Hydrophobization of Chitin Nanofibers by Grafting of Partially 2-Deoxygenated Amyloses Through Enzymatic Approach
Abstract
1. Introduction
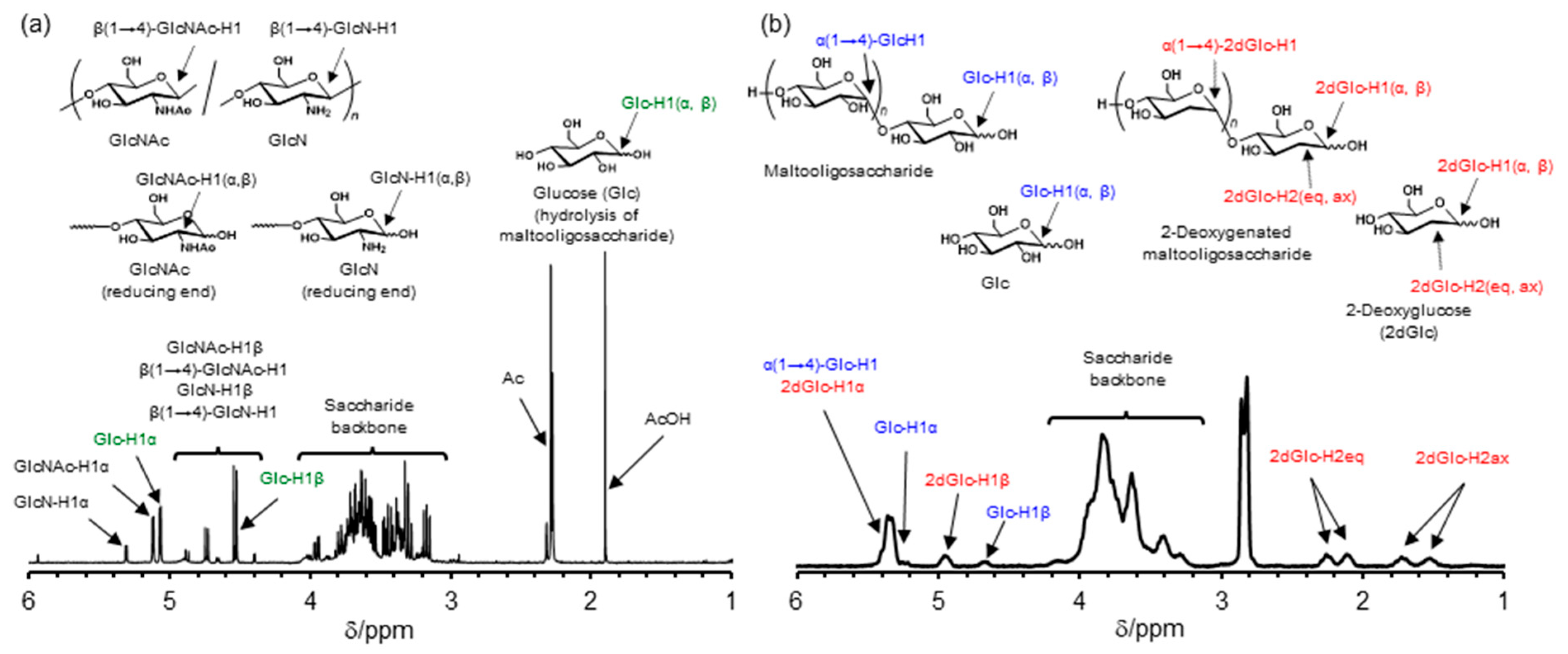
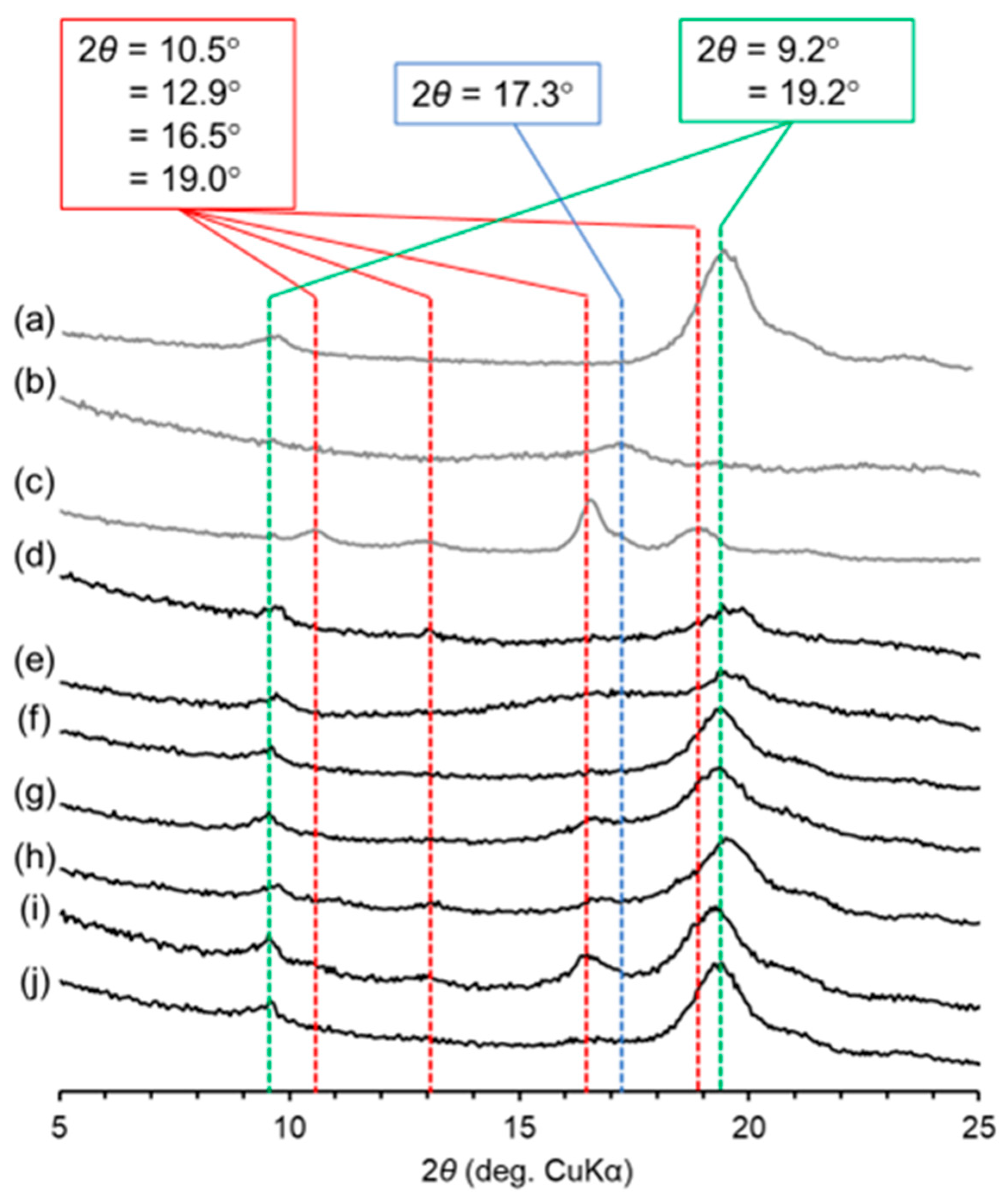
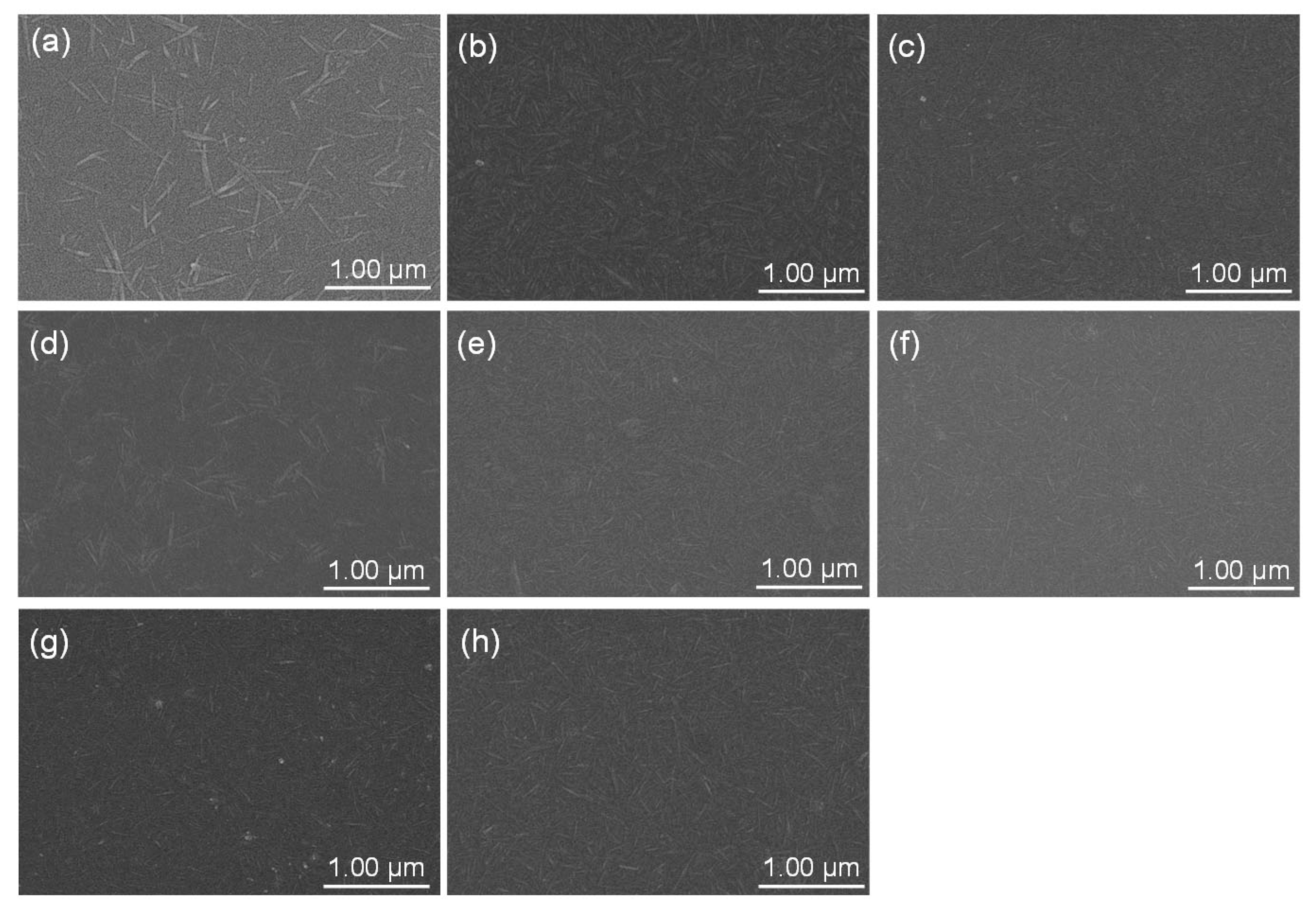
2. Results and Discussion
3. Materials and Methods
3.1. Materials
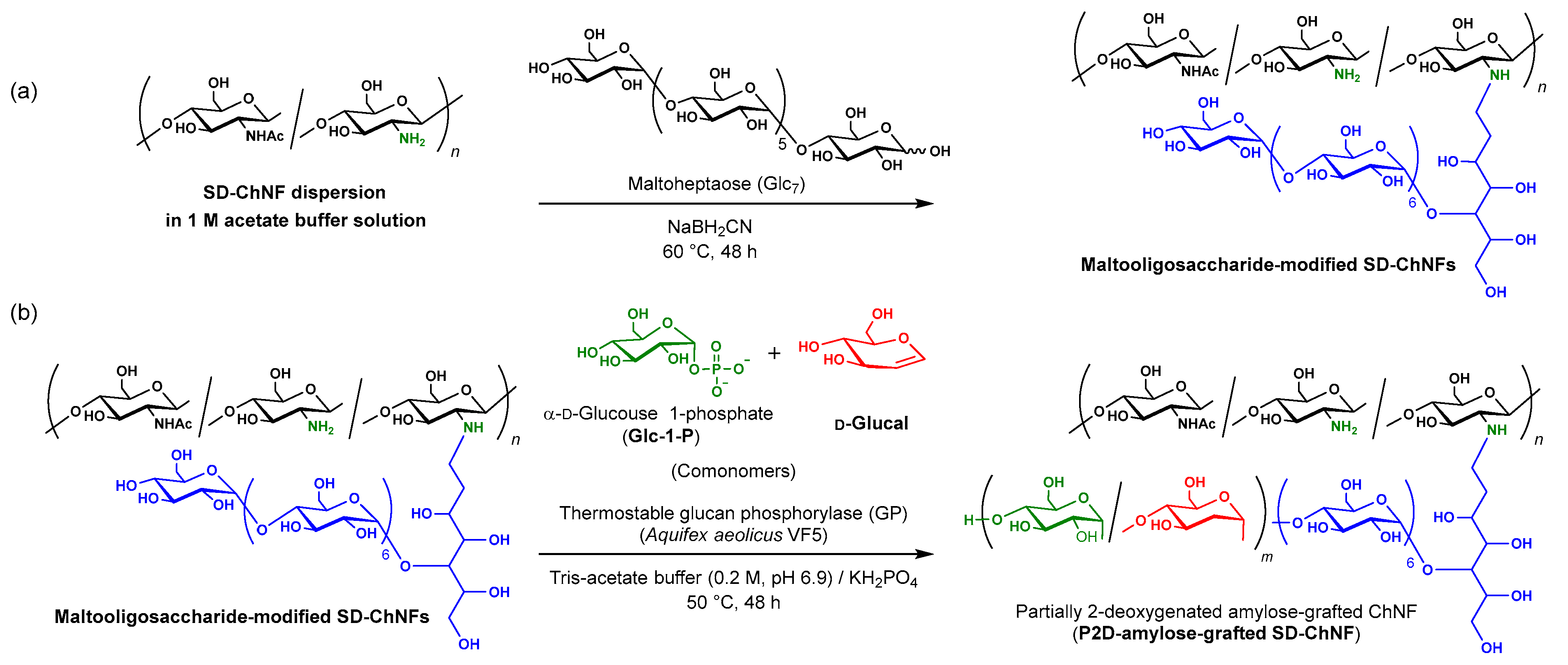
3.2. Preparation of Maltooligosaccharide-Modified SD-ChNFs (Scheme 1a)
3.3. Synthesis of Amylose-, P2D-Amylose-, and 2-Deoxyamylose-Grafted SD-ChNFs (Scheme 1b)
3.4. Dispersion of Amylose-, P2D-Amylose-, and 2-Deoxyamyloses-Grafted SD-ChNFs on Glass Substrates for SEM Observation
3.5. Preparation of Films from Amylose-, P2D-Amylose-, and 2-Deoxyamylose-Grafted SD-ChNFs
3.6. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and theientrylimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Shigenobu, Y.; Nakashima, A.; Kadokawa, J. Synthesis of thermoplastic mixed chitin esters. Biomacromolecules 2023, 24, 5175–5182. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Chigita, H.; Yamamoto, K. Chemoenzymatic synthesis of carboxylate-terminated maltooligosaccharides and their use for cross-linking of chitin. Int. J. Biol. Macromol. 2020, 159, 510–516. [Google Scholar] [CrossRef]
- Kaneko, Y.; Matsuda, S.; Kadokawa, J. Chemoenzymatic syntheses of amylose-grafted chitin and chitosan. Biomacromolecules 2007, 8, 3959–3964. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Chee, P.L.; Sathasivam, T.; Tan, Y.C.; Wu, W.; Leow, Y.; Lim, Q.R.T.; Yew, P.Y.M.; Zhu, Q.; Kai, D. Nanochitin for sustainable and advanced manufacturing. Nanoscale 2024, 16, 3269–3292. [Google Scholar] [CrossRef]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef]
- Ifuku, S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef]
- Nguyen, H.V.D.; de Vries, R.; Stoyanov, S.D. Chitin nanowhiskers with improved properties obtained using natural deep eutectic solvent and mild mechanical processing. Green Chem. 2022, 24, 3834–3844. [Google Scholar] [CrossRef]
- Harkin, C.; Mehlmer, N.; Woortman, D.V.; Brück, T.B.; Brück, W.M. Nutritional and additive uses of chitin and chitosan in the food industry. In Sustainable Agriculture Reviews; Crini, G., Lichtfouse, E., Eds.; Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Springer: Cham, Switzerland, 2019; Volume 36, pp. 1–43. [Google Scholar] [CrossRef]
- Ngasotter, S.; Sampath, L.; Xavier, K.A.M. Nanochitin: An update review on advances in preparation methods and food applications. Carbohydr. Polym. 2022, 291, 119627. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Schroën, K. Advances in chitin-based nanoparticle use in biodegradable polymers: A review. Carbohydr. Polym. 2023, 312, 120789. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. A Mini-Review: Fabrication of Polysaccharide Composite Materials Based on Self-Assembled Chitin Nanofibers. Materials 2024, 17, 1898. [Google Scholar] [CrossRef]
- Lee, S.; Hao, L.T.; Park, J.; Oh, D.X.; Hwang, D.S. Nanochitin and nanochitosan: Chitin nanostructure engineering with multiscale properties for biomedical and environmental applications. Adv. Mater. 2023, 35, 2203325. [Google Scholar] [CrossRef]
- Dong, X.; Shi, L.; Ma, S.; Chen, X.; Cao, S.; Li, W.; Zhao, Z.; Chen, C.; Deng, H. Chitin/chitosan nanofibers toward a sustainable future: From hierarchical structural regulation to functionalization applications. Nano Lett. 2024, 24, 12014–12026. [Google Scholar] [CrossRef]
- Gopalan Nair, K.; Dufresne, A.; Gandini, A.; Belgacem, M.N. Crab shell chitin whiskers reinforced natural rubber nanocomposites. 3. Effect of chemical modification of chitin whiskers. Biomacromolecules 2003, 4, 1835–1842. [Google Scholar] [CrossRef]
- Huang, Y.; He, M.; Lu, A.; Zhou, W.; Stoyanov, S.D.; Pelan, E.G.; Zhang, L. Hydrophobic modification of chitin whisker and Its potential application in structuring oil. Langmuir 2015, 31, 1641–1648. [Google Scholar] [CrossRef]
- Kadokawa, J. Preparation and grafting functionalization of self-assembled chitin nanofiber film. Coatings 2016, 6, 27. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, X.; Chang, Y.; Ren, L.; Zhou, J. Fabrication and characterization of chitin nanofibers through esterification and ultrasound treatment. Carbohydr. Polym. 2018, 180, 81–87. [Google Scholar] [CrossRef]
- Kadokawa, J.; Takegawa, A.; Mine, S.; Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydr. Polym. 2011, 84, 1408–1412. [Google Scholar] [CrossRef]
- Hashiguchi, T.; Yamamoto, K.; Kadokawa, J. Fabrication of highly flexible nanochitin film and its composite film with anionic polysaccharide. Carbohydr. Polym. 2021, 270, 118369. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. 8—Surface derivatization and grafting on self-assembled chitin nanofibers for modification, functionalization, and application. In Surface Treatment Methods of Natural Fibres and Their Effects on Biocomposites; Shahzad, A., Tanasa, F., Teaca, C.-A., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2022; pp. 187–202. [Google Scholar] [CrossRef]
- Kadokawa, J. Application of ionic liquids for the functional materialization of chitin. Mater. Adv. 2022, 3, 3355–3364. [Google Scholar] [CrossRef]
- Kadokawa, J. Hydrogelation from self-assembled and scaled-down chitin nanofibers by the modification of highly polar substituents. Gels 2023, 9, 432. [Google Scholar] [CrossRef]
- Kadokawa, J. Polymer nanocomposites based on nano chitin. In Chemical Physics of Polymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2024; pp. 199–227. [Google Scholar] [CrossRef]
- Watanabe, R.; Yamamoto, K.; Kadokawa, J. Hydrogelation from scaled-down chitin nanofibers by reductive amination of monosaccharide residues. J. Fiber Sci. Technol. 2022, 78, 10–17. [Google Scholar] [CrossRef]
- Kadokawa, J.; Egashira, N.; Yamamoto, K. Chemoenzymatic preparation of amylose-grafted chitin nanofiber network materials. Biomacromolecules 2018, 19, 3013–3019. [Google Scholar] [CrossRef]
- Kadokawa, J. Precision polysaccharide synthesis catalyzed by enzymes. Chem. Rev. 2011, 111, 4308–4345. [Google Scholar] [CrossRef]
- Shoda, S.; Uyama, H.; Kadokawa, J.; Kimura, S.; Kobayashi, S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 2016, 116, 2307–2413. [Google Scholar] [CrossRef]
- Ziegast, G.; Pfannemüller, B. Phosphorolytic syntheses with di-, oligo- and multi- functional primers. Carbohydr. Res. 1987, 160, 185–204. [Google Scholar] [CrossRef]
- Hatanaka, D.; Yamamoto, K.; Kadokawa, J. Preparation of chitin nanofiber-reinforced carboxymethyl cellulose films. Int. J. Biol. Macromol. 2014, 69, 35–38. [Google Scholar] [CrossRef]
- Totani, M.; Anai, T.; Kadokawa, J. Hydrophobization of surfaces on cellulose nanofibers by enzymatic grafting of partially 2-deoxygenated amylose. Carbohydr. Polym. 2024, 335, 122086. [Google Scholar] [CrossRef]
- Kadokawa, J. Synthesis of non-natural oligosaccharides by α-glucan phosphorylase-catalyzed Enzymatic glycosylations using analogue substrates of α-D-glucose 1-phosphate. Trends Glycosci. Glycotechnol. 2013, 25, 57–69. [Google Scholar] [CrossRef]
- Kadokawa, J. Enzymatic synthesis of non-natural oligo- and polysaccharides by phosphorylase-catalyzed glycosylations using analogue substrates. Green Polym. Chem. Biobased Mater. Biocatal. 2015, 1192, 87–99. [Google Scholar] [CrossRef]
- Kadokawa, J. Precision synthesis of functional polysaccharide materials by phosphorylase-catalyzed enzymatic reactions. Polymers 2016, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. α-Glucan phosphorylase-catalyzed enzymatic reactions using analog substrates to synthesize non-natural oligo- and polysaccharides. Catalysts 2018, 8, 473. [Google Scholar] [CrossRef]
- Kadokawa, J. α-Glucan phosphorylase-catalyzed enzymatic reactions to precisely synthesize non-natural polysaccharides. In Sustainability & Green Polymer Chemistry Volume 2: Biocatalysis and Biobased Polymers; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1373, pp. 31–46. [Google Scholar] [CrossRef]
- Kadokawa, J. Glucan phosphorylase-catalyzed enzymatic synthesis of unnatural oligosaccharides and polysaccharides using nonnative substrates. Polym. J. 2022, 54, 413–426. [Google Scholar] [CrossRef]
- Kadokawa, J.; Nakamura, S.; Yamamoto, K. Thermostable α-glucan Phosphorylase-catalyzed enzymatic copolymerization to produce partially 2-deoxygenated amyloses. Processes 2020, 8, 1070. [Google Scholar] [CrossRef]
- Abe, S.; Yamamoto, K.; Kadokawa, J. Hydrophobic polysaccharides: Partially 2-deoxygenated amyloses. Asian J. Org. Chem. 2022, 11, e202100763. [Google Scholar] [CrossRef]
- Uto, T.; Nakamura, S.; Yamamoto, K.; Kadokawa, J. Evaluation of artificial crystalline structure from amylose analog polysaccharide without hydroxy groups at C-2 position. Carbohydr. Polym. 2020, 240, 116347. [Google Scholar] [CrossRef]
- Kadokawa, J.; Abe, S.; Yamamoto, K. Hydrophobization of carboxymethyl cellulose by enzymatic grafting of partially 2-deoxygenated amyloses. Chem. Lett. 2022, 51, 646–649. [Google Scholar] [CrossRef]
- Anai, T.; Abe, S.; Shobu, K.; Kadokawa, J. Synthesis of hydrophobic poly(γ-glutamic acid) derivatives by enzymatic grafting of partially 2-deoxygenated amyloses. Appl. Sci. 2023, 13, 489. [Google Scholar] [CrossRef]
- Evers, B.; Thiem, J. Synthesis of 2-deoxy-maltooligosaccharides with phosphorylase and their degradation with amylases. Starch Stärke 1995, 47, 434–439. [Google Scholar] [CrossRef]
- Egi, Y.; Kontani, A.; Kadokawa, J. Fabrication of all-chitin composite films. Int. J. Biol. Macromol. 2023, 253, 127512. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.; Charlet, G. Chitin fractionation and characterization in N,N-dimethylacetamide/lithium chloride solvent system. Carbohydr. Polym. 2002, 50, 363–370. [Google Scholar] [CrossRef]
- Zhao, D.; Fei, Z.; Geldbach, T.J.; Scopelliti, R.; Laurenczy, G.; Dyson, P.J. Allyl-functionalised ionic liquids: Synthesis, characterisation, and reactivity. Helv. Chim. Acta 2005, 88, 665–675. [Google Scholar] [CrossRef]
- Braunmuehl, V.V.; Jonas, G.; Stadler, R. Enzymic grafting of amylose from poly(dimethylsiloxanes). Macromolecules 1995, 28, 17–24. [Google Scholar] [CrossRef]
- Kadokawa, J.; Setoguchi, T.; Yamamoto, K. Preparation of highly flexible chitin nanofiber-graft-poly(γ-l-glutamic acid) network film. Polym. Bull. 2013, 70, 3279–3289. [Google Scholar] [CrossRef]
- Kadokawa, J.; Obama, Y.; Yoshida, J.; Yamamoto, K. Gel formation from self-assembled chitin nanofiber film by grafting of poly(2-methyl-2-oxazoline). Chem. Lett. 2018, 47, 949–952. [Google Scholar] [CrossRef]

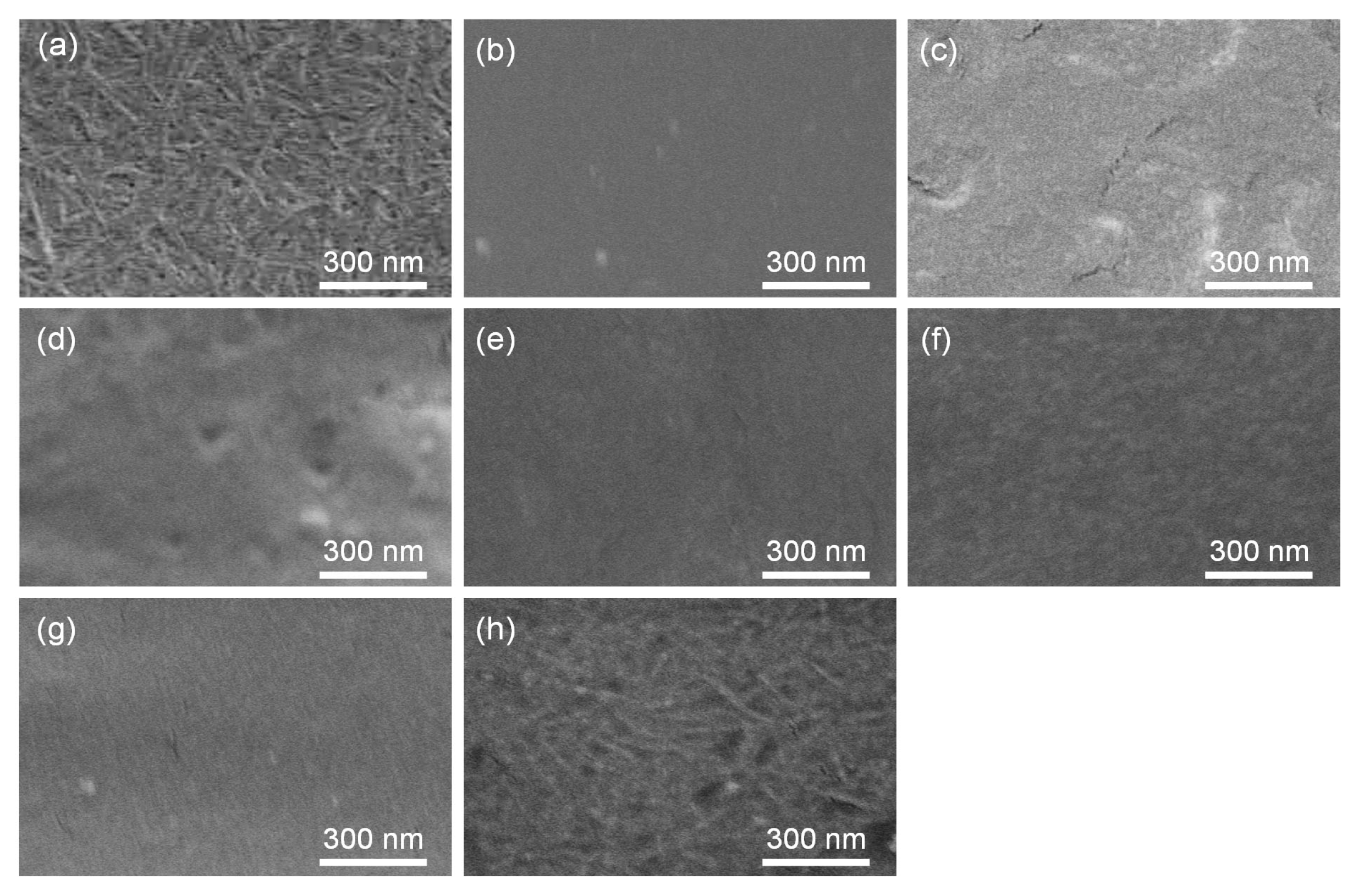
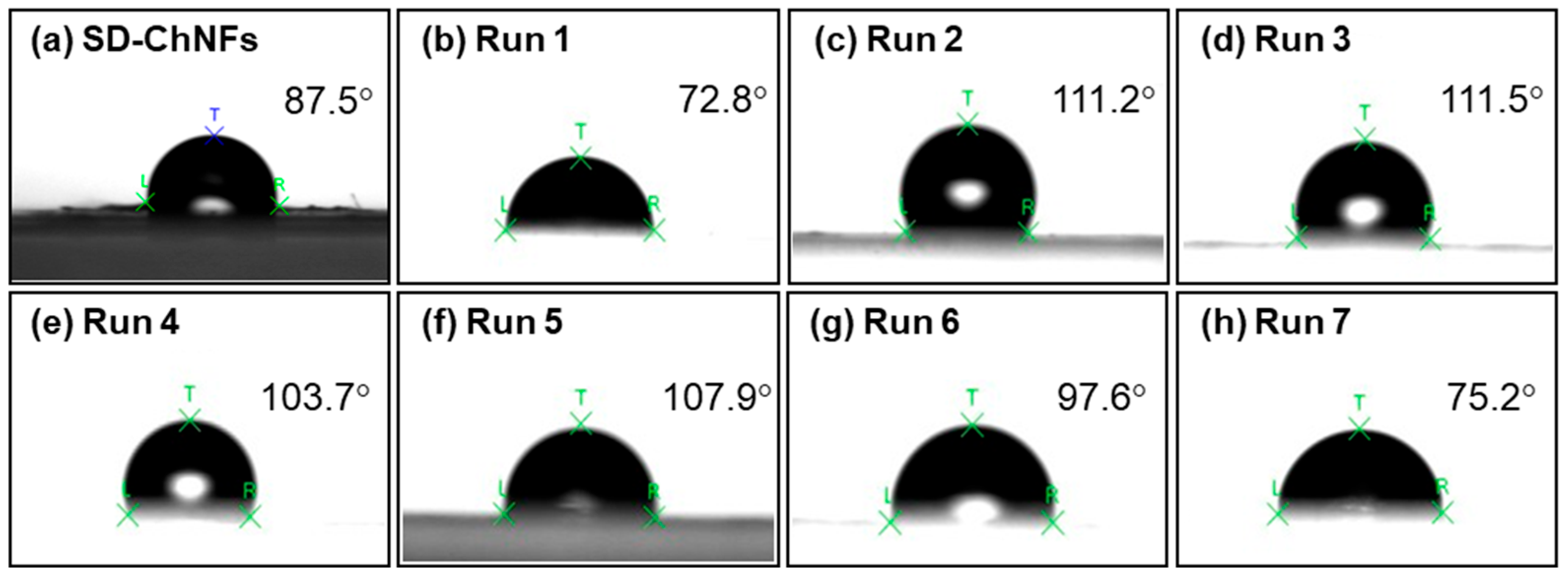
| Entry | Feed Ratio (Glc7/Glc-1-P/d-glucal) | Unit Ratio (Glc/2dGlc) (c) | DP (d) | Content of Elongated Chain (wt%) (e) | Thickness of Elongated Chain Layer/Radius of SD-ChNFs (=la/lb) (f) | ||
|---|---|---|---|---|---|---|---|
| 1 | 1/100/0 | 100 | / | 0 | 47 | 78.6 | 1.16 |
| 2 (b) | 1/50/50 | 74 | / | 26 | 25 | 67.2 | 0.75 |
| 3 | 1/25/75 | 50 | / | 50 | 30 | 68.0 | 0.78 |
| 4 | 1/20/80 | 48 | / | 52 | 28 | 67.0 | 0.74 |
| 5 | 1/15/85 | 43 | / | 57 | 27 | 63.3 | 0.65 |
| 6 | 1/10/90 | 38 | / | 62 | 22 | 61.4 | 0.61 |
| 7 | 1/0/100 | 0 | / | 100 | 3 | 15.0 | 0.08 |
 .
.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, N.; Totani, M.; Kadokawa, J.-i. Hydrophobization of Chitin Nanofibers by Grafting of Partially 2-Deoxygenated Amyloses Through Enzymatic Approach. Molecules 2025, 30, 16. https://doi.org/10.3390/molecules30010016
Yamamoto N, Totani M, Kadokawa J-i. Hydrophobization of Chitin Nanofibers by Grafting of Partially 2-Deoxygenated Amyloses Through Enzymatic Approach. Molecules. 2025; 30(1):16. https://doi.org/10.3390/molecules30010016
Chicago/Turabian StyleYamamoto, Naoki, Masayasu Totani, and Jun-ichi Kadokawa. 2025. "Hydrophobization of Chitin Nanofibers by Grafting of Partially 2-Deoxygenated Amyloses Through Enzymatic Approach" Molecules 30, no. 1: 16. https://doi.org/10.3390/molecules30010016
APA StyleYamamoto, N., Totani, M., & Kadokawa, J.-i. (2025). Hydrophobization of Chitin Nanofibers by Grafting of Partially 2-Deoxygenated Amyloses Through Enzymatic Approach. Molecules, 30(1), 16. https://doi.org/10.3390/molecules30010016








