Analysis of Differences in Metabolite and Antioxidant Activity in Highland Red Raspberry Pulp Based on Widely Targeted Metabolomics
Abstract
1. Introduction
2. Results
2.1. Metabolite Annotation and Comparative Analysis of Metabolite Profiles
2.2. OPLS-DA, and Permutation Test of Raspberry Pulp from Two Regions
2.3. Screening of Differential Metabolites
2.4. Major Differential Metabolites in Raspberry Pulp from Yunnan and Qinghai
2.5. Main Metabolic Pathways Involved in Differential Metabolites of Raspberry Pulp in Yunnan and Qinghai
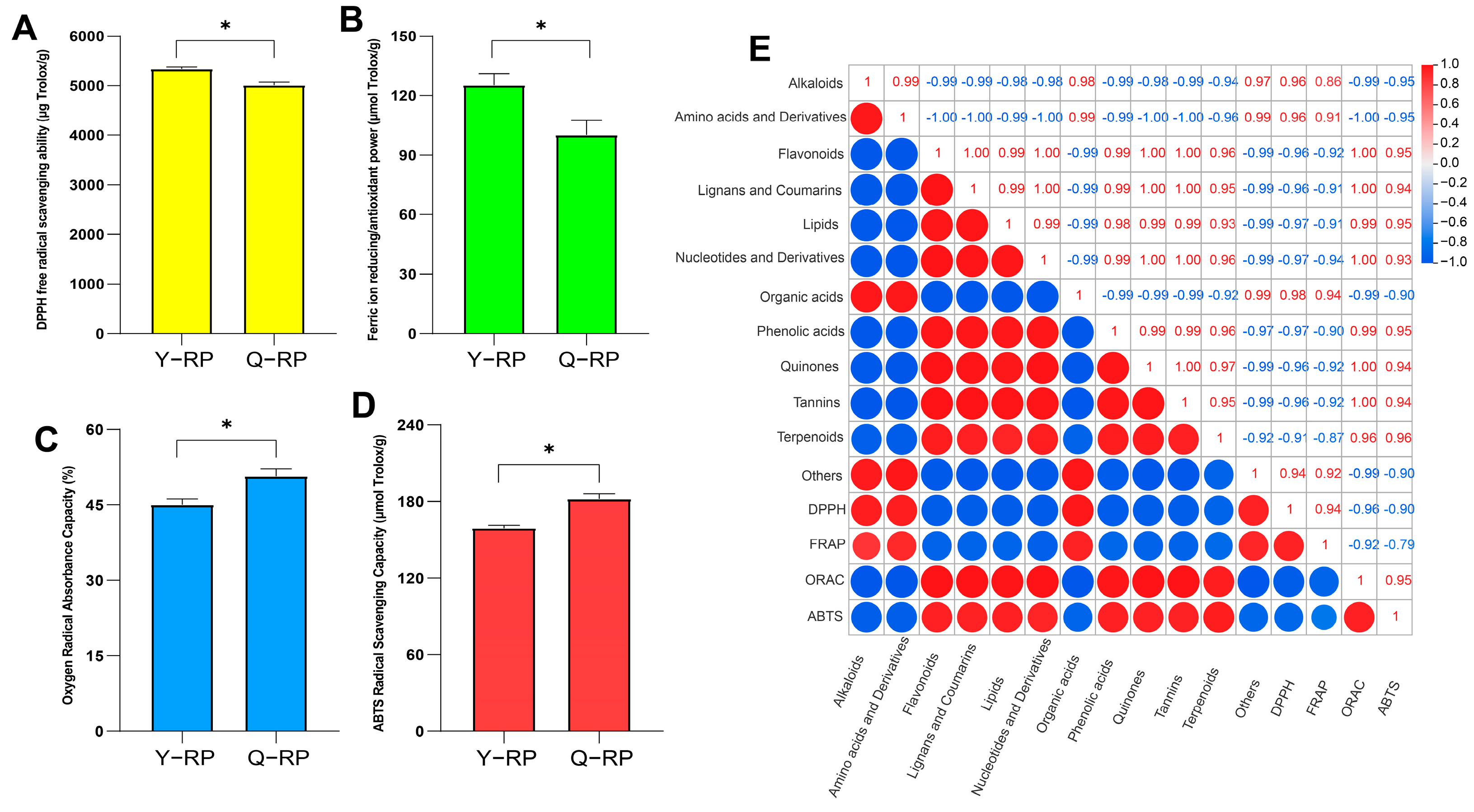
2.6. Correlation of Antioxidant Capacity Between Y-RP and Q-RP
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Research Methodology
4.2.1. Sample Preparation and Extraction
4.2.2. LC-ESI-MS/MS Analysis
4.2.3. ESI-QTRAP-MS/MS
4.2.4. Determination of Antioxidant Activity
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ispiryan, A.; Viškelis, J.; Viškelis, P. Red raspberry (Rubus idaeus L.) seed oil: A review. Plants 2021, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Davik, J.; Røen, D.; Lysøe, E.; Buti, M.; Rossman, S.; Alsheikh, M.; Aiden, E.L.; Dudchenko, O.; Sargent, D.J. A chromosome-level genome sequence assembly of the red raspberry (Rubus idaeus L.). PLoS ONE 2022, 17, e0265096. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lin, F.; Li, M.; Mei, Y.; Li, Y.; Bai, Y.; He, X.; Zheng, Y. Prediction of the potential distribution of a raspberry (Rubus idaeus) in China based on MaxEnt model. Sci. Rep. 2024, 14, 24438. [Google Scholar] [CrossRef] [PubMed]
- Bourmaud, M.; Zarka, M.; Le Cozannet, R.; Fança-Berthon, P.; Hay, E.; Cohen-Solal, M. Effect of Rubus idaeus extracts in murine chondrocytes and explants. Biomolecules 2021, 11, 245. [Google Scholar] [CrossRef]
- Adamczuk, N.; Krauze-Baranowska, M.; Ośko, J.; Grembecka, M.; Migas, P. Comparison of antioxidant properties of fruit from some cultivated varieties and hybrids of Rubus idaeus and Rubus occidentalis. Antioxidants 2025, 14, 86. [Google Scholar] [CrossRef]
- De Santis, D.; Carbone, K.; Garzoli, S.; Laghezza Masci, V.; Turchetti, G. Bioactivity and chemical profile of Rubus idaeus L. Leaves steam-distillation extract. Foods 2022, 11, 1455. [Google Scholar] [CrossRef]
- Klewicka, E.; Sójka, M.; Klewicki, R.; Kołodziejczyk, K.; Lipińska, L.; Nowak, A. Ellagitannins from raspberry (Rubus idaeus L.) fruit as natural inhibitors of geotrichum candidum. Molecules 2016, 21, 908. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.M.; Kondoleon, N.; Gutierrez, E.; Wolforth, J.; Bolling, S. The intake of red raspberry fruit is inversely related to cardiac risk factors associated with metabolic syndrome. J. Funct. Foods 2017, 41, 83–89. [Google Scholar] [CrossRef]
- Noratto, G.; Chew, B.P.; Ivanov, I. Red raspberry decreases heart biomarkers of cardiac remodeling associated with oxidative and inflammatory stress in obese diabetic db/db mice†. Food Funct. 2016, 7, 4944–4955. [Google Scholar] [CrossRef]
- Toshima, S.; Hirano, T.; Kunitake, H. Comparison of anthocyanins, polyphenols, and antioxidant capacities among raspberry, blackberry, and Japanese wild Rubus species. Sci. Hortic. 2021, 285, 110204. [Google Scholar] [CrossRef]
- Kostryco, M.; Chwil, M. Nectar abundance and Nectar composition in selected Rubus idaeus L. varieties. Agriculture 2022, 12, 1132. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Živković, U.; Avramov, S.; Miljković, D.; Barišić Klisarić, N.; Tubić, L.; Mišić, D.; Šiler, B.; Tarasjev, A. Genetic and environmental factors jointly impact leaf phenolic profiles of Iris variegata L. Plants 2021, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Marić, B.; Abramović, B.; Ilić, N.; Bodroža-Solarov, M.; Pavlić, B.; Oczkowski, M.; Wilczak, J.; Četojević-Simin, D.; Šarić, L.; Teslić, N. UHPLC-Triple-TOF-MS characterization, antioxidant, antimicrobial and antiproliferative activity of raspberry (Rubus idaeus L.) seed extracts. Foods 2022, 12, 161. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, X.; Li, Y.; Fan, Y.; Li, Y.; Cao, Y.; An, W.; Shi, Z.; Zhao, J.; Guo, S. Changes in metabolome and nutritional quality of lycium barbarum fruits from three typical growing areas of China as revealed by widely targeted metabolomics. Metabolites 2020, 10, 46. [Google Scholar] [CrossRef]
- Zhang, M.-Q.; Zhang, J.; Zhang, Y.-T.; Sun, J.-Y.; Prieto, M.A.; Simal-Gandara, J.; Putnik, P.; Li, N.-Y.; Liu, C. The link between the phenolic composition and the antioxidant activity in different small berries: A metabolomic approach. LWT 2023, 182, 114853. [Google Scholar] [CrossRef]
- Chwil, M.; Matraszek-Gawron, R.; Kostryco, M.; Różańska-Boczula, M. Nutritionally important pro-Health active ingredients and antioxidant properties of fruits and fruit juice of selected biennial fruiting Rubus idaeus L. Cultivars. Pharmaceuticals 2023, 16, 1698. [Google Scholar] [CrossRef]
- Mannino, G.; Serio, G.; Gaglio, R.; Busetta, G.; La Rosa, L.; Lauria, A.; Settanni, L.; Gentile, C. Phytochemical profile and antioxidant, antiproliferative, and antimicrobial properties of Rubus idaeus seed powder. Foods 2022, 11, 2605. [Google Scholar] [CrossRef]
- Kalischuk, M.L.; Kawchuk, L.M.; Leggett, F. First report of Rubus yellow net virus on Rubus idaeus in Alberta, Canada. Plant Dis. 2008, 92, 974. [Google Scholar] [CrossRef]
- Garjonyte, R.; Budiene, J.; Labanauskas, L.; Judzentiene, A. In vitro antioxidant and prooxidant activities of red raspberry (Rubus idaeus L.) stem extracts. Molecules 2022, 27, 4073. [Google Scholar] [CrossRef]
- Fu, M.; Jahan, M.S.; Tang, K.; Jiang, S.; Guo, J.; Luo, S.; Luo, W.; Li, G. Comparative analysis of the medicinal and nutritional components of different varieties of Pueraria thomsonii and Pueraria lobata. Front. Plant Sci. 2023, 14, 1115782. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, G.; Li, J.; Li, J.; Sun, X. Analysis of floral color differences between different ecological conditions of clematis tangutica (Maxim.) korsh. Molecules 2023, 28, 462. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, D.D.; Wang, L.; Lou, H.; Ren, D. Eriodictyol-7-O-glucoside, a novel Nrf2 activator, confers protection against cisplatin-induced toxicity. Food Chem. Toxicol. 2012, 50, 1927–1932. [Google Scholar] [CrossRef]
- Patel, D.K. Biological importance and therapeutic benefit of rhamnocitrin: A review of pharmacology and analytical aspects. Drug Metab. Bioanal. Lett. 2022, 15, 150–158. [Google Scholar] [CrossRef]
- Yoshida, K.; Oniduka, T.; Oyama, K.I.; Kondo, T. Blue flower coloration of Corydalis ambigua requires ferric ion and kaempferol glycoside. Biosci. Biotechnol. Biochem. 2021, 85, 61–68. [Google Scholar] [CrossRef]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef]
- Sudarshan, K.; Aidhen, I.S. Convenient Synthesis of 3-Glycosylated Isocoumarins. Eur. J. Org. Chem. 2016, 2017, 34–38. [Google Scholar] [CrossRef]
- Huang, Y.N.; Zhao, Y.L.; Gao, X.L.; Zhao, Z.F.; Jing, Z.; Zeng, W.C.; Yang, R.; Peng, R.; Tong, T.; Wang, L.F. Intestinal alpha-glucosidase inhibitory activity and toxicological evaluation of Nymphaea stellata flowers extract. J. Ethnopharmacol. 2010, 131, 306–312. [Google Scholar] [CrossRef]
- Dave, A.; Vaistij, F.E.; Gilday, A.D.; Penfield, S.D.; Graham, I.A. Regulation of Arabidopsis thaliana seed dormancy and germination by 12-oxo-phytodienoic acid. J. Exp. Bot. 2016, 67, 2277–2284. [Google Scholar] [CrossRef]
- Copp, W.; Karimi, A.; Yang, T.; Guarné, A.; Luedtke, N.W. Fluorescent molecular rotors detect O(6)-methylguanine dynamics and repair in duplex DNA. Chem. Commun. 2024, 60, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- .Moramarco, F.; Pezzicoli, A.; Salvini, L.; Leuzzi, R.; Pansegrau, W.; Balducci, E. A LONELY GUY protein of Bordetella pertussis with unique features is related to oxidative stress. Sci. Rep. 2019, 9, 17016. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, H.; Tan, J.; Wang, C.; Lin, H.; Zhu, H.; Liu, J.; Li, P.; Yin, J. Pharmacokinetic and metabolism studies of curculigoside C by UPLC-MS/MS and UPLC-QTOF-MS. Molecules 2018, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, A.; Bouhlel, I.; Skandrani, I.; Valenti, K.; Kadri, M.; Guiraud, P.; Steiman, R.; Mariotte, A.M.; Ghedira, K.; Laporte, F.; et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chem.-Biol. Interact. 2007, 165, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.X.; Li, P.P.; Jia, Y.J.; Wen, L.X.; Tang, Z.S.; Wang, Y.P.; Cui, F.; Hu, F.D. Integrated metabolomic and transcriptomic analysis of triterpenoid accumulation in the roots of Codonopsis pilosula var. modesta (Nannf.) L.T.Shen at different altitudes. Phytochem. Anal. 2025, 36, 358–368. [Google Scholar] [CrossRef]
- Bradish, C.M.; Perkins-Veazie, P.; Fernandez, G.E.; Xie, G.; Jia, W. Comparison of flavonoid composition of red raspberries (Rubus idaeus L.) grown in the southern United States. J. Agric. Food Chem. 2012, 60, 5779–5786. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Gao, Q.; Song, Y.; Liang, Y.; Li, Y.; Chang, Y.; Ma, R.; Cao, X.; Wang, S. Dynamics of physicochemical properties, functional compounds and antioxidant capacity during spontaneous fermentation of Lycium ruthenicum Murr. (Qinghai-Tibet Plateau) natural vinegar. Foods 2022, 11, 1344. [Google Scholar] [CrossRef]
- Martínez-Camacho, J.E.; Guevara-González, R.G.; Rico-García, E.; Tovar-Pérez, E.G.; Torres-Pacheco, I. Delayed senescence and marketability index preservation of blackberry fruit by preharvest application of chitosan and salicylic acid. Front. Plant Sci. 2022, 13, 796393. [Google Scholar] [CrossRef]
- Huang, G.; Mei, X.; Hu, J. The Antioxidant Activities of Natural Polysaccharides. Curr. Drug Targets 2017, 18, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Cör, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [PubMed]
- Lambelet, P.; Saucy, F.; Löliger, J. Chemical evidence for interactions between vitamins E and C. Experientia 1985, 41, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic profile and bioactive potential of stems and seed kernels of sweet cherry fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2011, 130, 928–936. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Rolnik, A.; Soluch, A.; Kowalska, I.; Olas, B. Antioxidant and hemostatic properties of preparations from Asteraceae family and their chemical composition—Comparative studies. Biomed. Pharmacother. 2021, 142, 111982. [Google Scholar] [CrossRef]
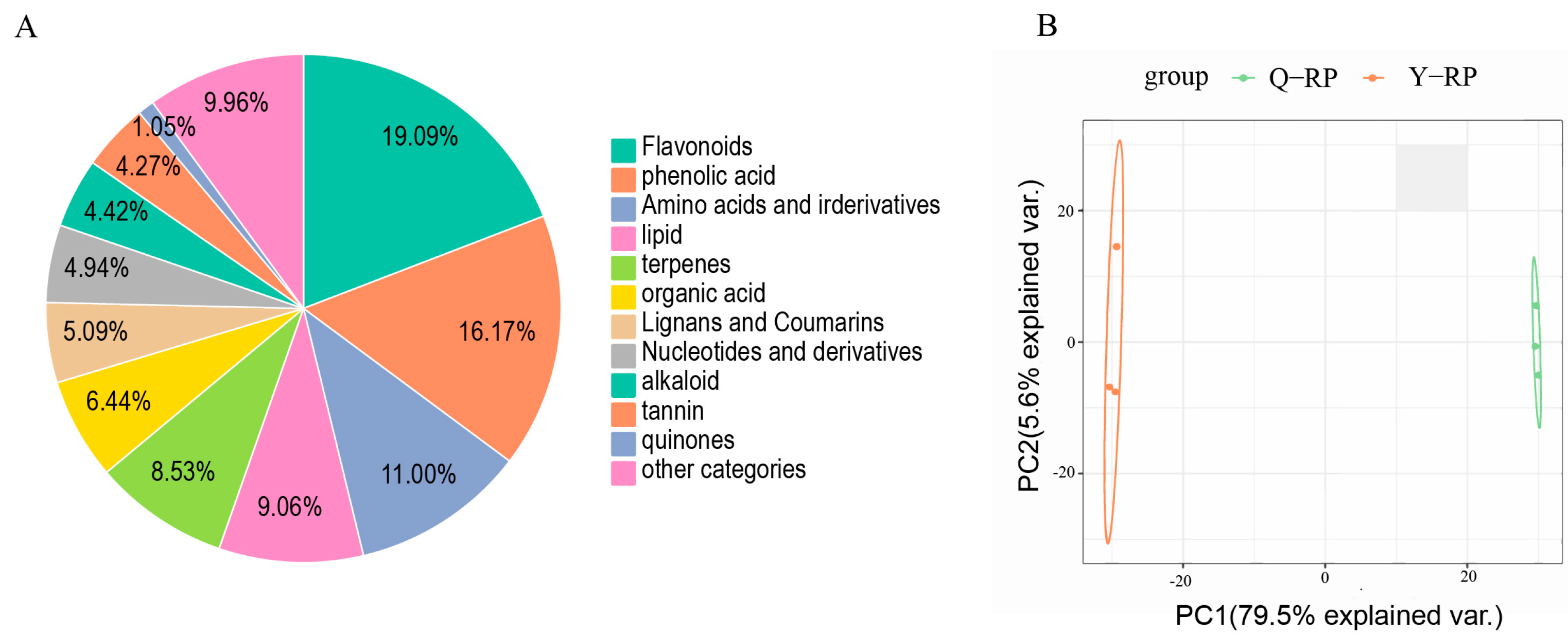
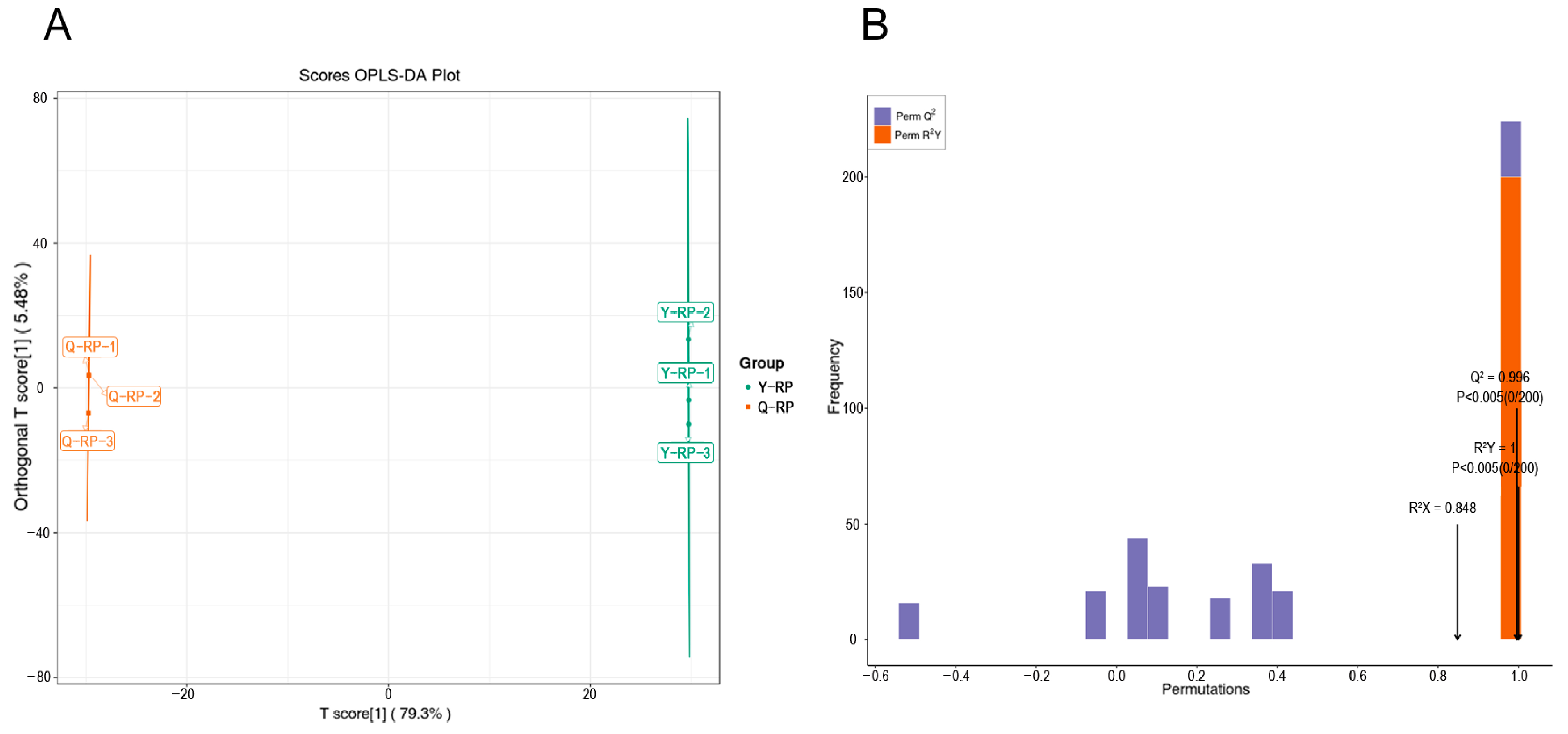

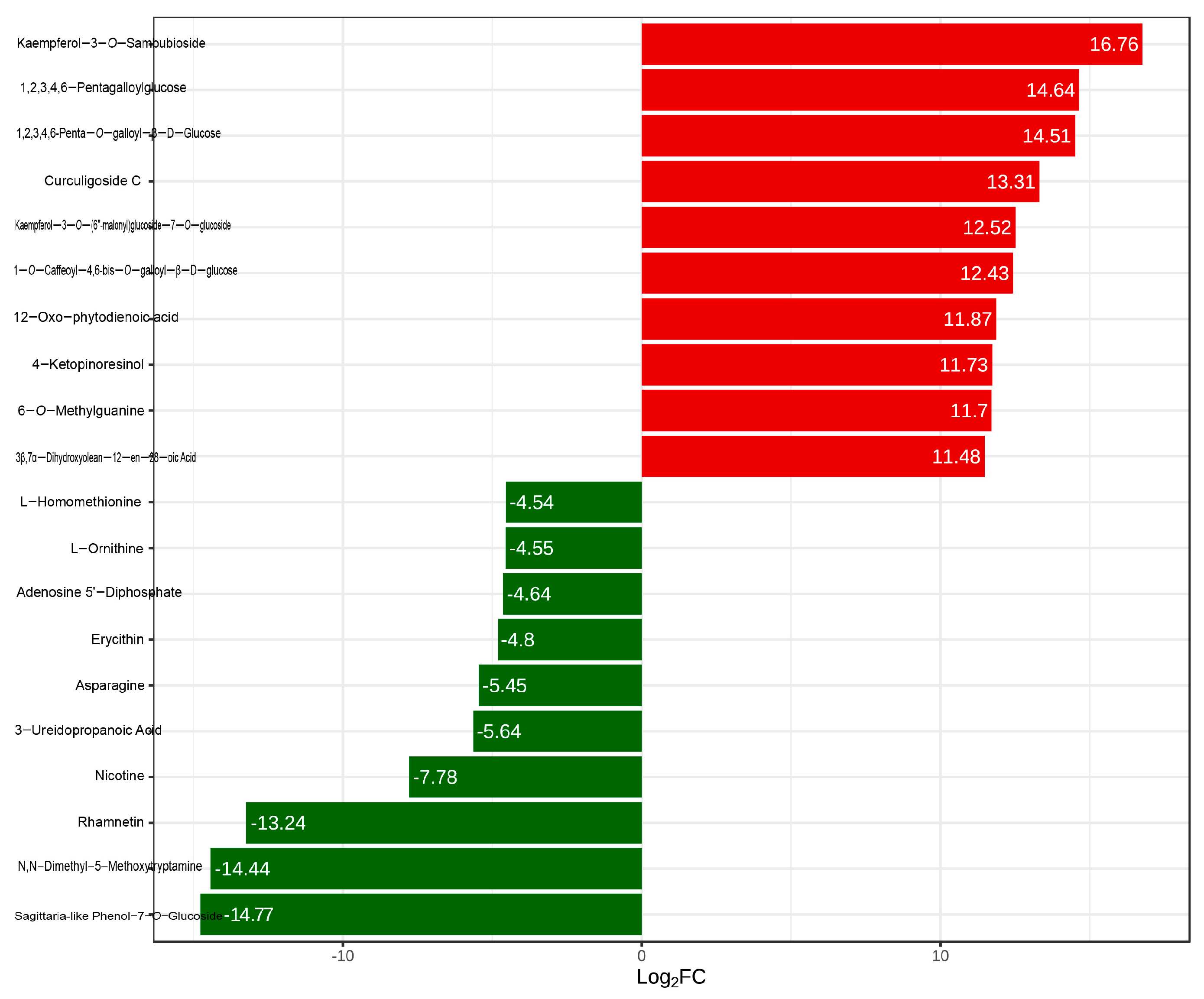
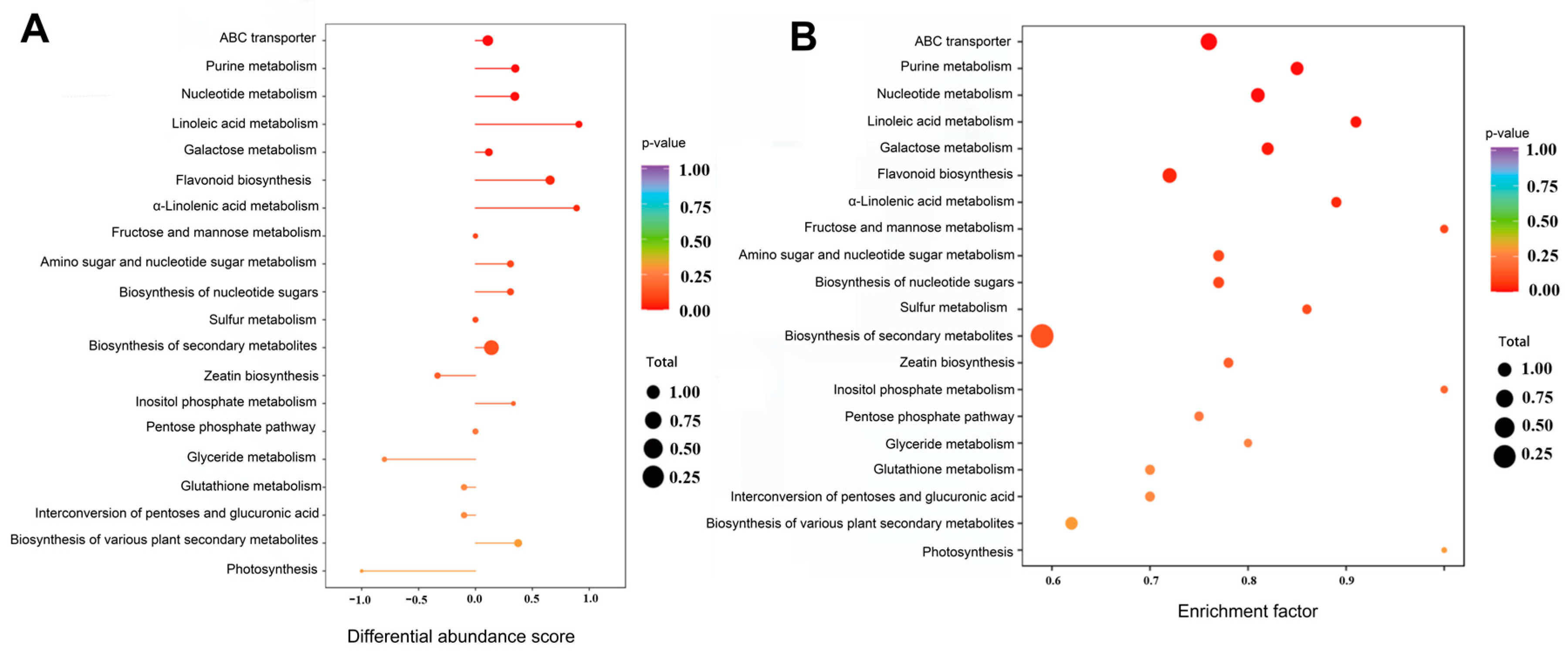
| Type | Total | Common | Y-RP | Q-RP |
|---|---|---|---|---|
| Flavonoids | 255 | 251 | Holy Grassinol-7-O-Glucoside, Rhamnus Limonin | Holy Grassinol-7-O-Glucoside, Rhamnus Limonin |
| Phenolic acid | 216 | 214 | - | 1,2,3,4,6-penta-O-galloyl-β-D-glucose, 1-O-caffeoyl-4,6-bis-O-galloyl-β-D-glucose |
| Amino acids and Irderivatives | 147 | 147 | - | - |
| Lipid | 121 | 117 | - | 2-linoleyl glycerides-1,3-di-O-glucoside, 1-linoleyl glycerides-2,3-di-O-glucoside, 2-alpha-linolenic acid glycerides-1-O-glucoside, 12-oxo-Phytodienoic acid |
| Terpenes | 114 | 113 | - | 3β, 7α-Dihydroxyoleanum-12-ene-28-acid |
| Organic acid | 86 | 86 | - | - |
| Lignans and Coumarins | 68 | 67 | - | 4-Ketoeugenol |
| Nucleotides and Derivatives | 66 | 65 | - | 6-O-Methylguanine |
| Alkaloid | 59 | 57 | N, N-Dimethyl-5-Methoxytryptamine | Isobutyryl carnitine |
| Tannin | 57 | 55 | - | Citronin C, 1,2,3,4,6-pentagalaryl glucose |
| Quinones | 14 | 14 | - | - |
| Other Categories | 133 | 133 | - | - |
| Total | 1336 | 1319 | 3 | 14 |
| Type | Total Number of Detections | Total Number of Differential Metabolites | Up-Regulated Metabolites in Y-RP | Up-Regulated Metabolites in Q-RP |
|---|---|---|---|---|
| Flavonoids | 255 | 166 | 16 | 150 |
| Phenolic acid | 216 | 116 | 14 | 102 |
| Terpenes | 114 | 75 | 4 | 71 |
| Lipid | 121 | 69 | 4 | 65 |
| Amino acids and Irderivatives | 147 | 66 | 32 | 34 |
| Nucleotides and Derivatives | 66 | 48 | 13 | 35 |
| Lignans and Coumarins | 68 | 42 | 7 | 35 |
| Tannin | 57 | 39 | 2 | 37 |
| Organic acid | 86 | 37 | 12 | 25 |
| Alkaloid | 59 | 26 | 14 | 12 |
| Quinones | 14 | 8 | 1 | 7 |
| Other categories | 133 | 68 | 23 | 45 |
| Total | 1336 | 760 | 142 | 618 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Wang, J.; Pu, X. Analysis of Differences in Metabolite and Antioxidant Activity in Highland Red Raspberry Pulp Based on Widely Targeted Metabolomics. Molecules 2025, 30, 2124. https://doi.org/10.3390/molecules30102124
Song Y, Wang J, Pu X. Analysis of Differences in Metabolite and Antioxidant Activity in Highland Red Raspberry Pulp Based on Widely Targeted Metabolomics. Molecules. 2025; 30(10):2124. https://doi.org/10.3390/molecules30102124
Chicago/Turabian StyleSong, Yangbo, Jie Wang, and Xiaojian Pu. 2025. "Analysis of Differences in Metabolite and Antioxidant Activity in Highland Red Raspberry Pulp Based on Widely Targeted Metabolomics" Molecules 30, no. 10: 2124. https://doi.org/10.3390/molecules30102124
APA StyleSong, Y., Wang, J., & Pu, X. (2025). Analysis of Differences in Metabolite and Antioxidant Activity in Highland Red Raspberry Pulp Based on Widely Targeted Metabolomics. Molecules, 30(10), 2124. https://doi.org/10.3390/molecules30102124






