denaturation study, thus identifying promising compounds for a combined PC/ERT therapy.
1. Introduction
Over the past twenty years, multivalency has gained significant importance in biomedicine and bioorganic synthesis due to its fundamental role in various biological phenomena. Although multivalency had been previously extensively studied essentially in carbohydrate–lectin interactions [1,2,3,4,5], its potential in enzyme inhibition started to be explored only since 2009, when iminosugar clusters were identified as potent inhibitors of enzyme Jack bean α-mannosidase [6]. The multivalent effect (MVE) refers to the increased binding affinity exhibited by a multivalent compound, which contains multiple bioactive units attached to a common scaffold, compared with a monovalent counterpart taken as reference. The relative potency (rp) of a multivalent compound is calculated by dividing the IC50 (or Ki) value of the monovalent reference compound by the IC50 (or Ki) value of the multivalent structure against the target enzyme, and further division by the number of bioactive units (n) quantifies the MVE of a multivalent ligand. It was defined that a positive MVE occurs when this ratio (rp/n) is higher than 1 [7].
Morquio A syndrome (or Mucopolysaccharidosis IVA) is a lysosomal storage disorder caused by the deficiency of the lysosomal enzyme N-acetylgalactosamine 6-sulfatase (GALNS, EC 3.1.6.4) that leads to the accumulation of two glycosaminoglycans (GAGs), keratan sulphate and chondroitin 6-sulphate, within lysosomes [8,9,10,11]. The global incidence of Morquio A ranges between 0.009 and 0.14 cases per 10,000 live births. The disease primarily manifests through skeletal abnormalities, connective tissue defects, and cognitive impairments in the more severe forms. Currently, enzyme replacement therapy (ERT) is used to treat Morquio A through the infusion of the recombinant enzyme VIMIZIM® (elosulfase alfa, BioMarin) [12,13], which unfortunately exhibits low conformational stability in vivo and thus limits its effectiveness. One promising approach to improve this treatment is the coadministration of recombinant human GALNS (rhGALNS) with a pharmacological chaperone (PC), a molecule that selectively binds to the misfolded protein, stabilizes its conformation, and assists in the restoration of the impaired function [14,15,16,17,18]. This combined PC/ERT therapy can reduce the required enzyme dosage and administration frequency, thereby minimizing hospitalization and enhancing patients’ quality of life. Successful applications of PC/ERT therapy have been reported for other lysosomal storage disorders [19,20,21,22,23,24].
For this reason, the identification of new compounds that bind rhGALNS is a crucial strategy for the development of enzyme stabilizers.
In addition to the field of lysosomal storage disorders, recent findings associated overexpression of GALNS with cancer [25] and degradation of perineural nets (composed of glycosaminoglycans (GAGs)) around neurons with neuropathic pain [26], thus increasing the interest of the scientific community toward this enzyme.
So far, three non-carbohydrate-based GALNS PCs (bromocriptine, ezetimibe, and pranlukast) [27,28] have been recognized through molecular docking-based virtual screening, and preliminary studies show their ability to increase the activity and thermostability of rhGALNS [29].
The X-ray structure of GALNS reveals a dimeric nature and a certain promiscuity in substrate recognition, with a large open depression on the surface of the enzyme that allows a wide variety of molecules access to the catalytic site [30]. The dimeric nature, in particular, which GALNS shares with other enzymes such as Jack Bean α-mannosidase, α-galactosidase, or β-glucocerebrosidase, has been shown to be prone to accepting multivalent substrates [7,31,32,33,34,35,36].
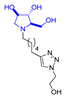
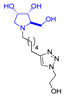
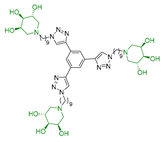
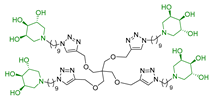
In our research group, we previously reported the synthesis of two nonavalent architectures, 1 and 2, characterized by a rigid aromatic core decorated with the natural 1,4-dideoxy-1,4-imino-d-arabinitol (DAB-1) iminosugar and one of its epimers at C-4, respectively [37]. Compounds 1 and 2 were the first multivalent iminosugar-based GALNS inhibitors identified in the literature. They showed IC50 values in the micromolar range (47–85 μM) and a positive MVE (rp/n 6.5–9.2) (Figure 1A).
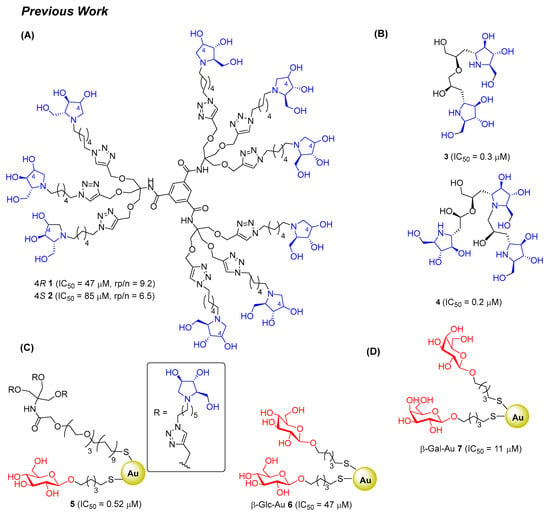
Figure 1.
Dendrimers and Au GNPs already known as GALNS inhibitors. (A) Nonavalent pyrrolidine architectures. (B) Dimer and trimer of DAB-1. (C) Glucose-based and mixed glucose-iminosugar gold nanoparticles. (D) Monosaccharide-based gold nanoparticles.
Afterward, it was found that dimerization and trimerization of DAB-1 obtained through a quite complex nitrone cycloaddition/ring-opening strategy afforded very potent GALNS inhibitors 3 and 4, with IC50 = 0.3 μM and IC50 = 0.2 μM, respectively (Figure 1B) [38].
Some of us also reported the first example of gold glyconanoparticles (Au GNPs) functionalized with DAB-1 moiety and 5-(thio)pentyl d-glucopyranoside (GlcC5S) that strongly inhibited GALNS in the low micromolar/high nanomolar range (compound 5, IC50 = 0.52 μM). Unexpectedly, the Au GNPs 6, functionalized with 100% of GlcC5S, showed a remarkable inhibition (IC50 = 47 μM) toward GALNS, corroborating GALNS promiscuity in substrate recognition and suggesting a synergistic effect of the sugar moiety and/or the scaffold with the iminosugar component in determining the inhibitory activity (Figure 1C) [39]. For this reason, we investigated a library of Au GNPs decorated with various monosaccharides (glucose, mannose, or galactose) with different anomeric configurations (α or β). Among the analyzed compounds, β-Gal-Au GNPs 7 (Figure 1D) was identified as the most effective inhibitor (IC50 = 11 μM). Interestingly, this compound was also able to recover enzyme activity after thermal denaturation when tested on the enzyme used for therapy [40].
Considering the above-mentioned results and with the aim of developing new potent and selective binders and stabilizers of the enzyme GALNS, we report herein the synthesis of new dendrimeric multivalent compounds that differ for (i) the bioactive unit (sugar or iminosugar), (ii) the valency, (iii) the linker between the scaffold and the bioactive unit, and (iv) the use of a protected or unprotected bioactive unit. The synthesis of a nonavalent pyrrolidine without hydroxy groups was also undertaken in order to evaluate an eventual synergistic effect of the scaffold in the binding to GALNS.
All synthesized dendrimers were tested as GALNS inhibitors on human leucocyte extracts. The most promising compounds were compared with the appropriate monovalent reference counterpart to quantitatively determine the relative potency (rp) and the multivalent effect (MVE, rp/n). The relative potency with respect to the appropriate corresponding scaffold, which lacked the hydroxy groups, was also evaluated to investigate synergistic effects. For the most promising compounds, the inhibitory activity toward the rhGALNS used for therapy and the ability to impart thermal stabilization to this enzyme were assessed.
2. Results and Discussion
We exploited the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction as the key synthetic step to obtain the multivalent compounds due to its efficiency and versatility [41,42,43]. CuAAC has proven to be an ultra-fast synthetic procedure, allowing the conjugation of a great number of azide-functionalized moieties onto a single alkynyl-armed multivalent scaffold with high yields and reduced reaction times.
Based on our previous experience in the synthesis of multivalent iminosugars and given the polarity of our azide components, the reaction conditions for the CuAAC that we employed in this study were the following: catalytic CuSO4 (0.3 equiv), sodium ascorbate (0.6 equiv), a 2:1 THF:H2O mixture as the solvent, microwave irradiation (MW 80 °C) for 45 min time, followed by flash column chromatography (FCC) or size exclusion chromatography (SEC) and eventual treatment with copper scavenger resins [44].
A series of scaffolds with different geometries and numbers of alkyne moieties, from trivalent and tetravalent to hexavalent and nonavalent, were selected (Figure 2). The trivalent and tetravalent 8 [45,46], 9 [47], and 10 [48] were obtained by propargylation of the corresponding alcohols (tris(hydroxymethyl)aminomethane, glycerol, and pentaerythritol), while the dendrimeric hexavalent and nonavalent 11 [49] and 12 [50] were prepared by coupling adipic acid and trimesoyl chloride, respectively, with tris[(propargyloxy)methyl]aminomethane 8. The new more basic and flexible scaffold 13 was also investigated in this study.
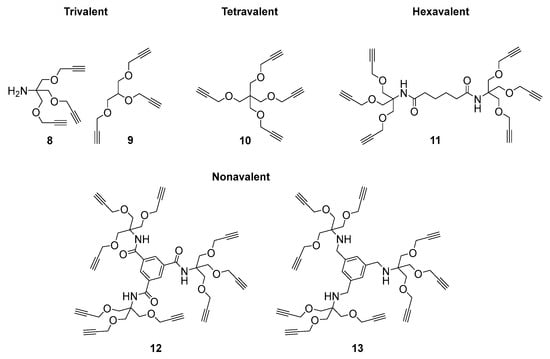
Figure 2.
Alkynyl-armed multivalent scaffold used in the CuAAC.
Concerning the synthesis of iminosugar-derived dendrimers, the initial attempts of CuAAC were successfully performed on the alkynyl-armed tetravalent 10 with the benzylated DAB-1 analog 14 [51] bearing a 6-azido hexane linker at the nitrogen atom. However, as revealed in our previous works, significant challenges arose during the deprotection of the perbenzylated adducts and purification of the crude reaction due to the high basicity and hydrophilicity of the polyhydroxylated compounds [51,52].
Purification could be achieved after per O-acetylation of the hydroxy groups after the debenzylation by catalytic hydrogenation to better characterize the final multivalent architectures, which gave us the opportunity to test also the peracetylated adducts (Scheme 1). The choice of the acetyls as the protecting group for the hydroxyls was motivated by the observation that peracetylated compounds can act as pro-drugs when tested on cells by facilitating cellular uptake, as previously reported by Compain and co-workers [53]. Catalytic hydrogenation of 15 [51] with Pd/C in acidic MeOH for 3 days followed by treatment with an excess of acetic anhydride and pyridine at room temperature for 16 h furnished the compound 16 in 49% yield over two steps (Scheme 1). To investigate the influence of a shorter linker between the polyhydroxypyrrolidines and the branch point of the multivalent architecture on GALNS inhibition, we selected the tetravalent scaffold 10 as it is easier to prepare compared with the other alkynes, along with the polyhydroxylated azido intermediate 17 bearing a 3-carbon-atom chain (Scheme 1). The CuAAC reaction of 10 with 17, carried out under the previously described conditions, afforded a product that could be used in the next step without further purification. The tetravalent pyrrolidine compound 18 was isolated in 86% of yield after FCC. The deprotection of the perbenzylated adduct 18 by catalytic hydrogenation in acidic MeOH, followed by acetylation of the crude with an excess of acetic anhydride in pyridine, provided peracetylated compound 19 in 38% yield over two steps. The final treatment of 19 with the strongly basic resin Ambersep 900 OH in MeOH for 16 h gave the pure polyhydroxylated multivalent iminosugar 20 in 95% yield (Scheme 1). We then synthesized the trivalent compound 23, which contained a free amine group on the scaffold and featured three pyrrolidines separated from the triazole moieties by a two-carbon aliphatic chain starting from azido-compound 21 (Scheme 1). To avoid undesired N-acetylation during the final acetylation step, the debenzylation procedure was modified by employing an acidic treatment with BCl3, followed by a basic workup [54]. The reaction of 22 [55] with BCl3 in CH2Cl2 at room temperature for 18 h followed by quenching with EtOH and treatment with the strongly basic Ambersep 900 OH resin for 30 min furnished 23 in 96% yield after filtration and purification over Sephadex LH-20 resin (Merk Life Science S.r.l., Merck KGaA, Germany) (Scheme 1).
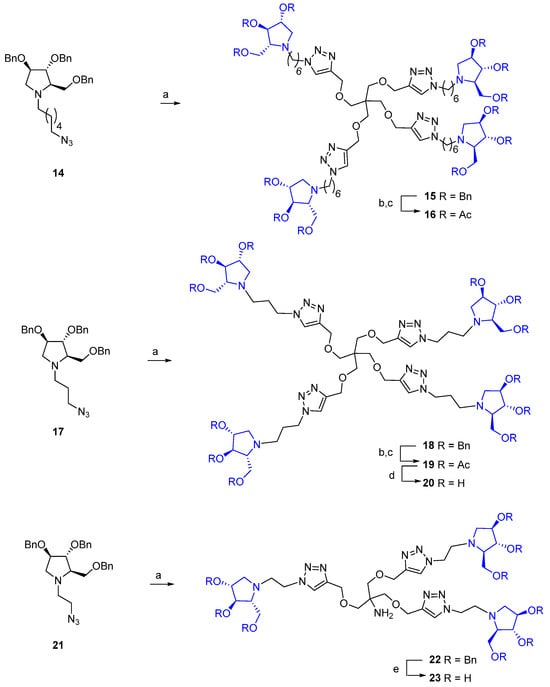
Scheme 1.
(a) 10, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 96% for 15 (Ref. [51]), 86% for 18, 68% for 22 (Ref. [55]). (b) H2, Pd/C, MeOH, HCl, r.t., 3 d. (c) Ac2O, pyridine, r.t., 16 h, 49% over 2 steps for 16, 38% over 2 steps for 19. (d) Ambersep 900 OH, MeOH, r.t., 16 h, 95%. (e) BCl3 1M in hexane, CH2Cl2, r.t., 18 h, then Ambersep 900 OH, EtOH, r.t., 30 min, quantitative.
To reduce the overall number of synthetic steps, we chose to employ the polyhydroxylated azido intermediate 24 instead of the benzylated derivative 14 in the CuAAC reactions depicted in Scheme 2.
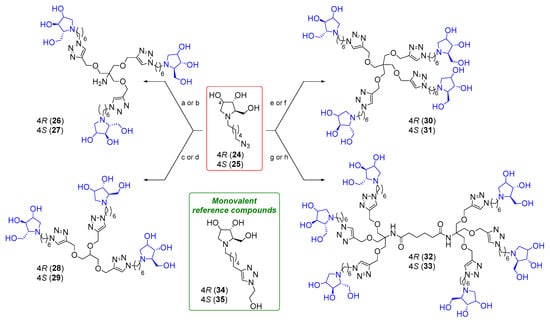
Scheme 2.
(a) From 24, reaction with 8, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 76% of 26 (Ref. [51]). (b) From 25, reaction with 8, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 62% of 27. (c) From 24, reaction with 9, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 78% of 28. (d) From 25, reaction with 9, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 67% of 29. (e) From 24, reaction with 10, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 48% of 30 (Ref. [51]). (f) From 25, reaction with 10, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 73% of 31. (g) From 24, reaction with 11, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 1 h 30 min, 73% of 32. (h) From 25, reaction with 11, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 72% of 33.
Copper-catalyzed cycloaddition of the deprotected azide 24 was run with the trivalent 9 and the hexavalent 11 in the conditions described above. After treatment with the copper scavenger resin Quadrasil MP® (Merk Life Science S.r.l., Merck KGaA, Germany) and purification by flash column chromatography (FCC) or size exclusion chromatography (SEC), the final multivalent iminosugars 28 and 32 were afforded in 78% and 73% yields, respectively (Scheme 2).
Analogous multivalent architectures 26 and 30 were previously reported by our research group with good to fair yields (76% and 48%, respectively) following the same synthetic strategy and tested against different types of α-mannosidases (Jack bean α-mannosidase (JBMan), Golgi α-mannosidase (GMII), and lysosomal α-mannosidase (bLAM)) [51]. To investigate the role of different C-4 configurations on the biological activity, the epimeric azide 25 underwent CuAAC reactions with the alkynyl-armed multivalent scaffolds 8–12 under the previously described conditions, leading to the synthesis of polyhydroxylated multivalent iminosugars 27, 29, 31, and 33.
After treatment with the copper scavenger resin Quadrasil MP® and purification upon FCC and/or by SEC, the polyhydroxylated compounds 27, 29, 31, and 33 were obtained in 62–73% yields (Scheme 2). Derivatives 34 and 35, which were previously synthesized by our research group, were used as monovalent reference compounds [37].
Given the promising inhibition data for the nonavalent architectures 1 and 2 [37], we synthesized two new nonavalent derivatives introducing some structural modifications to investigate their potential impact on the biological activity against the GALNS enzyme. First, we modified the scaffold carrying out the synthesis of the more basic and flexible compound 36, an analog of 1 that shows a stronger positive multivalent effect. In compound 36, the three amide bonds were replaced with amine moieties, while retaining the 4R configuration of the iminosugar ring (Scheme 3), which gave the best results (see Table 1, compound 1 vs. 2).
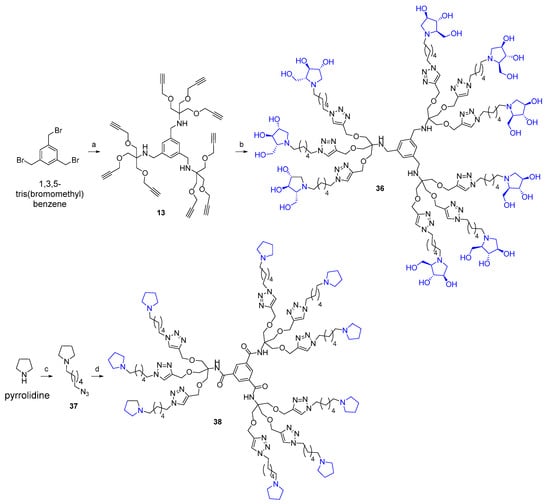
Scheme 3.
(a) 8, K2CO3, CH3CN, r.t., 18 h, 68%. (b) 24, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 82%. (c) 1-azido-6-bromohexane, K2CO3, CH3CN:H2O 5:1, MW 120 °C, 3 h, 36%. (d) 12, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 90%.

Table 1.
Inhibitory activities of the monovalent pyrrolidine derivatives 34 and 35 and of the multivalent compounds 1, 2, 36, and 38 toward N-acetylgalactosamine-6-sulfatase (GALNS).
Starting from 1,3,5-tris(bromomethyl)benzene, the substitution of the bromine groups with the amine 8 in the presence of K2CO3 in CH3CN at room temperature for 18 h afforded the alkynyl-armed nonavalent scaffold 13 in 68% yield.
The CuAAC reaction of 13 with the deprotected (4R) azide 24 in the conditions described above yielded the nonavalent compound 36 (82%) after treatment with the copper scavenger resin Quadrasil MP® and purification over Sephadex LH-20 resin (Scheme 3).
In addition, we synthesized a reference nonavalent compound, maintaining 12 as the scaffold but featured with simple pyrrolidines as inhitopes, in order to assess the role of polyhydroxyl groups and to examine the eventual synergistic role of the scaffold itself on the binding affinity toward GALNS enzyme. To our knowledge, the role of the scaffold has not been investigated so far in relation to multivalent iminosugars. The reaction of pyrrolidine with 1-azido-6-bromohexane [56] in the presence of K2CO3 in a 5:1 CH3CN:H2O mixture under MW irradiation at 120 °C for 3 h resulted in the alkylated compound 37 in 36% yield (Scheme 3). Hence, 37 was employed in the CuAAC reaction with the nonapropargylated scaffold 12 in the usual conditions described above to yield the compound 38 in 90% yield after treatment with QuadraSil® MP resin, followed by size exclusion chromatography (Sephadex LH-20 resin) (Scheme 3).
Since gold glyconanoparticles (Au GNPs) functionalized with sugar moieties (β-glucose and β-galactose) exhibited excellent GALNS inhibition [39,40], we synthesized new dendrimers decorated with β-glucose units, in both their acetylated and polyhydroxylated forms, to further investigate the role of the sugar component in the inhibition by multivalent compounds. To synthesize the sugar-derived 42 functionalized on the anomeric position with a six-carbon-atom chain ending with an azido moiety, we followed two synthetic routes starting from d-glucose. The azide 42 was first obtained by directly performing the glycosylation reaction on β-d-glucose pentaacetate 39 with 6-chlorohexanol, BF3·OEt2, and 3 Å molecular sieves in dry CH2Cl2, yielding both the α- and β-anomers 40 and 41 (Route I, Scheme 4A) in a 25% overall yield. The β:α anomer ratio was 1.17:1. The β-anomer 41 was subsequently treated with NaN3 in DMF at 50 °C for four days to give the azide 42 [57,58] in 79% yield. Alternatively, trichloroacetimidate 43 [59] underwent a glycosylation reaction with 6-chlorohexanol in the presence of BF3·OEt2 as Lewis acid and 3Å molecular sieves in dry CH2Cl2 affording selectively the corresponding β-anomer 41 in 58% yield. Compound 41 was then converted into the azide 42 (Route II, Scheme 4A). The overall yield of Route II was 19%. Although the first synthetic strategy (Route I) resulted in a slightly lower overall yield (8%) compared with Route II, it required fewer steps (Scheme 4A). In addition, Route I produced a mixture of two diastereoisomers (40 and 41), which complicated the purification of the intermediate step. Despite requiring more steps, Route II proceeded with a higher overall yield (19%), nearly double that of Route I. Given the substantially higher yield, Route II was the more favorable route as maximizing the yield of product 42 was a priority in this case.
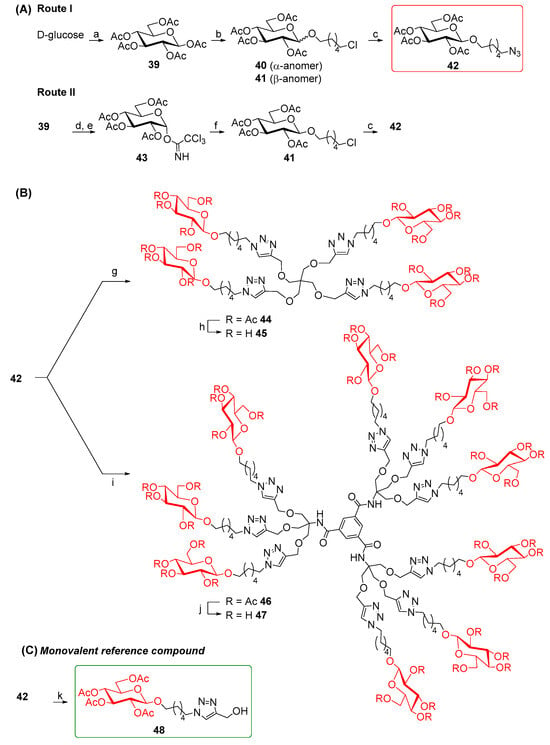
Scheme 4.
(A) Synthesis of glucose derivative 42. (B) Synthesis of multivalent glucose derivatives. (C) Synthesis of the monovalent reference compound 48. (a) Ac2O, NaOAc, reflux to r.t., 74%. (b) 6-chlorohexanol, BF3·OEt2, 3Å MS, dry CH2Cl2, 0 °C to r.t., 26 h, 25% (mixture of α and β anomers). (c) 41 (β-anomer), NaN3, dry DMF, 50 °C, 4 days, 79%. (d) 1,2-diamino ethane, AcOH, dry THF, r.t., 7 h, 86%. (e) Cl3CCN, DBU, dry CH2Cl2, 0 °C to r.t., 5 h, 65%. (f) 6-chlorohexanol, BF3·OEt2, 3Å MS, dry CH2Cl2,—78 °C to r.t., 18 h, 58%. (g) 10, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 79%. (h) Ambersep 900 OH, MeOH, r.t., 18 h, 88%. (i) 12, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 91%. (j) Na2CO3, MeOH, r.t., 18 h, 61%. (k) propargyl alcohol, CuSO4, sodium ascorbate, THF:H2O 2:1, r.t., 40 h, 61%.
The CuAAC reaction of the azido derivative 42 with the tetravalent scaffold 10 was carried out as usual to afford the acetylated tetravalent derivative 44 in 79% yield, after flash column chromatography (Scheme 4B). Deprotection of acetyl groups of 44 using the strongly basic resin Ambersep 900 OH in MeOH followed by purification over the size exclusion Bio-Beads SX-8 resin (Merk Life Science S.r.l., Merck KGaA, Germany) furnished the tetravalent dendrimer 45 in 88% yield. The CuAAC reaction of the nonavalent 12 and the sugar-derived azide 42 gave the acetylated nonavalent compound 46 in 91% yield after purification by FCC (Scheme 4B).
We faced some challenges in the cleavage of the acetyl groups of compound 46. The main attempts for the basic deprotection at room temperature are reported in the Supporting Information. Finally, the treatment of 46 with Na2CO3 for 18 h, followed by neutralization of the reaction mixture with 1% HCl in MeOH and subsequent filtration, afforded 47 in 61% yield. To evaluate the relative inhibitory activity enhancement of these newly derived sugar multivalent architectures, a corresponding monovalent counterpart was also synthesized. Starting from the azido derivative 42, a CuAAC reaction was performed with propargyl alcohol in the presence of CuSO4 and sodium ascorbate in THF:H2O 2:1 mixture at room temperature for 40 h, yielding the adduct 48 in 61% yield (Scheme 4C).
In this case as well, we synthesized a reference nonavalent compound, using 12 as the scaffold while incorporating tetrahydro-2H-pyran as the inhitope. This design aimed to evaluate the role of polyhydroxyl groups and investigate any potential synergistic effect of the scaffold itself on binding affinity toward the GALNS enzyme. The reaction of 3,4-dihydro-2H-pyran with azidohexan-1-ol [60] in the presence of Amberlyst 15 in a 1:1 Hexane:Et2O mixture at room temperature for 3 h yielded the acetal 49 with an 89% yield (Scheme 5). Subsequently, 49 underwent a CuAAC reaction with the nonapropargylated scaffold 12 under the previously described conditions, affording the desired product 50 in a 46% yield after treatment with QuadraSil® MP resin, followed by flash column chromatography (Scheme 5).
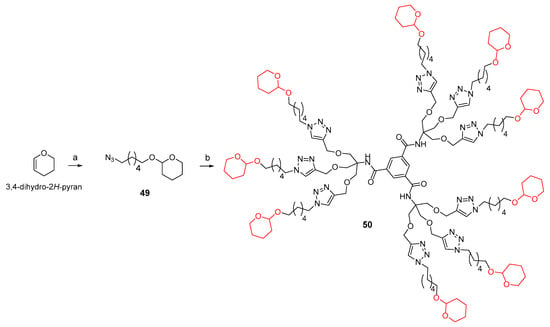
Scheme 5.
(a) 6-azidohexan-1-ol, Amberlyst 15, Hexane:Et2O 1:1, r.t., 3h, 89%. (b) 12, CuSO4, sodium ascorbate, THF:H2O 2:1, MW 80 °C, 45 min, 46%.
3. Biological Screening
A preliminary biological screening of the newly synthesized multivalent compounds at 1 mM inhibitor concentration was performed against GALNS in leukocytes isolated from healthy donors. Enzymatic assays were performed in triplicate by incubating leukocyte homogenate, compound, and substrate (4-methylumbelliferyl-β-galactoside-6-sulfate.Na) in Na-acetate/acetic acid buffer (pH 4.3) at 37 °C for 17 h, followed by the addition of β-galactosidase from Aspergillus oryzae and further incubation to release 4-methylumbelliferone. The fluorescence was measured, and the inhibition percentages were calculated relative to the control. The results are summarized in Table 1, Table 2, Table 3 and Table 4, along with new inhibition data toward GALNS of multivalent inhibitors whose synthesis was reported previously by our group (23, 26, 30, 51, 52–54). For compounds showing poor to negligible inhibitory activity, only the GALNS inhibition percentage at 1 mM was reported.

Table 2.
Inhibitory activities of the multivalent pyrrolidines 16, 19, 23, 26–29, 30–33, and 51 and of the multivalent pyrrolizidine compounds 52–54 toward N-acetylgalactosamine-6-sulfatase (GALNS).

Table 3.
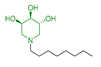
Inhibitory activities of the monovalent piperidine 55 and multivalent piperidines 56–58 toward N-acetylgalactosamine-6-sulfatase (GALNS).

Table 4.
Inhibitory activities of monovalent sugar compound 48 and multivalent sugar compounds 44–47 and multivalent compound 50 toward N-acetylgalactosamine-6-sulfatase (GALNS).
Conversely, IC50 values were calculated by fitting hydrolysis rates to a dose–response model using Origin for the best GALNS inhibitors (i.e., GALNS inhibition at 1 mM ≥ 75%), as well as for those compounds for which a numerical value was necessary to compare multivalent ligands with reference compounds (i.e., compounds 34, 35, and 38; Table 1 entries 1, 2, and 6). The only exception was represented by compound 33 (Table 2), but given the poor result (IC50 = 350 μM), we did not proceed further. Monovalent reference compounds 34 and 35 showed negligible inhibition against GALNS (IC50 = 3900 μM and 5000 μM, respectively; Table 1, entries 1, 2 [37]) and were used as monovalent reference compounds to evaluate the MVE for the multivalent derivatives.
In general, as previously reported in the literature for 1 and 2 [37], the nonavalent architectures were found to be most effective in inhibiting the GALNS enzyme, with rp/n values >1. Better results were obtained for the d-arabinose-configured compound (1) with respect to the d-ribose-configured one (2) (Table 1, entry 3 vs. entry 4). The GALNS preference for the d-arabinose vs. the d-ribose configuration was evident also considering the d-ribose-configured hexavalent compound 33, for which a little rp/n = 2.4 was calculated (Table 2).
Interestingly, the newly synthesized d-arabinose-configured 36 exhibited a very high inhibition value (Table 1, entry 5, 93%) at 1 mM with an IC50 in the low micromolar range (IC50 = 28 µM). Therefore, the replacement of the amide bonds in 1 with amine bonds in 36 almost doubled the inhibitory activity and showed an enhanced MVE (rp/n = 15.4 for 36 vs. rp/n = 9.2 for 1; Table 1, entry 5 vs. 3). We can only envisage, at this stage, that the improved inhibitory value with compound 36 was due to the higher basicity of the scaffold or its higher flexibility.
Conversely, the IC50 measured for the nonavalent compound 38 featured with simple pyrrolidines as inhitopes (IC50 = 70 μM; Table 1, entry 6) showed that the nonavalent scaffold itself played a key role in determining GALNS inhibition, even without the hydroxy groups, suggesting a synergistic effect between the iminosugar and the multivalent scaffold in imparting the inhibitory activity.
Taking 38 as a multivalent reference compound and by evaluating the relative potency inhibition of 1 with respect to 38, we calculated an rp = 1.5, which demonstrated that the presence of the iminosugars was still relevant to improve inhibition (Table 1, entry 3).
Instead, comparing the inhibitory activity of the d-ribose-configured 2 with respect to 38 resulted in no advantage resulting from the presence of the iminosugar (Table 1, entry 4 vs. 6), thus corroborating the preference of GALNS for the d-arabinose configuration.
Decreasing the valency of the multivalent compounds was not beneficial for the inhibitory properties toward GALNS. Indeed, the trivalent compounds 28, 29, 26 [51], 27, and 23 [55] derived from glycerol or from the tris[(propargyloxy)methyl]aminomethane amine 8 exhibited inhibition percentages lower than 75% (Table 2). Again, among the trivalent compounds with a six-carbon-atom linker 28, 29, 26, and 27, we observed that the d-arabinose-derived compounds 28 and 26 (with inhibition rates of 63% and 73% at 1 mM, respectively; Table 2) were preferred over the d-ribose-derived 29 and 27 (with inhibition rates of 18% and 46% at 1 mM, respectively; Table 2). This confirmed that changes in the absolute configuration at C-4 played a role in determining their affinity for GALNS. Coupling the free amine of 26 with 4-nitrobenzoyl chloride to afford the less polar derivative 51 [55] resulted in lower GALNS inhibition (49% for 51 vs. 73% for 26; Table 2). Similarly, reducing the linker between the polyhydroxy pyrrolidines and the triazole moieties (35% for 23 vs. 73% for 26; Table 2) resulted in lower inhibition. In analogy to the trivalent derivatives, also the tetravalent compounds resulted poor inhibitors. However, an opposite trend was observed with the d-ribose-configured 31 being twice as potent as the d-arabinose-derived 30 (inhibition of 73% for 31 vs. 35% for 30; Table 2). The acetylation of the hydroxy groups in 30 to yield compound 16 did not significantly improve the inhibition properties toward GALNS (35% for 30 vs. 47% for 16; Table 2). Similarly, the acetylated 19 with the shorter linker between the trihydroxy pyrrolidines and the triazole moieties exhibited a very poor 25% GALNS inhibition (Table 2).
Both the d-arabinose and d-ribose hexavalent 32 and 33 showed moderate inhibition properties (66% and 72% at 1 mM, respectively; Table 2) against GALNS. For the d-ribose-derived 33, an IC50 of 350 ± 80 μM was determined, which allowed the calculation of a low but positive multivalent effect rp/n of 2.4 when compared with its monovalent counterpart 35 (Table 1).
We then considered other multivalent compounds present in house, containing either pyrrolizidine or piperidine iminosugars as bioactive inhitopes.
The multivalent bicyclic pyrrolizidines [44] (compounds 52, 53, and 54; Table 2) did not overall improve GALNS inhibition compared with the corresponding multivalent pyrrolidine-armed compounds. Indeed, almost negligible inhibition was found for the tetravalent 54 (9% at 1 mM; Table 2), indicating again that a short linker between trihydroxy pyrrolidine and the triazole moiety (in analogy to compound 23; Table 2) had a detrimental effect on the inhibitory activity.
The longer linker in the trivalent compound 52 led to a mild improvement in its inhibitory activity (52% at 1 mM; Table 2). Increasing the number of iminosugar units and considering the same aromatic scaffold that yielded good results with the pyrrolidine series as in the nonavalent 53 led to a 63% inhibitory activity (Table 2). Considering these data and the more complicated and longer synthesis of pyrrolizidine iminosugars compared with the pyrrolidine derivatives [44], we deemed that it was not worthwhile to continue studying the GALNS inhibition properties with pyrrolizidine multivalent derivatives.
Conversely, the analysis of the previously synthesized monovalent and multivalent piperidine derivatives 55 [61], 56 [62], 57 [62], and 58 [52] (Table 3) revealed that all these compounds, regardless of the valency, showed inhibitory percentages no higher than 50% and even lower than that of the monovalent compound 55 taken as reference (57%; Table 3).
Among the newly synthesized glucose-derived dendrimers, the acetyl-protected dendrimers 44 and 46 showed good inhibitory activity against GALNS (66% and 78% inhibition, respectively; Table 4, entries 1 and 3), with again the nonavalent 46 (Table 4, entry 3) being better than the tetravalent 44 (Table 4, entry 1). In contrast, their corresponding deprotected counterparts 45 and 47 were almost negligible inhibitors showing only 17% and 27% inhibition, respectively (Table 4, entries 2 and 4). This disagreed with what we previously observed with the sugar-coated Au GNPs 6 and 7 (Figure 1), where the best inhibitory results were achieved using the corresponding deprotected carbohydrates with respect to the protected analogues [39,40].
The monovalent reference compound 48 exhibited very poor inhibition activity (16% inhibition; Table 4, entry 6) compared with its corresponding multivalent 44 and 46 as observed with the pyrrolidine derivatives discussed above. The nonavalent compound 50, featuring simple pyrans as inhitopes, exhibited the same inhibition percentage (~30%) as its glucose-containing analogue (Table 4, entries 4 and 5). This result highlights that hydroxyl groups are not crucial for the interaction with the enzyme. Instead, it reinforces the pivotal role of the nonavalent scaffold itself in driving GALNS inhibition, even in the absence of hydroxyl groups. Furthermore, when compared with the nonavalent 38, which incorporates pyrrolidines, the data suggest that tertiary amines contribute to the interaction, further influencing the inhibitory effect (Table 4, entry 5, vs. Table 1, entry 6).
The presence of pH-sensitive acetal groups in β-d-glucose-derived dendrimers led us to question whether these compounds would remain stable under the biological conditions of the screening assay. To assess their stability under the experimental conditions, we synthesized 2-(nonyloxy)tetrahydro-2H-pyran acetal following the literature procedure [63] as a control and conducted an NMR stability study. The NMR sample was prepared in D2O at pH* 4.3, where pH* refers to the pH measured in a D2O solution using an H2O-calibrated pH meter [64]. Spectra were recorded immediately after sample preparation and subsequently monitored over 17 h at 37 °C. The hydrolysis of the 2-(nonyloxy)tetrahydro-2H-pyran acetal yielded nonan-1-ol and 5-hydroxypentanal. The compound stability at pH* 4.3, assessed by ¹H-NMR experiments, is reported in the Supporting Information. The results indicated that acetal remained stable after 19 h at 37 °C and pH* 4.3, confirming the feasibility of the inhibitory assay for this class of β-d-glucose-derived dendrimers.
The promising IC50 values observed for 1 and 36 prompted us to assess their effects on the commercially available wild-type recombinant GALNS (rhGALNS VIMIZIM®). The results showed comparable IC50 values of 110 µM for 1 and 30 µM for 36, closely matching those obtained from leukocyte extracts (see Supporting Information). Building on these findings, we investigated the potential stabilizing effect of these compounds on the tertiary structure of GALNS through a thermal denaturation study. rhGALNS was preincubated with varying concentrations of the compounds before being subjected to heating at 48 °C for 40 and 60 min. The relative enzyme activity was expressed as the ratio between the activity measured after thermal denaturation at 48 °C and that obtained under physiological conditions at 37 °C. Notably, GALNS activity remained well preserved in the presence of 1 at the higher 500 µM concentration (around two-fold enzyme activity with respect to the control) across both time points (Figure 3A). In contrast, for compound 36, the highest relative enzyme activity was observed at 30 µM, with increased enzyme activity by 1.3-fold and 2.4-fold with respect to the control, after 40 and 60 min of denaturation, respectively (Figure 3B). These findings suggested that both compounds could serve as potential stabilizers for the recombinant enzyme, with 36 showing the highest stabilization after 60 min of denaturation at a lower concentration [40].
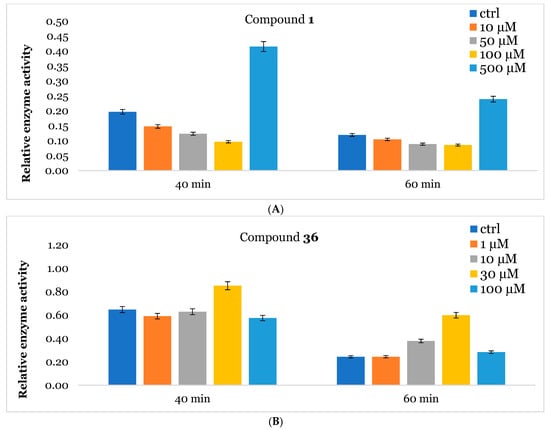
Figure 3.
Stabilization of rhGALNS VIMIZIM® in the presence of 1 (A) and 36 (B) was evaluated in vitro by using heat inactivation. Relative enzymatic activity after thermal treatment (48 °C) for 40 and 60 min at the indicated inhibitor concentrations (μM) with respect to the corresponding assay at 37 °C (no denaturation). Data for control were obtained as above except that no inhibitor was present. Experiments were performed in triplicate (see Supporting Information). Error bars reflect propagated uncertainties between two measurements.
4. Conclusions
The lysosomal enzyme N-acetylgalactosamine 6-sulfatase (GALNS) raised attention in relation to its important role in the field of lysosomal storage diseases and, more recently, due to its connection to cancer and neuropathic pain. Given its multimeric nature, GALNS is an enzyme prone to accepting multivalent ligands.
Following our ongoing interest in the identification of new GALNS inhibitors and stabilizers, we successfully synthesized in this work new iminosugar- and glucose-armed dendrimers through the CuAAC approach in quite good yields.
The new compounds, which differed for the type of the bioactive inhitope (pyrrolidine, pyrrolizidine, piperidine iminosugars, or carbohydrates), the valency, and the length of the linker between the bioactive inhitope and the triazole moiety, were preliminarily tested as GALNS inhibitors in leukocytes isolated from healthy donors, and their activity was compared with that of compounds previously synthesized in our group.
Among the screened compounds, the nonavalent dendrimers showed the highest inhibitory potency, and in particular, the nonavalent pyrrolidine compounds were confirmed as the best GALNS inhibitors since replacing them with other iminosugars (piperidine or pyrrolizidine) or with carbohydrates (either in their protected or deprotected form) did not improve the inhibitory properties of the multivalent adducts against GALNS. The observed superior performance of the nonavalent compounds with respect to lower-valent ones could be attributed to different effects, which are commonly invoked to explain the multivalent effect (statistical rebinding, chelating secondary binding, and cluster effects [34]). This was likely due to the specific spatial arrangement (presentation topology) of the bioactive units, which may enable the simultaneous engagement of multiple binding interactions or promote aggregation of the enzyme upon the binding. A deep investigation of which of these mechanisms are at work in this case is of great interest and will be the subject of future studies. Notably, we verified that the scaffold alone (compound 12) exhibited negligible inhibitory activity (25%; see Supporting Information), suggesting that the enhanced potency of the nonavalent compounds was only minimally due to the core structure itself but could rather be attributed to the multivalency and spatial arrangement of the bioactive moieties. The nonavalent 36 featured by a slightly modified scaffold with respect to the previously reported 1 resulted in the best inhibitor reported in this work, with an IC50 value in the low micromolar range (IC50 = 28 μM), which was lower than that reported for 1 (IC50 = 47 μM). This result shows that the choice of the scaffold is crucial in the identification of more potent inhibitors. Conversely, the nonavalent dendrimer 38, decorated with nine pyrrolidines and lacking the hydroxy groups, exhibited also a good inhibitory activity (IC50 = 70 μM), demonstrating the ability of the aromatic scaffold itself to inhibit GALNS enzyme and suggesting a synergistic effect between the nonavalent scaffold and the bioactive unit.
Building on the promising IC50 values of nonavalent dendrimers 1 and 36, we investigated their stabilizing effects on GALNS through a thermal denaturation study. Both compounds preserved enzyme activity after 40 and 60 min of denaturation, with 1 maintaining high activity (almost two-fold vs. the control) at 500 µM, while 36 showed stabilization peaks at a much lower concentration (namely, 30 µM) with 2.4-fold enhanced activity with respect to the control after 60 min. These findings underscore their dual role as inhibitors and stabilizers, with 36 exhibiting a remarkable stabilization effect at lower concentration, which can be in principle exploited in a combined PC/ERT therapy for Morquio A syndrome.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30102222/s1: The thorough experimental section for this manuscript is detailed in the Supporting Information Refs. [65,66] are cited in Supplementary Materials.
Author Contributions
Conceptualization, F.C. (Francesca Cardona) C.M. and F.C. (Francesca Clemente); methodology, M.G.D., F.C. (Francesca Clemente), G.D., M.M.-B. and A.M. (Alessio Morano); writing—original draft preparation, M.G.D. and F.C. (Francesca Cardona); writing—review and editing, M.G.D., F.C. (Francesca Clemente), F.C. (Francesca Cardona), C.M., A.M. (Amelia Morrone) and A.G.; funding acquisition, F.C. (Francesca Cardona). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Regione Toscana (Bando Salute 2018) with the project “Late onset Lysosomal Storage Disorders (LSDs) in the differential diagnosis of neurodegenerative diseases: development of new diagnostic procedures and focus on potential pharmacological chaperones (Acronym: Lysolate)”. F. Cardona also acknowledges #NEXTGENERATIONEU (NGEU) funded by the Ministry of University and Research (MUR) (PRIN: PROGETTI DI RICERCA DI RILEVANTE INTERESSE NAZIONALE—Bando 2022, Award Number: Project code 2022N9E847) for the project MULTIFUNCTIONAL COMPOUNDS FOR A MULTI-TARGET APPROACH AGAINST NEURODEGENERATIVE DISORDERS (MULTIFUN). A. Morrone also thanks PRIN: PROGETTI DI RICERCA DI RILEVANTE INTERESSE NAZIONALE—Bando 2022, Award Number: Project code 20228S5LWY. Financial support provided by the MUR—Dipartimenti di Eccellenza 2023–2027 (DICUS 2.0) to the Department of Chemistry “Ugo Schiff” of the University of Florence is acknowledged.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by Ethics Committee (Comitato Etico Regione Toscana—Area Vasta Centro) of Azienda Ospedaliero Universitaria Careggi (Assigned code: Lysolate “Late-onset Lysosomal Storage Disorders (LSDs) in the differential diagnosis of neurodegenerative diseases: development of new diagnostic procedures and focus on potential pharmacological chaperones (PCs)”, project ID code: 16774_bio, 5 May 2020).
Informed Consent Statement
In keeping with ethical guidelines, all blood and cell samples were obtained for storage and analyzed only after written informed consent of the patients (and/or their family members) was obtained, using a form approved by the local Ethics Committee. Control and patient samples were anonymized and used only for research purposes.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Prof. Paolo Paoli for fruitful discussions. We acknowledge CISM—Mass Spectrometry Centre, University of Florence, 50139 Florence, Italy—for the HRMS spectra performed.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wittmann, V.; Pieters, R.J. Bridging lectin binding sites by multivalent carbohydrates. Chem. Soc. Rev. 2013, 42, 4492–4503. [Google Scholar] [CrossRef] [PubMed]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K. Synthetic Multivalent Molecules: Concepts and Biomedical Applications; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-471-57770-6. [Google Scholar]
- González-Cuesta, M.; Ortiz Mellet, C.; García Fernández, J.M. Carbohydrate supramolecular chemistry: Beyond the multivalent effect. Chem. Commun. 2020, 56, 5207–5222. [Google Scholar] [CrossRef]
- Leslie, K.G.; Berry, S.S.; Miller, G.J.; Mahon, C.S. Sugar-Coated: Can Multivalent Glycoconjugates Improve upon Nature’s Design? J. Am. Chem. Soc. 2024, 146, 27215–27232. [Google Scholar] [CrossRef]
- Diot, J.; García-Moreno, M.I.; Gouin, S.G.; Ortiz Mellet, C.; Haupt, K.; Kovensky, J. Multivalent iminosugars to modulate affinity and selectivity for glycosidases. Org. Biomol. Chem. 2009, 7, 357–363. [Google Scholar] [CrossRef]
- Kanfar, N.; Bartolami, E.; Zelli, R.; Marra, A.; Winum, J.-Y.; Ulrich, S.; Dumy, P. Emerging trends in enzyme inhibition by multivalent nanoconstructs. Org. Biomol. Chem. 2015, 13, 9894–9906. [Google Scholar] [CrossRef]
- Peracha, H.; Sawamoto, K.; Averill, L.; Kecskemethy, H.; Theroux, M.; Thacker, M.; Nagao, K.; Pizarro, C.; Mackenzie, W.; Kobayashi, H.; et al. Molecular genetics and metabolism, special edition: Diagnosis, diagnosis and prognosis of Mucopolysaccharidosis IVA. Mol. Genet. Metab. 2018, 125, 18–37. [Google Scholar] [CrossRef]
- Algahim, M.F.; Almassi, G.H. Current and emerging management options for patients with Morquio A syndrome. Ther. Clin. Risk Manag. 2013, 9, 45–53. [Google Scholar]
- Solanki, G.A.; Martin, K.W.; Theroux, M.C.; Lampe, C.; White, K.K.; Shediac, R.; Lampe, C.G.; Beck, M.; Mackenzie, W.G.; Hendriksz, C.J.; et al. Spinal involvement in mucopolysaccharidosis IVA (Morquio-Brailsford or Morquio A syndrome): Presentation, diagnosis and management. J. Inher. Metab. Dis. 2013, 36, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, K.; Gonzalez, J.V.A.; Piechnik, M.; Otero, F.J.; Couce, M.L.; Suzuki, Y.; Tomatsu, S. Mucopolysaccharidosis IVA: Diagnosis, treatment, and management. Int. J. Mol. Sci. 2020, 21, 1517–1543. [Google Scholar] [CrossRef]
- Haddley, K. Elosulfase alfa. Drugs Today 2014, 50, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Sanford, M.; Lo, J.H. Elosulfase alfa: First global approval. Drugs 2014, 74, 713–718. [Google Scholar] [CrossRef]
- Liguori, L.; Monticelli, M.; Allocca, M.; Hay Mele, B.; Lukas, J.; Cubellis, M.V.; Andreotti, G. Pharmacological chaperones: A therapeutic approach for diseases caused by destabilizing missense mutations. Int. J. Mol. Sci. 2020, 21, 489–508. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; García Fernández, J.M.; Ortiz Mellet, C. Glycomimetic-based pharmacological chaperones for lysosomal storage disorders: Lessons from Gaucher, GM1-gangliosidosis and Fabry diseases. Chem. Commun. 2016, 52, 5497–5515. [Google Scholar] [CrossRef]
- Boyd, R.E.; Lee, G.; Rybczynski, P.; Benjamin, E.R.; Khanna, R.; Wustman, B.A.; Valenzano, K.J. Pharmacological Chaperones as Therapeutics for Lysosomal Storage Diseases. J. Med. Chem. 2013, 56, 2705–2725. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Andrade, P.B. Tuning protein folding in lysosomal storage diseases: The chemistry behind pharmacological chaperones. Chem. Sci. 2018, 9, 1740–1752. [Google Scholar] [CrossRef]
- Convertino, M.; Das, J.; Dokholyan, N.V. Pharmacological Chaperones: Design and Development of New Therapeutic Strategies for the Treatment of Conformational Diseases. ACS Chem. Biol. 2016, 11, 1471–1489. [Google Scholar] [CrossRef]
- Khanna, R.; Flanagan, J.J.; Feng, J.; Soska, R.; Frascella, M.; Pellegrino, L.J.; Lun, Y.; Guillen, D.; Lockhart, D.J.; Valenzano, K.J. The Pharmacological Chaperone AT2220 Increases Recombinant Human Acid α-Glucosidase Uptake and Glycogen Reduction in a Mouse Model of Pompe Disease. PLoS ONE 2012, 7, e40776. [Google Scholar] [CrossRef]
- Shen, J.-S.; Edwards, N.J.; Hong, Y.B.; Murray, G.J. Isofagomine increases lysosomal delivery of exogenous glucocerebrosidase. Biochem. Biophys. Res. Commun. 2008, 369, 1071–1075. [Google Scholar] [CrossRef][Green Version]
- Porto, C.; Cardone, M.; Fontana, F.; Rossi, B.; Tuzzi, M.R.; Tarallo, A.; Barone, M.V.; Andria, G.; Parenti, G. The pharmacological chaperone N-butyldeoxynojirimycin enhances enzyme replacement therapy in Pompe disease fibroblasts. Mol. Ther. 2009, 17, 964–971. [Google Scholar] [CrossRef]
- Benjamin, E.R.; Khanna, R.; Schilling, A.; Flannagan, J.J.; Pellegrino, L.J.; Brignol, N.; Lun, Y.; Guillen, D.; Ranes, B.E.; Frascella, M.; et al. Co-administration With the Pharmacological Chaperone AT1001 Increases Recombinant Human α-Galactosidase A Tissue Uptake and Improves Substrate Reduction in Fabry Mice. Mol. Ther. 2012, 20, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Parenti, G.; Fecarotta, S.; la Marca, G.; Rossi, B.; Ascione, S.; Donati, M.A.; Morandi, L.O.; Ravaglia, S.; Pichiecchio, A.; Ombrone, D.; et al. chaperone enhances blood α-glucosidase activity in Pompe disease patients treated with enzyme replacement therapy. Mol. Ther. 2014, 22, 2004–2012. [Google Scholar] [CrossRef]
- Schoser, B.; Roberts, M.; Byrne, B.J.; Sitaraman, S.; Jiang, H.; Laforêt, P.; Toscano, A.; Castelli, J.; Díaz-Manera, J.; Goldman, M.; et al. Safety and efficacy of cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo in late-onset Pompe disease (PROPEL): An international, randomised, double-blind, parallel-group, phase 3 trial. Lancet Neurol. 2021, 20, 1027–1037. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Feferman, L.; Tobacman, J.K. Dihydrotestosterone inhibits arylsulfatase B and Dickkopf Wnt signaling pathway inhibitor (DKK)-3 leading to enhanced Wnt signaling in prostate epithelium in response to stromal Wnt3A. Prostate 2019, 79, 689–700. [Google Scholar] [CrossRef]
- Müller, C.E.; Claff, T. Activated microglia nibbling glycosaminoglycans from spinal cord perineural nets: A new mechanism for neuropathic pain. Signal Transduct. Target. Ther. 2022, 7, 333–335. [Google Scholar] [CrossRef]
- Olarte-Avellaneda, S.; Cepeda Del Castillo, J.; Rojas-Rodriguez, A.F.; Sánchez, O.; Rodríguez-López, A.; Suárez García, D.A.; Salazar Pulido, L.M.; Alméciga-Díaz, C.J. Bromocriptine as a Novel Pharmacological Chaperone for Mucopolysaccharidosis IV. ACS Med. Chem. Lett. 2020, 11, 1377–1385. [Google Scholar] [CrossRef]
- Alméciga, C.J.; Hidalgo, O.A.; Olarte-Avellaneda, S.; Rodríguez-López, A.; Guzman, E.; Garzón, R.; Pimentel-Vera, L.N.; Puentes-Tellez, M.A.; Rojas-Rodriguez, A.F.; Gorshkov, K.; et al. Identification of Ezetimibe and Pranlukast as Pharmacological Chaperones for the Treatment of the Rare Disease Mucopolysaccharidosis Type IVA. J. Med. Chem. 2019, 62, 6175–6189. [Google Scholar] [CrossRef]
- Losada Díaz, J.C.; Cepeda del Castillo, J.; Rodriguez-López, E.A.; Alméciga-Díaz, C.J. Advances in the Development of Pharmacological Chaperones for the Mucopolysaccharidoses. Int. J. Mol. Sci. 2020, 21, 232–251. [Google Scholar] [CrossRef]
- Rivera-Colón, Y.; Schutsky, E.K.; Kita, A.Z.; Garman, S.C. The Structure of Human GALNS Reveals the Molecular Basis for Mucopolysaccharidosis IV A. J. Mol. Biol. 2012, 423, 736–751. [Google Scholar] [CrossRef]
- Compain, P.; Bodlenner, A. The multivalent effect in glycosidase inhibition: A new, rapidly emerging topic in glycoscience. Chem. Bio. Chem. 2014, 15, 1239–1251. [Google Scholar] [CrossRef]
- Gouin, S.G. Multivalent inhibitors for carbohydrate-processing enzymes: Beyond the “lock-and-key” concept. Chem. Eur. J. 2014, 20, 11616–11628. [Google Scholar] [CrossRef] [PubMed]
- Zelli, R.; Longevial, J.-F.; Dumy, P.; Marra, A. Synthesis and biological properties of multivalent iminosugars. New J. Chem. 2015, 30, 5050–5074. [Google Scholar] [CrossRef]
- Matassini, C.; Parmeggiani, C.; Cardona, F.; Goti, A. Are enzymes sensitive to the multivalent effect? Emerging evidence with glycosidases. Tetrahedron Lett. 2016, 57, 5407–5415. [Google Scholar] [CrossRef]
- Compain, P. Multivalent effect in glycosidase inhibition: The end of the beginning. Chem. Rec. 2020, 20, 10–22. [Google Scholar] [CrossRef]
- Martínez-Bailén, M.; Carmona, A.T.; Cardona, F.; Matassini, C.; Goti, A.; Kubo, M.; Kato, A.; Robina, I.; Moreno-Vargas, A.J. Synthesis of multimeric pyrrolidine iminosugar inhibitors of human β-glucocerebrosidase and α-galactosidase A: First example of a multivalent enzyme activity enhancer for Fabry disease. Eur. J. Med. Chem. 2020, 192, 112173. [Google Scholar] [CrossRef]
- D’Adamio, G.; Matassini, C.; Parmeggiani, C.; Catarzi, S.; Morrone, A.; Goti, A.; Paoli, P.; Cardona, F. Evidence for a multivalent effect in inhibition of sulfatases involved in lysosomal storage disorders (LSDs). RSC Adv. 2016, 6, 64847–64851. [Google Scholar] [CrossRef]
- Matassini, C.; D’Adamio, G.; Vanni, C.; Goti, A.F. Cardona Studies for the Multimerization of DAB-1-Based Iminosugars through Iteration of the Nitrone Cycloaddition/Ring-Opening/Allylation Sequence. Eur. J. Org. Chem. 2019, 4897–4905. [Google Scholar] [CrossRef]
- Matassini, C.; Vanni, C.; Goti, A.; Morrone, A.; Marradi, M.; Cardona, F. Multimerization of DAB-1 onto Au GNPs affords new potent and selective N-acetylgalactosamine-6-sulfatase (GALNS) inhibitors. Org. Biomol. Chem. 2018, 16, 8604–8612. [Google Scholar] [CrossRef]
- Buco, F.; Matassini, C.; Vanni, C.; Clemente, F.; Paoli, P.; Carozzini, C.; Beni, A.; Cardona, F.; Goti, A.; Moya, S.E.; et al. Gold nanoparticles decorated with monosaccharides and sulfated ligands as potential modulators of the lysosomal enzyme N-acetylgalactosamine-6-sulfatase (GALNS). Org. Biomol. Chem. 2023, 21, 9362–9371. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.C.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- D’Adamio, G.; Parmeggiani, C.; Goti, A.; Moreno-Vargas, A.J.; Moreno-Clavijo, E.; Robina, I.; Cardona, F. 6-Azido hyacinthacine A2 gives a straightforward access to the first multivalent pyrrolizidine architectures. Org. Biomol. Chem. 2014, 12, 6250–6266. [Google Scholar] [CrossRef]
- Chabre, Y.M.; Contino-Pépin, C.; Placide, V.; Shiao, T.C.; Roy, R. Expeditive Synthesis of Glycodendrimer Scaffolds Based on Versatile TRIS and Mannoside Derivatives. J. Org. Chem. 2008, 73, 5602–5605. [Google Scholar] [CrossRef]
- Segura, M.; Sansone, F.; Casnati, A.; Ungaro, R. Synthesis of Lower Rim Polyhydroxylated Calix [4]arenes. Synthesis 2001, 2105–2112. [Google Scholar] [CrossRef]
- Mourer, M.; Hapiot, F.; Tilloy, S.; Monflier, E.; Menuel, S. Easily Accessible Mono-and Polytopic β-Cyclodextrin Hosts by Click Chemistry. Eur. J. Org. Chem. 2008, 5723–5730. [Google Scholar] [CrossRef]
- Papp, I.; Dernedde, J.; Enders, S.; Haag, R. Modular synthesis of multivalent glycoarchitectures and their unique selectin binding behavior. Chem. Commun. 2008, 5851–5853. [Google Scholar] [CrossRef]
- Martínez-Bailén, M.; Jiménez-Ortega, E.; Carmona, A.T.; Robina, I.; Sanz-Aparicio, J.; Talens-Perales, D.; Polaina, J.; Matassini, C.; Cardona, F.; Moreno-Vargas, A.J. Structural basis of the inhibition of GH1 β-glucosidases by multivalent pyrrolidine iminosugars. Bioorg. Chem. 2019, 89, 103026. [Google Scholar] [CrossRef]
- Chabre, Y.M.; Giguère, D.; Blanchard, B.; Rodrigue, J.; Rocheleau, S.; Neault, M.; Rauthu, S.; Papadopoulos, A.; Arnold, A.A.; Imberty, A.; et al. Combining glycomimetic and multivalent strategies toward designing potent bacterial lectin inhibitors. Chem. Eur. J. 2011, 17, 6545–6562. [Google Scholar] [CrossRef]
- Mirabella, S.; D’Adamio, G.; Matassini, C.; Goti, A.; Delgado, S.; Gimeno, A.; Robina, I.; Moreno-Vargas, A.J.; Šesták, S.; Jiménez-Barbero, J.; et al. Mechanistic Insight into the Binding of Multivalent Pyrrolidines to α-Mannosidases. Chem. Eur. J. 2017, 23, 14585–14596. [Google Scholar] [CrossRef]
- Matassini, C.; Mirabella, S.; Goti, A.; Robina, I.; Moreno-Vargas, A.J.; Cardona, F. Exploring architectures displaying multimeric presentations of a trihydroxypiperidine iminosugar. Beilstein J. Org. Chem. 2015, 11, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
- Joosten, A.; Decroocq, C.; de Sousa, J.; Schneider, J.; Etamé, E.; Bodlenner, A.; Butters, T.D.; Compain, P. A systematic investigation of iminosugar click clusters as pharmacological chaperones for the treatment of Gaucher disease. ChemBioChem 2014, 15, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Desvergnes, S.; Vallée, Y.; Py, S. Novel Polyhydroxylated Cyclic Nitrones and N-Hydroxypyrrolidines through BCl3-Mediated Deprotection. Org. Lett. 2008, 10, 2967–2970. [Google Scholar] [CrossRef]
- Ferhati, X.; Matassini, C.; Fabbrini, M.G.; Goti, A.; Morrone, A.; Cardona, F.; Moreno-Vargas, A.J.; Paoli, P. Dual targeting of PTP1B and glucosidases with new bifunctional iminosugar inhibitors to address type 2 diabetes. Bioorg. Chem. 2019, 87, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Coutrot, F.; Busseron, E. Controlling the Chair Conformation of a Mannopyranose in a Large-Amplitude [2] Rotaxane Molecular Machine. Chem. Eur. J. 2009, 15, 5186–5190. [Google Scholar] [CrossRef]
- Miyagawa, A.; Yamamura, H. Synthesis of β-1,3-glucan mimics by β-1,3-glucan trisaccharyl monomer polymerization. Carbohydr. Polym. 2020, 227, 115105–115113. [Google Scholar] [CrossRef]
- Kushwaha, D.; Xu, P.; Kováč, P. Carbohydrates as potentially versatile core subcarriers for multivalent immunogens. RSC Adv. 2017, 7, 7591–7603. [Google Scholar] [CrossRef]
- Van Scherpenzeel, M.; Van den Berg, R.; Donker-Koopman, W.E.; Liskamp, R.M.J.; Aerts, J.M.F.G.; Overkleeft, H.S.; Pieters, R.J. Nanomolar affinity, iminosugar-based chemical probes for specific labeling of lysosomal glucocerebrosidase. Bioorg. Med. Chem. 2010, 18, 267–273. [Google Scholar] [CrossRef]
- Higa, C.M.; Tek, A.T.; Wojtecki, R.J.; Braslau, R. Nonmigratory Internal Plasticization of Poly(Vinyl Chloride) via Pendant Triazoles Bearing Alkyl or Polyether Esters. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2397–2411. [Google Scholar] [CrossRef]
- Matassini, C.; Mirabella, S.; Xhenti, F.; Faggi, C.; Robina, I.; Goti, A.; Moreno-Clavijo, E.; Moreno-Vargas, A.J.; Cardona, F. Polyhydroxyamino-Piperidine-Type Iminosugars and Pipecolic Acid Analogues from a D-Mannose-Derived Aldehyde. Eur. J. Org. Chem. 2014, 5419–5432. [Google Scholar] [CrossRef]
- Vanni, C.; Clemente, F.; Paoli, P.; Morrone, A.; Matassini, C.; Goti, A.; Cardona, F. 3,4,5-Trihydroxypiperidine Based Multivalent Glucocerebrosidase (GCase) Enhancers. ChemBioChem 2022, 23, e202200077. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, K.; Suzuki, T. Aniline–terephthalaldehyde resin p-toluenesulfonic acid (ATRT) salt as efficient mild polymeric solid acid catalyst. Tetrahedron Letters 2013, 54, 6740–6743. [Google Scholar] [CrossRef]
- Krężel, A.; Bal, W. A formula for correlating pKa values determined in D2O and H2O. J. Inorg. Biochem. 2004, 98, 161–166. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A.J. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Michihata, N.; Kaneko, Y.; Kasai, Y.; Tanigawa, K.; Hirokane, T.; Higasa, S.; Yamada, H. High-yield total synthesis of (-)-strictinin through intramolecular coupling of gallates. J. Org. Chem. 2013, 78, 4319–4328. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).