An Experimental and Quantum Chemical Calculation Study on the Performance of Different Types of Ester Collectors
Abstract
1. Introduction
2. Results and Discussion
2.1. Microflotation Tests Results
2.2. Microcalorimetric Measurement Results
2.3. Molecular Structure and Properties of Collectors
2.3.1. Difference in C=S Bond in Collectors
2.3.2. Frontier Orbital, Atomic Charge, and Electrostatic Potential of Molecules
2.3.3. Polarizability, First Ionization Potential, and Electrophilic Nucleophile Index of Collectors
2.3.4. Coordination Model of Collector and Chalcopyrite Surface
3. Experimental and Computational Methods
3.1. Microflotation Tests
3.2. Microcalorimetric Measurement
3.3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jena, S.S.; Tripathy, S.K.; Mandre, N.R.; Venugopal, R.; Farrokhpay, S. Sustainable Use of Copper Resources: Beneficiation of Low-Grade Copper Ores. Minerals 2022, 12, 545. [Google Scholar] [CrossRef]
- Xian-zhong, B.U.; Feng, Y.Y.; Xue, J.W.; Yang, L.; Zhang, C.H. Effective recovery of chalcopyrite at low temperatures using modified ester collector. Trans. Nonferrous Met. Soc. China 2022, 32, 296–306. [Google Scholar] [CrossRef]
- Mkhonto, P.P.; Zhang, X.R.; Lu, L.; Xiong, W.; Zhu, Y.G.; Han, L.; Ngoepe, P.E. Adsorption mechanisms and effects of thiocarbamate collectors in the separation of chalcopyrite from pyrite minerals: DFT and experimental studies. Miner. Eng. 2022, 176, 107–318. [Google Scholar] [CrossRef]
- Harris, G.H.; Fischback, B.C. Process for the Manufacture of Dialkyl Thionocarbamates. U.S. Patent No. 2,691,635, 12 October 1954. [Google Scholar]
- Fairthorne, G.; Fornasiero, D.; Ralston, J. Solution properties of thionocarbamate collectors. Int. J. Miner. Process. 1996, 46, 137–153. [Google Scholar] [CrossRef]
- Huang, Z.R.; Zhong, H.; Wang, S.; Liu, G.Y. Progress of flotation technology and collectors for chalcopyrite. Appl. Chem. Ind. 2013, 42, 2048–2051. [Google Scholar] [CrossRef]
- Falvey; Joseph, J. Dialkyl Dithiocarbamates as Collectors in Froth Flotation. US Patent US3464551A, 2 September 1969. [Google Scholar]
- Ackerman, P.K.; Harris, G.H.; Klimpel, R.R.; Aplan, F.F. Effect of alkyl substituents on performance of thionocarbamates as copper sulphide and pyrite collectors. In Reagents in the Minerals Industry; Springer: London, UK, 1984; pp. 69–78. [Google Scholar]
- Liu, G.Y.; Zhong, H.; Dai, T.G.; Xia, L.Y. Investigation of the effect of N-substituents on performance of thionocarbamates as selective collectors for copper sulfides by ab initio calculations. Miner. Eng. 2008, 21, 1050–1054. [Google Scholar] [CrossRef]
- Mkhonto, P.P.; Zhang, X.; Lu, L.; Zhu, Y.; Han, L.; Ngoepe, P.E. Unravelling the performance of oxycarbonyl-thiocarbamate collectors on chalcopyrite using first-principles calculations and micro-flotation recoveries. Appl. Surf. Sci. 2021, 563, 150332. [Google Scholar] [CrossRef]
- Huang, X.; Huang, K.; Jia, Y.; Wang, S.; Cao, Z.; Zhong, H. Investigating the selectivity of a xanthate derivative for the flotation separation of chalcopyrite from pyrite. Chem. Eng. Sci. 2019, 205, 220–229. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, G.; Zhong, H.; Huang, Y.; Cao, Z. The flotation behavior and adsorption mechanism of O-isopropyl-S-[2-(hydroxyimino) propyl] dithiocarbonate ester to chalcopyrite. J. Taiwan Inst. Chem. Eng. 2017, 71, 38–46. [Google Scholar] [CrossRef]
- Liu, S.; Xie, L.; Liu, G.; Zhong, H.; Wang, Y.; Zeng, H. Hetero-difunctional reagent with superior flotation performance to chalcopyrite and the associated surface interaction mechanism. Langmuir 2019, 35, 4353–4363. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Density functional theory. Phys. Rev. 1964, 136, 864–876. [Google Scholar] [CrossRef]
- Ziegler, T. Approximate density functional theory as a practical tool in molecular energetics and dynamics. Chem. Rev. 1991, 91, 651–667. [Google Scholar] [CrossRef]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density functional theory of electronic structure. J. Phys. Chem. A 1996, 100, 12974–12980. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.F.; Chen, J.H.; Chen, Y. Effect of content and spin state of iron on electronic properties and floatability of iron-bearing sphalerite: A DFT+U study. Int. J. Min. Sci. Technol. 2023, 33, 1563–1571. [Google Scholar] [CrossRef]
- Huang, X.; Huang, K.; Wang, S.; Cao, Z.; Zhong, H. Synthesis of 2-hydroxyethyl dibutyldithiocarbamate and its adsorption mechanism on chalcopyrite. Appl. Surf. Sci. 2019, 476, 460–467. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.; Long, X. Study of H2O adsorption on sulfides surfaces andthermokinetic analysis. J. Ind. Eng. Chem. 2014, 20, 605–609. [Google Scholar] [CrossRef]
- Lan, L.H.; Chen, J.H.; Li, Y.Q. Microthermokinetic study of xanthate adsorption on impurity-doped galena. Trans. Nonferrous Met. Soc. China 2016, 26, 272–281. [Google Scholar] [CrossRef]
- Fukui, K.; Koga, N.; Fujimoto, H. Interaction frontier orbitals. J. Am. Chem. Soc. 1981, 103, 196–197. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, N.; Feng, H.Q.; Wang, Y.H.; Cui, M.; Li, Y. A new combined collector of sodium oleate and 1,12-dodecanediamine for efficient flotation separation of spodumene from feldspar. Appl. Surf. Sci. 2024, 673, 160905. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.Q.; Shen, Z.Y. Effect of oxygen-containing functional groups on the wettability of coal through DFT and MD simulation. Arab. J. Chem. 2023, 16, 104606. [Google Scholar] [CrossRef]
- Ding, M.Y.; Liu, Y.C.; Chen, J.H.; Li, Y.Q. DFT study on the effects of substituents on the structure and properties of dithiophosphate collectors. Int. J. Quantum Chem. 2024, 124, e27355. [Google Scholar] [CrossRef]
- CHEN, J.H.; WANG, J.M.; Long, X.H.; Guo, J. First-principle theory on electronic structure of copper sulfides. J. Cent. South Univ. (Sci. Technol.) 2011, 42, 3612–3617. [Google Scholar]
- Bayly, C.I.; Cieplak, P.; Cornell, W.D.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Chen, J.H. The interaction of flotation reagents with metal ions in mineral surfaces: A perspective from coordination chemistry. Miner. Eng. 2021, 171, 107067. [Google Scholar] [CrossRef]
- Li, Y.Q.; Liu, Y.C.; Chen, J.H.; Zhao, C.H.; Cui, W.Y. Comparison study of crystal and electronic structures for chalcopyrite (CuFeS2) and pyrite (FeS2). Physicochem. Probl. Miner. Process. 2020, 57, 100–111. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R. Ab initio study of solvated molecules: A new implementation of the polarizable continuum mode. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Miertus, S.; Tomasi, J. Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
- Yekeler, H.; Yekeler, M. Reactivities of some thiol collectors and their interactions with Ag (+1) ion by molecular modeling. Appl. Surf. Sci. 2004, 236, 435–443. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, G.; Xia, L.; Lu, Y.; Hu, Y.; Zhao, S.; Yu, X. Flotation separation of diaspore from kaolinite, pyrophyllite and illite using three cationic collectors. Miner. Eng. 2008, 21, 12–14. [Google Scholar] [CrossRef]
- Yekeler, H.; Yekeler, M. Predicting the efficiencies of 2-mercaptobenzothiazole collectors used as chelating agents in flotation processes: A density-functional study. J. Mol. Model. 2006, 12, 763–768. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.Y.; Sun, B.L.; Guo, J.Y.; Li, B. Study on low-rank coal flotation collector screening and performance based on molecular polarity index. J. Mol. Model. 2024, 30, 329. [Google Scholar] [CrossRef]
- Yang, X.L.; Liu, S.; Liu, G.Y.; Liu, H.Z. A DFT study on the structure–reactivity relationship of aliphatic oxime derivatives as copper chelating agents and malachite flotation collectors. J. Ind. Eng. Chem. 2017, 46, 404–415. [Google Scholar] [CrossRef]
- de Miranda, D.B.; Quintal, S.; Ferreira, G.B. Theoretical studies of Zn2+ complexes with alkyl xanthate ligands: A thermochemical, electronic energy decomposition, and natural bond orbital analysis. J. Mol. Model. 2023, 29, 203. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F.W. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Comput. Chem. 2024, 161, 082503. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; François, J.P.; Deleuze, M.S. Theoretical study of the internal elimination reactions of xanthate precursors. J. Comput. Chem. 2003, 24, 2023–2031. [Google Scholar] [CrossRef] [PubMed]

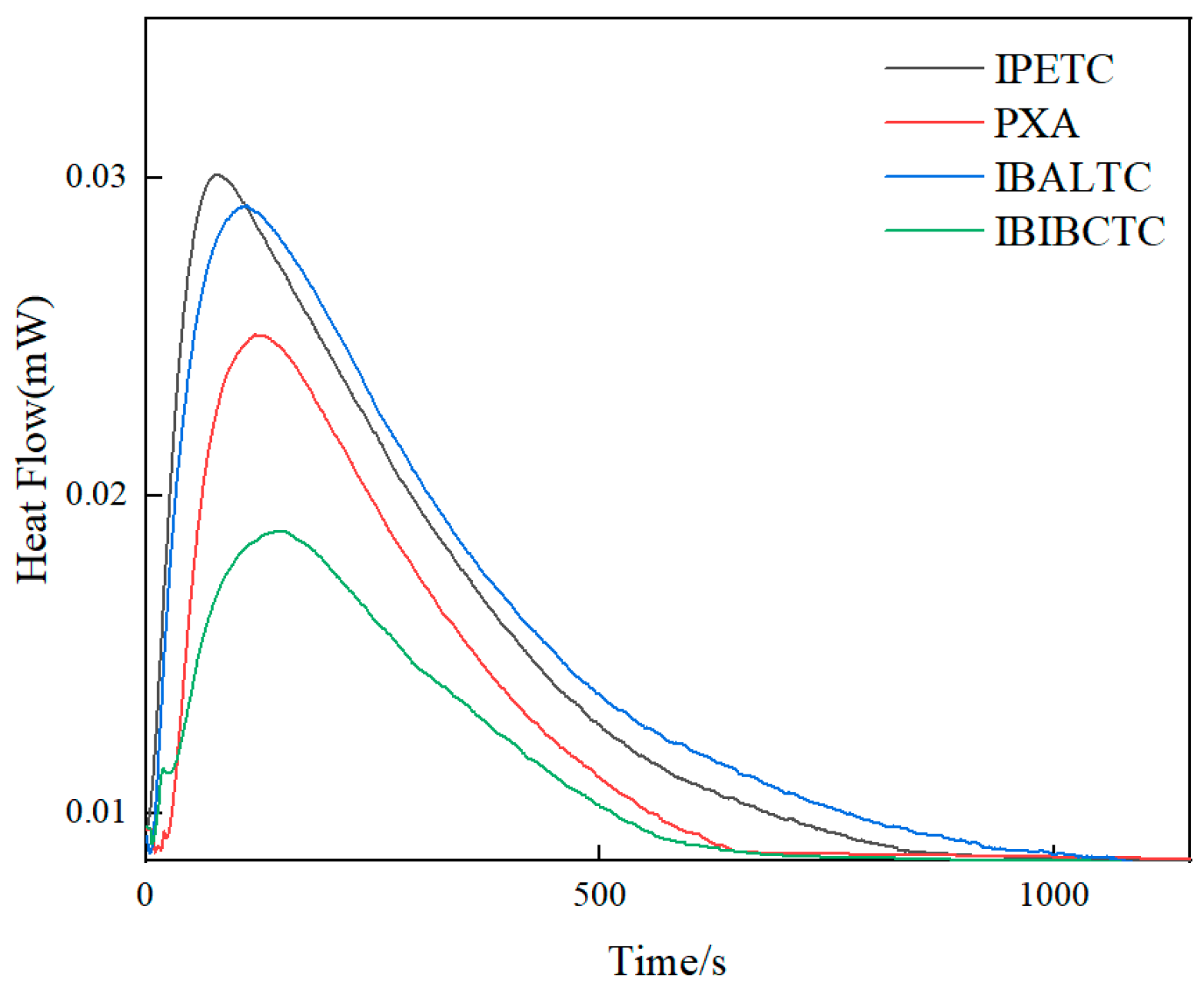
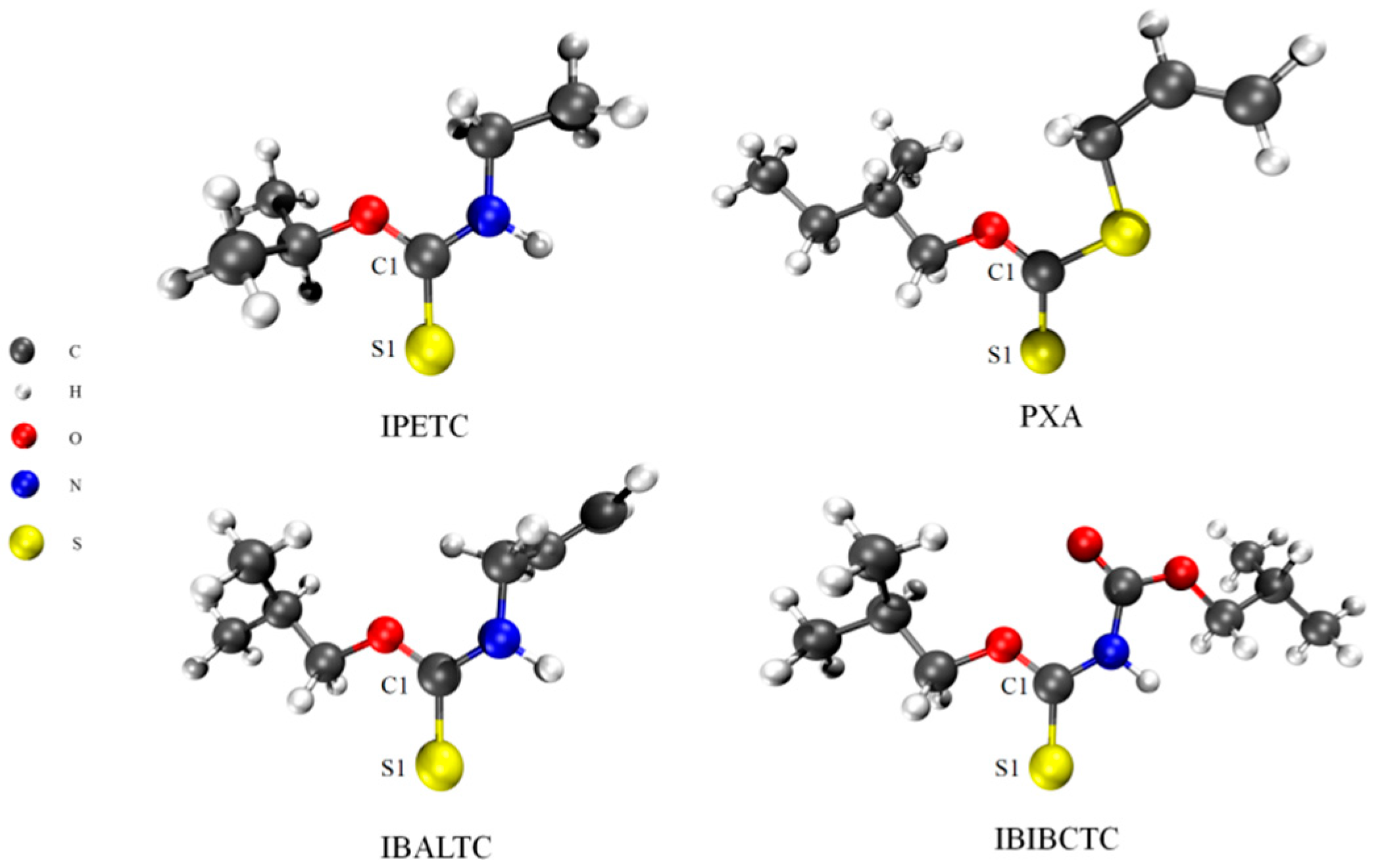
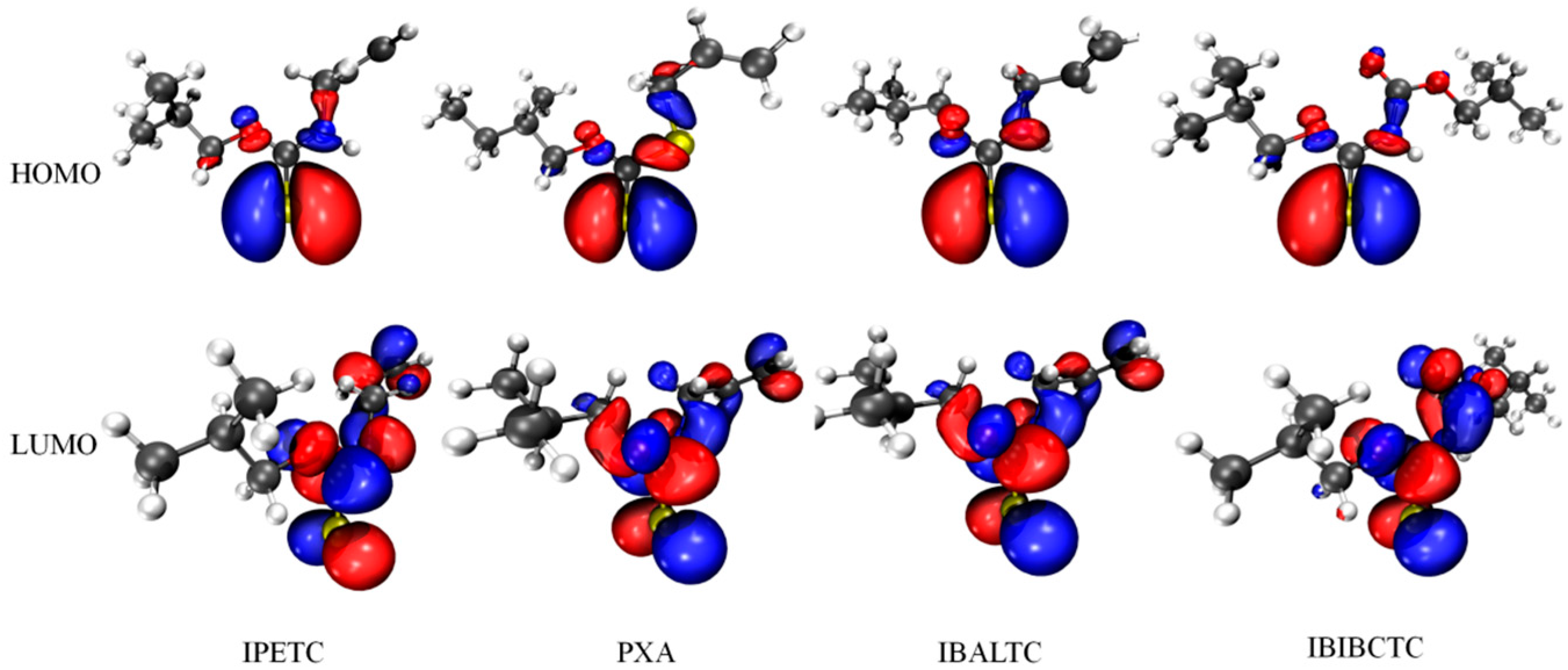
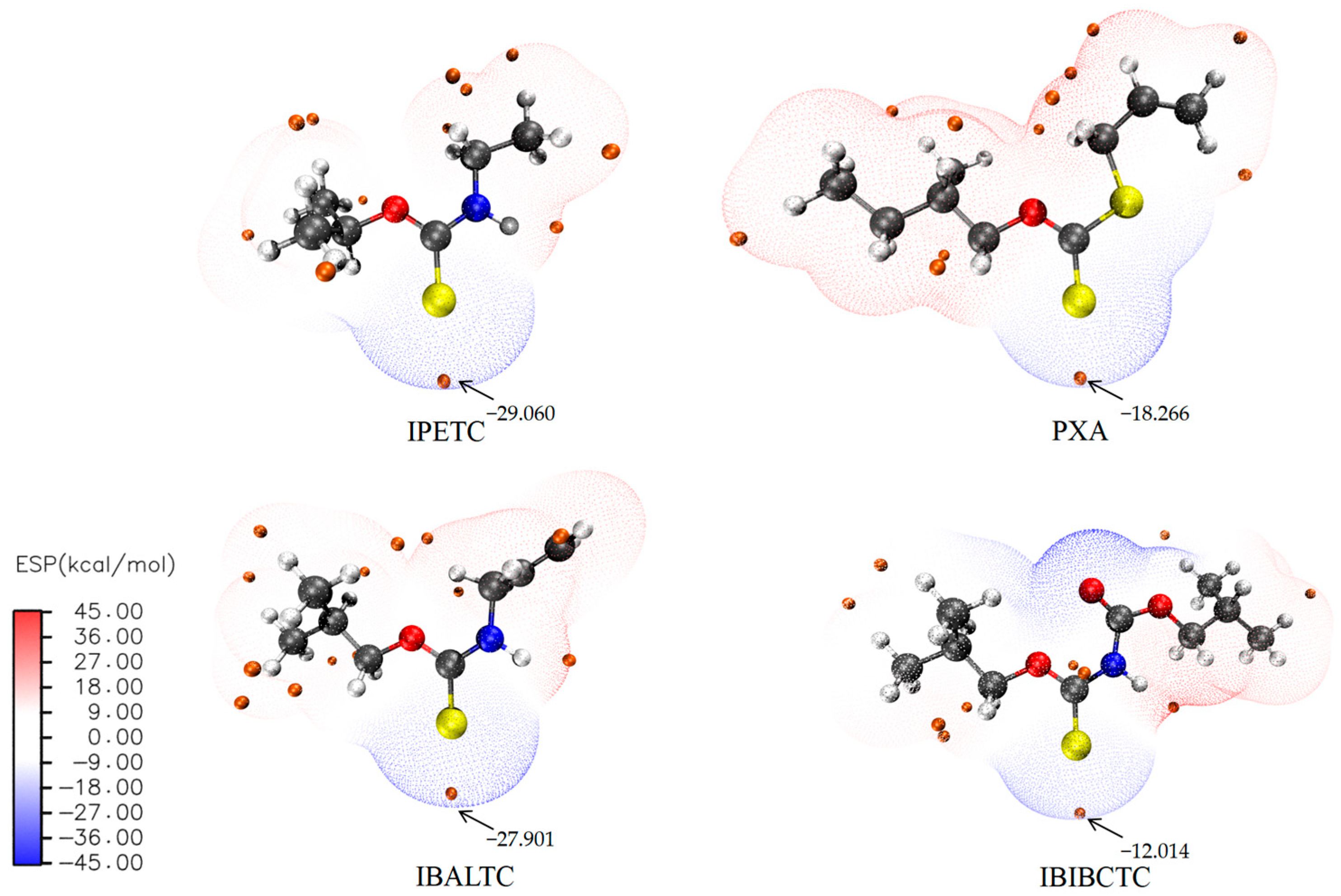


| Collector | Q (mJ) | k (×10−3 s−1) | n |
|---|---|---|---|
| IPETC | 218.12 | 3.70 | 0.86 |
| PXA | 165.08 | 2.93 | 0.64 |
| IBALTC | 349.34 | 1.66 | 0.97 |
| IBIBCTC | 132.54 | 3.45 | 0.70 |
| Collector | C1=S1 Bond Length (Å) | C1=S1 Mayer Bond Order |
|---|---|---|
| IPETC | 1.701 | 1.454 |
| PXA | 1.664 | 1.611 |
| IBALTC | 1.697 | 1.463 |
| IBIBCTC | 1.674 | 1.602 |
| Collector | HOMO and LUMO Energies (eV) | (eV) | (eV) | RESP Atomic Charges (e−) | |
|---|---|---|---|---|---|
| HOMO | LUMO | ||||
| IPETC | −6.34 | −0.51 | 1.46 | 5.11 | −0.61 |
| PXA | −6.69 | −1.65 | 1.81 | 3.97 | −0.43 |
| IBALTC | −6.37 | −0.68 | 1.49 | 4.94 | −0.58 |
| IBIBCTC | −6.74 | −1.58 | 1.86 | 4.04 | −0.46 |
| CuFeS2 | −5.62 | −4.88 | — | — | — |
| Collector | HOMO and LUMO energies (eV) | (eV) | (eV) | RESP atomic charges (e−) | |
| Collector | Polarizability of S Atom (a.u.) | First Ionization Potential of S Atom (eV) |
|---|---|---|
| IPETC | 19.55 | 6.19 |
| PXA | 18.93 | 6.42 |
| IBALTC | 20.54 | 6.08 |
| IBIBCTC | 19.42 | 6.59 |
| Collector | |||
|---|---|---|---|
| IPETC | 0.65 | 0.34 | −0.31 |
| PXA | 0.40 | 0.38 | −0.02 |
| IBALTC | 0.67 | 0.34 | −0.33 |
| IBIBCTC | 0.64 | 0.35 | −0.29 |
| Element | Cu | Fe | S |
|---|---|---|---|
| chalcopyrite | 32.15 | 32.97 | 33.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Chen, J.; Li, Y. An Experimental and Quantum Chemical Calculation Study on the Performance of Different Types of Ester Collectors. Molecules 2025, 30, 147. https://doi.org/10.3390/molecules30010147
Wu D, Chen J, Li Y. An Experimental and Quantum Chemical Calculation Study on the Performance of Different Types of Ester Collectors. Molecules. 2025; 30(1):147. https://doi.org/10.3390/molecules30010147
Chicago/Turabian StyleWu, Di, Jianhua Chen, and Yuqiong Li. 2025. "An Experimental and Quantum Chemical Calculation Study on the Performance of Different Types of Ester Collectors" Molecules 30, no. 1: 147. https://doi.org/10.3390/molecules30010147
APA StyleWu, D., Chen, J., & Li, Y. (2025). An Experimental and Quantum Chemical Calculation Study on the Performance of Different Types of Ester Collectors. Molecules, 30(1), 147. https://doi.org/10.3390/molecules30010147





