PRMT5 Inhibitor EPZ015666 Decreases the Viability and Encystment of Entamoeba invadens
Abstract
1. Introduction
2. Results
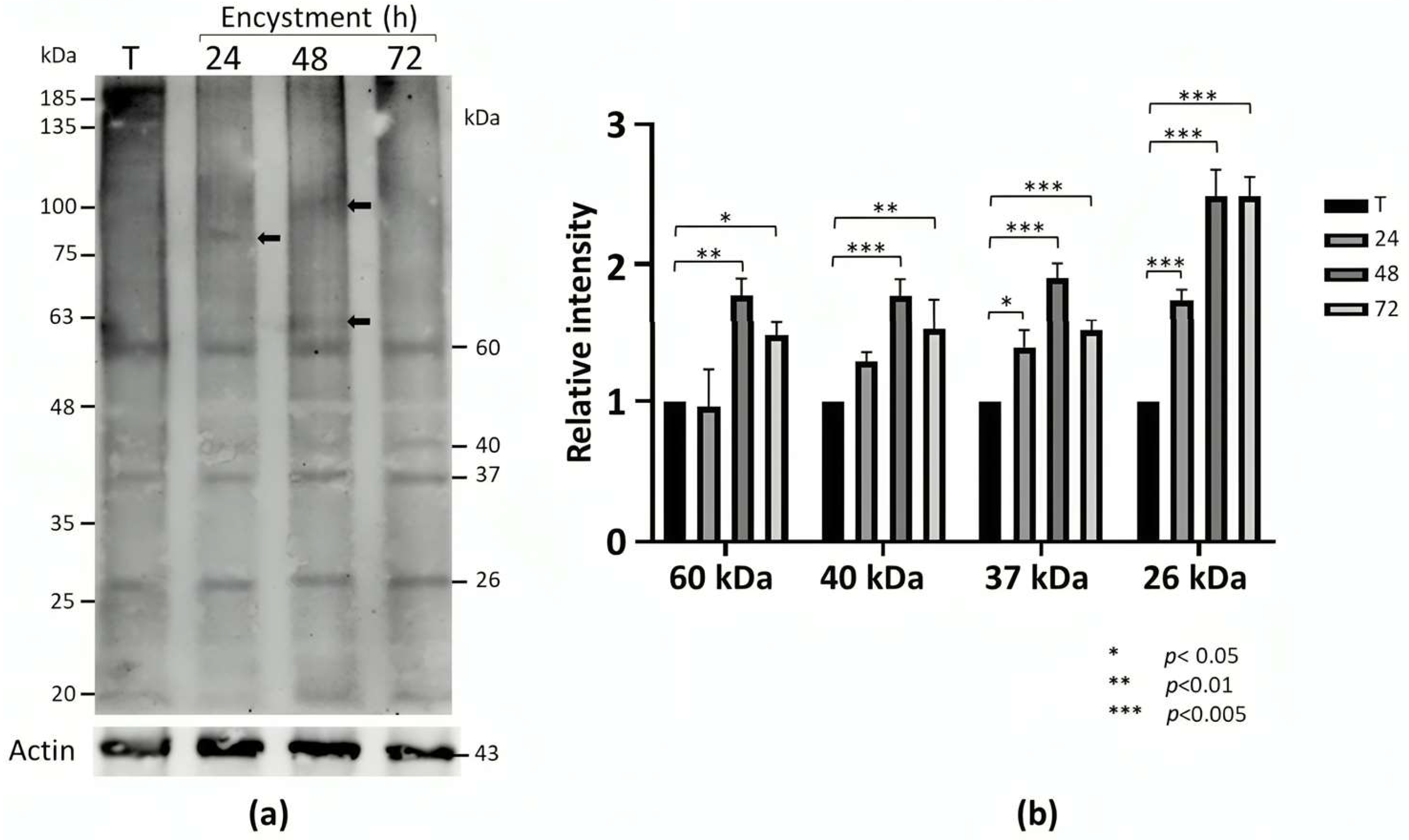
2.1. sDMA Occurs in E. invadens and Increases During Encystment
2.2. E. invadens Has Only One Type II PRMT
2.3. EiPRMT5 Retains the Characteristic Domains of the PRMT5 Family
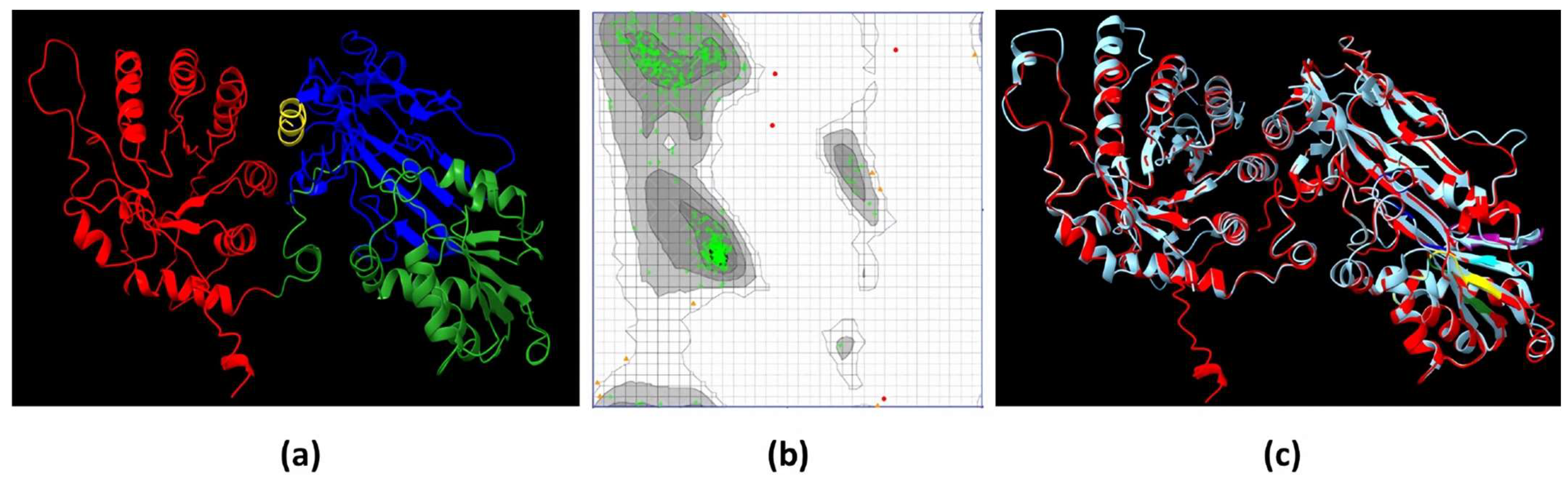
2.4. The 3D Model of EiPRMT5 Showed Homology with the X. laevis PRMT5 Crystal
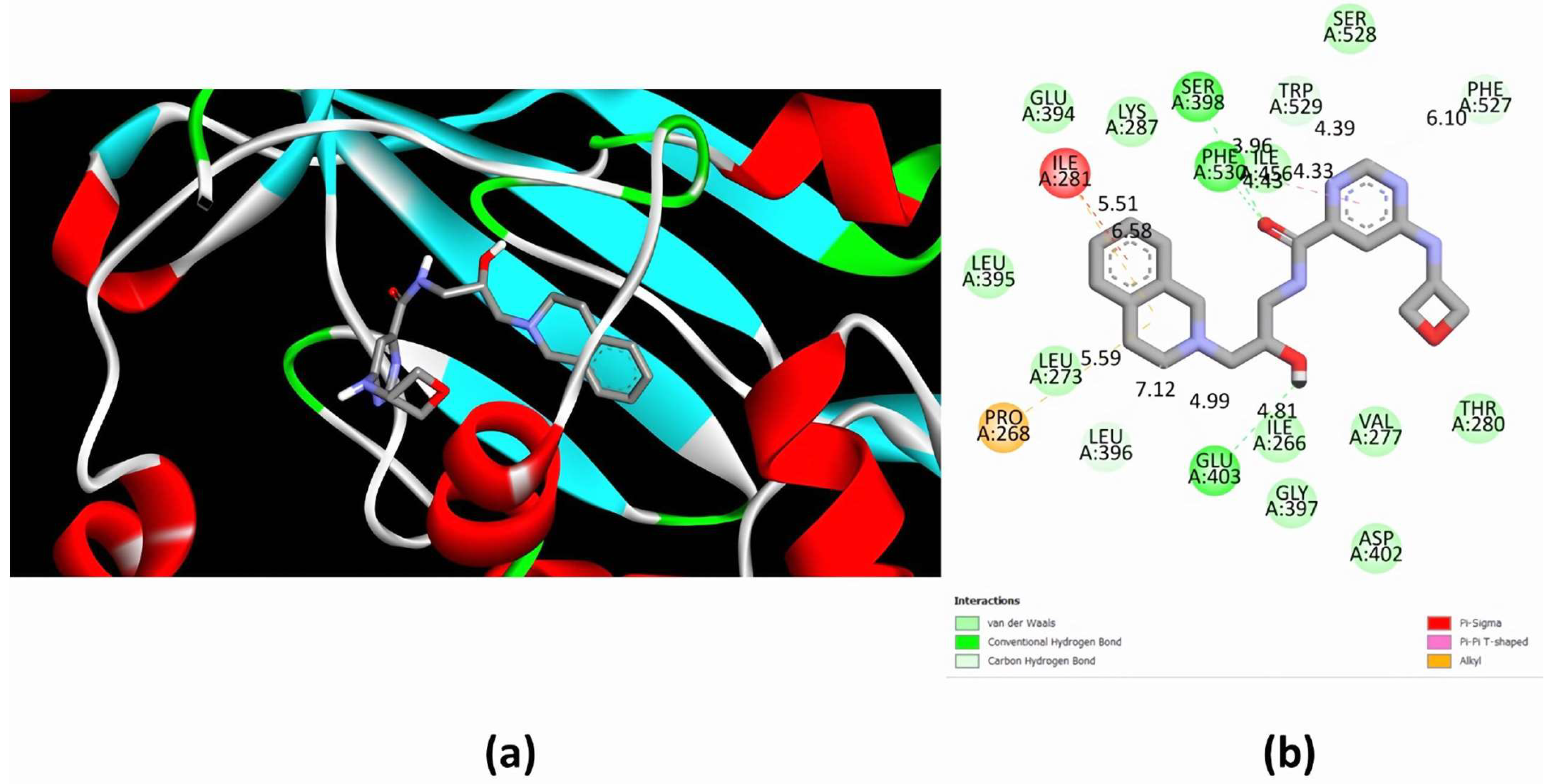
2.5. Molecular Docking Predicts the Binding of EPZ015666 to the Active Site of EiPRMT5
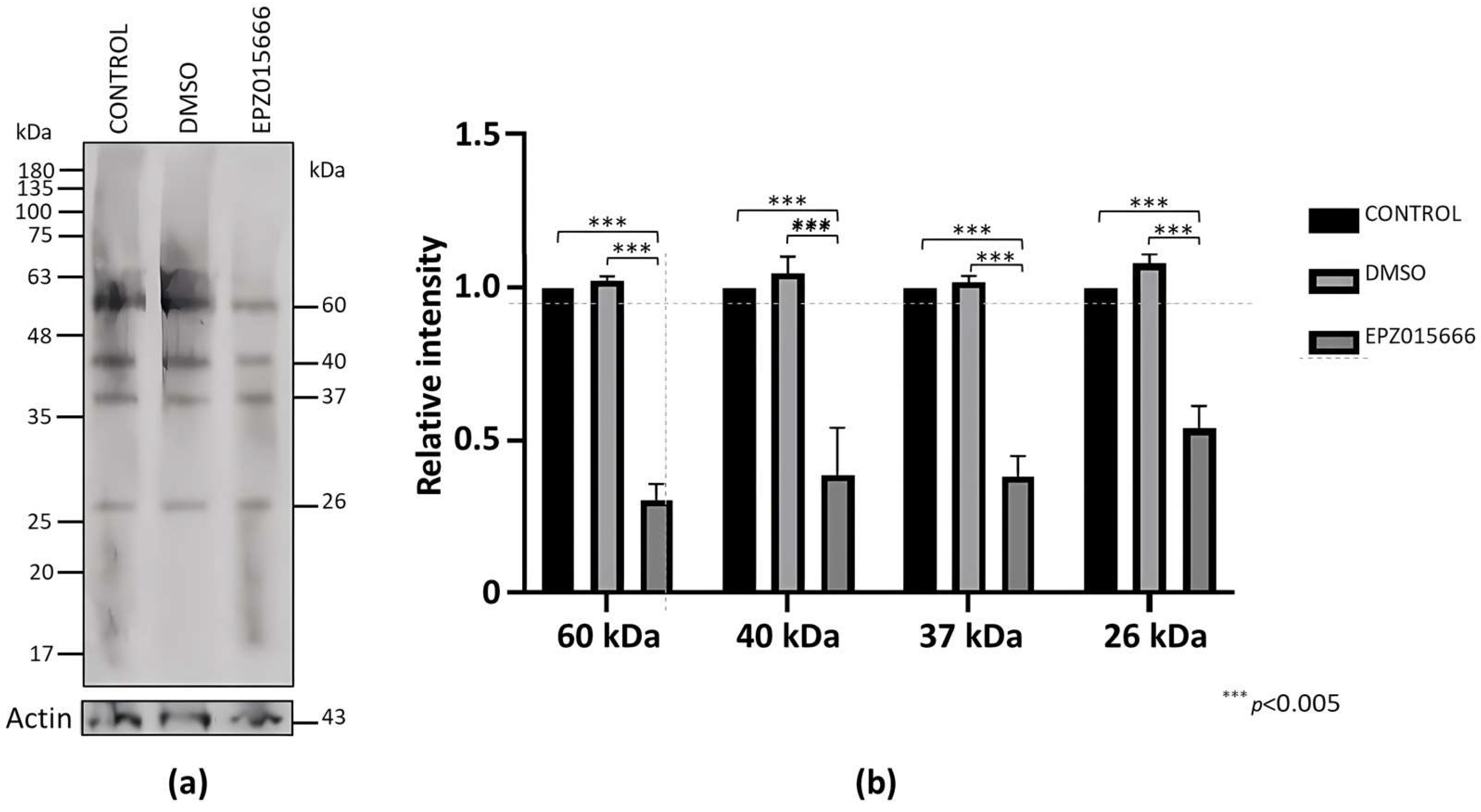
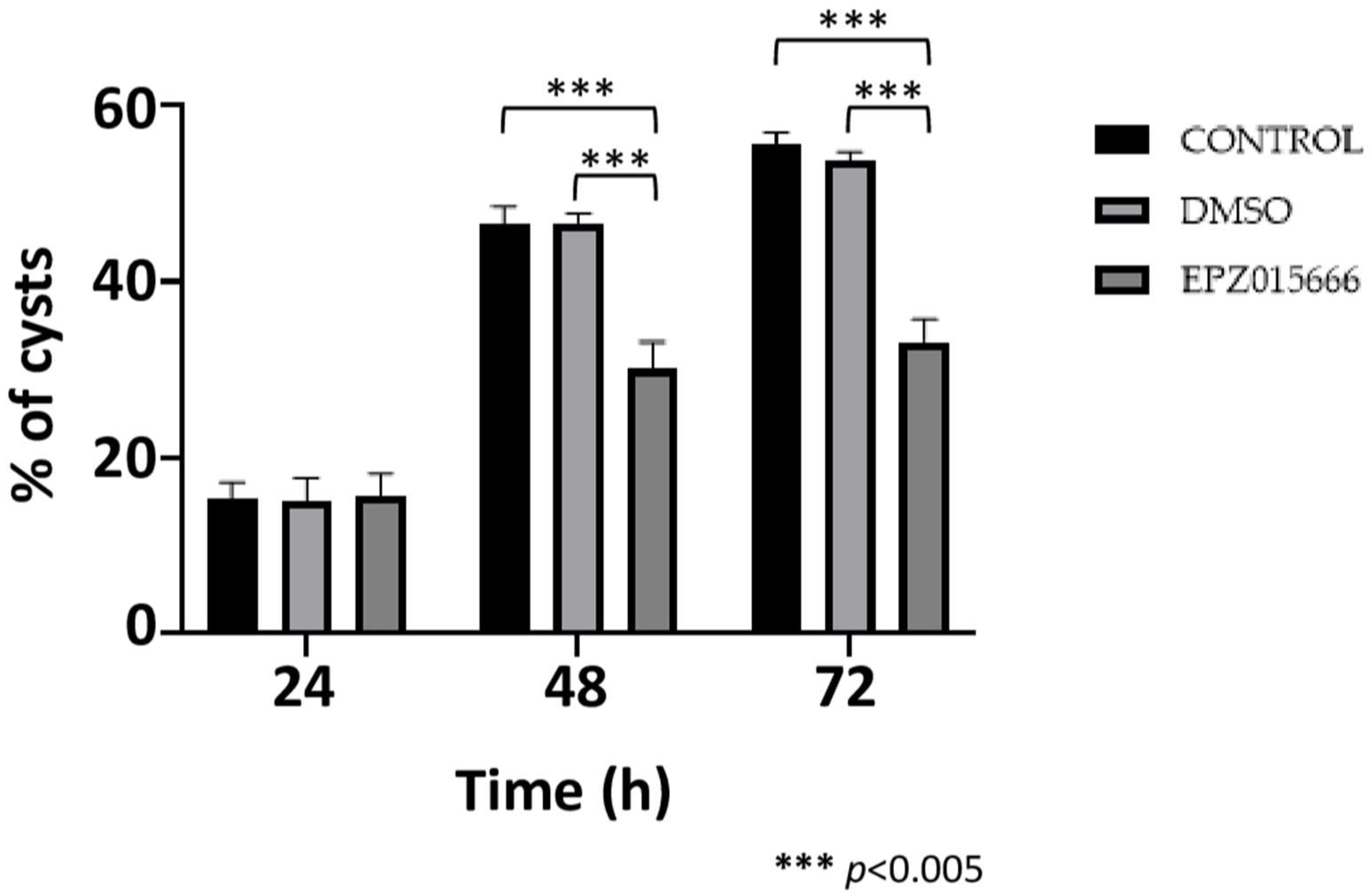
2.6. EPZ015666 Affects the Viability and Encystment of E. invadens
3. Discussion
4. Materials and Methods
4.1. Culture of E. invadens Trophozoites and In Vitro Encystment
4.2. Western Blot Assays
4.3. Immunofluorescence and Confocal Microscopy
4.4. Identification of E. invadens PRMTs and In Silico Characterization of EiPRMT5
4.5. EiPRMT5 Modeling
4.6. Molecular Docking Analysis of the Interaction Between EiPRMT5 and EPZ015666
4.7. Effect of EPZ015666 on E. invadens Trophozoites
4.8. Effect of EPZ015666 on Encystment
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanc, R.S.; Richard, S. Arginine Methylation: The Coming of Age. Mol. Cell 2017, 65, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, J.; Clancy, K.W.; Thompson, P.R. Chemical Biology of Protein Arginine Modifications in Epigenetic Regulation. Chem. Rev. 2015, 115, 5413–5461. [Google Scholar] [CrossRef] [PubMed]
- Ernzen, K.; Melvin, C.; Yu, L.; Phelps, C.; Niewiesk, S.; Green, P.L.; Panfil, A.R. The PRMT5 Inhibitor EPZ015666 Is Effective against HTLV-1-Transformed T-Cell Lines in Vitro and in Vivo. Front. Microbiol. 2023, 14, 1101544. [Google Scholar] [CrossRef] [PubMed]
- Gullà, A.; Hideshima, T.; Bianchi, G.; Fulciniti, M.; Kemal Samur, M.; Qi, J.; Tai, Y.T.; Harada, T.; Morelli, E.; Amodio, N.; et al. Protein Arginine Methyltransferase 5 Has Prognostic Relevance and Is a Druggable Target in Multiple Myeloma. Leukemia 2018, 32, 996–1002. [Google Scholar] [CrossRef]
- Chan-Penebre, E.; Kuplast, K.G.; Majer, C.R.; Boriack-Sjodin, P.A.; Wigle, T.J.; Johnston, L.D.; Rioux, N.; Munchhof, M.J.; Jin, L.; Jacques, S.L.; et al. A Selective Inhibitor of PRMT5 with in Vivo and in Vitro Potency in MCL Models. Nat. Chem. Biol. 2015, 11, 432–437. [Google Scholar] [CrossRef]
- Fisk, J.C.; Read, L.K. Protein Arginine Methylation in Parasitic Protozoa. Eukaryot. Cell 2011, 10, 1013–1022. [Google Scholar] [CrossRef]
- Rodriguez, M.A. Protein Arginine Methyltransferases in Protozoan Parasites. Parasitology 2022, 149, 427–435. [Google Scholar] [CrossRef]
- Liu, M.; Li, F.-X.; Li, C.-Y.; Li, X.-C.; Chen, L.-F.; Wu, K.; Yang, P.-L.; Lai, Z.-F.; Liu, T.; Sullivan, W.J.; et al. Characterization of Protein Arginine Methyltransferase of TgPRMT5 in Toxoplasma gondii. Parasit. Vectors 2019, 12, 221. [Google Scholar] [CrossRef]
- Moon, E.-K.; Kong, H.-H.; Hong, Y.; Lee, H.-A.; Quan, F.-S. Identification and Characterization of Protein Arginine Methyltransferase 1 in Acanthamoeba castellanii. Korean J. Parasitol. 2017, 55, 109–114. [Google Scholar] [CrossRef]
- Ximénez, C.; Morán, P.; Rojas, L.; Valadez, A.; Gómez, A. Reassessment of the Epidemiology of Amebiasis: State of the Art. Infection, Genet. Evol. 2009, 9, 1023–1032. [Google Scholar] [CrossRef]
- Mousa, E.A.A.; Sakaguchi, M.; Nakamura, R.; Abdella, O.H.; Yoshida, H.; Hamano, S.; Mi-Ichi, F. The Dynamics of Ultrastructural Changes during Entamoeba invadens Encystation. Parasitology 2020, 147, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Antonysamy, S.; Bonday, Z.; Campbell, R.M.; Doyle, B.; Druzina, Z.; Gheyi, T.; Han, B.; Jungheim, L.N.; Qian, Y.; Rauch, C.; et al. Crystal Structure of the Human PRMT5:MEP50 Complex. Proc. Natl. Acad. Sci. USA 2012, 109, 17960–17965. [Google Scholar] [CrossRef]
- Sun, L.; Wang, M.; Lv, Z.; Yang, N.; Liu, Y.; Bao, S.; Gong, W.; Xu, R.M. Structural Insights into Protein Arginine Symmetric Dimethylation by PRMT5. Proc. Natl. Acad. Sci. USA 2011, 108, 20538–20543. [Google Scholar] [CrossRef]
- Tewary, S.K.; Zheng, Y.G.; Ho, M.-C. Protein Arginine Methyltransferases: Insights into the Enzyme Structure and Mechanism at the Atomic Level. Cell Mol. Life Sci. 2019, 76, 2917–2932. [Google Scholar] [CrossRef]
- Motolani, A.; Martin, M.; Sun, M.; Lu, T. The Structure and Functions of Prmt5 in Human Diseases. Life 2021, 11, 1074. [Google Scholar] [CrossRef]
- Zhu, K.; Shao, J.; Tao, H.; Yan, X.; Luo, C.; Zhang, H.; Duan, W. Rational Design, Synthesis and Biological Evaluation of Novel Triazole Derivatives as Potent and Selective PRMT5 Inhibitors with Antitumor Activity. J. Comput. Aided Mol. Des. 2019, 33, 775–785. [Google Scholar] [CrossRef]
- Lucky, A.B.; Wang, C.; Liu, M.; Liang, X.; Min, H.; Fan, Q.; Siddiqui, F.A.; Adapa, S.R.; Li, X.; Jiang, R.H.Y.; et al. A Type II Protein Arginine Methyltransferase Regulates Merozoite Invasion in Plasmodium Falciparum. Commun. Biol. 2023, 6, 659. [Google Scholar] [CrossRef]
- Lorenzon, L.; Quilles, J.C.; Campagnaro, G.D.; Azevedo Orsine, L.; Almeida, L.; Veras, F.; Miserani Magalhães, R.D.; Alcoforado Diniz, J.; Rodrigues Ferreira, T.; Kaysel Cruz, A. Functional Study of Leishmania Braziliensis Protein Arginine Methyltransferases (PRMTs) Reveals That PRMT1 and PRMT5 Are Required for Macrophage Infection. ACS Infect. Dis. 2022, 8, 516–532. [Google Scholar] [CrossRef]
- Min, H.; Lucky, A.B.; Madsen, J.J.; Chim-Ong, A.; Li, X.; Cui, L.; Miao, J. Onametostat, a PfPRMT5 Inhibitor, Exhibits Antimalarial Activity to Plasmodium Falciparum. Antimicrob. Agents Chemother. 2024, 68, e00176-24. [Google Scholar] [CrossRef]
- Ho, M.-C.; Wilczek, C.; Bonanno, J.B.; Xing, L.; Seznec, J.; Matsui, T.; Carter, L.G.; Onikubo, T.; Kumar, P.R.; Chan, M.K.; et al. Structure of the Arginine Methyltransferase PRMT5-MEP50 Reveals a Mechanism for Substrate Specificity. PLoS ONE 2013, 8, e57008. [Google Scholar] [CrossRef]
- Pasternack, D.A.; Sayegh, J.; Clarke, S.; Read, L.K. Evolutionarily Divergent Type II Protein Agrinine Methyltransferase in Trypanosoma brucei. Eukaryot Cell 2007, 6, 1665–1681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holmes, B.; Benavides-Serrato, A.; Saunders, J.T.; Landon, K.A.; Schreck, A.J.; Nishimura, R.N.; Gera, J. The Protein Arginine Methyltransferase PRMT5 Confers Therapeutic Resistance to mTOR Inhibition in Glioblastoma. J. Neurooncol 2019, 145, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Liu, F.; Veazey, K.J.; Gao, G.; Das, P.; Neves, L.F.; Lin, K.; Zhong, Y.; Lu, Y.; Giuliani, V.; et al. Genetic Deletion or Small-Molecule Inhibition of the Arginine Methyltransferase PRMT5 Exhibit Anti-Tumoral Activity in Mouse Models of MLL-Rearranged AML. Leukemia 2018, 32, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Stopa, N.; Krebs, J.E.; Shechter, D. The PRMT5 Arginine Methyltransferase: Many Roles in Development, Cancer and Beyond. Cell Mol. Life Sci. 2015, 72, 2041–2059. [Google Scholar] [CrossRef]
- Liu, X.; He, J.Z.; Mao, L.; Zhang, Y.; Cui, W.W.; Duan, S.; Jiang, A.; Gao, Y.; Sang, Y.; Huang, G. EPZ015666, a Selective Protein Arginine Methyltransferase 5 (PRMT5) Inhibitor with an Antitumour Effect in Retinoblastoma. Exp. Eye Res. 2021, 202, 108286. [Google Scholar] [CrossRef]
- Lozano-Amado, D.; Herrera-Solorio, A.M.; Valdés, J.; Alemán-Lazarini, L.; Almaraz-Barrera, M.D.J.; Luna-Rivera, E.; Vargas, M.; Hernández-Rivas, R. Identification of Repressive and Active Epigenetic Marks and Nuclear Bodies in Entamoeba histolytica. Parasit. Vectors 2016, 9, 19. [Google Scholar] [CrossRef]
- Eichinger, D. Encystation of Entamoeba Parasites. BioEssays 1997, 19, 633–639. [Google Scholar] [CrossRef]
- Nielsen, A.B.; Holder, A.J. Gauss View 5.0, User’s Reference; Scientific Research: Pittsurgh, PA, USA, 2009. [Google Scholar]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- López-Blanco, J.R.; Aliaga, J.I.; Quintana-Ortí, E.S.; Chacón, P. IMODS: Internal Coordinates Normal Mode Analysis Server. Nucleic Acids Res. 2014, 42, W271–W276. [Google Scholar] [CrossRef]




| Human PRMT5 a | E. invadens PRMT5 |
|---|---|
| L319 | L273 |
| T323 | V277 |
| F327 | I281 |
| E435 | E344 |
| L437 | L396 |
| --- | S398 |
| E444 | E403 |
| W579 | W529 |
| F582 | F530 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Hernández, R.; Millán-Casarrubias, E.J.; Bolaños, J.; Munguía-Robledo, S.; Vázquez-Calzada, C.; Azuara-Licéaga, E.; Valdés, J.; Rodríguez, M.A. PRMT5 Inhibitor EPZ015666 Decreases the Viability and Encystment of Entamoeba invadens. Molecules 2025, 30, 62. https://doi.org/10.3390/molecules30010062
Ortiz-Hernández R, Millán-Casarrubias EJ, Bolaños J, Munguía-Robledo S, Vázquez-Calzada C, Azuara-Licéaga E, Valdés J, Rodríguez MA. PRMT5 Inhibitor EPZ015666 Decreases the Viability and Encystment of Entamoeba invadens. Molecules. 2025; 30(1):62. https://doi.org/10.3390/molecules30010062
Chicago/Turabian StyleOrtiz-Hernández, Rigoberto, Elmer Joel Millán-Casarrubias, Jeni Bolaños, Susana Munguía-Robledo, Carlos Vázquez-Calzada, Elisa Azuara-Licéaga, Jesús Valdés, and Mario Alberto Rodríguez. 2025. "PRMT5 Inhibitor EPZ015666 Decreases the Viability and Encystment of Entamoeba invadens" Molecules 30, no. 1: 62. https://doi.org/10.3390/molecules30010062
APA StyleOrtiz-Hernández, R., Millán-Casarrubias, E. J., Bolaños, J., Munguía-Robledo, S., Vázquez-Calzada, C., Azuara-Licéaga, E., Valdés, J., & Rodríguez, M. A. (2025). PRMT5 Inhibitor EPZ015666 Decreases the Viability and Encystment of Entamoeba invadens. Molecules, 30(1), 62. https://doi.org/10.3390/molecules30010062








