Synthesis of CF3-Indazoles via Rh(III)-Catalyzed C-H [4+1] Annulation of Azobenzenes with CF3-Imidoyl Sulfoxonium Ylides
Abstract
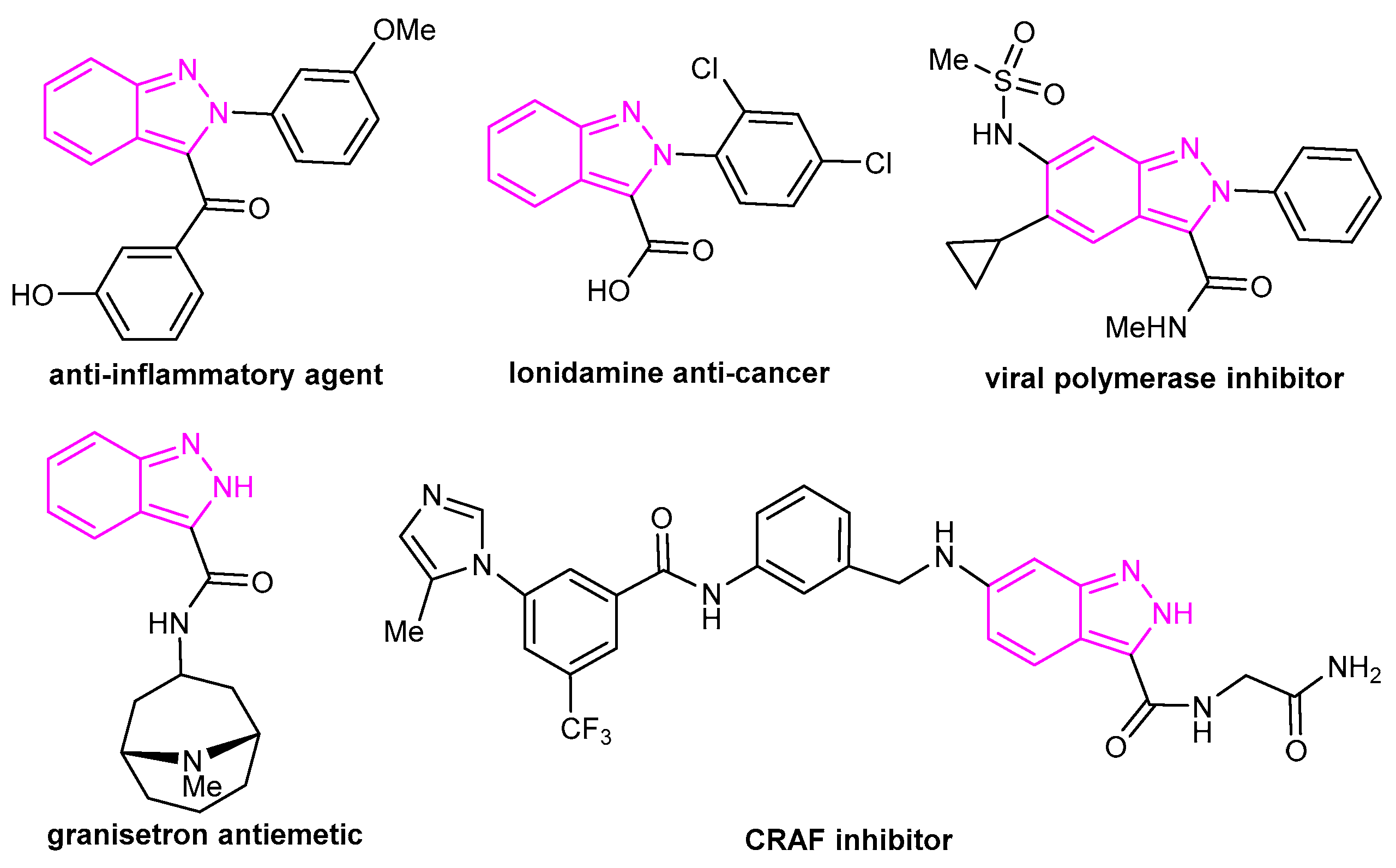
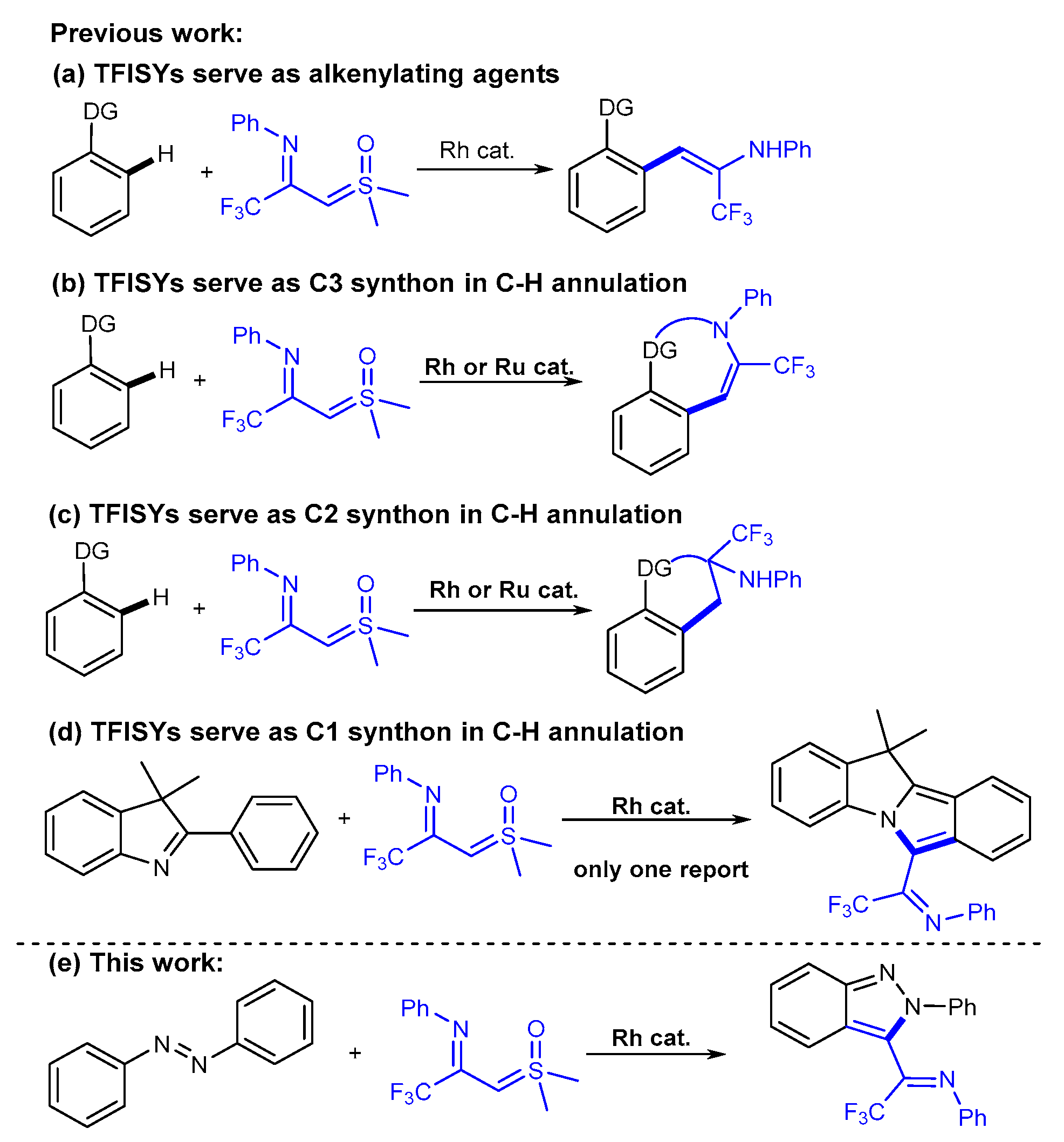
1. Introduction
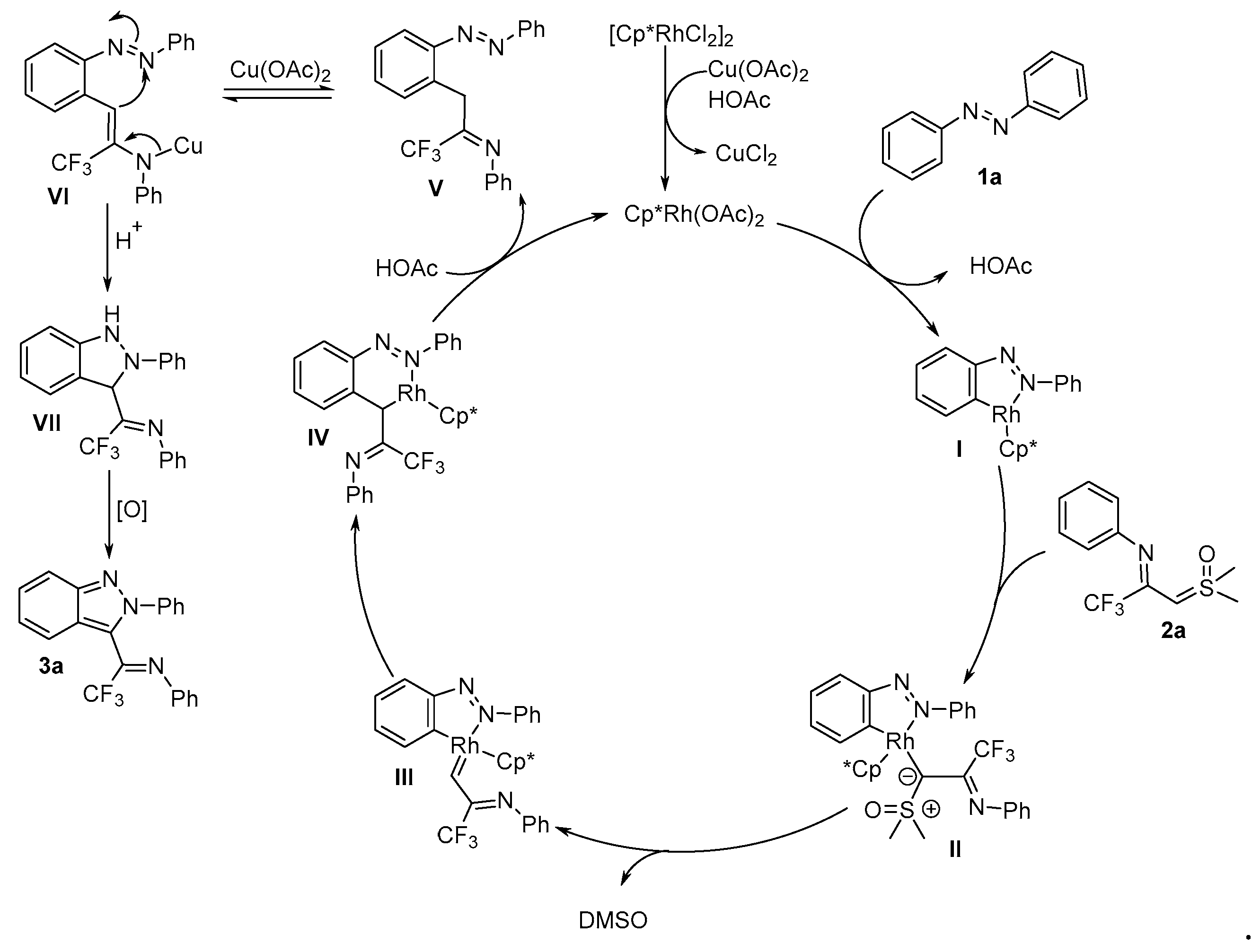
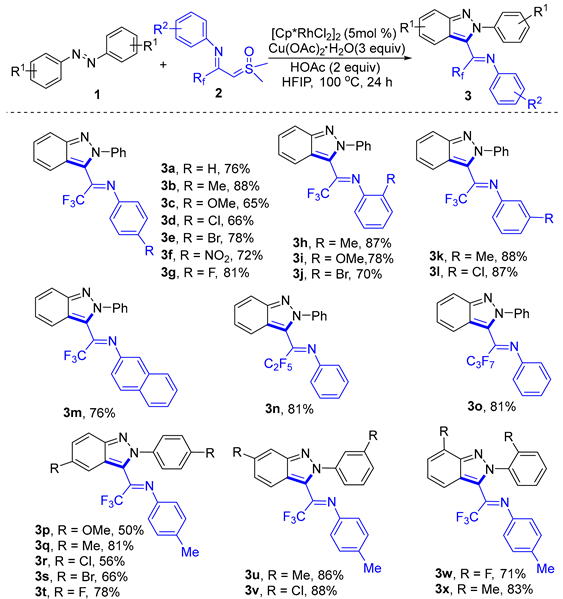
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. The General Procedure
3.3. Characterization Data of Novel Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Sun, S.; Cheng, J. Rh(III)-catalyzed [4+1]-annulation of azobenzenes with α-carbonyl sulfoxonium ylides toward 3-acyl-(2H)-indazoles. Tetrahedron Lett. 2018, 59, 2284–2287. [Google Scholar] [CrossRef]
- Oh, H.; Han, S.; Pandey, A.K.; Han, S.H.; Mishra, N.K.; Kim, S.; Chun, R.; Kim, H.S.; Park, J.; Kim, I.S. Synthesis of (2H)-Indazoles through Rh(III)-Catalyzed Annulation Reaction of Azo-benzenes with Sulfoxonium Ylides. J. Org. Chem. 2018, 83, 4070–4077. [Google Scholar] [CrossRef]
- Shinde, V.N.; Rangan, K.; Kumar, D.; Kumar, A. Rhodium(III)-Catalyzed Dehydrogenative Annulation and Spirocyclization of 2-Arylindoles and 2-(1H-Pyrazol-1-yl)-1H-indoles with Maleimides: A Facile Access to Isogranulatimide Alkaloid Analogues. J. Org. Chem. 2021, 86, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Shah, T.A.; Maharana, P.K.; Talukdar, K.; Das, B.K.; Punniyamurthy, T. Transi-tion-Metal-Catalyzed Directing Group Assisted (Hetero)aryl C-H Functionalization: Construction of C-C/C-Heteroatom Bonds. Chem. Rec. 2021, 21, 3758–3778. [Google Scholar] [CrossRef]
- Hu, D.; Pi, C.; Hu, W.; Han, X.; Wu, Y.; Cui, X. Ru(III)-catalyzed construction of variously substituted quinolines from 2-aminoaromatic aldehydes (ketones) and isoxazoles: Isoxazoles as cyclization reagent and cyano sources. Chin. Chem. Lett. 2022, 33, 4064–4068. [Google Scholar] [CrossRef]
- Hummel, J.R.; Ellman, J.A. Cobalt(III)-Catalyzed Synthesis of Indazoles and Furans by C-H Bond Functionaliza-tion/Addition/Cyclization Cascades. J. Am. Chem. Soc. 2015, 137, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Wang, C. Rhenium-Catalyzed [4+1] Annulation of Azobenzenes and Aldehydes via Isolable Cyclic Rhenium(I) Complexes. Org. Lett. 2015, 17, 2434–2437. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.; Han, S.H.; Han, S.; Sharma, S.; Park, J.; Lee, J.S.; Kwak, J.H.; Jung, Y.H.; Kim, I.S. Access to 3-Acyl-(2H)-indazoles via Rh(III)-Catalyzed C-H Addition and Cyclization of Azobenzenes with α-Keto Aldehydes. Org. Lett. 2016, 18, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Lin, S.; Yi, X.; Xi, C. Substrate-controlled Transformation of Azobenzenes to Indazoles and Indoles via Rh(III)-catalysis. J. Org. Chem. Res. 2017, 82, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, Y.; Yang, Z.; Yao, Z.; Wang, L.; Cui, X. Rh(III)-catalyzed Annulation of Azobenzenes and α-Cl Ketones toward 3-Acyl-2H-indazoles. Chin. Chem. Lett. 2021, 32, 1709–1712. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Acena, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluo-rine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Iqbal, N.; You, Y.; Cho, E.J. Controlled Fluoroalkylation Raections by Visible-Light Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 2284–2294. [Google Scholar] [CrossRef]
- Pattanayak, P.; Nikhitha, S.; Halder, D.; Ghosh, B.; Chatterjee, T. Exploring the impact of trifluoromethyl (-CF3) functional group on the anti-cancer activity of isoxazole-based molecules: Design, synthesis, biological evaluation and molecular docking analysis. RSC Adv. 2024, 14, 18856–18870. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, H.; Wang, B.; Li, B. Cascade C-H Activation and Defluorinative Annulation of 2-Arylbenzimidazoles with α-Trifluoromethyl-α-diazoketones: Modular Assembly of 6-Fluorobenzimidazo[2,1-a]isoquinolines. Org. Lett. 2023, 25, 4770–4775. [Google Scholar] [CrossRef]
- Li, H.; Shen, M.; Li, B.; Zhang, X.; Fan, X. Solvent-Dependent Selective Synthesis of CF3-Tethered Indazole De-rivatives Based on Multiple Bond Activations. Org. Lett. 2023, 25, 720–725. [Google Scholar] [CrossRef]
- Li, H.; Yan, S.; Xu, Y.; Ma, C.; Zhang, X.; Fan, X. Synthesis of functionalized quinolines from the cascade reactions of N-aryl amidines with two CF3-ynones via C-H/N-H/C-N/C-C bond cleavage. Org. Chem. Front. 2024, 11, 1917–1923. [Google Scholar] [CrossRef]
- Liu, J.; Tan, H.; Zhang, Y.; Zhou, L. Copper-Catalyzed Defluorinative [3+2] Cyclization of Amidines and Trifluoromethyl Carbenoids for the Synthesis of 5-Fluoroimidazoles. Org. Lett. 2024, 26, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Kazi, A.A.; Manjuladevi, N.; Kumar, S.S.; Sharma, A.; Singh, L.R. A quick access to CF3-containing functionalized benzofuranyl, benzothiophene and indolyl heterocycles under catalyst-free conditions. Chem. Commun. 2024, 60, 9376–9379. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tang, J.; Li, C.; Chen, Z.; Wu, X.-F. Rhodium(III)-catalyzed regioselective C2-alkenylation of indoles with CF3-imidoyl sulfoxonium ylides to give multi-functionalized enamines using a migratable directing group. Chem. Commun. 2023, 59, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, Z.; Chen, Z. Rhodium(III)-Catalyzed Coupling of Quinolin-8-carboxaldehydes with CF3-Imidoyl Sulfoxonium Ylides by Chelation-Assisted C(sp2)-H Bond Activation for the Synthesis of Trifluoromethyl-Substituted Enaminones. J. Org. Chem. 2024, 89, 10736–10747. [Google Scholar] [CrossRef]
- Wen, S.; Zhang, Y.; Tian, Q.; Chen, Y.; Cheng, G. Ruthenium(II)-catalyzed synthesis of CF3-isoquinolinones via C-H activa-tion/annulation of benzoic acids and CF3-imidoyl sulfoxonium ylides. Org. Chem. Front. 2022, 9, 4388–4393. [Google Scholar] [CrossRef]
- Duan, Y.; Lu, S.-N.; Yang, Z.; Chen, Z.; Wu, X.-F. Rh(III)-catalyzed C-H activation/annulation of N-carbamoylindoles with CF3-imidoyl sulfoxonium ylides for the divergent synthesis of trifluoromethyl-substituted (dihydro)pyrimidoindolones. Org. Chem. Front. 2023, 10, 3843–3848. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, S.; Li, P.; Chen, Z.; Wu, X.-F. Rh(III)-Catalyzed Dual C-H Activation/Cascade Annulation of Benzimidates and CF3-Imidoyl Sulfoxonium Ylides for the Synthesis of Trifluoromethyl-Decorated Ben-zo[de][1,8]naphthyridines. Org. Lett. 2022, 24, 8864–8869. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, P.; Chen, Z.; Wu, X.-F. Rh(III)-catalyzed C-H/C-C ac-tivation of benzoylacetonitriles and cyclization with CF3-imidoyl sulfoxonium ylides to 3-trifluoromethyl-isoquinolones. J. Catal. 2023, 427, 115098. [Google Scholar] [CrossRef]
- Wang, P.; Wu, H.; Zhang, X.-P.; Huang, G.; Crabtree, R.H.; Li, X. Sigma-Bond Metathesis as an Unusual Asymmetric Induction Step in Rhodium-Catalyzed Enantiodivergent Synthesis of C-N Axially Chiral Biaryls. J. Am. Chem. Soc. 2023, 145, 8417–8429. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, J.; Chen, Z.; Wu, X.-F. Ruthenium-Catalyzed Hydroxyl-Directed peri-Selective C-H Activation and Annulation of 1-Naphthols with CF3-Imidoyl Sulfoxonium Ylides for the Synthesis of 2-(Trifluoromethyl)-2,3-dihydrobenzo[de]chromen-2-amines. Org. Lett. 2022, 24, 7288–7293. [Google Scholar] [CrossRef]
- Liang, M.; Yan, S.; Xu, Y.; Ma, C.; Zhang, X.; Fan, X. Synthesis of CF3-Isoquinolinones and Imidazole-Fused CF3-Isoquinolinones Based on C-H Activation-Initiated Cascade Reactions of 2-Aryloxazolines. J. Org. Chem. 2024, 89, 10180–10196. [Google Scholar] [CrossRef]
- Zhou, Q.; Yan, S.; Ma, C.; Gong, Y.; Zhang, X.; Fan, X. Divergent Synthesis of (CF3)-Indazolo [3,2-a]Isoquinolines with Potent Photophysical Property and Anticancer Activity from 3-Aryl-1H-Indazoles and Sulfoxonium Ylides. Adv. Synth. Catal. 2024, 366, 5190–5196. [Google Scholar] [CrossRef]
- Liao, J.; Zhai, R.; Gao, X.; Wu, H.; Kong, D.; Wang, S.; Chen, X. Rh(III)-Catalyzed C-H Cyclization of 2-Arylbenzimidazoles with CF3-Imidoyl Sulfoxonium Ylides and Further Sc(III)-Catalyzed Deaminative Hydroxylation. Adv. Synth. Catal. 2024. [Google Scholar] [CrossRef]
- Yang, Z.; Li, P.; Chen, Z.; Wu, X.-F. Rh(III)-Catalyzed Cascade Cyclization of 2-Aryl-3H-indoles and CF3-Imidoyl Sulfoxonium Ylides Toward Trifluoroacetimidoyl-Substituted 11H-Isoindolo [2,1-a]indoles. Adv. Synth. Catal. 2023, 365, 3855–3860. [Google Scholar] [CrossRef]
- Wan, Q.; Huang, C.-Y.; Hou, Z.-W.; Jiang, H.; Wang, L. Organophotoelectrochemical silylation cyclization for the synthesis of silylated 3-CF3-2-oxindoles. Org. Chem. Front. 2023, 10, 3585–3590. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Yang, S.; Yan, Y.; Zhang, X. Enantioselective intramolecular tandem cyclization of o-alkynylbenzamides: Generation of enantioenriched CF3-containing spi-ro-isoindoli-none-indoles. Org. Chem. Front. 2024, 11, 2591–2599. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Q.; Ge, J.; Wu, X.; Li, Z.; Cheng, G. Recent advances in the synthesis of trifluoromethyl-containing heterocyclic compounds via trifluoromethyl building blocks. Org. Biomol. Chem. 2024, 22, 6246–6276. [Google Scholar] [CrossRef]
- Wang, M.; Yan, S.; Li, B.; Hou, H.; Ma, C.; Zhang, X.; Fan, X. Synthesis of CF3-Substituted N-Heterocyclic Compounds Based on C-H Activation-Initiated Formal [2+3] Annulation Featuring with a Latent Nucleophilic Site. J. Org. Chem. 2024, 89, 7828–7842. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Qiu, J.; Wang, Z.; Li, X.; Pan, Y.-M.; Hou, C.; Li, H. Synthesis of 2-Pyrazolines with a CF3- and Alkyne-Substituted Quaternary Carbon Center via [3+2] Cycloaddition of β-CF3-1,3-enynes and Diazoacetates. J. Org. Chem. 2022, 87, 12477–12481. [Google Scholar] [CrossRef]
- Liang, Y.; Jiao, N. Cationic Cobalt(III)-Catalyzed Indole Synthesis: The Regioselective Intermolecular Cyclization of N-Nitrosoanilines and Alkynes. Angew. Chem. Int. Ed. 2016, 55, 4035–4039. [Google Scholar] [CrossRef]
- Caiuby, C.A.D.; de Jesus, M.P.; Burtoloso, A.C.B. α-Imino iridium carbenes from imidoyl sulfoxonium ylides: Application in the one-step synthesis of indoles. J. Org. Chem. 2020, 85, 7433–7445. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Tian, Q.; Chen, Y.; Zhang, Y.; Cheng, G. Annulation of CF3–Imidoyl Sulfoxonium Ylides with 1, 3-Dicarbonyl Compounds: Access to 1,2,3-Trisubstituted 5-Trifluoromethylpyrroles. Org. Lett. 2021, 23, 7407–7411. [Google Scholar] [CrossRef]
- Wen, S.; Chen, Y.; Tian, Q.; Zhang, Y.; Cheng, G. Transition-metal-, additive-, and solvent-free [3+3] annulation of RCF2-imidoyl sulfoxonium ylides with cyclopropenones to give multifunctionalized CF3-pyridones. J. Org. Chem. 2022, 87, 1124–1132. [Google Scholar] [CrossRef]
- Pan, M.; Tong, Y.; Qiu, X.; Zeng, X.; Xiong, B. One-pot synthesis of 3-trifluoromethylbenzo [b][1,4] oxazines from CF3-imidoyl sulfoxonium ylides with 2-bromophenols. Chem. Commun. 2022, 58, 12443–12446. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Y.; Wang, T.; Dou, Q.; Zhou, K.; Zhai, H.; Cheng, B. Synthesis of 4-(Trifluoromethyl)-2, 3-Dihydrothiazoles from α-Enolic Dithioesters and Imidoyl Sulfoxonium Ylides. Adv. Synth. Catal. 2023, 365, 88–95. [Google Scholar] [CrossRef]
- Fu, T.; Yang, J.; Sun, H.; Zhang, C.; Xiang, H.; Zhou, X. Rh(III)-Catalyzed C-H Amination of Azobenzenes with Anthranils. Asian J. Org. Chem. 2018, 7, 1844–1848. [Google Scholar] [CrossRef]
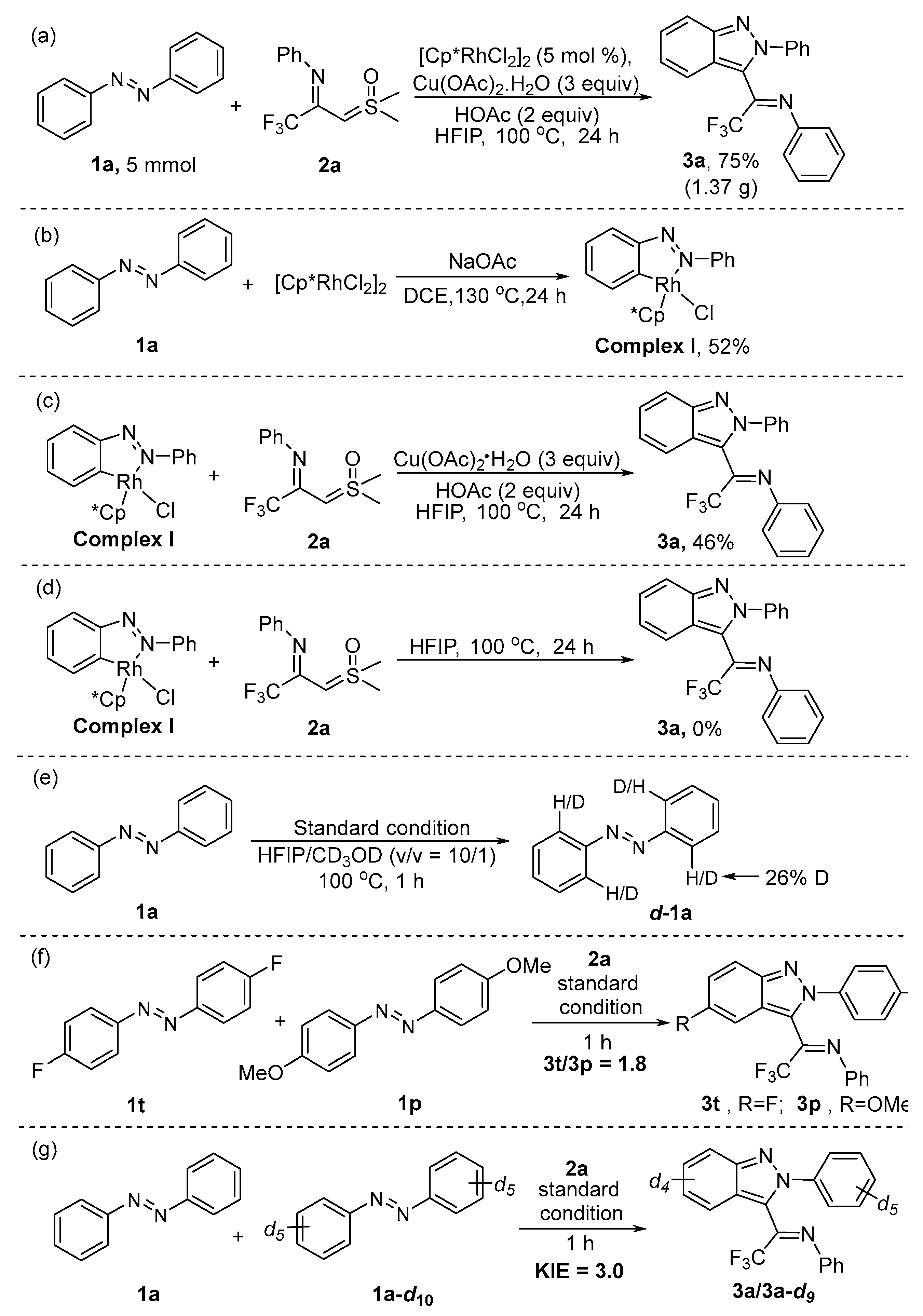
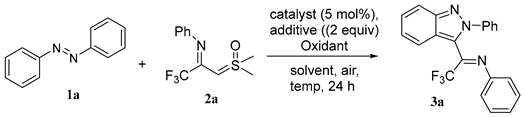
| Entry | Catalyst | Additive | Oxidant | Solvent | Yield [b] |
|---|---|---|---|---|---|
| 1 | [Cp*RhCl2]2 | HOAc | AgOAc | DCE | 27 |
| 2 | [Cp*RhCl2]2 | HOAc | AgOAc | MeCN | 21 |
| 3 | [Cp*RhCl2]2 | HOAc | AgOAc | TFE | 45 |
| 4 | [Cp*RhCl2]2 | HOAc | AgOAc | HFIP | 50 |
| 5 | [Cp*RhCl2]2 | HOAc | AgOAc | DMF | 0 |
| 6 | [Cp*RhCl2]2 | HOAc | AgOAc | THF | 0 |
| 7 | [Cp*RhCl2]2 | HOAc | AgOAc | Dioxane | 0 |
| 8 | Cp*Co(CO)I2 | HOAc | AgOAc | HFIP | 0 |
| 9 | Pd(OAc)2 | HOAc | AgOAc | HFIP | 0 |
| 10 | [Cp*IrCl2]2 | HOAc | AgOAc | HFIP | 0 |
| 11 | [Ru(p-cymene)Cl2]2 | HOAc | AgOAc | HFIP | 12 |
| 12 | none | HOAc | AgOAc | HFIP | 0 |
| 13 | [Cp*RhCl2]2 | none | AgOAc | HFIP | 42 |
| 14 | [Cp*RhCl2]2 | NaOAc | AgOAc | HFIP | 36 |
| 15 | [Cp*RhCl2]2 | HOAc | Ag2CO3 | HFIP | 54 |
| 16 | [Cp*RhCl2]2 | HOAc | Cu(OAc)2·H2O | HFIP | 58 |
| 17 | [Cp*RhCl2]2 | HOAc | K2S2O8 | HFIP | 0 |
| 18 | [Cp*RhCl2]2 | HOAc | PhI(OAc)2 | HFIP | 0 |
| 19 | [Cp*RhCl2]2 | HOAc | O2 | HFIP | 0 |
| 20 | [Cp*RhCl2]2 | HOAc | Cu(OAc)2·H2O | HFIP | 43 [c] |
| 21 | [Cp*RhCl2]2 | HOAc | Cu(OAc)2·H2O | HFIP | 65 [d] |
| 22 | [Cp*RhCl2]2 | HOAc | Cu(OAc)2·H2O | HFIP | 57 [e] |
| 23 | [Cp*RhCl2]2 | HOAc | Cu(OAc)2·H2O | HFIP | 76 [f] |
| 24 | [Cp*RhCl2]2 | HOAc | Cu(OAc)2·H2O | HFIP | 71 [g] |
 |
| [a] Reaction conditions: 1 (0.2 mmol), 2 (0.3 mmol), [Cp*RhCl2]2 (5 mol%), HOAc (2 equiv), Cu(OAc)2·H2O (3 equiv), HFIP (2 mL), air, 100 °C, 24 h; [b] isolated yield. |
| Compound | IC50 (μM) | ||
|---|---|---|---|
| A549 | Hela | HepG2 | |
| 3a | 12.8 | 26.8 | 51.2 |
| 3b | 29.4 | 57.3 | 37.3 |
| 3g | 25.4 | 10.2 | 2.1 |
| 3i | 34.0 | 42.6 | 81.2 |
| 3l | 12.3 | 79.8 | 11.6 |
| 3m | 20.3 | 87.6 | 5.7 |
| 3o | 19.9 | 40.3 | 53.0 |
| 3p | 39.4 | 107.0 | 100.2 |
| 3s | 41.2 | 42.6 | 89.5 |
| 3t | 63.8 | 30.0 | 4.3 |
| 3v | 51.2 | 100.5 | 25.7 |
| 3y | 55.7 | 164.0 | 22.4 |
| Gefitinib | 12.8 | 12.8 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, Y.; Li, C.; Wang, G.; Yao, G.; Wu, H.; Chen, X.; Zhai, R. Synthesis of CF3-Indazoles via Rh(III)-Catalyzed C-H [4+1] Annulation of Azobenzenes with CF3-Imidoyl Sulfoxonium Ylides. Molecules 2025, 30, 183. https://doi.org/10.3390/molecules30010183
Shang Y, Li C, Wang G, Yao G, Wu H, Chen X, Zhai R. Synthesis of CF3-Indazoles via Rh(III)-Catalyzed C-H [4+1] Annulation of Azobenzenes with CF3-Imidoyl Sulfoxonium Ylides. Molecules. 2025; 30(1):183. https://doi.org/10.3390/molecules30010183
Chicago/Turabian StyleShang, Yilong, Chen Li, Guiqiu Wang, Guiwei Yao, Hongliang Wu, Xun Chen, and Ruirui Zhai. 2025. "Synthesis of CF3-Indazoles via Rh(III)-Catalyzed C-H [4+1] Annulation of Azobenzenes with CF3-Imidoyl Sulfoxonium Ylides" Molecules 30, no. 1: 183. https://doi.org/10.3390/molecules30010183
APA StyleShang, Y., Li, C., Wang, G., Yao, G., Wu, H., Chen, X., & Zhai, R. (2025). Synthesis of CF3-Indazoles via Rh(III)-Catalyzed C-H [4+1] Annulation of Azobenzenes with CF3-Imidoyl Sulfoxonium Ylides. Molecules, 30(1), 183. https://doi.org/10.3390/molecules30010183






