Synthesis of Branched Cyclo-Olefin Copolymers Using Neutral α-Sulfonate-β-Diimine Nickel Catalyst
Abstract
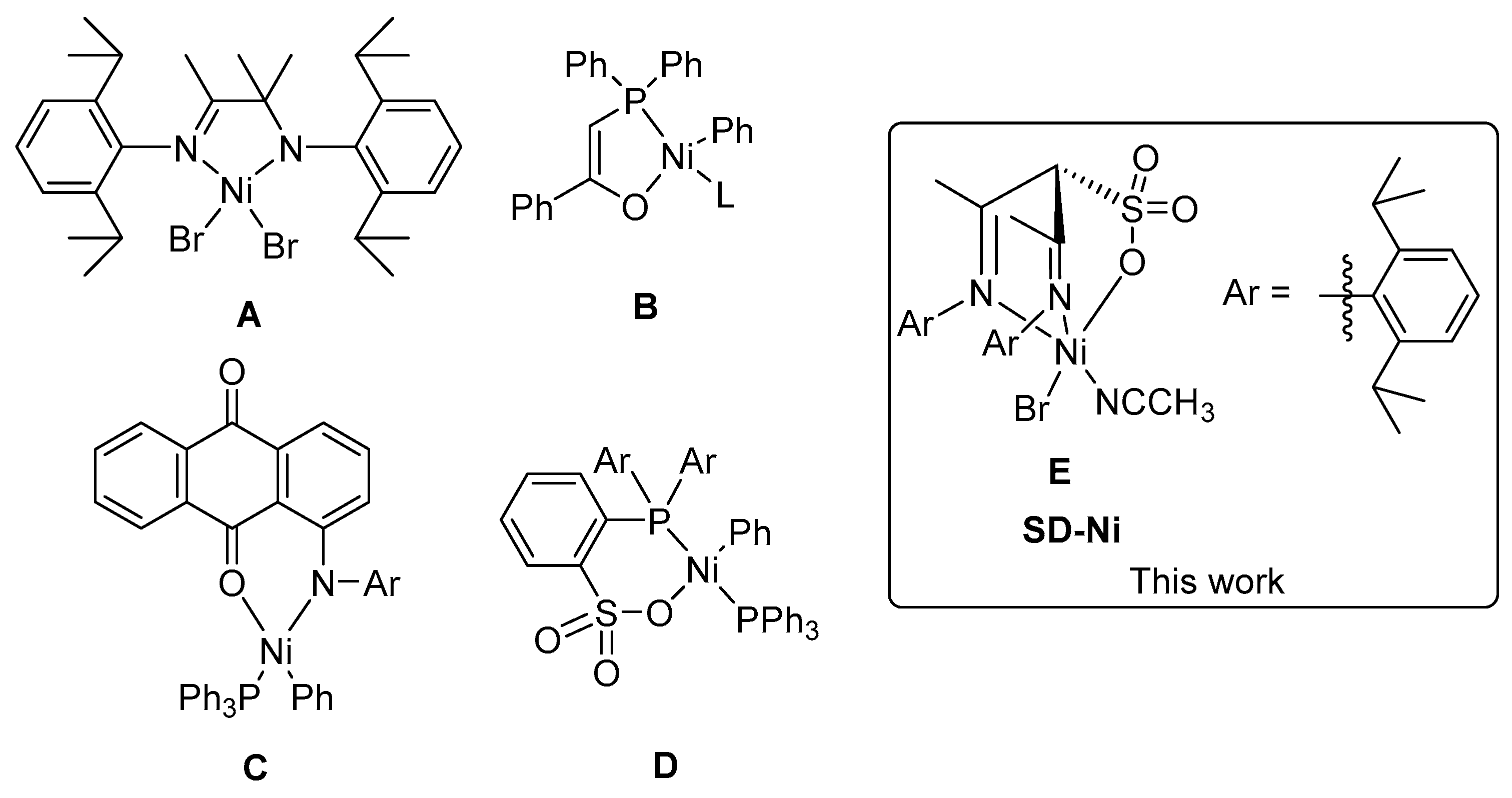
1. Introduction
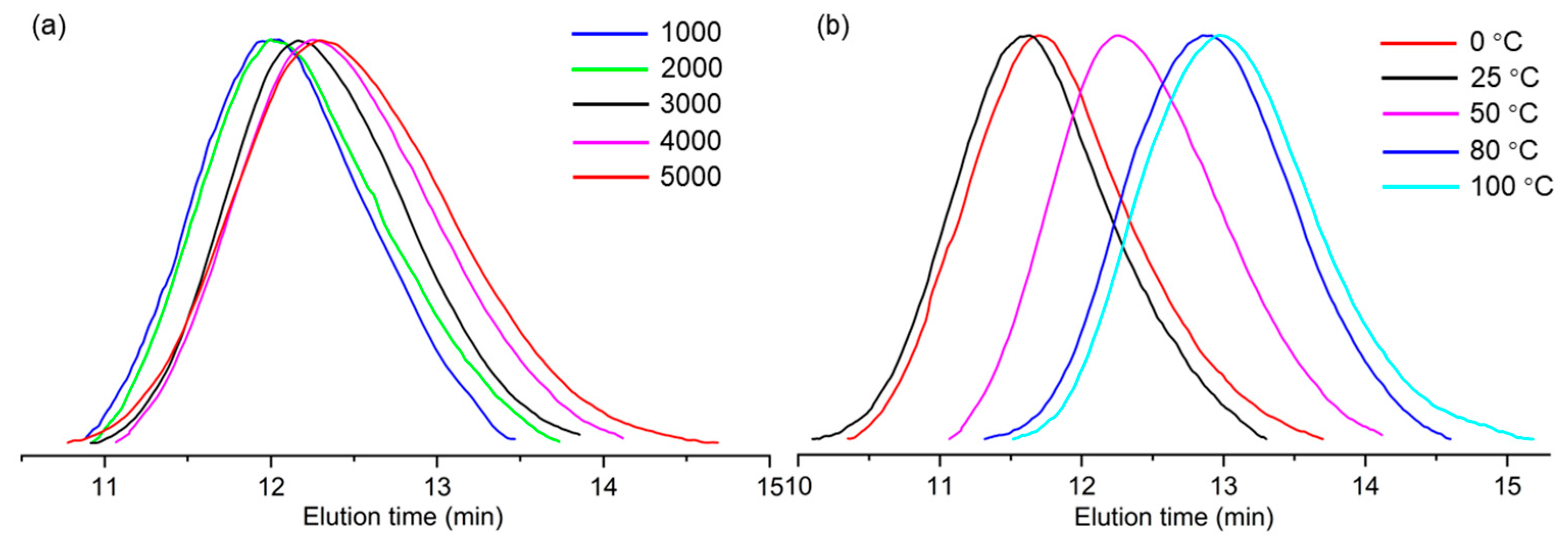
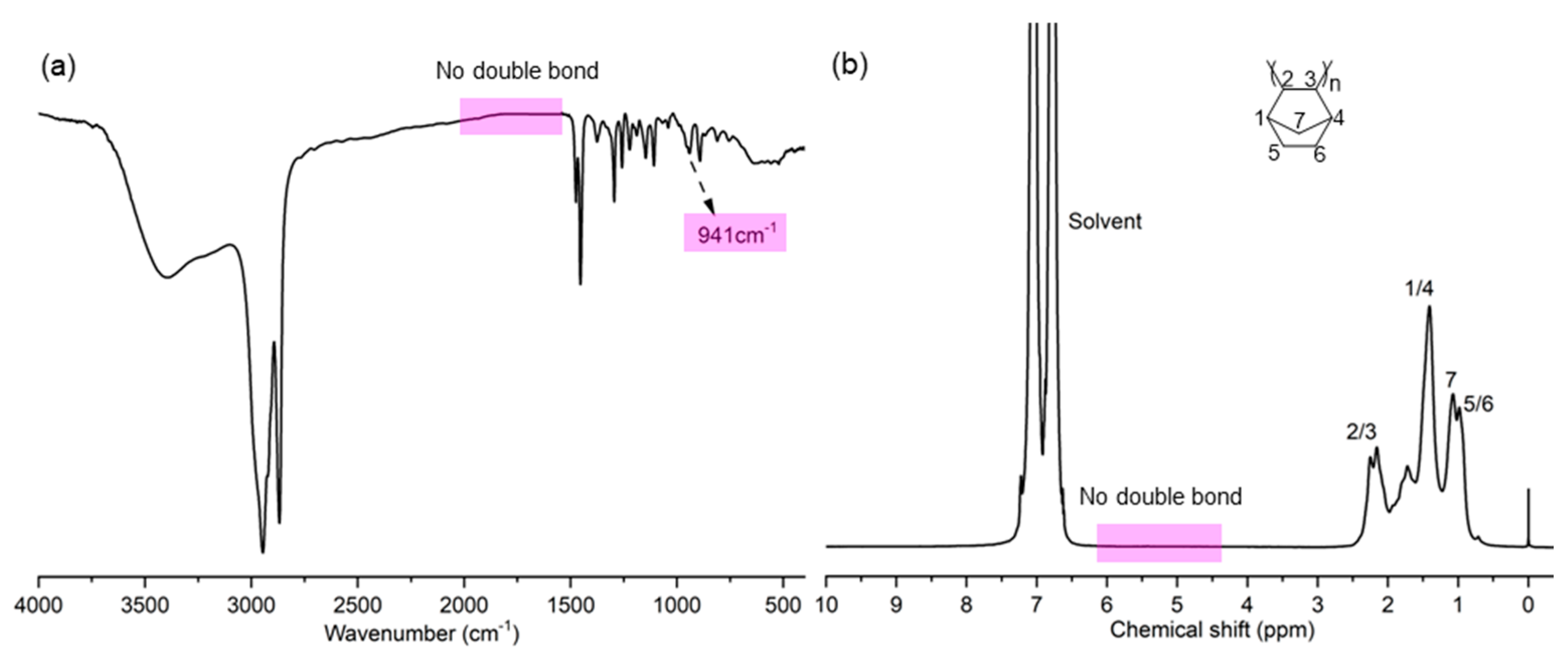
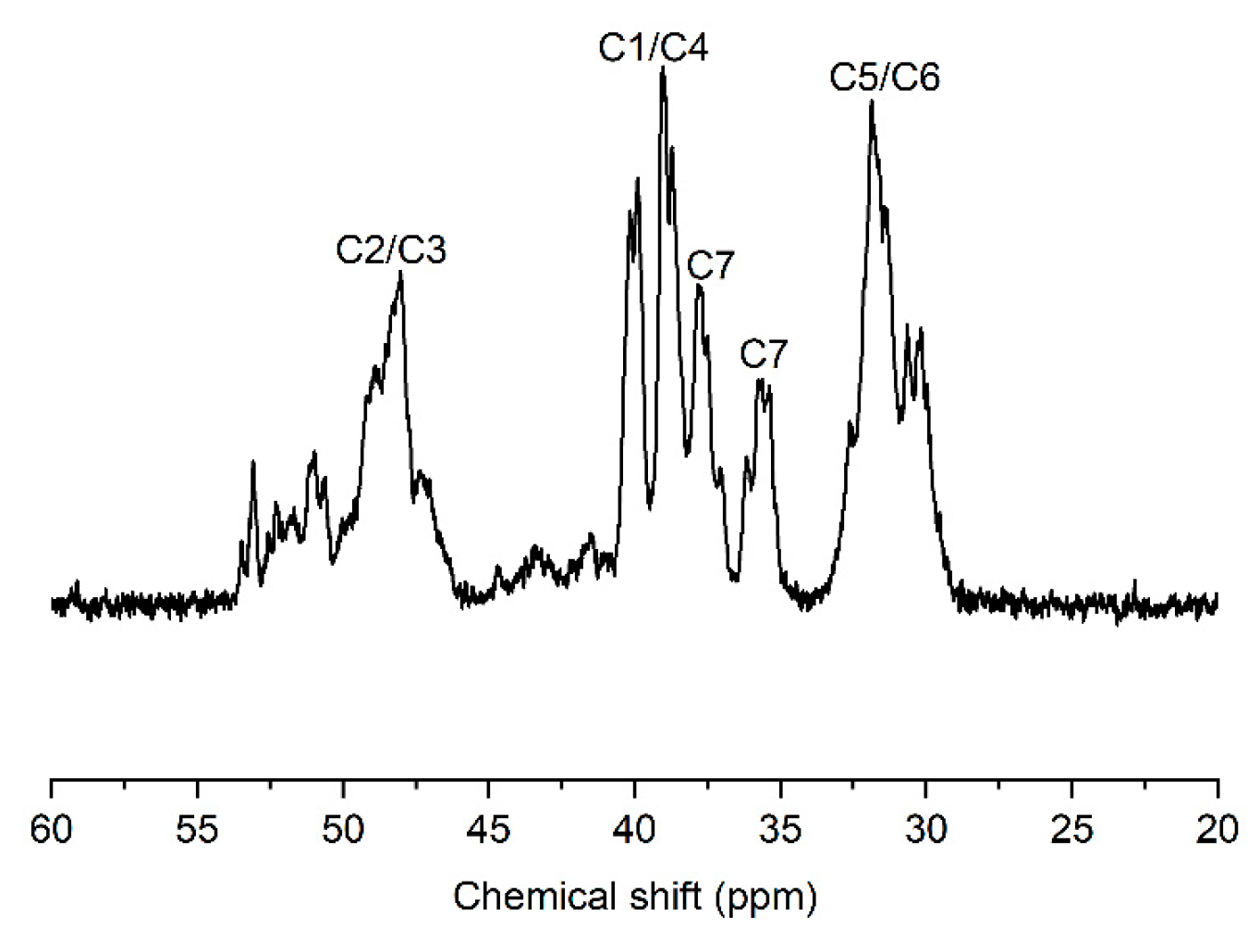
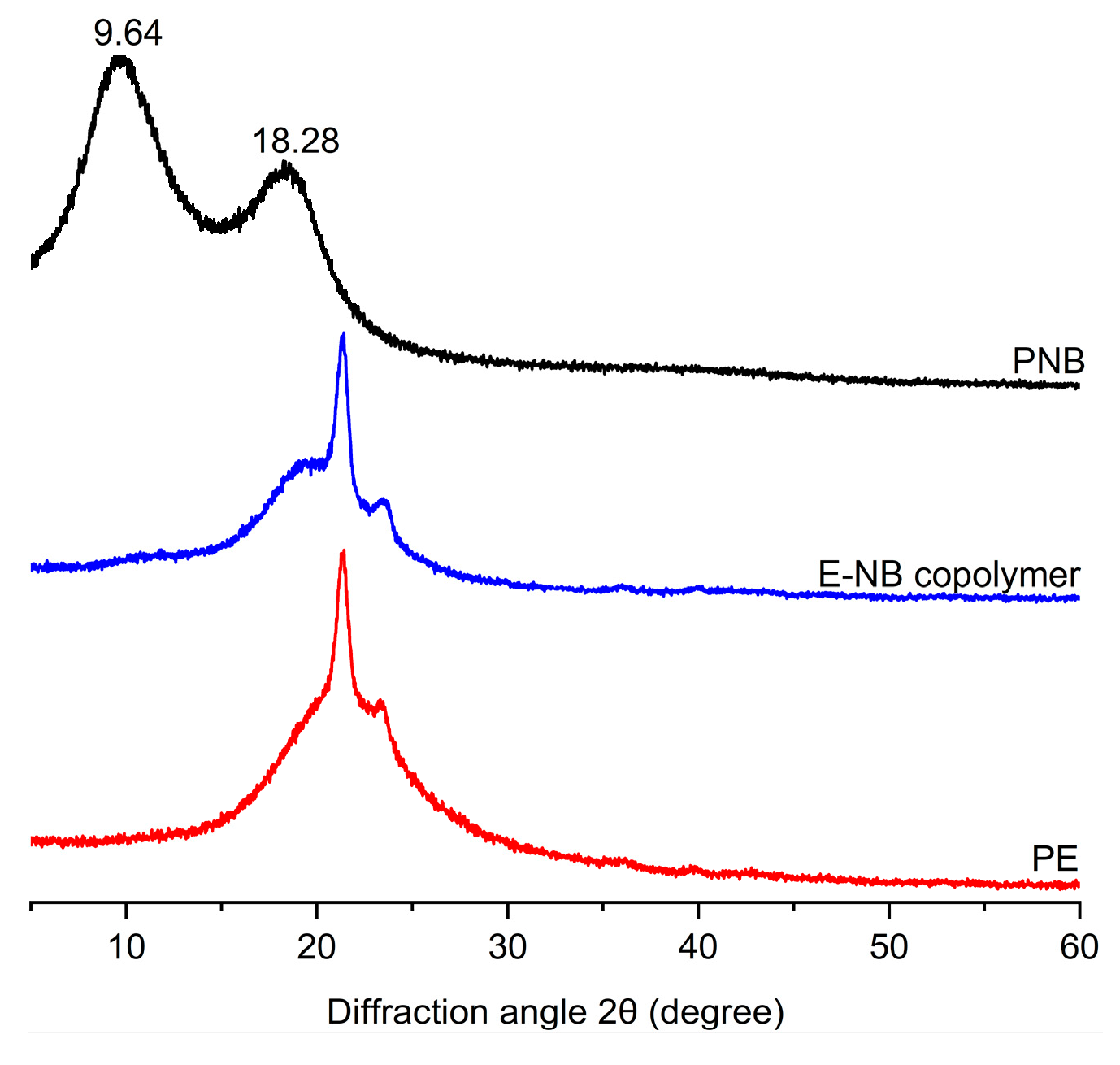
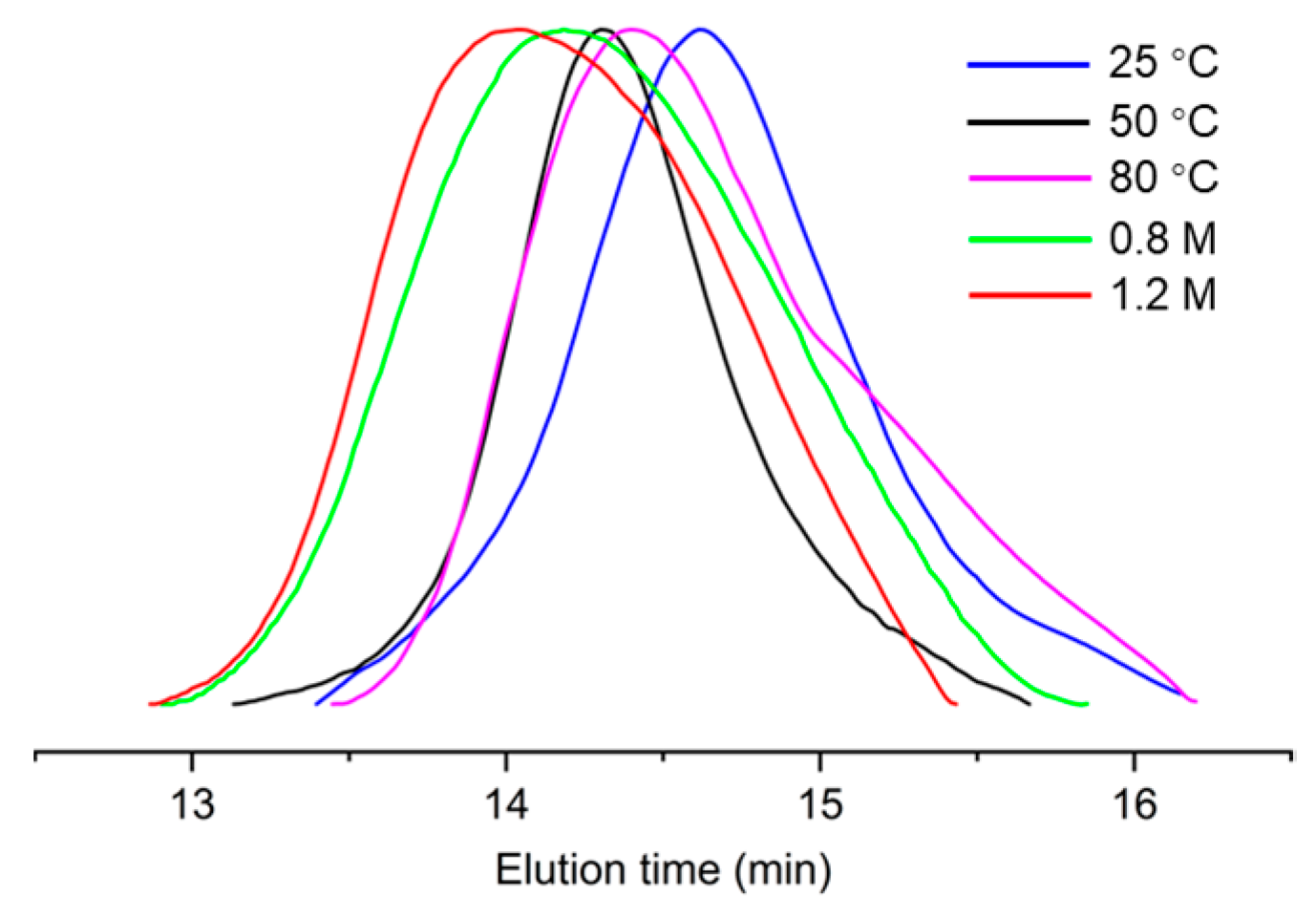
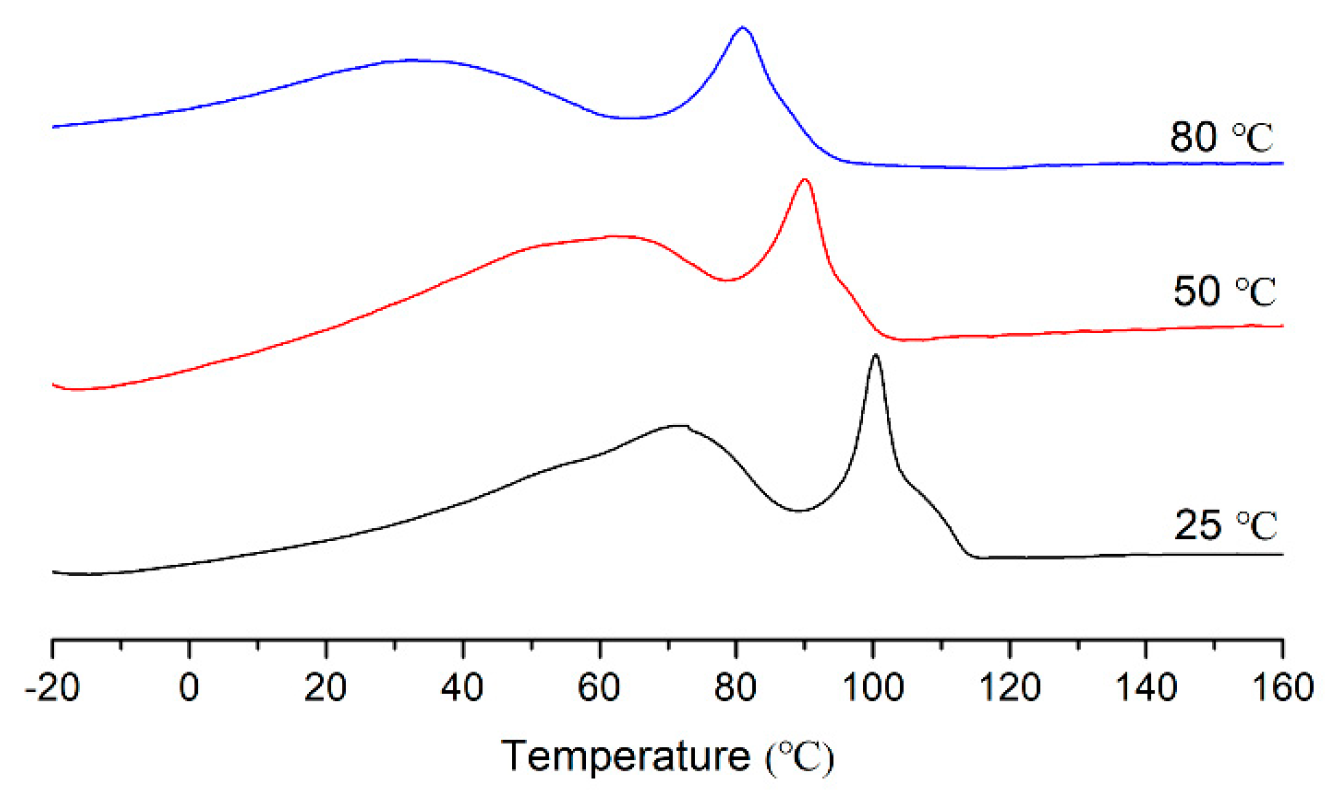
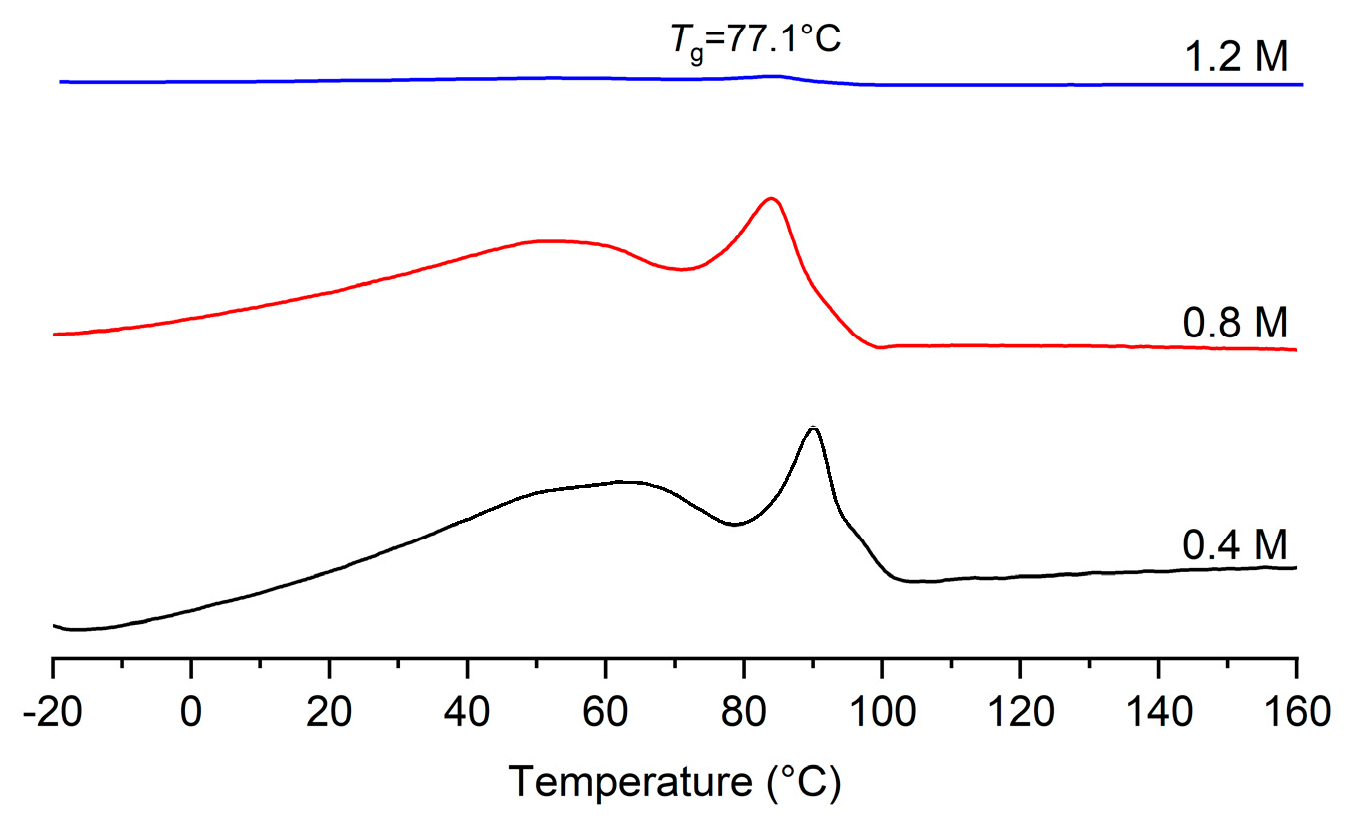
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Materials
3.3. Measurements
3.4. Norbornene Homopolymerization
3.5. Copolymerization of Norbornene with Ethylene
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Hou, Z. Organometallic Catalysts for Copolymerization of Cyclic Olefins. Coord. Chem. Rev. 2008, 252, 1842–1869. [Google Scholar] [CrossRef]
- Bermeshev, M.V.; Chapala, P.P. Addition Polymerization of Functionalized Norbornenes as a Powerful Tool for Assembling Molecular Moieties of new Polymers with Versatile Properties. Prog. Polym. Sci. 2018, 84, 1–46. [Google Scholar] [CrossRef]
- Wang, W.; Qu, S.; Li, X.; Chen, J.; Guo, Z.; Sun, W. Transition Metal Complex Catalysts Promoting Copolymers of Cycloolefin with Propylene/higher Olefins. Coord. Chem. Rev. 2023, 494, 215351. [Google Scholar] [CrossRef]
- Makovetskii, K.L. Catalytic Addition Polymerization of Norbornene and its Derivatives and Copolymerization of Norbornene with Olefins. Polym. Sci. Ser. C 2008, 50, 22–38. [Google Scholar] [CrossRef]
- Zhao, W.; Nomura, K. Design of Efficient Molecular Catalysts for Synthesis of Cyclic Olefin Copolymers (COC) by Copolymerization of Ethylene and α-Olefins with Norbornene or Tetracyclododecene. Catalysts 2016, 6, 175. [Google Scholar] [CrossRef]
- Li, M.; Fang, Y.; Cai, Z.; Eisen, M.S. Nickel- and Palladium-Catalyzed Copolymerizations of Norbornene with Polar α-Olefins. ChemCatChem 2024, 16, e202301731. [Google Scholar] [CrossRef]
- Tritto, I.; Boggioni, L.; Ferro, D.R. Metallocene Catalyzed Ethene- and Propene co-Norbornene Polymerization: Mechanisms from a Detailed Microstructural Analysis. Coord. Chem. Rev. 2006, 250, 212–241. [Google Scholar] [CrossRef]
- Lago, W.S.R.; Aymes-Chodur, C.; Ahoussou, A.P.; Yagoubi, N. Physico-chemical Ageing of Ethylene-norbornene Copolymers: A Review. J. Mater. Sci. 2017, 52, 6879–6904. [Google Scholar] [CrossRef]
- Kaminsky, W.; Bark, A.; Arndt, M. New Polymers by Homogenous Zirconocene/aluminoxane Catalysts. Macromol. Symp. 1991, 47, 83–93. [Google Scholar] [CrossRef]
- Blank, F.; Janiak, C. Metal catalysts for the vinyl/addition polymerization of norbornene. Coord. Chem. Rev. 2009, 253, 827–861. [Google Scholar] [CrossRef]
- Ma, R.; Hou, Y.; Gao, J.; Bao, F. Recent Progress in the Vinylic Polymerization and Copolymerization of Norbornene Catalyzed by Transition Metal Catalysts. Polym. Rev. 2009, 49, 249–287. [Google Scholar] [CrossRef]
- Suslov, D.S.; Bykov, M.V.; Kravchenko, O.V. Norbornene Addition Polymerization with Catalysts Based on Transition Metal Compounds: 2008–2018. Polym. Sci. Ser. C 2019, 61, 145–173. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-Cyclopentadienyl-Amido Catalysts for Olefin Polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W. Precise Control of Polyolefin Stereochemistry Using Single-Site Metal Catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, J.; Li, Y.; Liu, S.; Li, Y. High-Temperature Living Copolymerization of Ethylene with Norbornene by Titanium Complexes Bearing Bidentate [O, P] Ligands. Macromolecules 2009, 42, 8566–8570. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Jiang, H.; Chen, A.; Zou, C. Bidentate Pyridyl-Amido Hafnium Catalysts for Copolymerization of Ethylene with 1-Octene and Norbornene. Eur. J. Inorg. Chem. 2023, 26, e202200725. [Google Scholar] [CrossRef]
- Gao, H.; Hu, H.; Wu, Q. High Norbornene Incorporation in Ethylene-norbornene Copolymerization with a Bis(α-alkyloxoimine) Titanium-MAO Catalyst. Sci. China Chem. 2010, 53, 1634–1640. [Google Scholar] [CrossRef]
- Terao, H.; Iwashita, A.; Ishii, S.; Tanaka, H.; Yoshida, Y.; Mitani, M.; Fujita, T. Ethylene/Norbornene Copolymerization Behavior of Bis(phenoxy-imine)Ti Complexes Combined with MAO. Macromolecules 2009, 42, 4359–4361. [Google Scholar] [CrossRef]
- Gao, M.; Wang, C.; Sun, X.; Qian, C.; Ma, Z.; Bu, S.; Tang, Y.; Xie, Z. Ethylene-Norbornene Copolymerization by New Titanium Complexes bearing Tridentate ligands. Sidearm Effects on Catalytic Activity. Macromol. Rapid Commun. 2007, 28, 1511–1516. [Google Scholar] [CrossRef]
- Gao, J.; Luo, Z.; Wang, Z.; Song, H.; Li, Q.; Li, H.; Hu, Y. Vanadium (III) Catalysts with Bulky Bis-NHCs Ligands for Ethylene-norbornene (Co)polymerization. Appl. Catal. A 2023, 661, 119263. [Google Scholar] [CrossRef]
- Liu, Y.; Cong, R.; Pan, Y.; Chen, M.; Xu, M. Efficient Copolymerization of Ethylene with Norbornene Mediated by Phosphine-Sulfonate Palladium Catalysts. Eur. Polym. J. 2024, 203, 112674. [Google Scholar] [CrossRef]
- Ravasio, A.; Boggioni, L.; Tritto, I. Copolymerization of Ethylene with Norbornene by Neutral Aryl Phosphine Sulfonate Palladium Catalyst. Macromolecules 2011, 44, 4180–4186. [Google Scholar] [CrossRef]
- Meng, S.; Liao, D.; Li, C.; Xu, M. Synthesis of Partially Fluorinated Polyolefins via Copolymerization of Ethylene with Fluorinated Norbornene-based Comonomers. Polym. Chem. 2024, 15, 1642–1647. [Google Scholar] [CrossRef]
- Ruchatz, D.; Fink, G. Ethene-Norbornene Copolymerization with Homogeneous Metallocene and Half-Sandwich Catalysts: Kinetics and Relationships between Catalyst Structure and Polymer Structure. 4. Development of Molecular Weights. Macromolecules 1998, 31, 4684–4686. [Google Scholar] [CrossRef] [PubMed]
- Pong, F.Y.; Mandal, S.; Sen, A. Steric and Electronic Effects in Ethene/Norbornene Copolymerization by Neutral Salicylaldiminato-Ligated Palladium(II) Catalysts. Organometallics 2014, 33, 7044–7051. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Li, G.; Xiao, Z.; Liang, G.; Wu, Q. Catalytic Synthesis of Polyethylene-block-Polynorbornene Copolymers using a Living Polymerization Nickel Catalyst. Polym. Chem. 2014, 5, 6012–6018. [Google Scholar] [CrossRef]
- Benedikt, G.M.; Elce, E.; Goodall, B.L.; Kalamarides, H.A.; McIntosh, L.H.; Rhodes, L.F.; Selvy, K.T.; Andes, C.; Oyler, K.; Sen, A. Copolymerization of Ethene with Norbornene Derivatives Using Neutral Nickel Catalysts. Macromolecules 2002, 35, 8978–8988. [Google Scholar] [CrossRef]
- Makovetskii, K.L.; Bykov, V.I.; Finkel’shtein, E.S. Nickel Catalysts for the Addition Polymerization of Norbornene and its Derivatives and for their Copolymerization with Ethylene. Kinet. Catal. 2006, 47, 241–244. [Google Scholar] [CrossRef]
- Cheng, H.; Cai, Z. (Anilino)anthraquinone Nickel-Catalyzed Random Copolymerization of Norbornene and Ethylene. ChemCatChem 2018, 10, 497–500. [Google Scholar] [CrossRef]
- Chen, M.; Zou, W.; Cai, Z.; Chen, C. Norbornene Homopolymerization and Copolymerization with Ethylene by Phosphine-Sulfonate Nickel Catalysts. Polym. Chem. 2015, 6, 2669–2676. [Google Scholar] [CrossRef]
- Song, G.; Pang, W.; Li, W.; Chen, M.; Chen, C. Phosphine-Sulfonate-based Nickel Catalysts: Ethylene Polymerization and Copolymerization with Polar-Functionalized Norbornenes. Polym. Chem. 2017, 8, 7400–7405. [Google Scholar] [CrossRef]
- Gao, H.; Chen, S.; Du, B.; Dai, Z.; Lu, X.; Zhang, K.; Pan, L.; Li, Y.; Li, Y. Cyclic Olefin Copolymers Containing both Linear Polyethylene and Poly(ethylene-co-norbornene) Segments Prepared from Chain Shuttling Copolymerization of Ethylene and Norbornene. Polym. Chem. 2022, 13, 245–257. [Google Scholar] [CrossRef]
- Hasan, T.; Ikeda, T.; Shiono, T. Highly Efficient Ti-Based Catalyst Systems for Vinyl Addition Polymerization of Norbornene. Macromolecules 2004, 37, 7432–7436. [Google Scholar] [CrossRef]
- Johnson, L.K.; Mecking, S.; Brookhart, M. Copolymerization of Ethylene and Propylene with Functionalized Vinyl Monomers by Palladium(II) Catalysts. J. Am. Chem. Soc. 1996, 118, 267–268. [Google Scholar] [CrossRef]
- Guo, L.; Dai, S.; Sui, X.; Chen, C. Palladium and Nickel Catalyzed Chain Walking Olefin Polymerization and Copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Pei, L.; Liu, F.; Liao, H.; Gao, J.; Zhong, L.; Gao, H.; Wu, Q. Synthesis of Polyethylenes with Controlled Branching with α-Diimine Nickel Catalysts and Revisiting Formation of Long-Chain Branching. ACS Catal. 2018, 8, 1104–1113. [Google Scholar] [CrossRef]
- Du, W.; Zheng, H.; Li, Y.; Cheung, C.S.; Li, D.; Gao, H.; Deng, H.; Gao, H. Neutral Tridentate α-Sulfonato-β-diimine Nickel Catalyst for (Co)polymerizations of Ethylene and Acrylates. Macromolecules 2022, 55, 3096–3105. [Google Scholar] [CrossRef]
- Gao, H.; Pei, L.; Li, Y.; Zhang, J.; Wu, Q. Vinyl Polymerization of Norbornene with Nickel Catalysts bearing [N, N] Six-membered Chelate Ring: Important Influence of Ligand Structure on Activity. J. Mol. Catal. A Chem. 2008, 280, 81–86. [Google Scholar] [CrossRef]
- Gao, H.; Guo, W.; Bao, F.; Gui, G.; Zhang, J.; Zhu, F.; Wu, Q. Synthesis, Molecular Structure, and Solution-Dependent Behavior of Nickel Complexes Chelating Anilido-Imine Donors and Their Catalytic Activity toward Olefin Polymerization. Organometallics 2004, 23, 6273–6280. [Google Scholar] [CrossRef]
- Li, D.; Zheng, H.; Gao, H.; Wang, X.; Gao, H. Ion Pair Effects of Zwitterionic Ni/Pd Allyl Complexes Bearing an α-Sulfonate-β-diimine Ligand by Binding of B(C6F5)3 on Norbornene Polymerization. Organometallics 2024, 43, 2527–2536. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, X.; Zhang, H.; Cao, L.; Li, Y.; Cai, Z. Synthesis of 1,2-Bis(imidazolidin-2-imine)benzene Nickel Complexes and their Application for Norbornene (co)polymerization with Styrene. Eur. Polym. J. 2021, 150, 110426. [Google Scholar] [CrossRef]
- Huo, P.; Li, J.; Liu, W.; Mei, G.; He, X. A Highly Active and Thermally Stable 6,13-Dihydro-6,13-ethanopentacene-15,16-diimine Nickel(II) Complex as Catalyst for Norbornene Polymerization. RSC Adv. 2017, 7, 51858–51863. [Google Scholar] [CrossRef]
- Li, L.; Gomes, P.T.; Lemos, M.A.N.D.A.; Lemos, F.; Fan, Z. Polymerization of Norbornene Catalyzed by Highly Active Tetradentate Chelated α-Diimine Nickel Complexes. Macromol. Chem. Phys. 2011, 212, 367–374. [Google Scholar] [CrossRef]
- Li, M.; Cai, Z.; Eisen, M.S. Rational Design of Aldimine Imidazolidin-2-imine/guanidine Nickel Catalysts for Norbornene (Co)polymerizations with Enhanced Catalytic Performance. J. Catal. 2023, 420, 58–67. [Google Scholar] [CrossRef]
- Pei, L.; Tang, Y.; Gao, H. Homo- and Copolymerization of Ethylene and Norbornene with Anilido-imine Chromium Catalysts. Polymers 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Boglia, A.; Boccia, A.C.; Zetta, L.; Famulari, A.; Meille, S.V. New Stereoregularity in the Stereospecific Polymerization of Bulky Strained Olefins: Diheterotactic Polynorbornene. Macromolecules 2008, 41, 3109–3113. [Google Scholar] [CrossRef]
- Huang, W.; Chang, F.; Chu, P.P.J. Solid-state NMR study of Cyclo-Olefin Copolymer (COC). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2554–2563. [Google Scholar] [CrossRef]
- Chu, P.P.J.; Cheng, M.H.; Huang, W.J.; Chang, F.C. Conformational Conversion and Local Packing of Cyclic Olefin Copolymers. Macromolecules 2000, 33, 9360–9366. [Google Scholar] [CrossRef]
- Zhao, C.T.; do Rosario Ribeiro, M.; de Pinho, M.N.; Subrahmanyam, V.S.; Gil, C.L.; de Lima, A.P. Structural Characteristics and Gas Permeation Properties of Polynorbornenes with Retained Bicyclic Structure. Polymer 2001, 42, 2455–2462. [Google Scholar] [CrossRef]
- Wilks, B.R.; Chung, W.J.; Ludovice, P.J.; Rezac, M.R.; Meakin, P.; Hill, A.J. Impact of Average Free-volume Element Size on Rransport in Stereoisomers of Polynorbornene. I. Properties at 35 °C. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2185–2199. [Google Scholar] [CrossRef]
- Wei, C.; Gao, Y.; Zu, F.; Cui, C. Synthesis of Si-O-Si Bridged Diamido Group 4 Complexes and the Applications to Polymerization of Olefins. Appl. Organomet. Chem. 2023, 37, e7048. [Google Scholar] [CrossRef]
- Tritto, I.; Marestin, C.; Boggioni, L.; Zetta, L.; Provasoli, A.; Ferro, D.R. Ethylene-Norbornene Copolymer Microstructure. Assessment and Advances Based on Assignments of 13C NMR Spectra. Macromolecules 2000, 33, 8931–8944. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, N.; Wang, L.; Chen, L.; Li, C.; Jiang, Y.; Wang, J. Norbornene Polymerization and Copolymerization with Ethylene by Titanium Complexes bearing Pyridinium Imide Ligand. Transit. Met. Chem. 2023, 48, 11–20. [Google Scholar] [CrossRef]
- Li, X.; Baldamus, J.; Hou, Z. Alternating Ethylene-Norbornene Copolymerization Catalyzed by Cationic Half-sandwich Scandium Complexes. Angew. Chem. Int. Ed. 2005, 44, 962–965. [Google Scholar] [CrossRef] [PubMed]

| Entry | Al/Ni | T (°C) | Yield (g) | Act. b | Mn c (kg/mol) | Mw c (kg/mol) | Mw/Mn c |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 50 | - | - | - | - | - |
| 2 | 1000 | 50 | 0.782 | 0.78 | 632.9 | 1025.4 | 1.62 |
| 3 | 2000 | 50 | 1.123 | 1.12 | 549.6 | 961.9 | 1.75 |
| 4 | 3000 | 50 | 1.402 | 1.40 | 489.6 | 788.3 | 1.61 |
| 5 | 4000 | 50 | 2.672 | 2.67 | 446.2 | 736.2 | 1.65 |
| 6 | 5000 | 50 | 2.396 | 2.40 | 388.8 | 734.9 | 1.89 |
| 7 | 4000 | 0 | 1.356 | 1.36 | 613.9 | 1141.9 | 1.86 |
| 8 | 4000 | 25 | 1.868 | 1.87 | 774.4 | 1339.7 | 1.73 |
| 9 | 4000 | 80 | 3.413 | 3.41 | 256.5 | 451.4 | 1.76 |
| 10 | 4000 | 100 | 3.083 | 3.08 | 194.7 | 375.7 | 1.93 |
| Entry | T (°C) | [NB] (mol/L) | Yield (g) | Act. b | INB c (mol %) | Mw d | Mw/Mn d | Tm/Tg e |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | - | 0.916 | 45.8 | - | 207.1 | 1.43 | 82.3, 103.5/- |
| 2 | 25 | 0.4 | 0.353 | 17.6 | 2.3 | 32.2 | 1.53 | 70.3, 100.5/- |
| 3 | 50 | 0.4 | 0.485 | 24.2 | 4.8 | 44.1 | 1.41 | 61.6, 90.1/- |
| 4 | 80 | 0.4 | 0.213 | 10.7 | 7.2 | 39.8 | 1.61 | 40.5, 79.5/- |
| 5 | 50 | 0.8 | 0.352 | 17.6 | 10.6 | 62.8 | 1.64 | 53.3, 84.3/- |
| 6 | 50 | 1.2 | 0.285 | 14.3 | 22.1 | 74.1 | 1.52 | -/77.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Pei, L.; Du, W.; Xiao, X.; Gao, H.; Zheng, H.; Gao, H. Synthesis of Branched Cyclo-Olefin Copolymers Using Neutral α-Sulfonate-β-Diimine Nickel Catalyst. Molecules 2025, 30, 157. https://doi.org/10.3390/molecules30010157
Li D, Pei L, Du W, Xiao X, Gao H, Zheng H, Gao H. Synthesis of Branched Cyclo-Olefin Copolymers Using Neutral α-Sulfonate-β-Diimine Nickel Catalyst. Molecules. 2025; 30(1):157. https://doi.org/10.3390/molecules30010157
Chicago/Turabian StyleLi, Donghui, Lixia Pei, Wenbo Du, Xieyi Xiao, Heng Gao, Handou Zheng, and Haiyang Gao. 2025. "Synthesis of Branched Cyclo-Olefin Copolymers Using Neutral α-Sulfonate-β-Diimine Nickel Catalyst" Molecules 30, no. 1: 157. https://doi.org/10.3390/molecules30010157
APA StyleLi, D., Pei, L., Du, W., Xiao, X., Gao, H., Zheng, H., & Gao, H. (2025). Synthesis of Branched Cyclo-Olefin Copolymers Using Neutral α-Sulfonate-β-Diimine Nickel Catalyst. Molecules, 30(1), 157. https://doi.org/10.3390/molecules30010157







