Alkaloids in Tibetan Medicine Corydalis conspersa Maxim. and Their Hepatoprotective Effect Against Acute Liver Injury
Abstract
:1. Introduction
2. Results
2.1. Alkaloids in Corydalis conspersa Maxim.
2.2. NP Prediction Results
2.2.1. Possible Targets of ALI Affected by the Chemical Components of Corydalis conspersa Maxim.
2.2.2. Construction of Protein–Protein Interaction (PPI) Network
2.2.3. Construction of Drug-Active Ingredient-Target Network Diagram
2.2.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
2.2.5. Molecular Docking Verification Results
2.3. Experimental Results of Hepatoprotective Effect
2.3.1. Changes in Serum Biochemical Indicators of Mice
2.3.2. Changes in C-Reactive Protein (CRP) Levels in Mouse Liver
2.3.3. Results of H&E-Stained Sections
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.1.1. Experimental Medicinal Materials
4.1.2. Animals and Drugs
4.1.3. Reagents and Instruments
4.2. Methods
4.2.1. Extraction and Separation Methods
4.2.2. Research Methods of NP
4.2.3. Pharmacological Experimental Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full name of compound |
| CCl4 | Carbon tetrachloride |
| ALI | Acute liver injury |
| NP | Network pharmacology |
| ACE | Acetylcorynoline |
| BBC | Bulbocorynoline |
| ISO | Isocorynidine |
| KM | Kunming |
| PPI | Protein–Protein Interaction |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| NP | Network pharmacology |
| BP | Biological process |
| CC | Cellular component |
| MF | Molecular function |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| ALP | Alkaline phosphatase |
| CON | Control |
| SIL | Silymarin |
| CRP | C-reactive protein |
| MeOH | Methanol |
| PE | Petroleum ether |
| EtOAc | Ethyl acetate |
| CHCl3 | Chloroform |
| HCl | Hydrochloric acid |
| EtOH | Anhydrous ethanol |
| SGCC | Silica gel column chromatography |
| LPS | Lipopolysaccharide |
| D-GaIN | D-Galactosamine |
References
- Bungau, S.; Popa, V.-C. Between Religion and Science: Some Aspects: Concerning Illness and Healing in Antiquity. Transylv. Rev. 2015, XXIV, 3–19. [Google Scholar]
- Du, Q.; Cai, Y.; Chen, Z.; Wei, D.; Cao, Y.; Chen, Y.; Yu, S.; Zhao, Q.; Wu, J.; Liu, M. Determination of Trace Elements in Corydalis conspersa and Corydalis linarioides by ICP-AES. J. Chem. 2020, 2020, 6567015. [Google Scholar] [CrossRef]
- Que, S.; Chen, W.-D.; Yang, S.-P.; Lin, P.-C.; Ye, J. Investigation of Tibetan medicine resources of Corydalis conspersa Maxim. from Qinghai. J. Chin. Med. Mater. 2017, 40, 2551–2556. [Google Scholar]
- Wang, Z.-m.; Wang, Q.; Lei, H.-F.; Fu, Y.-X.; Wang, X.-L. Antioxidant and Antimicrobial Activities of Different Polar Parts ofTibetan Medicine Corydalis Conspersa Maxim. Guangzhou Chem. Ind. 2024, 52, 78–82. [Google Scholar]
- Que, S.; Chen, W.-D.; Wu, J.; Lin, P.-C. Chemical constituents from Corydalis conspersa. Chin. Tradit. Pat. Med. 2016, 38, 2405–2408. [Google Scholar]
- Wu, J.; Zhang, M.; Lin, P.-C. Study on chemical constituents and pharmacological activities from the Tibetan medicine Corydalis conspersa Maxim. China J. Tradit. Chin. Med. Pharm. 2023, 38, 3773–3779. [Google Scholar]
- Que, S.; Da, L.-J.; Zeng, Q.-Y.; Lin, P.-C.; Chen, W.-D. Chemical constituents of the volatile component from Corydalis conspersaMaxim by supercritical CO2 fluid extraction and their antibacterial activity. Chin. J. New Drugs 2017, 26, 203–207. [Google Scholar]
- Li, J.; Song, D.; Zhang, B.; Guo, J.; Li, W.; Zhang, X.; Zhao, Q. Hepatoprotective Effects of Heracleum candicans Against Carbon Tetrachloride-Induced Acute Liver Injury in Rats. Dose Response 2021, 19, 15593258211029510. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abo-El-Sooud, K.; Aleya, L.; Bungǎu, S.G.; Najda, A.; Saluja, R. Alleviation of Drugs and Chemicals Toxicity: Biomedical Value of Antioxidants. Oxid. Med. Cell. Longev. 2018, 2018, 6276438. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Zakhary, N.I.; Aleya, L.; Bungǎu, S.G.; Bohara, R.A.; Siddiqi, N.J. Aging, Metabolic, and Degenerative Disorders: Biomedical Value of Antioxidants. Oxid. Med. Cell. Longev. 2018, 2018, 2098123. [Google Scholar] [CrossRef]
- Li, S.-Y.; Wang, L.-S.; You, L.-P.; Lin, J.-C.; Kong, X.-N.; Gao, Y.-Q.; Sun, X.-H. Mechanism of Sophocarpine in Improving Acetaminophen-Induced Acute Liver Injury in Mice. Pharmacol. Clin. Chin. Mater. Med. 2023, 39, 46–52. [Google Scholar]
- Zhou, Z.; Chen, B.; Chen, S.; Lin, M.; Chen, Y.; Jin, S.; Chen, W.; Zhang, Y. Applications of Network Pharmacology in Traditional Chinese Medicine Research. Evid. Based Complement. Alternat. Med. 2020, 2020, 1646905. [Google Scholar] [CrossRef]
- Liu, B.; Fang, Y.; Yi, R.; Zhao, X. Preventive Effect of Blueberry Extract on Liver Injury Induced by Carbon Tetrachloride in Mice. Foods 2019, 8, 48. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhang, M.; Chen, Y.; Zhu, T.; Wang, J. Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice. Mar. Drugs 2018, 16, 300. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Z.; Zhang, J.; Mu, J.; Zhou, X.; Zhao, X. Liver Injury Induced by Carbon Tetrachloride in Mice Is Prevented by the Antioxidant Capacity of Anji White Tea Polyphenols. Antioxidants 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Dan, X.; Du, X.X.; Ran, C.L.; Lu, X.; Ren, S.J.; Tang, Z.T.; Yin, L.Z.; He, C.L.; Yuan, Z.X.; et al. Protective Effects of Salidroside against Carbon Tetrachloride (CCl(4))-Induced Liver Injury by Initiating Mitochondria to Resist Oxidative Stress in Mice. Int. J. Mol. Sci. 2019, 20, 3187. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, C.-J.; Zhong, M.-L.; Jiang, X.-C.; Liu, G.-F. Chemical Constituents from Corydalis bungeana Turcz. Nat. Prod. Res. Dev. 2013, 25, 1665–1668+1689. [Google Scholar]
- Wangchuk, P.; Keller, P.A.; Pyne, S.G.; Willis, A.C.; Kamchonwongpaisan, S. Antimalarial alkaloids from a Bhutanese traditional medicinal plant Corydalis dubia. J. Ethnopharmacol. 2012, 143, 310–313. [Google Scholar] [CrossRef]
- Kim, A.H.; Jang, J.H.; Woo, K.W.; Park, J.E.; Lee, K.H.; Jung, H.K.; An, B.; Jung, W.S.; Ham, S.H.; Cho, H.W. Chemical constituents of Dicentra spectabilis and their anti-inflammation effect. J. Appl. Biol. Chem. 2018, 61, 39–46. [Google Scholar] [CrossRef]
- Liao, H.-P.; Ouyang, H.; Huang, L.-Q.; Huang, W.-P.; Feng, Y.-L.; Li, Z.-F.; Wu, B.; Yang, S.-L. Chemical constituents of Corydalis decumbens. Chin. Tradit. Herb. Drugs 2014, 45, 3067–3070. [Google Scholar]
- Sun, G.-F.; Chen, F.-Z.; Tian, C.; Cheng, Y.; Li, S.-H. Isolation and identificatian of the alkaloids from rhizomes of Stephania macrantha. Guihaia 2023, 43, 2106–2112. [Google Scholar]
- Yang, Y.M.; Cho, Y.E.; Hwang, S. Crosstalk between Oxidative Stress and Inflammatory Liver Injury in the Pathogenesis of Alcoholic Liver Disease. Int. J. Mol. Sci. 2022, 23, 774. [Google Scholar] [CrossRef] [PubMed]
- Ingawale, D.K.; Mandlik, S.K.; Naik, S.R. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): A critical discussion. Environ. Toxicol. Pharmacol. 2014, 37, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zhang, S.; Yin, L.; Tang, X.; Xu, Y.; Han, X.; Qi, Y.; Peng, J. Protective effects of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013, 62, 120–130. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Z.; Zhao, Y.; Li, X.; Jiang, C.; He, H.; Huo, J.; Xiao, Q.; Wu, J.; Zhu, F.; et al. Acetylcorynoline alleviates acute liver injury via inhibiting TLR4/JNK/NF-ĸB pathway Based on RNA-seq and molecular docking in vivo and in vitro. Int. Immunopharmacol. 2024, 143, 113550. [Google Scholar] [CrossRef]
- Shen, C.-Z.; Wang, X.-H.; Li, C.; Shen, P.-P.; Xie, H.-T. Exploration on the Mechanism of Schisandra chinensisin the Treatment of Acute Liver Injury Based on Network Pharmacology and Molecular Docking. J. Hubei Minzu Univer.(Nat. Sci. Ed.) 2022, 40, 33–39. [Google Scholar]
- Feng, P.-W.; Han, J.-C.; Li, D.-F.; Wang, F.-H.; Fang, X.; Zheng, Q.-S. Hepatoprotective Effect of Corynoline on Carbon Tetrachloride-induced Hepatotoxicity in Mice. Nat. Prod. Res. Dev 2017, 29, 224–228. [Google Scholar]
- Chen, Z.-H.; Zhang, Z.-X.; Wang, M.-D. Pharmacological and toxicity studies of Isocorydine. Pharmacol. Clin. Chin. Mater. Med. 1985, 00, 181–182. [Google Scholar]
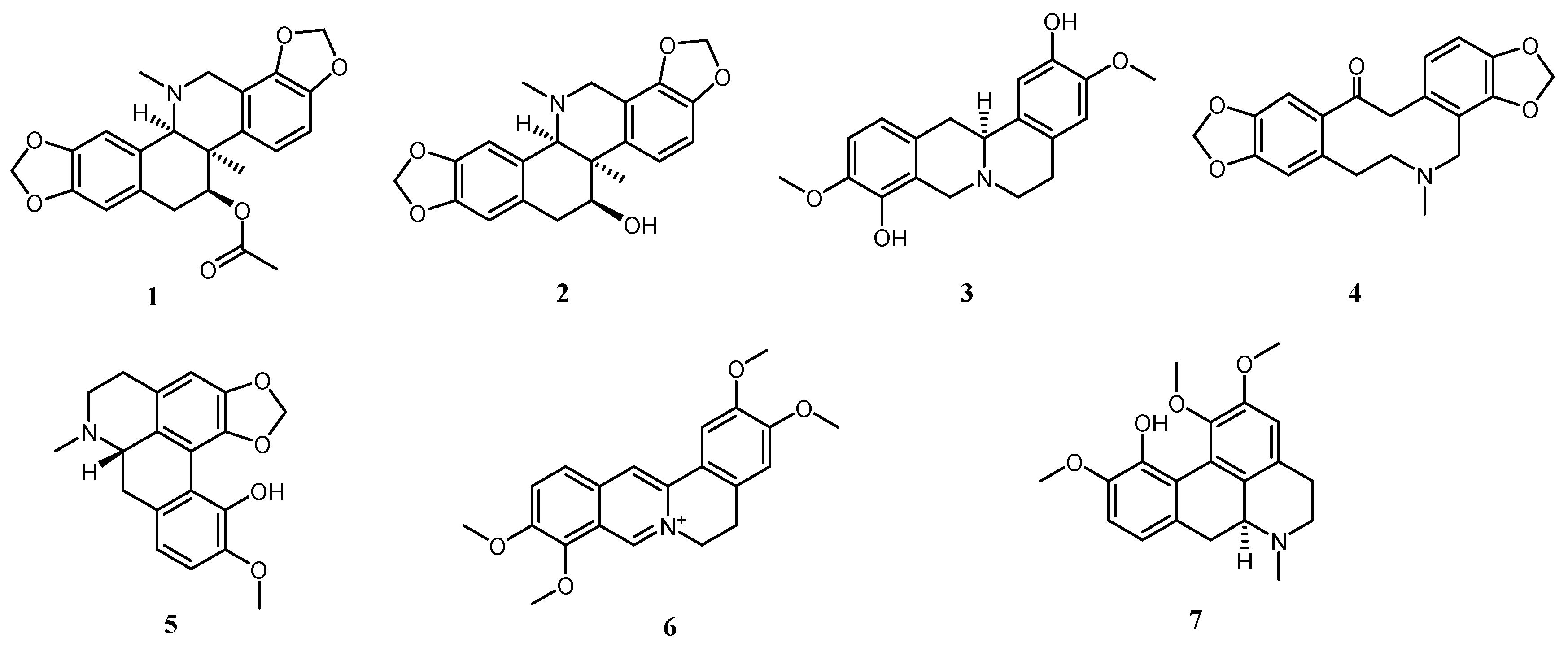
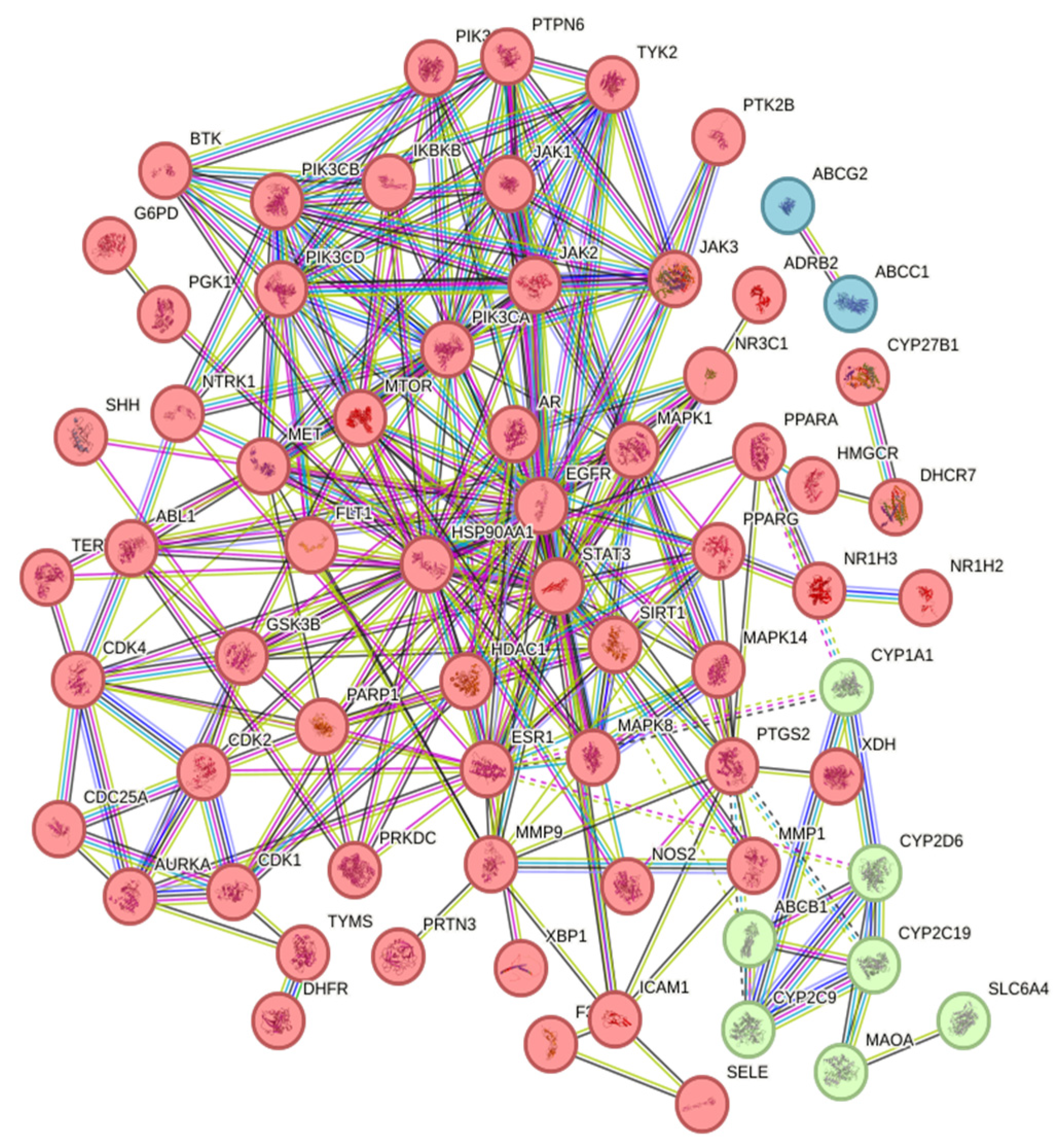
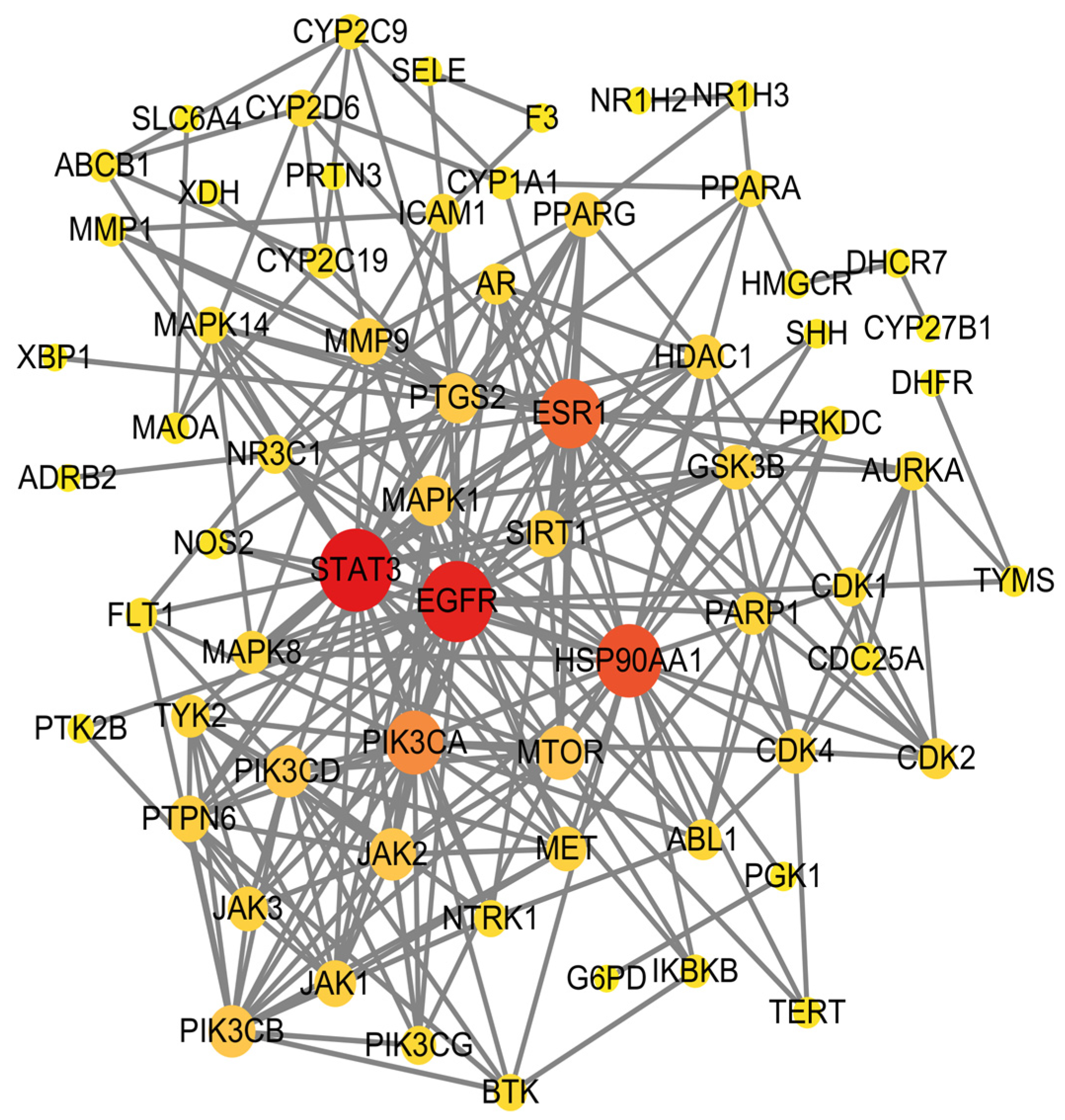
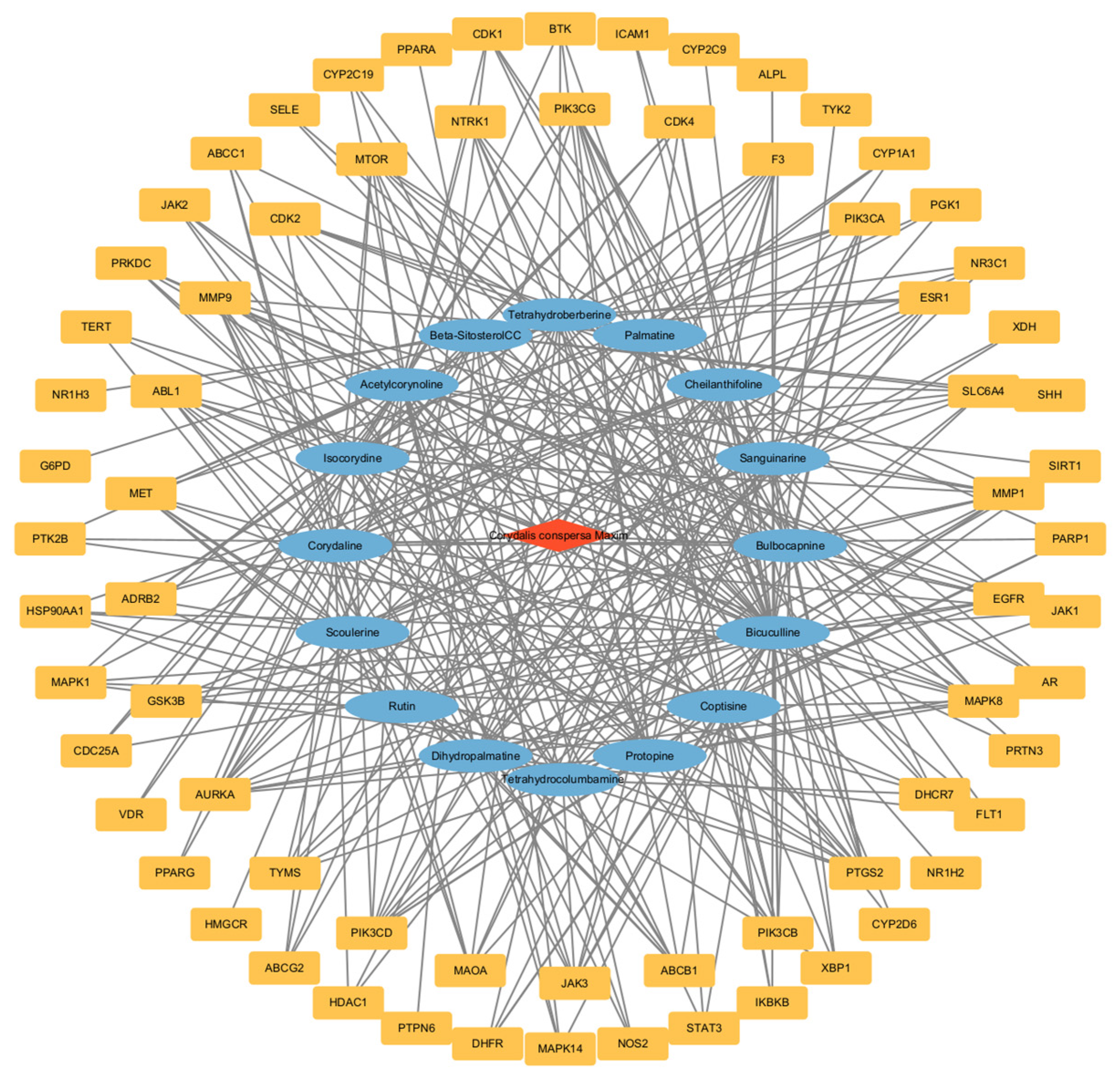
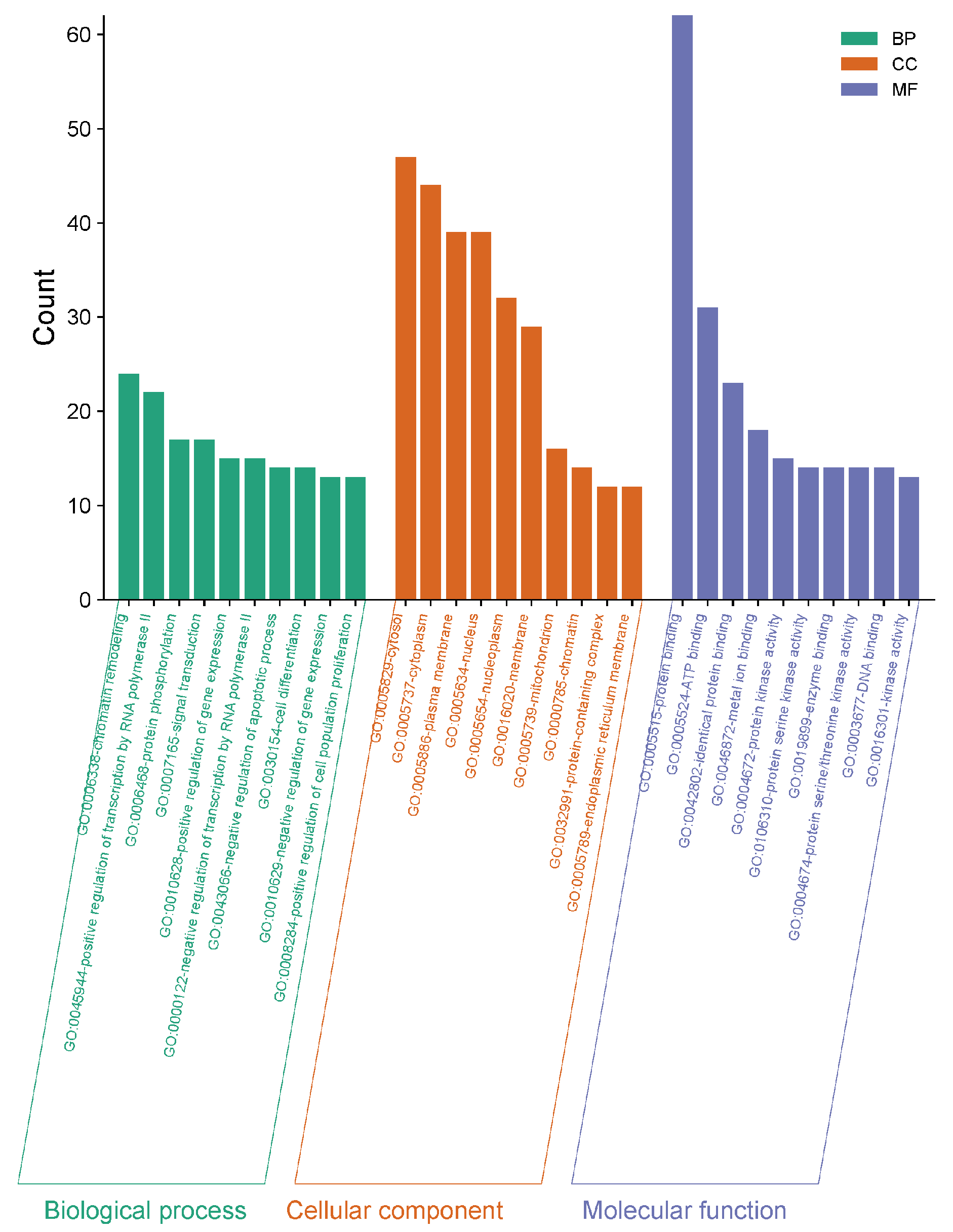
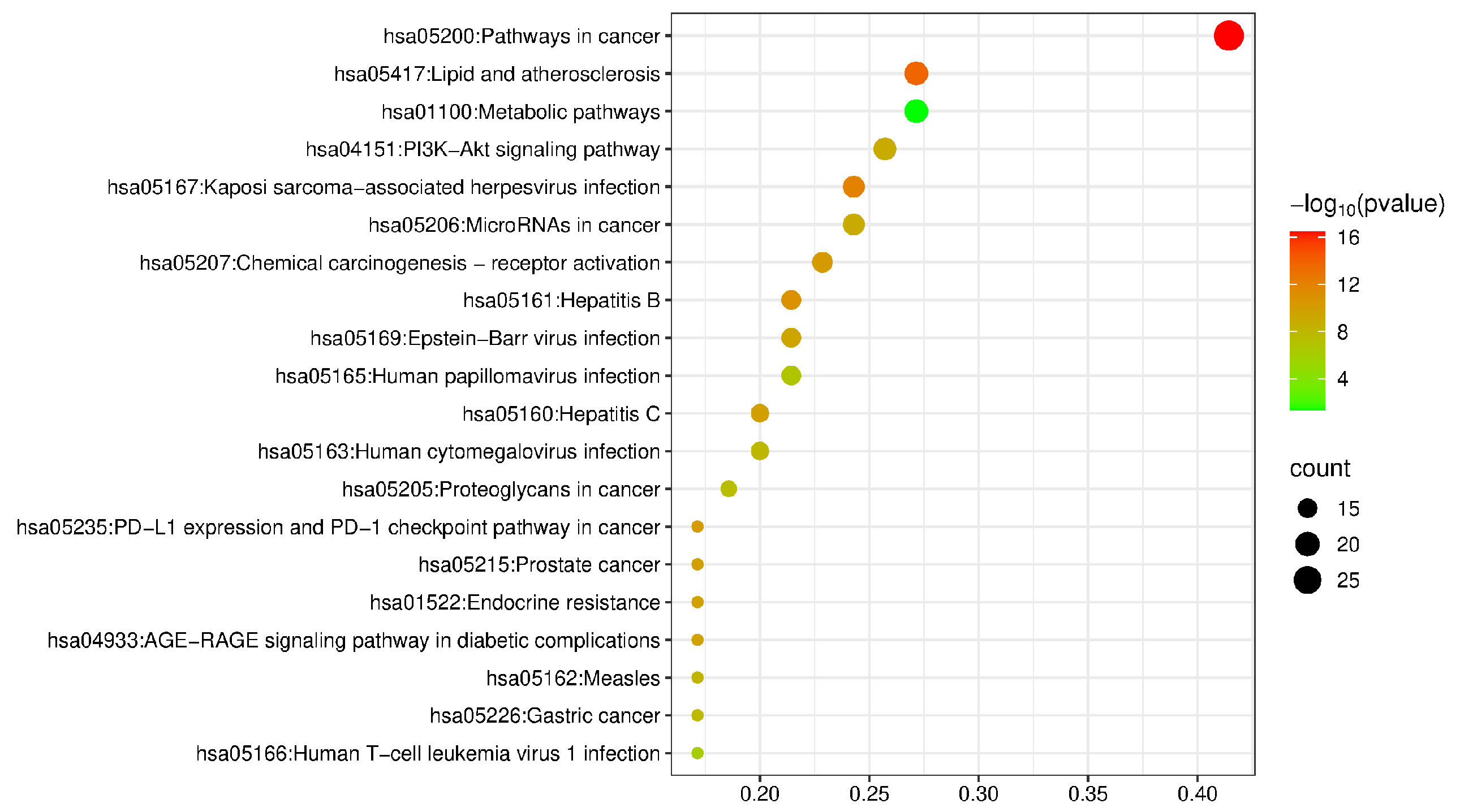
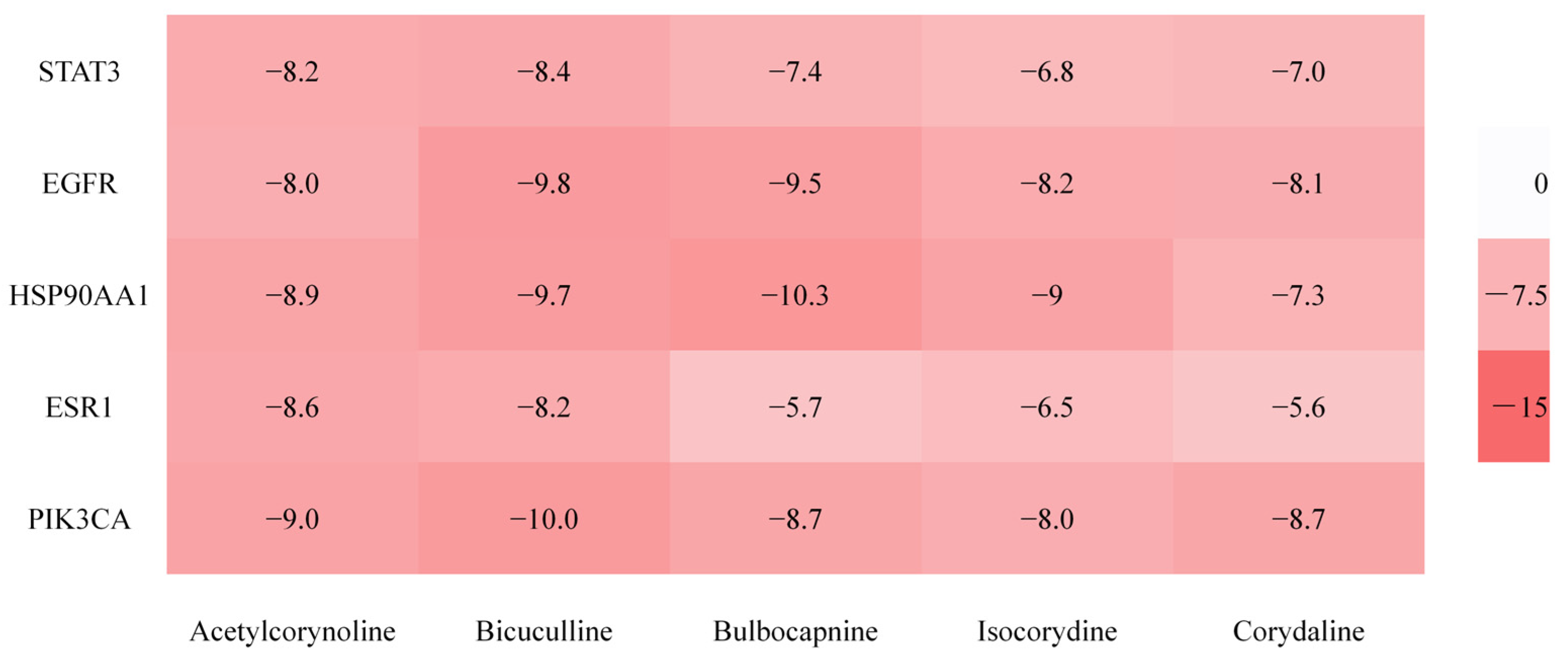


| Order Number | Gene | Degree |
|---|---|---|
| 1 | STAT3 | 30 |
| 2 | EGFR | 29 |
| 3 | HSP90AA1 | 25 |
| 4 | ESR1 | 23 |
| 5 | PIK3CA | 20 |
| Number | Name | CAS or Pubchem ID | Degree |
|---|---|---|---|
| 1 | Bicuculline | 485-49-4 | 43 |
| 2 | Acetylcorynoline | 18797-80-3 | 30 |
| 3 | Corydaline | 6451-73-6 | 25 |
| 4 | Bulbocapnine | 298-45-3 | 24 |
| 5 | Isocorydine | 475-67-2 | 24 |
| Groups | AST/(U·L−1) | ALT/(U·L−1) | ALP/(U·L−1) |
|---|---|---|---|
| CON | 132.43 ± 12.06 | 42.80 ± 7.18 | 56.60 ± 5.51 |
| CCl4 | 405.50 ± 18.34 ### | 205.60 ± 13.93 ### | 255.23 ± 12.44 ### |
| ACE 5 mg/kg | 331.93 ± 15.95 *** | 169.97 ± 11.51 ** | 223.43 ± 14.54 ** |
| ACE 10 mg/kg | 279.23 ± 12.58 *** | 131.30 ± 12.35 *** | 175.20 ± 12.87 *** |
| ACE 20 mg/kg | 184.07 ± 14.45 *** | 91.23 ± 6.09 *** | 92.08 ± 10.06 *** |
| BBC 5 mg/kg | 316.23 ± 12.55 *** | 173.13 ± 10.27 ** | 217.87 ± 13.08 *** |
| BBC 10 mg/kg | 246.93 ± 9.11 *** | 149.60 ± 8.22 *** | 151.60 ± 10.98 *** |
| BBC 20 mg/kg | 169.63 ± 18.23 *** | 101.50 ± 12.46 *** | 104.07 ± 8.67 *** |
| ISO 5 mg/kg | 345.64 ± 13.31 *** | 178.61 ± 9.72 ** | 238.52 ± 10.29 * |
| ISO 10 mg/kg | 291.34 ± 10.72 *** | 152.93 ± 12.18 *** | 189.97 ± 11.73 *** |
| ISO 20 mg/kg | 223.58 ± 16.29 *** | 126.27 ± 11.24 *** | 131.82 ± 8.18 *** |
| TTA 50 mg/kg | 326.81 ± 11.84 *** | 164.59 ± 9.41 ** | 229.39 ± 9.92 ** |
| TTA 100 mg/kg | 261.39 ± 13.05 *** | 138.67 ± 8.08 *** | 162.21 ± 8.06 *** |
| TTA 200 mg/kg | 208.67 ± 9.12 *** | 93.25 ± 12.57 *** | 110.41 ± 6.27 *** |
| SIL 10 mg/kg | 167.28 ± 16.17 *** | 83.18 ± 8.29 *** | 112.26 ± 7.69 *** |
| Groups | CRP/ (μg·L−1) |
|---|---|
| CON | 541.93 ± 34.84 |
| CCl4 | 1792.50 ± 147.36 ### |
| ACE 5 mg/kg | 1509.87 ± 136.31 * |
| ACE 10 mg/kg | 1183.27 ± 104.47 *** |
| ACE 20 mg/kg | 729.20 ± 112.17 *** |
| BBC 5 mg/kg | 1558.87 ± 134.30 * |
| BBC 10 mg/kg | 1241.40 ± 121.32 *** |
| BBC 20 mg/kg | 796.53 ± 91.47 *** |
| ISO 5 mg/kg | 1631.92 ± 135.68 |
| ISO 10 mg/kg | 1425.64 ± 118.29 ** |
| ISO 20 mg/kg | 947.56 ± 124.64 *** |
| TTA 50 mg/kg | 1438.81 ± 141.21 ** |
| TTA 100 mg/kg | 1221.34 ± 128.63 *** |
| TTA 200 mg/kg | 803.91 ± 99.71 *** |
| SIL 10 mg/kg | 857.81 ± 81.82 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Jin, Y.; Fan, F.; Feng, X.; Yin, X.; Wang, X.; Geng, Z. Alkaloids in Tibetan Medicine Corydalis conspersa Maxim. and Their Hepatoprotective Effect Against Acute Liver Injury. Molecules 2025, 30, 2127. https://doi.org/10.3390/molecules30102127
Wang Q, Jin Y, Fan F, Feng X, Yin X, Wang X, Geng Z. Alkaloids in Tibetan Medicine Corydalis conspersa Maxim. and Their Hepatoprotective Effect Against Acute Liver Injury. Molecules. 2025; 30(10):2127. https://doi.org/10.3390/molecules30102127
Chicago/Turabian StyleWang, Qiu, Yingrui Jin, Fangyan Fan, Xueting Feng, Xuemei Yin, Xiaoling Wang, and Zangjia Geng. 2025. "Alkaloids in Tibetan Medicine Corydalis conspersa Maxim. and Their Hepatoprotective Effect Against Acute Liver Injury" Molecules 30, no. 10: 2127. https://doi.org/10.3390/molecules30102127
APA StyleWang, Q., Jin, Y., Fan, F., Feng, X., Yin, X., Wang, X., & Geng, Z. (2025). Alkaloids in Tibetan Medicine Corydalis conspersa Maxim. and Their Hepatoprotective Effect Against Acute Liver Injury. Molecules, 30(10), 2127. https://doi.org/10.3390/molecules30102127






